Abstract
Angiopoeitin-2 (Ang-2) antagonizes Angiopeitin-1 (Ang-1) -mediated Tie-2 signaling. Ang-1 is reported to up-regulate anti-apoptotic Survivin expression. Here, we investigated the interplay between Ang-2 and Survivin in response to oxidized low density lipoprotein (OxLDL)-induced apoptosis. We demonstrate that treatment of human aortic endothelial cells (HAEC) with 100μg/ml of OxLDL down-regulated Ang-2 expression as early as 4 hours after treatment and persisted up to 24 hours (p<0.05, n=3), but did not down-regulate Survivin until the 24 hour point. Further, treatment of HAEC with recombinant Ang-2 up-regulated Survivin expression (at Ang-2 >= 200ng/ml, p<0.05, n=3) and attenuated the OxLDL-mediated down-regulation of Survivin (p<0.05, n=3). Knockdown of Ang-2 further down-regulated Survivin expression, whereas over-expression of Survivin attenuated OxLDL-induced HAEC apoptosis (p<0.05, n=3). Hence, Ang-2 mediated Survivin expression in response to OxLDL-induced endothelial apoptosis.
Keywords: Atherosclerosis, Survivin, Angiopoietin-2 (Ang-2), inhibitor of apoptosis protein (IAP), Endothelial Cells, Oxidized Low Density Lipoprotein (OxLDL)
Introduction
Apoptosis of vascular cells is intimately related to atherosclerosis. Survivin, a Wnt/β-catenin target protein, is a member of the inhibitors of apoptosis protein (IAP) family, and plays an important role in cytokinesis and tumorigenesis [1,2]. While IAP protein blocks apoptosis via inhibition of effector caspases, oxidized low density lipoprotein (OxLDL) differentially regulates the members of IAP family [3,4]. OxLDL has been reported to decrease the expression of cellular inhibitor of apoptosis protein 1 (cIAP-1) in endothelial cells [5], and prolonged OxLDL treatment down-regulated Survivin expression in macrophages [6]. In advanced atherosclerotic lesions, Survivin expression is absent despite an increase in both x-linked inhibitor of apoptosis protein (XIAP) and cellular inhibitor of apoptosis protein 2 (cIAP2) [6]. Whether OxLDL induces apoptosis via down-regulation of Survivin expression in vascular endothelial cells remains unknown.
Angiopoeitin-1 (Ang-1) and Angiopoietin-2 (Ang-2) are antagonistic ligands that bind to Tie-2 receptors [7,8] [9]. Ang-1 is constitutively released from pericytes and smooth muscle cells, promoting endothelial cell survival via Akt/Survivin signaling pathway [10], whereas Ang-2 stored in the endothelial Weibel-Palade bodies is rapidly released upon inflammatory responses, disrupting protective Tie-2 signaling [10,11]. However, the role of Ang-2 on Survivin expression has not been elucidated.
In this study, we examined the interplay between Ang-2 and Survivin in response to OxLDL in human aortic endothelial cells (HAEC). We demonstrate that OxLDL treatment downregulated the expression of Ang-2 expression in HAEC as early as 4 hours post treatment, whereas Survivin was not down-regulated until 24 hours. Ang-2 modulated Survivin expression and over-expression of survivin attenuated OxLDL induced apoptosis. Our findings indicate that Ang-2 mediates Survivin expression in OxLDL-induced apoptosis in HAEC.
Materials and Methods
Cell Culture
Human aortic endothelial cells (HAEC) were cultured with endothelial cell growth media (Cell Application). The cells were utilized between passages 5 and 11. OxLDL was prepared as previously described [12]. HAEC were incubated with or without specific concentration of OxLDL in DMEM (Invitrogen Inc)/1% FBS (Phenix Research) for specified intervals.
