Abstract
Background
The prostate component of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial was undertaken to determine whether there is a reduction in prostate cancer mortality from screening using serum prostate-specific antigen (PSA) testing and digital rectal examination (DRE). Mortality after 7–10 years of follow-up has been reported previously. We report extended follow-up to 13 years after the trial.
Methods
A total of 76 685 men, aged 55–74 years, were enrolled at 10 screening centers between November 1993 and July 2001 and randomly assigned to the intervention (organized screening of annual PSA testing for 6 years and annual DRE for 4 years; 38 340 men) and control (usual care, which sometimes included opportunistic screening; 38 345 men) arms. Screening was completed in October 2006. All incident prostate cancers and deaths from prostate cancer through 13 years of follow-up or through December 31, 2009, were ascertained. Relative risks (RRs) were estimated as the ratio of observed rates in the intervention and control arms, and 95% confidence intervals (CIs) were calculated assuming a Poisson distribution for the number of events. Poisson regression modeling was used to examine the interactions with respect to prostate cancer mortality between trial arm and age, comorbidity status, and pretrial PSA testing. All statistical tests were two-sided.
Results
Approximately 92% of the study participants were followed to 10 years and 57% to 13 years. At 13 years, 4250 participants had been diagnosed with prostate cancer in the intervention arm compared with 3815 in the control arm. Cumulative incidence rates for prostate cancer in the intervention and control arms were 108.4 and 97.1 per 10 000 person-years, respectively, resulting in a relative increase of 12% in the intervention arm (RR = 1.12, 95% CI = 1.07 to 1.17). After 13 years of follow-up, the cumulative mortality rates from prostate cancer in the intervention and control arms were 3.7 and 3.4 deaths per 10 000 person-years, respectively, resulting in a non-statistically significant difference between the two arms (RR = 1.09, 95% CI = 0.87 to 1.36). No statistically significant interactions with respect to prostate cancer mortality were observed between trial arm and age (Pinteraction = .81), pretrial PSA testing (Pinteraction = .52), and comorbidity (Pinteraction = .68).
Conclusions
After 13 years of follow-up, there was no evidence of a mortality benefit for organized annual screening in the PLCO trial compared with opportunistic screening, which forms part of usual care, and there was no apparent interaction with age, baseline comorbidity, or pretrial PSA testing.
CONTEXT AND CAVEATS
Prior knowledge
The previous report from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening trial showed that there was no mortality benefit of prostate cancer screening after up to 7–10 years of follow-up.
Study design
Men were randomly assigned to the screening (intervention) arm, where annual serum prostate-specific antigen testing was done for 6 years and annual digital rectal examination for 4 years, and usual care (control) arm, where men sometimes underwent opportunistic screening. In this follow-up report, incident prostate cancers and deaths from prostate cancer were followed through 13 years, and cumulative incidence and mortality rates were calculated.
Contribution
Although a 12% relative increase in the incidence rates of prostate cancer was observed in the intervention arm compared with the control arm, there was no difference in mortality between the two arms.
Implication
Prostate-specific antigen and digital rectal examination screening for prostate cancer has no effect on mortality after 13 years of follow-up.
Limitation
Random errors in death attribution and bias cannot be ruled out.
From the Editors
The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial is a multicenter, randomized two-arm trial designed to evaluate the effect of screening for prostate, lung, colorectal, and ovarian cancer on disease-specific mortality. Randomization began in November 1993 and ended in June 2001, with 154 901 men and women enrolled. The study design has been previously described (1). All participants signed informed consent documents approved by both the National Cancer Institute and local institutional review boards. Men in the intervention arm underwent testing for serum prostate-specific antigen (PSA) and digital rectal examination (DRE) to screen for prostate cancer. The PLCO trial enrolled participants aged 55–74 years who reported no previous personal history of prostate, lung, colorectal, or ovarian cancer. Criteria for exclusion included 1) current treatment for cancer except non-melanoma skin cancer; 2) previous surgical removal of the entire prostate, one lung, or the entire colon; 3) participation in another cancer screening or primary prevention study; and 4) use of finasteride in the previous 6 months. Beginning in April 1995, the PLCO trial also excluded men reporting more than one PSA blood test in the previous 3 years and men reporting any lower gastrointestinal diagnostic procedure in the previous 3 years.
In our first report on the results of screening for prostate cancer in the PLCO trial (1), we presented findings based on the data that had accrued to 10 years. However, in the first report from the European Randomized Trial of Screening for Prostate Cancer (ERSPC), follow-up was extended to 14 years; the findings in the last 2 years of follow-up coming only from Belgium and Sweden that enrolled subjects from 1991 (2).
