Abstract
The increasing prevalence of insecticide resistance in Anopheles sinensis, a major vector of malaria in Jiangsu province in eastern China, threatens to compromise the successful use of insecticides in malaria control strategies. It is therefore vital to understand the insecticide resistance status of An. sinensis in the region. This study examined the nucleotide diversity of the para-sodium channel and knockdown resistance (kdr) in five field populations of adult An. sinensis mosquitoes collected in Jiangsu province, identifying the L1014F and L1014C substitutions for the first time. Competitive polymerase chain reaction (PCR) amplification of specific allele (cPASA) and polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) for resistance diagnosis were developed and validated. Comparing the results with direct sequencing revealed that the PCR-RFLP method was more sensitive and specific whereas the cPASA method was more convenient and suitable. The significant positive correlation between kdr allele frequency and bioassay-based resistance phenotype demonstrates that the frequency of L1014F and L1014C substitutions in the kdr gene provides a useful molecular marker for monitoring beta-cypermethrin resistance in natural populations of An. sinensis. Our results point to the L1014F substitution as the key mutation associated with beta-cypermethrin resistance. The high resistance and mutation frequency detected in the five populations also suggest cross-resistance with other pyrethroids may occur in An. sinensis, highlighting the need for further surveys to map insecticide resistance in China and the adoption of a rational management of insecticide application for resistance management and mosquito vector control.
Introduction
The main vectors of malaria in China are An. sinensis, An. anthropophagus, An. minimus and An. dirus. Of these, An. sinensis is the principal malaria vector in Jiangsu province. The impregnation of bed nets [1]–[3] and indoor residual spraying with pyrethroids [4]–[6] are the primary methods used for vector control in China, but the extensive use of insecticides tends to induce resistance in mosquito populations. Indeed, resistance to pyrethroids and DDT has already been detected in An. sinensis and An. anthropophagus and is widespread from Jiangsu, Henan, Sichuan and Fujian provinces to the county of Motuo (Tibet) [1]–[2], [7]–[13]. Malaria control programs in Jiangsu would therefore greatly benefit from a better understanding of the status of insecticide resistance in the local An. sinensis population and the development of appropriate resistance diagnostic tools.
Pyrethroid insecticides are known to act by modifying the gating kinetics of the para-type sodium channels in insect' neurocytes by slowing both the activation and inactivation of the channels [14]. However, modifications in the sodium channel structure such as point mutations or substitutions resulting from single nucleotide polymorphisms [SNP] can dramatically lower sensitivity to DDT and pyrethroids in the sodium channels of the insect's nervous system by reducing or even eliminating the binding affinity of the insecticides to proteins [15], thus diminishing the toxic effects of the insecticides and conferring insecticide resistance [16]. Reduced target-site sensitivity of sodium channels is known to be one of the major mechanisms of pyrethroid resistance and is referred to as knockdown resistance (kdr) [17]. Kdr was first identified in the house-fly Musca domestica L. [18]. Comparisons of partial and complete sequences from 15 susceptible and kdr and kdr-like resistant housefly strains revealed two point mutations (L1014F and/or M918T) associated with knockdown resistance [19]–[21]. The L1014F substitution has also been reported in many pyrethroid-resistant pest species, including An. gambiae [22], Cx. p. pallens [23], Blattella germanica [20], [24], Hematobia irritans [25], Plutella xylostella [26], Leptinotarsa decemlineata [27], and Myzus persicae [28].
Two different kdr mutations, L1014F and L1014S, resulting from single nucleotide polymorphisms in the 6th segment of domain II (IIS6) of the para-type sodium channels, have been found in the African malaria vector An. gambiae [22], [29]. Importantly, both African kdr mutations (L1014F and L1014S) were detected in the same individuals in field populations of An. gambiae and An. arabiensis collected in Uganda by Verhaeghen [30]. Co-occurring kdr mutations in the same allele have also been reported in Cx. p. pallens mosquitoes in eastern China, where they were linked to pyrethroid resistance [31].