Apoptotic Assay by FACS
The apoptosis of HAEC was examined by FACS analysis with PE-Annexin-V (BD Biosciences). HAEC were treated with or without OxLDL for 24 hours. The cells were then trypsinized and collected. After incubation with PE-Annexin-V, cells were re-suspended in PBS/2% paraformaldehyde, and analyzed with LSR II flow cytometer (BD Biosciences). The percentage of PE-positive cells was quantified as the degree of apoptosis. In the apoptotic assay with Survivin over-expression, percentages of PE-positive cells in GFP positive cells were also quantified.
Caspase-3 Activity Assay
HAEC were treated with or without OxLDL for 24 hours or with 10 μM of Camptothecin (CPT, positive control) for 4 hours. The cells were then lysed with passive lysis buffer (PLB, Promega). After centrifugation, the clear lysate was used for Caspase-3 activity assay (Caspase-3 colorimetric assay kit: Genscript) performed according to the manufacturer instructions. The protein concentration of the lysate was measured and the relative Caspase-3 activities were normalized to protein concentration.
Western Blot
HAEC were grown to confluence and treated with or without OxLDL for 6 hours for Ang-2 expression (Anti-Angiopoietin-2 and Anti-β-Tubulin: Millipore) or for 24 hours for Survivin expression (Ant-Survivin: Cell Signaling) in DMEM/1% FBS. Cell lysate preparation and western blot were performed as previously described [12].
Quantitative RT-PCR
The expression of Survivin and Ang-2 mRNA were quantified (qRT-PCR: Applied Biological Materials Inc). Total RNA was isolated (Bio-Rad kit), and potential genomic DNA contamination was removed with on-column DNase I digestion (R&D System). Total RNA (0.5–1 μg) was reverse transcribed (Bio-Rad's iScript cDNA synthesis kit), and qRT-PCR was performed as previously described [13]. The following primers were used for qRT-PCR: for Survivin: forward: 5′ -CCTGGCAGCCCTTTCTCAAGGACCA-3′, reverse: 5′ -CCAGCCTTCCAGCTCCTTGAAGCAG-3′; for Ang-2: forward: 5′- GACCACGAGACTTGAACTTCAG-3′, reverse: 5′- GGATGATGTGCTTGTCTTCCATAG -3′; for GAPDH: forward 5'- CCTCAAGACATCAGCAATGCCTCCT -3', reverse 5'- GGTCATGAGTCCTTCCACGATACCAA -3'. The differences in CT values versus control were used to determine the relative expression of genes of interest normalized to GAPDH.
siRNA Transfection
siRNA Transfection was performed with Lipofectamine RNAiMax (Invitrogen). HAEC were plated in 6 well plates without antibiotics on the day prior to transfection. The cells were transfected with 50 nM Ang-2 siRNA (Qiagen). Transfection media were changed to normal growth media after 5 hours of transfection. Cells were used for confirmation of gene knockdown or function assay at 48 hours after transfection.
Survivin Over-Expression
Survivin over-expression was established with transduction of Adenovirus-GFP-Survivin (Adv-Survivin). Adenovirus-GFP (Adv-GFP) was used as control. HAEC were infected with Adv-Survivin or Adv-GFP at MOI (Multiple of Infection) 100 for 24 hours. The cells were then used for expression measurement or treatment with or without OxLDL for apoptosis assay. Survivin recombinant adenovirus was kindly provided by Dr. Altieri of University of Massachusetts Medical School.
Statistical Analysis
Experiments were performed in three or more trials. Data were expressed as mean ± standard deviation (SD). For comparison between two groups, student t-test was used. For comparison among multiple values, one-way analysis of variance (ANOVA) was performed. A p value < 0.05 was considered statistically significant.