Our study (1) was criticized for having a relatively short follow-up (3). At that time, vital status was known for 98% of men through 7 years and for 67% of men through 10 years. We now present the results available through 13 years of follow-up. We also address the issue of whether the efficacy of PSA and DRE screening is affected by the presence or absence of substantial comorbidity at baseline, as suggested by Crawford et al. (4). Because compliance with screening in the intervention arm and the extent of opportunistic PSA screening in the usual care arm (contamination) were fully reported previously (1), we shall not present the data again in this study.
Methods
Study Subjects and Screening Methods
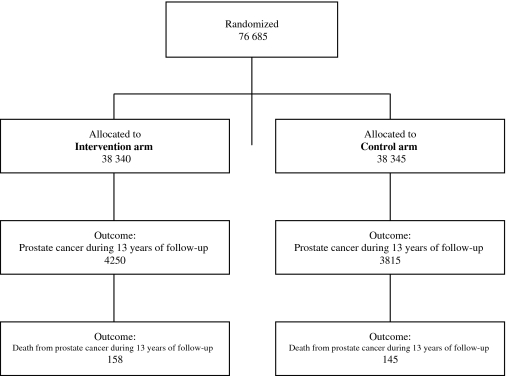
We have previously described the methods of recruitment for the PLCO trial (ClinicalTrials.gov identifier: NCT00002540), randomization, follow-up, and determination of primary and secondary endpoints (1). The diagram for the participant flow in the trial is presented as Figure 1. In brief, 76 685 men aged 55–74 years were randomly assigned to the intervention arm (38 340 subjects) and to the control (usual care) arm (38 345 subjects) at 10 screening centers (in Birmingham, AL; Denver, CO; Detroit, MI; Honolulu, HI; Marshfield, WI; Minneapolis, MN; Pittsburgh, PA; Salt Lake City, UT; St Louis, MO; and Washington, DC) between 1993 and 2001. Each center obtained annual approval from its institutional review board to carry out the study, and all participants provided written informed consent. Men who were randomly assigned to the intervention arm were offered screening with annual PSA tests for 6 years and DRE for 4 years. Screening was completed in October 2006. A positive test was defined as a PSA value greater than 4 ng/mL or a suspicious DRE. Participants and health-care providers received the results; they decided upon the method by which abnormal screening test results were evaluated. Usual care sometimes included opportunistic screening when a test was requested by a participant or recommended by a doctor. All diagnosed cancers, deaths, and causes of death were ascertained by annual follow-up questionnaire and periodic linkage to the National Death Index. Follow-up was through December 31, 2009, or to 13 years from trial entry. Clinical stage was determined from the clinical assessment of the extent of tumor involvement by using the TNM staging system. Tumor stage was categorized according to the fifth edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (5). Gleason grade was determined using the biopsy Gleason score (range 2–10); high-grade cancer was defined as a Gleason score of 8–10, and non–high-grade cancer as a Gleason score of 2–7. The underlying cause of death was determined in a uniform and unbiased manner from the death certificate and relevant medical records, as has been described in detail previously (6). Subjects completed a baseline questionnaire near the time of enrollment, which inquired about demographics, medical history, and past screening practices.
Figure 1.
Flow diagram for male participants in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial.
Assessment of Comorbidity
To assess whether comorbidity status influenced the mortality effect of screening (ie, whether there was an interaction of comorbidity with trial arm), we used the baseline questionnaire responses to calculate a modified Charlson comorbidity score (0 = no comorbidity, ≥1 = one or more comorbid conditions) (7). Because of the scope of the medical history section of the baseline questionnaire, a complete Charlson score could not be determined. The modified Charlson score contained the following conditions found in the Charlson score: myocardial infarction, stroke, diabetes, cancer, pulmonary disease (bronchitis and/or emphysema), and liver disease (cirrhosis and/or hepatitis); not included in the modified score were congestive heart failure, peripheral vascular disease, connective tissue disease, hemiplegia, HIV, renal disease, ulcer disease, and dementia. The modified score is expected to identify the great majority of PLCO subjects with a positive (true) Charlson score at baseline; it has been used previously in PLCO (8). We also examined whether there was an interaction of trial arm with age (55–64 vs 65–74 years) and with use of PSA tests before entering the PLCO trial.
Statistical Analysis
The primary analysis was an intention-to-screen comparison of prostate cancer–specific mortality rates between the two trial arms. Comparison of prostate cancer–specific incidence rates was a secondary analysis. Event rates were defined as the ratio of the number of events (deaths or diagnoses) in a given time period to the person-years at risk for the event. Person-years were measured from randomization to the date of death or censoring (whichever came first) for death rates, and to the date of diagnosis, death or date of censoring (whichever came first) for incidence rates. Relative risks (RRs) were estimated as rate ratios and were derived as the ratio of event rates in the two arms. The 95% confidence intervals (CIs) for rate ratios were calculated with the use of asymptotic methods, assuming a normal distribution for the logarithm of the rate ratio and a Poisson distribution for the number of events (9). The statistical significance of interactions between trial arm and covariates was assessed using Poisson regression. Specifically, the P value for the interaction was derived from the likelihood ratio of a saturated (four-parameter) model vs the linear model with baseline, trial arm, and covariate effect. Analyses were performed using SAS software (SAS Institute, Cary, NC). All P values presented are two-sided, and those less than .05 were considered statistically significant.