Due to the increasing incidence of knockdown resistance to pyrethroids in pest populations and the disequilibrium of vector control in malaria prevention, better monitoring of knockdown resistance in An. sinensis populations is becoming vital. Larval bioassays are generally used for insecticide resistance monitoring in Chinese satellite CDC branches although it is acknowledged that the construction of an insecticide resistance phenotype may differ between the aquatic and adult life stages and that insecticide resistance in larvae is not always transferred through to the adult stage and vice versa. Larvae bioassay was done in this study in order to determine the correlation between the resistance phenotype and knockdown resistance frequencies so as to enhance the work of malaria control in grassroots labs. At present, there is little or no information on the spread of kdr mutations in An. sinensis. In order to pinpoint the precise genotypic composition and frequency of the kdr mutations and link these findings with the insecticide resistance status as defined by bioassays at the end of the intervention period in the five wild populations collected for this study, two approaches were utilized for this research, namely polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) and cPASA, to identify the kdr genotypes in An. sinensis mosquitoes in Jiangsu province.
Materials and Methods
Statement of ethical approval
No ethical approval was required as no regulated animals were used in this study. Pre-permission (April 2009–November 2010) was granted for the mosquito observation, adult mosquito collection and field studies in Jiangsu province for this study, which is part of the Infective Diseases Prevention and Cure Project (No: 2008ZX10402). All the field studies on Anopheles were authorized by the Committee for Animal Welfare and Animal Ethics in the CDC of Jiangsu province, China (address: 172 Jiangsu Road, Nanjing, Jiangsu province, P. R. China).
Mosquito strains
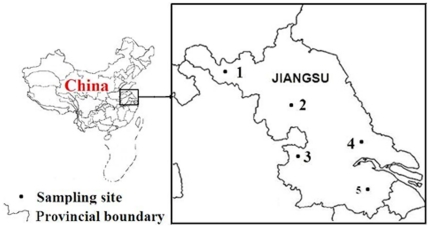
A total of five populations of An. sinensis adult mosquitoes were collected from July to September 2009 from five sites close to the cities of Xuzhou, Huaiyin, Nanjing, Changshu, and Suzhou. The mosquito populations were named XZ (XuZhou, rural), HY (HuaiYin, rural), NJ (NanJing, rural), CS (ChangShu, rural) and SZ (SuZhou, rural) (Figure 1).
Figure 1. Locations of five blood fed An. sinensis field populations.
Abbreviations: (1) XZ, XuZhou (34°09′18″N, 117°18′75″E);(2) HY, HuaiYing (33°01′181″N, 118°34′541″E);(3) NJ,NanJing (32°01′18″N,118°28′54″E);(4) CS, ChangShu(31°03′63″N, 119°50′90″E);(5) SZ, SuZhou (31°21′36″N, 120°20′26″E).
All the mosquitoes were collected in their adult form in rural areas (primarily milk cow cowsheds and pigsties) and transported to insectaria (Insectaria of Huadong Research Institute for Medicine and Biotechnics in Nanjing, Jiangsu, China). The numbers of adults collected were very high from XZ and SZ, 38 adults from HY, 44 adults from NJ, and 49 adults from CS. Most were blood fed when caught. The larvae were supplied with baker's yeast until oviposition occurred. Larvae were kept under the room temperature of 25–27°C and 12 h indoor illumination every day. The density of the larvae was kept at 400–800 larvae/L. After becoming adult, part of each population was stored at −20°C for molecular assay and part for bioassay. One susceptible strain that had been protected from contact with insecticides for 20 years and routinely reared under laboratory conditions was used as a reference for diagnostic tests.
Specimen identification
Two Anopheles species, An. sinensis and An. anthropophagus, co-exist in Jiangsu province. But it is difficult to distinguish the two species by dichotomous keys. In order to correlate the dose response with kdr genotype frequencies for these specific species, molecular identification by PCR of the 290 specimen samples was carried out using primers designed based on the sequence differences in ribosomal DNA internal transcribed spacer 2 of An. sinensis and An. anthropophagus [32]. The diagnostic lengths of specific fragments were 425-bp in An. sinensis based on the primers UP and PS and 253-bp in An. anthropophagus based on the primers UP and PA (Table 1).