Results
OxLDL induced HAEC apoptosis
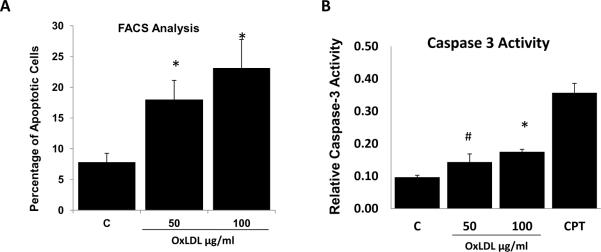
To induce HAEC apoptosis, we treated primary HAEC with high-dose OxLDL (50 and 100 μg/ml) for 24 hours. Flow cytometry analysis revealed that OxLDL at 50 and 100 μg/ml increased apoptotic (PE-Annexin V positive) cells from 7.8% (control) to 18% and 23%, respectively (n=3, p<0.01) (Fig. 1A). OxLDL also significantly induced caspase-3 activities (Control = 0.097± 0.006; at 50 μg/ml: 0.144±0.025; at 100 μg/ml: 0.175±0.008; p < 0.05, n=3) (Fig. 1B).
Fig. 1. OxLDL induced apoptosis of human aortic endothelial cells (HAEC).
HAEC were treated with OxLDL at the indicated concentration for 24 hours. (A) FACS analysis with PE-Annexin V staining revealed that OxLDL induced apoptosis. (B) Caspase-3 activities were measured as described in Methods. Camptothecin (CPT) at 10 μM for 4 hour treatment was used as positive control for apoptosis (C= control. * vs. C, p < 0.01, n=3; # vs. C, p < 0.05, n=3).
OxLDL down-regulated Survivin and Ang-2 expression
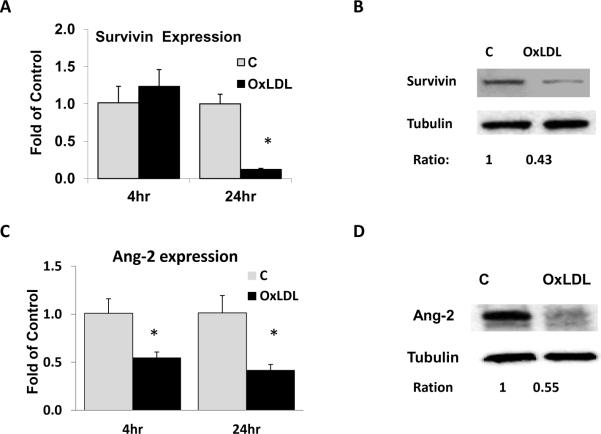
OxLDL decreased Survivin mRNA expression in a dose-dependent manner with significant effects at OxLDL ≥ 50 μg/ml (Data not shown). OxLDL at 100μg/ml down-regulated Survivin mRNA by 85% (p < 0.05, n=3) and protein by 57%, respectively (Figs. 2A & B). These trends were consistent with those of OxLDL-induced apoptosis in Figure 1.
Fig. 2. OxLDL down-regulated Survivin and Angiopoietin-2 expression.
HAEC were treated with OxLDL at 100 μg/ml for 4 hours or 24 hours. (A) Survivin and (C) Ang-2 mRNA expression was measured by quantitative RT-PCR. (B) Survivin protein expression in HAEC was measured by Western blot after 24 hour treatment. (D) Ang-2 protein expression was down-regulated 6 hours after OxLDL treatment at 100 μg/ml (C= control. * vs C, p<0.05, n=3).
OxLDL also decreased Ang-2 expression. While Survivin expression was significantly down-regulated at 24 hours (Fig. 2A), Ang-2 mRNA expression was down-regulated by 46% as early as 4 hours and 59% at 24 hours (p < 0.05, n=3) (Fig. 2C). OxLDL also down-regulated Ang-2 protein expression by 45% at 6 hours (Fig. 2D).