Results
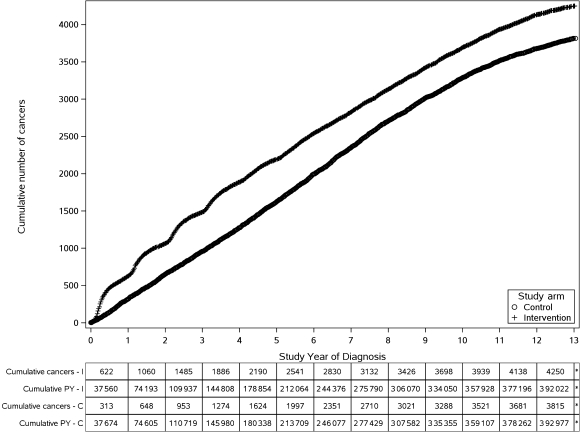
As of December 31, 2009 (the cutoff date for this analysis), the vital status of 92% of the trial participants was known at 10 years and of 57% of the participants at 13 years. The cumulative number of prostate cancers in the intervention and control arms from year 1 to year 13 are shown in Figure 2. At 13 years, 4250 of the 38 340 participants had been diagnosed with prostate cancer in the intervention arm and 3815 of the 38 345 participants were diagnosed in the control arm. The cumulative incidence rates for prostate cancer in the intervention and control arms were 108.4 and 97.1 per 10 000 person-years, respectively, resulting in a statistically significant 12% relative increase in the intervention arm (RR = 1.12, 95% CI = 1.07 to 1.17). A total of 401 participants were diagnosed with high-grade (Gleason score 8–10) prostate cancer in the intervention arm compared with 454 participants in the control arm (not shown in the figure), showing a non-statistically significant decrease in the incidence of high-grade prostate cancer in the intervention arm (RR = 0.89, 95% CI = 0.77 to 1.01).
Figure 2.
Cumulative number of prostate cancers in the intervention and control arms from year 1 to year 13. C = control arm; I = intervention arm; PY = person-years.
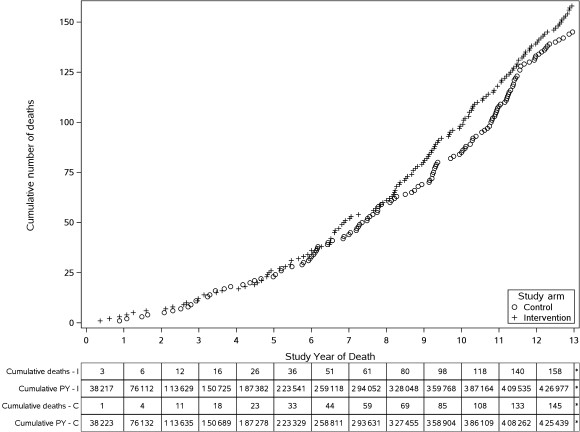
The cumulative number of deaths from prostate cancer in the intervention and control arms from year 1 to year 13 are shown in Figure 3. At 13 years, a total of 158 deaths occurred in the intervention arm compared with 145 deaths in the control arm. The cumulative mortality rates from prostate cancer were 3.7 and 3.4 deaths per 10 000 person-years, respectively, resulting in a non-statistically significant difference between the two arms (RR = 1.09, 95% CI = 0.87 to 1.36). The difference in number of deaths from all causes other than prostate, lung, and colorectal cancers between the two arms was of borderline statistical significance; 5783 in the intervention arm compared with 5982 in the control arm (RR = 0.96, 95% CI = 0.93 to 1.00). Among these deaths, intervention and control arms showed 23% and 22% deaths, respectively, from non-PLCO cancers, and 21% and 19% deaths, respectively, from ischemic heart disease.
Figure 3.
Cumulative deaths from prostate cancer in the intervention and control arms from year 1 to year 13. C = control arm; I = intervention arm; PY = person-years.