Table 1. PCR primers used in this study.
| Name | Sequence (5′→3′) | |
| D1 | AAR YTN GCN AAR TCT TGG CC | (Martinez-Torres, 1998) |
| Dg2 | GCD ATY TTR TTN GTN TCR TTR TC | (Martinez-Torres, 1998) |
| CN1 | TGG CCN ACG CTG AAY TTA CTC | |
| CN2 | CCG AAATTG GAC AAA AGC AAA G | |
| CP1 | TGATCGTGTTTCGCGTGCTG | |
| CP2 | GCGTCTCGTTATCCGCCGTT | |
| CD1 | TGATCGTGTTTCGCGTGCTG | |
| CD2 | GTCTCGTTATCCGCCGTTGG | |
| Cgd3 | CCCGGTGGTAATTGGAAACTTG | |
| Cgd4 | TGCGGTGGTAATTGGAAACTTT | |
| Cgd5 | TGCGGTGGTAATTGGAAACTGT | |
| UP | CCA TGACGTACACA TACTTG | (Ma et al. 1998) |
| PA | GCTCCA TCTACACACA GCGT | (Ma et al. 1998) |
| PS | GTTGTCCA GCCCGCTAACA T | (Ma et al. 1998) |
| EP1 | gcggtcccaaaagggtcagtTAGCCACTGTGGTAATTGGAAgCT | |
| EP2 | gcggtcccaaaagggtcagtTGGTGCAGAGAGCGATGATG | |
| EP3 | gcggtcccaaaagggtcagtGGAGTGGATCGAATCAATGTGG | |
| EP4 | gcggtcccaaaagggtcagtTGTCGTCCTGCAGTTACTCAtCAC |
Bioassays
In order to correlate the kdr genotypes with their resistance phenotypic outcomes, larvae bioassays were performed on the six populations. Beta-cypermethrin powder (Tianjin Pesticide Co. Ltd, China), the mostly widely used insecticide in the malaria control effort in Jiangsu province, was completely dissolved in acetone. The stock solution, with a final concentration of 20 ppm, was stored at 4°C for less than 2 months. The stock solution was diluted in seven concentrations along a gradient from multiproportion. Bioassays were made using late third and early fourth instar larvae. Batches of ninety larvae per concentration were respectively put into three parallel cups. Each cup contained 199 ml distilled water and 1 ml of insecticide solution and this provided a range from 0 to 100% mortality. Mortality was recorded after a 24-hour exposure. The temperature was maintained at 26±1°C during the bioassay. The mortality of a control group exposed to water and 1 ml of acetone never exceeded 4%.
Partial sequencing of the sodium channel cDNA
The IIS4-IIS6 coding region sequences of the sodium channel gene were sequenced from a susceptible strain (SS) and three resistant wild specimens from the HY (HuaiYin) strain. Total RNA was extracted with Trizol (Invitrogen, USA) from a single mosquito specimen in each of the five samples. The first-strand cDNA synthesis was performed using the SuperScript™ III First-Strand Synthesis System for RT-PCR (Invitrogen, USA) and an oligo (dT) adapter primer. Two steps of PCR were performed. Primary PCR on the single-stranded cDNA was carried out with the degenerate primers D1 and Dg2 [22]. One unit of Taq polymerase (TaKaRa) in buffer (supplied by the manufacturer), 200 ng of each primer and 0.2 mM dNTP were used in a 25-µl total volume PCR reaction consisting of 1 cycle of 94°C for 3 min and 35 cycles at 94°C for 1 min, 50°C for 30 sec, 72°C for 1 min and a final extension step at 72°C for 7 min. The secondary step of PCR was then carried out based on the primary product with nested inner primers of CN1 and CN2 (Table 1) based on the partial sequencing of para-type sodium channel of M. domestica [21], B. germanica [20], An. gambiae [22] and Cx. P. pallens [23]. The amplified fragment (Genbank accession number: JN002364) was then recovered by Wizard PCR preps DNA Kit and used as a template for direct sequencing with the automated ABI PRISM Dye Terminator Cycle Sequencing Kit.