Survivin as a target gene of Ang-2
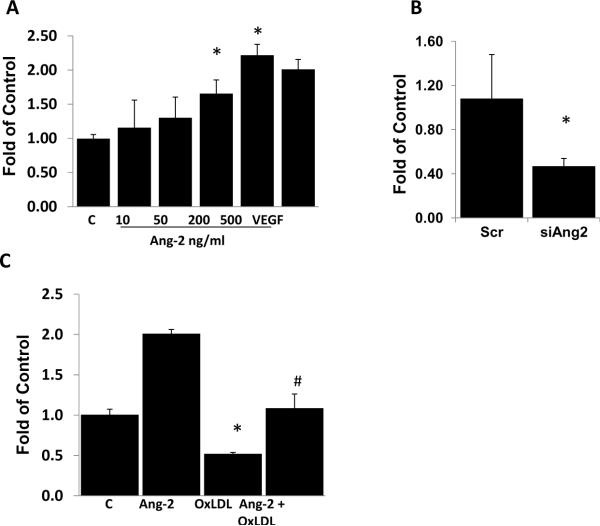
We investigated whether Ang-2 was an upstream cytokine of Survivin expression. Treatment of HAEC with recombinant Ang-2 dose-dependently increased Survivin mRNA expression (significant at Ang-2 >= 200ng/ml, p<0.05, n=3, Fig. 3A). Knockdown of Ang-2 expression by siRNA (siAng2) inhibited Ang-2 expression (data not shown) and Survivin mRNA by 59% and 53%, respectively (p < 0.05, n=3, Fig. 3B). These findings support the notion that Survivin is a target gene of Ang-2.
Fig. 3. Survivin as a target gene of Ang-2.
(A) Ang-2 up-regulated Survivin expression. HAEC were treated with different concentrations of Ang-2 for 6 hours and VEGF was used as a positive control. Survivin mRNA expression was measured by qRT-PCR. Ang-2 dose-dependently up-regulated Survivin expression. (B) Down-regulation of Ang-2 influenced Survivin expression. HAEC were transfected with 50 nM of control (Scr) or Ang-2 siRNA (siAng2) for 48 hours. Survivin expression was measured. (C) OxLDL down-regulated Survivin expression via Ang-2. HAEC were treated with 100 μg/ml of OxLDL for 24 hours in the presence or absence of 1 μg/ml of Ang-2. Ang-2 significantly attenuated OxLDL-induced down-regulation in Survivin mRNA expression (C= control. * vs. C, p < 0.05, n=3; # OxLDL vs. Ang-2 + OxLDL: p < 0.05, n=3).
Ang-2 mediated Survivin expression in response to OxLDL
To further examine the interplay between Ang-2 and Survivin, we assessed the effect of recombinant Ang-2 in OxLDL-treated HAEC. Recombinant Ang-2 significantly attenuated OxLDL-mediated down-regulation in Survivin expression (control = 1.0±0.07, Ang-2 = 2.01±0.52, OxLDL = 0.52±0.02, Ang-2 + OxLDL = 1.09±0.18; p < 0.05 for Ang-2 + OxLDL vs. OxLDL, n=3) (Fig. 3C). This finding suggests that Ang-2 is implicated in down-regulation in Survivin expression in response to OxLDL.
Survivin over-expression attenuated OxLDL-induced apoptosis
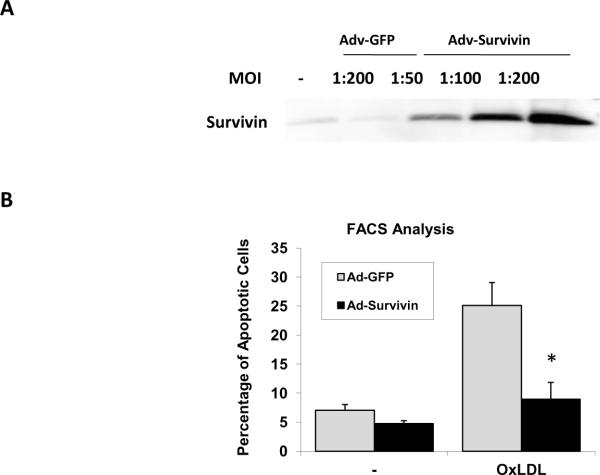
Using recombinant Survivin adenovirus (Adv-Survivin) that also expresses green fluorescent protein (GFP), we over-expressed Survivin in HAEC (Fig. 4A), and used GFP-adenovirus (Adv-GFP) as a control. Over-expression of Survivin significantly attenuated OxLDL-induced HAEC apoptosis (PE-Annexin V positive) from 25.1± 3.96% to 8.97±2.88% (p < 0.05, n=3) (Fig. 4B). Survivin further reduced background level of apoptosis (Fig. 4B). These findings corroborate that OxLDL induces apoptosis via down-regulation of Survivin expression in vascular endothelial cells.