We also examined mortality rates per 10 000 person-years and relative risks of prostate cancer mortality by age, comorbidity status, and pretrial PSA testing (Table 1). The relative risk of mortality in intervention vs control arm for men aged 55–64 years was 1.19 (95% CI = 0.83 to 1.72) compared with a relative risk of 1.02 (95% CI = 0.77 to 1.37) for men aged 65–74 years (Pinteraction = .81). The relative risk among those with no comorbidities (modified Charlson score = 0) was 1.00 (95% CI = 0.76 to 1.31) compared with a relative risk of 1.11 (95% CI = 0.72 to 1.71) for those with comorbidities (modified Charlson score = ≥1) (Pinteraction = .68). Finally, the relative risk for no pretrial PSA testing was 1.18 (95% CI = 0.85 to 1.64) compared with a relative risk of 1.02 (95% CI = 0.71 to 1.46) for any previous pretrial PSA testing (Pinteraction = .52). Thus, there were no statistically significant interactions between trial arm and any of the above covariates, which means the relative risks of prostate cancer–specific mortality were similar in the intervention and control arms between the subgroups defined by age or comorbidity score or pretrial PSA testing.
Table 1.
Relative risk of mortality by age, comorbidity status, and number of PSA tests before enrollment in the PLCO trial*
| Covariate | Intervention arm | Control arm | RR (95% CI) | Pinteraction† | ||
| No. of deaths (person-years) | Deaths per 10 000 person-years | No. of deaths (person-years) | Deaths per 10 000 person-years | |||
| Age, y | ||||||
| 55–64 | 65 (276 170) | 2.35 | 54 (274 314) | 1.97 | 1.19 (0.83 to 1.72) | |
| 65–74 | 93 (150 807) | 6.17 | 91 (151 125) | 6.02 | 1.02 (0.77 to 1.37) | .81 |
| Modified Charlson score‡ | ||||||
| 0 | 104 (299 994) | 3.47 | 100 (286 992) | 3.48 | 1.00 (0.76 to 1.31) | |
| ≥1 | 44 (116 404) | 3.78 | 39 (114 366) | 3.41 | 1.11 (0.72 to 1.71) | .68 |
| No. of pretrial PSA tests§ | ||||||
| 0 | 80 (190 214) | 4.21 | 64 (179 367) | 3.57 | 1.18 (0.85 to 1.64) | |
| ≥1 | 60 (190 924) | 3.14 | 59 (191 082) | 3.09 | 1.02 (0.71 to 1.46) | .52 |
CI = confidence interval; PLCO = Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; PSA = prostate-specific antigen; RR = relative risk.
P values were calculated a using two-sided Wald test in a Poisson regression model.
Score 0 means no comorbid conditions. Score at least 1 means at least one comorbid condition. Sixteen subjects dying from prostate cancer (10 in the intervention arm and six in the control arm) had no comorbidity information available and were excluded from the analysis.
PSA test in the 3-year period before entry into the PLCO trial.
The previously reported treatment data (1) for all clinical cancer stages of prostate cancer (stages I–IV) has also now been updated for all incident prostate cancers through 13 years (Table 2). Within each stage, the distribution of primary treatment of the prostate cancers in each trial arm is displayed. For each stage, the treatment distribution was very similar across trial arms.
Table 2.
Primary treatment of prostate cancers diagnosed through 13 years by clinical stage and trial arm in the PLCO trial
| Clinical stage† | Trial arm | All prostate cancers |
|||||||
| No. | Primary treatment* |
||||||||
| Prostatectomy | Radiation | Radiation and hormone | Hormone | Other ablative with curative intent | No known curative intent | Not available | |||
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |||
| Stage I | Intervention | 19 | 3 (15.8) | 3 (15.8) | — | — | — | 13 (68.4) | — |
| Control | 17 | 2 (11.8) | 3 (17.6) | — | — | — | 12 (70.6) | — | |
| Stage II (T1 or T1A) | Intervention | 49 | 7 (14.3) | 2 (4.1) | 3 (6.1) | 1 (2.0) | — | 35 (71.4) | 1 (2.0) |
| Control | 50 | 10 (20.0) | 4 (8.0) | 1 (2.0) | 1 (2.0) | — | 34 (68.0) | — | |
| Stage II (T1B or T1C) | Intervention | 2530 | 1022 (40.4) | 584 (23.1) | 461 (18.2) | 134 (5.3) | 28 (1.1) | 282 (11.1) | 19 (0.8) |
| Control | 2265 | 859 (37.9) | 519 (22.9) | 454 (20.0) | 133 (5.9) | 36 (1.6) | 249 (11.0) | 15 (0.7) | |
| Stage II (T2, T2A, T2B, or T2C) | Intervention | 1477 | 646 (43.7) | 296 (20.0) | 275 (18.6) | 86 (5.8) | 23 (1.6) | 149 (10.1) | 2 (0.1) |
| Control | 1269 | 484 (38.1) | 257 (20.3) | 301 (23.7) | 108 (8.5) | 24 (1.9) | 92 (7.2) | 3 (0.2) | |
| Stage III | Intervention | 58 | 5 (8.6) | 13 (22.4) | 28 (48.3) | 8 (13.8) | 2 (3.4) | 2 (3.4) | — |
| Control | 65 | 14 (21.5) | 10 (15.4) | 34 (52.3) | 7 (10.8) | — | — | — | |
| Stage IV | Intervention | 96 | 1 (1.0) | 5 (5.2) | 14 (14.6) | 71 (74.0) | — | 4 (4.2) | 1 (1.0) |
| Control | 111 | 1 (0.9) | 1 (0.9) | 24 (21.6) | 77 (69.4) | — | 8 (7.2) | — | |
| Not available | Intervention | 21 | 16 (76.2) | — | — | 2 (9.5) | — | 2 (9.5) | 1 (4.8) |
| Control | 38 | 26 (68.4) | 1 (2.6) | — | 3 (7.9) | — | 8 (21.1) | — | |