Intron sequence determination
Genomic DNA of the five strains, XZ, SZ, NJ, CS and HY, was respectively extracted from a single mosquito of each using Universal Genomic DNA Extraction Kit Ver 3.0 (TaKaRa) according to the method of Collins [33]. The genomic region containing the intron sequence of interest, which was located 2 bp downstream of the kdr mutation, was PCR amplified on 10–50 ng of genomic DNA using primers CP1 and CP2 (Table 1). One unit of KOD plus polymerase (TOYOBO) and 100 ng of each primer were used in a 50-µl total PCR volume. Amplification was performed as follows: 1 cycle of 94°C for 3 min and 35 cycles at 94°C for 30 sec , 54°C for 30 sec, and 68°C for 1 min with a final extension step at 68°C for 7 min. After sequencing, one intron manifested its size.
Assay for kdr genotype
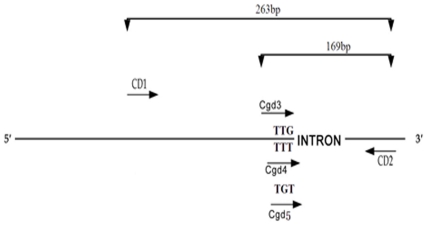
Based on the sequence data for An. sinensis and the methods of competitive PASA (cPASA) described by Jamroz et al. [34], Martinez-Torres et al. [22], Zhang et al. [35] and Song et al. [36], with some modifications, a test method involving three PCR reactions for each specimen was developed to diagnose the kdr genotypes (Figure 2). The three PCR reactions were almost the same except that one contained a sense-specific primer (cdg3) ending with the two bases “TG” in the 3′end position to detect the susceptible codon “TTG”, the second contained a sense-specific primer (cdg4) ending with the two bases “TT”in the 3′end position to detect the mutation codon “TTT”, and the third reaction contained the third sense-specific primer (cdg5) ending with the two bases “GT”in the 3′end position to detect the mutation codon “TGT”. Thus, a total of three allele-specific inner primers were designed: sensitive sense-primer Cgd3 and two resistant sense-primers Cgd4 and Cgd5 (Table 1). Two additional nonspecific outer primers were based on the sequence immediately downstream from the mutation site and one sense primer upstream of the site, thus bracketing the mutation points. The two allele-nonspecific outer primers were sense primer CD1 and anti-sense primer CD2 (Table 1).
Figure 2. Schematic diagram of cPASA strategy for detecting L1014F and L1014C substitutions and predicting the size of PCR products in the para sodium channel gene in An. sinensis.
CD1-CD2 and Cdg3-Cdg5 indicate PCR primers whose sequences are reported in Table 1. Primer pair Cdg3 and CD2 amplifies a 169-bp fragment for the wildtype susceptible allele (for codon TTG). Primer pair Cdg4 and CD2 yields a 169-bp fragment for resistant L1014F allele (codon TTT). Similarly, primer pair Cdg5 and CD2 leads to amplification of a 169-bp fragment diagnostic to the L1014C resistant allele (codon TGT). The primer pair CD1 and CD2 is two allele-nonspecific outer primers.
A PCR diagnostic test was performed in accordance with the standard procedure using a total volume of 50 µl, consisting of 10× Buffer 5 µl, 80–100 ng genomic DNA 4 µl, 50 mM MgCl2 2 µl, 10 mM each dNTP 2 µl ,1 µl KOD plus polymerase, 2 µl primers (1 µl of each primer), 34 µl dH2O. For each template from an individual strain, there were three PCR reactions, of which the first reaction included the primers CD1, Cgd3 and CD2, the second CD1, Cgd4 and CD2, and the third CD1, Cgd5 and CD2. The PCR conditions were one cycle of 93°C for 4 min, then 35 cycles of 94°C for 1 min, 55°C for 30 sec and 68°C for 1 min, followed by one cycle of 68°C for 7 min. PCR products were checked by electrophoresis on 1.2% agarose gel in TAE buffer. The resulting bands were visualized by ethidium bromide staining. The diagnostic PCR products for the kdr alleles were 169-bp and those for the allele-nonspecific outer primers were 263-bp.