Fig. 4. Over-expression of Survivin significantly attenuated OxLDL-induced endothelial apoptosis.
(A) HAEC were infected with recombinant Survivin adenoviruses (Adv-Survivin) or GFP adenovirus (Adv-GFP, control) for 24 hours at different MOI. Survivin expression was measured by Western blot. (B) HAEC were infected with Adv-Survivin or Adv-GFP at MOI 100 for 24 hours and the cells were treated with or without 100 μg/ml of OxLDL. Over-expression of Survivin significantly inhibited OxLDL-induced apoptosis (* OxLDL+Survivin vs. OxLDL+GFP: p < 0.05, n=3).
Discussion
This study presents the novel finding that Ang-2 expression is intimately concordant with Survivin expression. We demonstrate that OxLDL down-regulated Ang-2 as early as 4 hours post-treatment, followed by Survivin at 24 hours. Recombinant Ang-2 significantly attenuated OxLDL-mediated down-regulation in Survivin expression. Furthermore, Survivin over-expression attenuated OxLDL-induced apoptosis. In this context, our findings support the notion that Survivin is a target gene of Ang-2.
Survivin is highly expressed in breast, lung, colorectal, and prostate cancer[14]. However, Survivin expression is developmentally regulated in healthy tissues, and its expression is relatively low in the vast majority of terminally differentiated tissues [1,14,15]. Increasing evidence reveals that Survivin is implicated in both regulatory [14] and survival mechanisms [16,17]. In atherosclerosis-prone areas, the rate of endothelial cell turnover and apoptosis is significantly elevated [18]. Moran et al. reported an elevated Survivin, cIAP2, and XIAP expression in smooth muscle cells from patients with carotid stenosis[19]. The immuno-activity of Survivin expression was present in CD68 positive monocyte/macrophages in the endoluminal fatty streaks, and was also elevated in the fibrous cap of atherosclerotic lesions [19]. However, Survivin expression was down-regulated in the advanced lesions [6]. Our finding of OxLDL-mediated down-regulation of Survivin may support the absence of Survivin in the advanced lesions.
Ang-1 and Ang-2 are well-characterized ligands binding to Tie-2 receptor [20,21]. Both in vitro and animal models establish Ang-1 as the natural activator of Tie-2, and Ang-2 as the antagonist to Ang-1[9,22]. Ang-1 was reported to up-regulated Survivin expression via Akt activation. Here we report Ang-2 also stimulated Survivin expression. While Ang-2 is an antagonist to Tie-2 in the presence of Ang-1, several studies support the notion that Ang-2 may also be an agonist to Tie-2. For instance, Ang-2 was implicated as a Tie-2 activator for post-natal retinal vascular remodeling in the Ang-2 knockout mouse model [23]. Furthermore, both Ang-1 and Ang-2 were reported to induce HUVEC tube formation via Tie-2 receptor, suggesting that both Ang-1 and Ang-2 activate Tie-2 signaling in the absence of pericytes or smooth muscle cells [24].