| Total | Intervention | 4250 | 1700 (40.0) | 903 (21.2) | 781 (18.4) | 302 (7.1) | 53 (1.2) | 487 (11.5) | 24 (0.6) |
| Control | 3815 | 1396 (36.6) | 795 (20.8) | 814 (21.3) | 329 (8.6) | 60 (1.6) | 403 (10.6) | 18 (0.5) | |
| Total | 8065 | 3096 (38.4) | 1698 (21.1) | 1595 (19.8) | 631 (7.8) | 113 (1.4) | 890 (11.0) | 42 (0.5) | |
“Prostatectomy” consists of radical prostatectomies. “Radiation” and “Hormone” consist of unspecified radiation and hormonal treatments (data were not collected on the specific type of radiation and hormonal treatment administered). “Other ablative with curative intent” includes cryosurgery and radiofrequency ablation. “No known curative intent” includes no treatment, transurethral resection of prostate, subtotal prostatectomy, laser prostatectomy, and other treatments. “Not available” indicates that medical record abstraction could not be completed. — = no entries in those cells, or zeros.
Staging is based on the fifth edition of the American Joint Committee on Cancer (AJCC) Staging Manual (5).
Discussion
This article updates with more person-years of follow-up our previously reported finding of no reduction in mortality from prostate cancer in the intervention arm compared with the control arm to 10 years, with no indication of a reduction in prostate cancer mortality to 13 years. We observed a statistically significant 12% relative increase in the incidence of prostate cancer and a non-statistically significant decrease in the incidence of high-grade prostate cancer in the intervention arm. There was no apparent prostate cancer mortality interaction of trial arm with age, baseline comorbidity (defined by a modified Charlson index), and pretrial PSA testing.
In contrast, the ERSPC trial reported a 20% reduction in prostate cancer mortality in their core age group (men aged 55–69 years) that largely occurred after 10 years of follow-up, although a non-statistically significant reduction of 15% was noted in all men (aged 50–74 years) randomly assigned (2). There were major differences between the PLCO and ERSPC trials. One relates to the extent of opportunistic PSA screening that occurred in the control arms. In the PLCO trial, 45% of those randomly assigned had at least one PSA test in the 3 years preceding randomization, and PSA screening in the control arm was estimated to be 52% during the time period of the last round of screening in the intervention arm (1). In a more detailed analysis, the intensity of PSA screening in the control arm was estimated to be approximately half of that in the intervention arm (10). Nevertheless, the level of screening in the intervention arm was substantially greater than that in the control arm throughout the trial screening period. In the ERSPC trial, the degree of contamination was probably less, although details have only been reported from one center (11).
A possible reason for the difference in the mortality results between the PLCO and ERSPC trials is differences in the application of treatment for prostate cancer. In a recent publication from the ERSPC trial investigators, it was reported that men in the screened arm who were diagnosed with prostate cancer were more likely to be treated at an academic center than men who were diagnosed in the control arm (12). The difference in place of treatment favored the screening arm to the extent that outcomes after major surgery are better in major referral centers than in community hospitals. Furthermore, trial arm was associated with treatment choice, especially in men with high-risk localized prostate cancer. Thus, a control arm subject with high-risk prostate cancer was more likely than a screened arm subject to receive radiotherapy (odds ratio [OR] = 1.43, 95% CI = 1.01 to 2.05), expectant management (OR = 2.92, 95% CI = 1.33 to 6.42), or hormonal treatment (OR = 1.77, 95% CI = 1.07 to 2.94) instead of radical prostatectomy (12). These differences are potentially important given a recent report that radical prostatectomy is associated with improved mortality in young men with aggressive cancers (13). In contrast, the policy in the PLCO trial not to mandate specific therapies after screen detection resulted in substantial similarity in initial treatment by stage between the two arms (Table 2). A planned combined evaluation of the PLCO and ERSPC trials, using mathematical models specifically developed with regard to prostate cancer (14–16), may help to resolve some of the current uncertainties.
Improvements in prostate cancer treatment are probably at least in part responsible for declining prostate cancer mortality rates (17). Even if life is only prolonged by therapy, the opportunities for competing causes of death increase, especially among older men.