PCR-RFLP protocol for kdr genotype discrimination and direct sequencing of the kdr gene
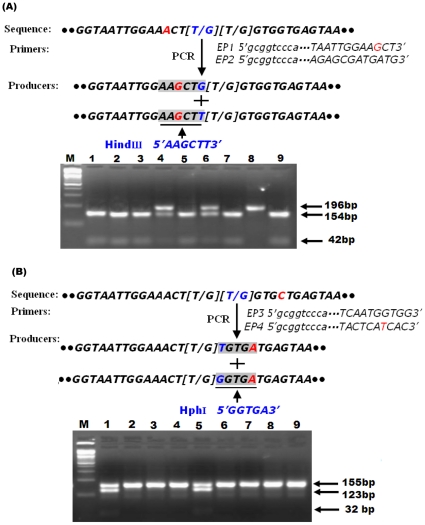
Two approaches, namely PCR-RFLP (Figure 3) and direct sequencing, were used to further confirm the kdr genotype. We developed a PCR-RFLP method for genotyping both mutations using two PCR reactions followed by two restriction digests and agarose gel electrophoresis. Two sets of primers were used to identify both mutation sites. In this study, the point mutation in L1014 in domain IIS4- IIS6 of the sodium channel gene was present in the form T (T/G)(T/G). The first PCR reaction was used to detect the first “T/G” based on the primers EP1and EP2. An “A” to “G” mismatch at the EP1-primer (forward) at the 3rd base position from 3′ end terminus (shown in small letter in primer sequence) was designed. The terminal AAgCT-3′ sequence with the first (T/G) may create a recognition site for the restriction enzyme HindIII AAGCTT at the 42-bp position in the PCR fragment. The second PCR reaction was used to detect the second “T/G” based on the primers EP3 and EP4. A “C” to “T” mismatch at the EP4-primer (reverse) at the 4rd base position from 3′ end terminus (shown in small letter in primer sequence) was designed. The terminal tCAC-3′ sequence with the second (T/G) may create a recognition site for the restriction enzyme HphIGGTGA at the 32-bp position of the PCR fragment. Both PCR reactions were done with the previously described PCR conditions, briefly 10×Taq buffer 1.5 µl, 1.2 µl MgCl2, 0.1 µl Taq polymerase (TaKaRa), 0.3 µl dNTP mixture, 0.4 µl primers (each primer 0.2 µl), 0.5 µl template DNA and 11 µl dH2O in a 15-µl total volume, PCR reaction consisting of 1 cycle of 94°C for 3 min, 35 cycles at 94°C for 30 sec, 66°C for 30 sec, 72°C for 60 sec. Then the PCR product was stored on ice at −0.4°C until the final stage in the process, which consisted of another 20 cycles at 95°C for 30 sec and 68°C for 60 sec and a final extension step at 72°C for 6 min. The restriction digest reaction involved 0.2 µl (10 U/ml) enzyme and 15 µl PCR reactant in a 20-µl total digest volume. The electrophoresis was performed with 4% agarose at 150 V for 90 min.
Figure 3. Schematic diagram of PCR-RFLP strategy for detecting L1014F and L1014C substitutions and predicting the size of PCR-RFLP products in the para sodium channel gene in An. sinensis.
A: A“A”to “G” substitution at the EP1-primer causes the formation of a diagnostic HindIII recognition site (gray shade) at position 42-bp of the PCR fragment.“M” is a marker. The substitution can be inferred from the appearance of the agarose gel electrophoresis: “1,2,3,5,7,9” are (T/T), “4,6” are (T/G), “8” is (G/G). B:A“C”to “T” substitution at the EP4-primer causes the formation of a diagnostic HphI recognition site (gray shade) at position 32-bp of the PCR fragment.“M” is a marker. The substitution can be inferred from the appearance of the agarose gel electrophoresis: “2,3,4,6,7,8,9” are (T/T), “1,5” are (T/G).