Both Ang-1 and Ang-2 promote endothelial cell survival [11,25,26,27]. Ang-2 has protective effects in stressed endothelial cells, whereas down-regulation of Ang-2 in endothelial cells promotes apoptosis [28]. While Ang-1 is primarily expressed in pericytes and smooth muscle cells, Ang-2 is selectively expressed in endothelial cells [9,22]. Our findings suggest that Ang-2 may play an autocrine role in mediating Survivin expression. In sum, we provide a new molecular insight into the interplay between Ang-2 and Survivin in response to OxLDL-induced apoptosis.
*Highlights
OxLDL down-regulated the expression of Ang-2 and Survivin.
Survivin is a target gene of Ang-2.
Ang-2 attenuates the OxLDL-mediated down-regulation of Survivin.
Overexpression of Survivin attenuated OxLDL-induced HAEC apoptosis.
Acknowledgements
The authors are grateful to Dr. Altieri (University of Massachusetts Medical School) for providing with the GFP and Survivin-GFP recombinant adenoviruses. This project was supported by National Institutes of Health, National Heart Lung and Blood Institute (HL083015, TKH and HL091302 TKH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- [2].Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14:5000–5005. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- [3].Altieri DC. Survivin and IAP proteins in cell-death mechanisms. Biochem J. 2010;430:199–205. doi: 10.1042/BJ20100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fulda S. Targeting inhibitor of apoptosis proteins (IAPs) for cancer therapy. Anticancer Agents Med Chem. 2008;8:533–539. doi: 10.2174/187152008784533107. [DOI] [PubMed] [Google Scholar]
- [5].Chen J, Mehta JL, Haider N, Zhang X, Narula J, Li D. Role of caspases in Ox-LDL-induced apoptotic cascade in human coronary artery endothelial cells. Circ Res. 2004;94:370–376. doi: 10.1161/01.RES.0000113782.07824.BE. [DOI] [PubMed] [Google Scholar]
- [6].Blanc-Brude OP, Teissier E, Castier Y, Leseche G, Bijnens AP, Daemen M, Staels B, Mallat Z, Tedgui A. IAP survivin regulates atherosclerotic macrophage survival. Arterioscler Thromb Vasc Biol. 2007;27:901–907. doi: 10.1161/01.ATV.0000258794.57872.3f. [DOI] [PubMed] [Google Scholar]
- [7].Reiss Y. Angiopoietins. Recent Results Cancer Res. 2010;180:3–13. doi: 10.1007/978-3-540-78281-0_2. [DOI] [PubMed] [Google Scholar]
- [8].Thomas M, Augustin HG. The role of the Angiopoietins in vascular morphogenesis. Angiogenesis. 2009;12:125–137. doi: 10.1007/s10456-009-9147-3. [DOI] [PubMed] [Google Scholar]
- [9].Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- [10].Papapetropoulos A, Fulton D, Mahboubi K, Kalb RG, O'Connor DS, Li F, Altieri DC, Sessa WC. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275:9102–9105. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- [11].Kim I, Kim HG, So JN, Kim JH, Kwak HJ, Koh GY. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3'-Kinase/Akt signal transduction pathway. Circ Res. 2000;86:24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- [12].Takabe W, Li R, Ai L, Yu F, Berliner JA, Hsiai TK. Oxidized low-density lipoprotein-activated c-Jun NH2-terminal kinase regulates manganese superoxide dismutase ubiquitination: implication for mitochondrial redox status and apoptosis. Arterioscler Thromb Vasc Biol. 2010;30:436–441. doi: 10.1161/ATVBAHA.109.202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li R, Ning Z, Cui J, Khalsa B, Ai L, Takabe W, Beebe T, Majumdar R, Sioutas C, Hsiai T. Ultrafine particles from diesel engines induce vascular oxidative stress via JNK activation. Free Radic Biol Med. 2009;46:775–782. doi: 10.1016/j.freeradbiomed.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fukuda S, Pelus LM. Survivin, a cancer target with an emerging role in normal adult tissues. Mol Cancer Ther. 2006;5:1087–1098. doi: 10.1158/1535-7163.MCT-05-0375. [DOI] [PubMed] [Google Scholar]