A statistically significant interaction of trial arm by comorbidity status has recently been reported by Crawford et al. (4) using the PLCO prostate mortality data through 10 years. The reported hazard ratio (intervention vs control arms) was 0.56 (22 deaths in the intervention arm vs 38 deaths in the control arm) among those with no comorbidity vs 1.43 (62 deaths in the intervention arm vs 42 deaths in the control arm) among those with a comorbidity. The primary explanation for the difference in analysis between Crawford et al. (4) and this study lies in the definitions of comorbidity used. Crawford et al. (4) used an expanded definition of comorbidity, which included, in addition to the Charlson conditions, obesity and hypertension (plus a few other comorbid conditions). Hypertension was included as a comorbid condition whether or not it was well controlled. This increased the proportion of subjects classified as having a comorbidity to 64% (4), from the 30% based on the modified Charlson index used in this study. With the 13-year data, the relative risks of mortality in the intervention arm vs control arm were 0.73 and 1.26 for those without and with comorbidity as defined in Crawford et al. (4), ratios both closer to unity than their reported values of 0.56 and 1.43, although the interaction was still statistically significant (P = .03). Furthermore, to validate our observations, we used only the additional deaths and follow-up time through 13 years that were not included in the analysis by Crawford et al. (4). A total of 123 additional prostate cancer deaths were available for this analysis since the 164 deaths reported in the original analysis (4). We found no evidence of interaction between screening arm and comorbidity status because the prostate cancer mortality relative risks comparing trial arms were very similar (RR = 0.99 in the no comorbidity group and RR = 1.06 in the comorbidity group).
In a more detailed exploration of the 13-year data, the highest relative risk of 1.40 was observed for those men with comorbidity according to the definition by Crawford et al. (4) but with a modified Charlson score of 0. This compares to the relative risk of 0.73 for men with no comorbidity according to the definition by Crawford et al. and a relative risk of 1.11 for men with comorbidity according to the definitions of both Crawford et al. and Charlson. Combining the rates from which the 1.40 and the 0.73 rate ratios for the Charlson scores were calculated, 0 men gives the observed ratio of 1.0 for these men. In any case, the biological plausibility of the interaction reported by Crawford et al. (4) seems questionable, because the cohort was relatively healthy to start with (18) and because those men in the 1.40 rate ratio group primarily reported obesity and/or hypertension, which would seem to convey minimal extra risks associated with treatment and minimal differences in treatment options. Thus, the interaction between screening effect and baseline comorbidity is sensitive to the definition of comorbidity. Further, Bach and Vickers (19) concluded that the data do not support the notion that an elevated degree of comorbidity attenuates the benefit of PSA screening in the PLCO study. They advised caution in the interpretation of the analysis of Crawford et al. (4).
A report of follow-up through 14 years of the Goteborg component of ERSPC included findings from some subjects who were not part of the ERSPC analysis (20). Comparing the earlier ERSPC report (2) with this report, it seems that 60% of the Goteborg cohort was included in the core age group (55–69 years) of ERSPC. Of the 122 deaths from prostate cancer reported in the Goteborg trial, 109 (89%) occurred in those aged 55–69 years at entry. Although the extent of the overlap in deaths is unclear, it seems reasonable to assume that most or all of these 109 were included in the core group analysis of the ERSPC. Indeed, the overall ERSPC result without the Goteborg (Swedish) component did not quite reach statistical significance (RR = 0.84, 95% CI = 0.70 to 1.01) (2). Thus, we conclude that the major finding of the Goteborg study (20) concerning reduction in prostate cancer mortality from screening seems largely derived from previously reported data from the ERSPC trial. Furthermore, as the control group in the Goteborg trial was followed passively, it is possible that differences in treatment had an impact on the reported results.
This study has certain limitations. For example, the borderline statistically significant lower all-cause (excluding PLCO cancers) mortality in the intervention arm compared with the control arm raises the question as to whether a reduction in prostate cancer mortality in the present analysis has somehow been masked by problems in death attribution, the sticking diagnosis effect (21), even though a death review process has been in operation throughout the trial (6). There is no single cause of death that could account for the difference in all-cause mortality. The deaths in excess in the control arm are from cerebrovascular accidents, other circulatory diseases; respiratory illnesses; infectious diseases; endocrine, nutritional, metabolic and immune diseases; diseases of the nervous system; accidents; and other causes. The deaths in excess in the intervention arm are from non-PLCO neoplasms, ischemic heart disease, and digestive diseases. As anticipated, the majority of those diagnosed with prostate cancer died of other causes. Of the 4250 prostate cancer case patients diagnosed in the intervention arm, 455 (10.7%) had died of causes other than prostate, lung, and colorectal cancer by 13 years, the corresponding numbers for the usual care arm being 3815 prostate cancer case patients and 377 deaths (9.9%), respectively. Thus, a higher percentage of deaths from other causes rather than a deficit occurred among the prostate cancer patients diagnosed in the intervention arm, an indication of the overdiagnosis associated with PSA screen detection (14). We conclude that error in cause of death attribution does not account for the excess in prostate cancer deaths in the intervention arm; random errors and bias cannot be ruled out as explanations for the discrepant outcomes.