Results
Species identification and beta-cypermethrin resistance of Anopheles populations
Two hundred and ninety specimens of Anopheles complex mosquitoes were collected during the survey and tested with a Polymerase Chain Reaction (PCR) adapted from Ma et al. [32] to distinguish the An. sinensis and An. anthropophagus. PCR assay gave the 452-bp species-specific fragments of An. sinensis, no amplification of the 253-bp fragment signifying the absence of An. anthropophagus. All the tested mosquitoes were therefore deemed to be An. sinensis in agreement with the known geographic distribution of species within the An. anthropophagus complex in Jiangsu province, eastern China. In the bioassays, the five populations all showed high resistance to beta-cypermethrin (Table 2). Resistance ratios of LC50 for the five populations were found to range from 700 to 2100-fold, much higher than the 3.5- to 5-fold reported for An. sinensis mosquitoes from Liaoning province [37].
Table 2. Values of 50% lethal concentration (LC50) for the five An. sinensis resistant field populations compared to the susceptible lab strain in response to beta-cypermethrin.
| Mosquito Population | LC50(ppm) | 95% confidence interval | R/S* |
| SS | 0.001 | 0.0002–0.001 | 1 |
| XZ | 0.9 | 0.3–3.5 | 900 |
| HY | 1.3 | 0.2–11 | 1300 |
| NJ | 1.7 | 0.4–3.3 | 1700 |
| CS | 2.1 | 1.3–3.1 | 2100 |
| SZ | 0.7 | 0.46–1.0 | 700 |
*R/S is the ratios of LC50 of the test population to the SS population.
Partial sequencing of the sodium channel cDNA
A 357-bp cDNA sequence of individual mosquitoes was amplified from the susceptible strain and the wild resistant strain from XZ (XuZhou). The nucleotide sequences were the same except a difference at position 1014, where TTG (Leu) in the SS strain sequence was replaced with TTT (Phe) or TGT (Cys) in the XZ strain sequences at amino acid 1014 (Figure 4).
Figure 4. The sequences of cDNA and amino acids from the susceptible SS strain and resistant XZ populations.
The sequences XZ1 and XZ2 come from cloning An. sinensis individuals from the XZ population. The sequence of the SS strain amino acid was translated from the DNA sequence of the An. sinensis SS strains. XZ1 and XZ2 amino acid sequences were translated from XZ cDNA sequences by cloning.
Intron sequence determination
Blast comparison of the partial cDNA sequence of the sodium channel gene and comparing the results with those given on the website (http://genome.ucsc.edu/cgi-bin/hgBlat), the results showed that the intron was located in the domain II region, just at the conserved positions for Cx. P. pallens [22]. One intron was located downstream of the kdr mutation and the knowledge of its sequence length was necessary in order to discern the size of the allele using the PASA test. The PCR products amplified by CP1 and CP2 from individual genomic DNA extracted from the five strains were sequenced. No polymorphism was found in this intron after comparing several specimens.
Optimization of kdr mutation diagnostic assays
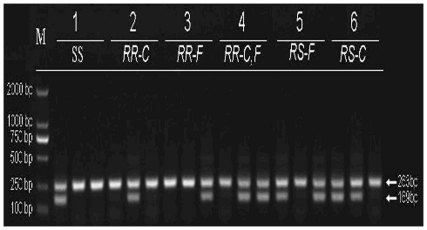
After optimization, the PCR diagnostic test was capable of discerning the kdr homozygous (RR) and heterozygous (RS) genotypes for the L1014F and L1014C substitutions in the sodium channel a-subunit gene para. The product amplified by two non-specific outer primers CD1 and CD2 was 263-bp. The product amplified by three specific paired-primers was 169-bp (Figure 5).
Figure 5. Schematic diagram of CPASA detection gel for L1014F and L1014C sodium channel substitutions in An. sinensis.
The allele-specific kdr diagnostic test developed by Martinez-Torres et al. [22] was adapted to detect kdr genotypes of individuals from 6 typical wild specimens. M: marker. The 263-bp fragment was the out control and the 169-bp fragment was the kdr specific allele. Genotype results:specimen 1:TTG/TTG;specimen 2:TGT/TGT;specimen 3:TTT/TTT;specimen 4:TGT/TTT;specimen 5:TTG/TTT;specimen 6:TTG/TGT.