- [15].Blanc-Brude OP, Mesri M, Wall NR, Plescia J, Dohi T, Altieri DC. Therapeutic targeting of the survivin pathway in cancer: initiation of mitochondrial apoptosis and suppression of tumor-associated angiogenesis. Clin Cancer Res. 2003;9:2683–2692. [PubMed] [Google Scholar]
- [16].Mesri M, Morales-Ruiz M, Ackermann EJ, Bennett CF, Pober JS, Sessa WC, Altieri DC. Suppression of vascular endothelial growth factor-mediated endothelial cell protection by survivin targeting. Am J Pathol. 2001;158:1757–1765. doi: 10.1016/S0002-9440(10)64131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zwerts F, Lupu F, De Vriese A, Pollefeyt S, Moons L, Altura RA, Jiang Y, Maxwell PH, Hill P, Oh H, Rieker C, Collen D, Conway SJ, Conway EM. Lack of endothelial cell survivin causes embryonic defects in angiogenesis, cardiogenesis, and neural tube closure. Blood. 2007;109:4742–4752. doi: 10.1182/blood-2006-06-028068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xu Q. Disturbed flow-enhanced endothelial turnover in atherosclerosis. Trends Cardiovasc Med. 2009;19:191–195. doi: 10.1016/j.tcm.2009.12.002. [DOI] [PubMed] [Google Scholar]
- [19].Moran EP, Agrawal DK. Increased expression of inhibitor of apoptosis proteins in atherosclerotic plaques of symptomatic patients with carotid stenosis. Exp Mol Pathol. 2007;83:11–16. doi: 10.1016/j.yexmp.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Thurston G. Role of Angiopoietins and Tie receptor tyrosine kinases in angiogenesis and lymphangiogenesis. Cell Tissue Res. 2003;314:61–68. doi: 10.1007/s00441-003-0749-6. [DOI] [PubMed] [Google Scholar]
- [21].Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- [22].Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- [23].Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- [24].Teichert-Kuliszewska K, Maisonpierre PC, Jones N, Campbell AI, Master Z, Bendeck MP, Alitalo K, Dumont DJ, Yancopoulos GD, Stewart DJ. Biological action of angiopoietin-2 in a fibrin matrix model of angiogenesis is associated with activation of Tie2. Cardiovasc Res. 2001;49:659–670. doi: 10.1016/s0008-6363(00)00231-5. [DOI] [PubMed] [Google Scholar]
- [25].Hayes AJ, Huang WQ, Mallah J, Yang D, Lippman ME, Li LY. Angiopoietin-1 and its receptor Tie-2 participate in the regulation of capillary-like tubule formation and survival of endothelial cells. Microvasc Res. 1999;58:224–237. doi: 10.1006/mvre.1999.2179. [DOI] [PubMed] [Google Scholar]
- [26].Kwak HJ, So JN, Lee SJ, Kim I, Koh GY. Angiopoietin-1 is an apoptosis survival factor for endothelial cells. FEBS Lett. 1999;448:249–253. doi: 10.1016/s0014-5793(99)00378-6. [DOI] [PubMed] [Google Scholar]
- [27].Kim I, Kim JH, Moon SO, Kwak HJ, Kim NG, Koh GY. Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3'-kinase/Akt signal transduction pathway. Oncogene. 2000;19:4549–4552. doi: 10.1038/sj.onc.1203800. [DOI] [PubMed] [Google Scholar]
- [28].Daly C, Pasnikowski E, Burova E, Wong V, Aldrich TH, Griffiths J, Ioffe E, Daly TJ, Fandl JP, Papadopoulos N, McDonald DM, Thurston G, Yancopoulos GD, Rudge JS. Angiopoietin-2 functions as an autocrine protective factor in stressed endothelial cells. Proc Natl Acad Sci U S A. 2006;103:15491–15496. doi: 10.1073/pnas.0607538103. [DOI] [PMC free article] [PubMed] [Google Scholar]