A systematic review and meta-analysis of all randomized screening trials for prostate cancer has been reported (22), including the data from PLCO (1) and ERSPC (2) trials in 2009, data from the Goteborg and French components that were not part of the ERSPC 2009 report, and the earlier Quebec and Norwegian trials. In this meta-analysis, there was no statistically significant effect of screening on prostate cancer mortality (RR = 0.88, 95% CI = 0.71 to 1.09) and no effect on overall mortality (RR = 0.99, 95% CI = 0.97 to 1.01).
We plan to update the mortality findings from the prostate component of the PLCO when follow-up data through 15 years are available. In PLCO, the screening that occurred in the usual care arm was not enough to eliminate the expected impacts of the annual screening in the intervention arm, such as earlier diagnosis and a persistent excess of cases. Therefore, the trial was evaluating the effect of adding an organized component of annual screening to the opportunistic screening already in place, and as far as the follow-up has continued to date (13 years), there is no evidence of a benefit. Indeed, there is evidence of harms, in part associated with the false-positive tests, but also with the overdiagnosis inseparable from PSA screening, especially in older men.
Caution is required in determining whether the efficacy of screening is influenced by comorbidity in the relatively healthy population of men that is generally targeted for screening. Using an approach to classify comorbidity as has been previously applied in the trial, we did not find any evidence for an interaction of comorbidity with trial arm, in contrast to what has been recently reported.
Funding
This work was supported by contracts from the National Cancer Institute at the National Institutes of Health (N01-CN-25514 to E.D.C.; N01-CN-25522 to C.I.; N01-CN-25515 to L.A.Y.; N01-CN-25512 to P.A.K.; N01-CN-25513 to T.R.C.; N01-CN-25511 to J.L.W.; N01-CN-25524 to S.S.B.; N01-CN-25518 to D.J.R.; N01-CN-25516 to G.L.A. and R.L.G.; N01-CN-75022 to M.N.F.; N01-CN-25404 to D.C.; and N01-CN-25476 to B.O’B and L.R.R.).
Appendix
The following persons are either current or former members of the Data and Safety Monitoring Board of the PLCO Cancer Screening Trial.
Current Members: J. E. Buring (chair), Brigham and Women's Hospital; D. Alberts, Arizona Cancer Center; H. B. Carter, Johns Hopkins School of Medicine; G. Chodak, Midwest Prostate and Urology Health Center; E. Hawk, M.D. Anderson Cancer Center; H. Malm, Loyola University; R.J. Mayer, Dana–Farber Cancer Institute; S. Piantadosi, Cedars–Sinai Medical Center; G. A. Silvestri, Medical University of South Carolina; I. M. Thompson, University of Texas Health Sciences Center at San Antonio; C. L. Westhoff, Columbia University. Former Members: J. P. Kahn, Medical College of Wisconsin; B. Levin, M.D. Anderson Cancer Center; D. DeMets, University of Wisconsin; J. R. O’Fallon, Mayo Clinic; A. T. Porter, Harper Hospital; M. M. Ashton, Edina, MN; W. C. Black, Dartmouth–Hitchcock Medical Center.
The following persons are either current or former members of the endpoint verification team.
Current Members: P. C. Albertsen (chair), University of Connecticut Health Center; J. H. Edmonson, Rochester, MN; W. Lawrence, Medical College of Virginia; R. Fontana, Rochester, MN; A. Rajput, Roswell Park Cancer Institute. Former Members: A. B. Miller, University of Toronto; M. Eisenberger, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins; I. Jatoi, National Naval Medical Center; E. Glatstein, University of Pennsylvania Medical Center; H. G. Welch, Dartmouth Medical School.
Footnotes
Conflict of interest declarations: G. L. Andriole serves as a Consultant/Advisor for Amgen, Augmenix, Bayer, Cambridge Endo, Caris, France Foundation, GenProbe, GlaxoSmithKline, Myriad Genetics, Steba Biotech, and Ortho Clinical Diagnostics. He serves as Medical Director of Envisioneering Medical and Viking Medical. He is the recipient of grant support from the National Cancer Institute and the National Institute of Diabetes and Digestive and Kidney Diseases. R. L. Grubb conducts research sponsored by GlaxoSmithKline. J. D. Clapp has a financial interest in Human Genome Sciences, Inc, which conducts research and development in the treatment of hepatitis, lupus, and cancer. The Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial is a federally funded registered clinical trial (ClinicalTrials.gov identifier NCT00002540). We thank the study participants for their contributions in making this study possible. The authors are solely responsible for the study design, data collection, analysis and interpretation of the data, writing the article, and decision to submit the article for publication.
References
- 1.Andriole GL, Grubb RL, Buys SS, et al. for the PLCO Project Team. Mortality results from a randomized prostate-cancer screening trial. New Eng J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schröder FH, Hugosson J, Roobol MJ, et al. for the ERSPC Investigators. Screening and prostate-cancer mortality in a randomized European study. New Eng J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 3.Catalona WJ. Screening for prostate cancer. New Eng J Med. 2009;361:202. doi: 10.1056/NEJMc090849. [DOI] [PubMed] [Google Scholar]
- 4.Crawford ED, Grubb R, Black A, et al. Comorbidity and mortality results from a randomized prostate cancer screening trial. J Clin Oncology. 2011;29:355–361. doi: 10.1200/JCO.2010.30.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleming ID, Cooper JS, Henson DE, et al., editors. AJCC Cancer Staging Manual. 5th ed. Philadelphia, PA: Lippincott-Raven; 1997. [Google Scholar]
- 6.Miller AB, Yurgalevitch S, Weissfeld JL. Death review process in the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial. Control Clin Trials. 2000;21:400S–406S. doi: 10.1016/s0197-2456(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 7.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 8.Schoen RE, Pinsky PF, Weissfeld J, et al. Utilization of surveillance colonoscopy in community practice. Gastroenterology. 2010;138:73–81. doi: 10.1053/j.gastro.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahlbom A. Biostatistics for Epidemiologists. Boca Raton, FL: CRC Press; 1993. [Google Scholar]
- 10.Pinsky PF, Black A, Kramer BS, et al. Assessing contamination and compliance in the prostate component of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Clinical Trials. 2010;7:303–311. doi: 10.1177/1740774510374091. [DOI] [PubMed] [Google Scholar]
- 11.Roobol MJ, Kerkhof M, Schröder FH, et al. Prostate cancer mortality reduction by prostate-specific antigen–based screening adjusted for nonattendance and contamination in the European Randomised Study of Screening for Prostate Cancer (ERSPC) Eur Urol. 2009;56:584–591. doi: 10.1016/j.eururo.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Wolters T, Roobol MJ, Steyerberg EW, et al. The effect of study arm on prostate cancer treatment in a large screening trial (ERSPC) Int J Cancer. 2010;126:2387–2393. doi: 10.1002/ijc.24870. [DOI] [PubMed] [Google Scholar]
- 13.Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364:1708–1717. doi: 10.1056/NEJMoa1011967. [DOI] [PubMed] [Google Scholar]
- 14.Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374–383. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Etzioni R, Gulati R, Falcon S, Penson DF. Impact of PSA screening on the incidence of advanced stage prostate cancer in the United States: a surveillance modeling approach. Med Decis Making. 2008;28:323–331. doi: 10.1177/0272989X07312719. [DOI] [PubMed] [Google Scholar]
- 16.Tsodikov A, Szabo A, Wegelin J. A population model of prostate cancer incidence. Stat Med. 2006;25:2846–2866. doi: 10.1002/sim.2257. [DOI] [PubMed] [Google Scholar]
- 17.Etzioni R, Feuer E. Studies of prostate cancer mortality: caution advised. Lancet Oncol. 2008;9:407–409. doi: 10.1016/S1470-2045(08)70112-8. [DOI] [PubMed] [Google Scholar]
- 18.Pinsky PF, Miller A, Kramer BS, et al. Evidence of a healthy volunteer effect in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Am J Epidemiol. 2007;165:874–881. doi: 10.1093/aje/kwk075. [DOI] [PubMed] [Google Scholar]
- 19.Bach PB, Vickers AJ. Do the data support the comorbidity hypothesis for the Prostate, Lung, Colorectal, and Ovarian cancer screening trial results? J Clin Oncol. 2011;29:e387. doi: 10.1200/JCO.2011.34.9027. [DOI] [PubMed] [Google Scholar]
- 20.Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Goteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11:725–732. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Black WC, Haggstrom DA, Welch HG. All-cause mortality in randomized trials of cancer screening. J Natl Cancer Inst. 2002;94:167–173. doi: 10.1093/jnci/94.3.167. [DOI] [PubMed] [Google Scholar]
- 22.Djulbegovic M, Beth RJ, Neuberger MM, et al. Screening for prostate cancer: systematic review and metaanalysis of randomised controlled trials. BMJ. 2010;341:c4543. doi: 10.1136/bmj.c4543. doi:10.1136/bmj.c4543. [DOI] [PMC free article] [PubMed] [Google Scholar]