Results of PCR-RFLP and direct sequencing
After the first PCR reaction based on the primers EP1 and EP2 and digested with HindIII, the presence of the PCR products in the electrophoresis could be identified as kdr genotypes. A sequence with G/G in the first (T/G) position could be inferred by the appearance of a single 196-bp fragment, T/T was inferred by the appearance of a 154-bp and a 42-bp fragment and G/T was inferred by the appearance of three fragments, of 196-bp, 154-bp and 42-bp. In the same way, the second (T/G) involving (T/T), (G/G) and (G/T), was inferred by the three fragments of 155-bp, 123-bp and 32-bp, respectively (Figure 3). Direct sequencing was used to validate the genotyping results of cPASA and PCR-RFLP. The sensitivity and specificity of the two genotype methods was found to exceed 94% in all cases (Table 3).
Table 3. Sensitivity of the cPASA and PCR-RFLP methods in reference to allele sequence data for identification of kdr genotypes in Anopheles sinensis.
| Detection methods | |||
| Sequencing(n = 50) | cPASA(n = 50) | PCR-RFLP(n = 50) | |
| Genotype frequency | |||
| TTG/TTG | 0.00 | 0.00 | 0.00 |
| TTT/TTT | 68 | 72 | 68 |
| TGT/TGT | 4.0 | 2.0 | 6.0 |
| TTG/TTT | 2.0 | 2.0 | 4.0 |
| TTT/TGT | 20 | 22 | 20 |
| TTG/TGT | 6.0 | 2.0 | 2.0 |
| Sensitivity (%) | 94 | 96 | |
| Specificity (%) | 94 | 96 | |
The 50 Anopheles sinensis individuals used in this assay were from the XZ (XuZhou) population.
Distribution of kdr allele frequencies in natural populations from eastern China
A total of five field populations, 290 specimens collected from rural locations close to the cities of XZ, SZ, CS, NJ and HY, plus the susceptible SS strain were tested with cPASA. Table 4 summarized the results of the cPASA assays. Based on the presence or absence of the kdr alleles, individual mosquitoes were genotyped as homozygous susceptible (SS), homozygous resistant (RR), or heterozygous (RS). No L1014C (TTG/TCG) genotype was found in any of the mosquito samples tested. Only one specimen from XZ (XuZhou) was found to be homozygous susceptible (SS). None of the two resistant alleles were present in any of the specimens from the laboratory reference strain. PCR assays revealed clear differences in overall kdr allelic frequency between the resistant and susceptible strains. All the SS strain specimens were homozygous susceptible but kdr frequencies in the wild resistant strains ranged from 16% to 84%. The kdr alleles existed mainly in the kdr-F/F and kdr-F/C genotype, the resistant homozygous form, and only a small portion (1–4%) of the mosquitoes possessed kdr-C homozygous (RR-C) kdr alleles, with 3–5% of the kdr-C and kdr-F heterozygous (RS-C and RS-F) kdr genotype in the five populations. Kdr allelic frequency ranged from 74% to 84% on the kdr-F, and 16% to 24% on kdr-C. The genotype frequency of RR-F and RR-F/C ranged from 51% to 69% and 29% to 47%, respectively.
Table 4. Frequencies (in percentages) of kdr alleles and genotypes in relation to the five An. sinensis populations from east-China monitored by cPASA.
| Populations | SS | XZ | HY | NJ | CS | XZ |
| Sample size (n) | 50 | 84 | 38 | 44 | 49 | 75 |
| Frequency of kdr allele (%) | ||||||
| TTG (L1014) | 100 | 5 | 1 | 1 | 1 | 0 |
| TTT (1014F) | 0 | 75 | 75 | 77 | 76 | 84 |
| TGT (1014C) | 0 | 20 | 24 | 22 | 23 | 16 |
| Frequency of kdr genotype (%) | ||||||
| L/L (TTG/TTG) | 100 | 1 | 0 | 0 | 0 | 0 |
| L/F (TTG/TTT) | 0 | 5 | 3 | 2 | 2 | 0 |
| L/C (TTG/TGT) | 0 | 4 | 0 | 0 | 0 | 0 |
| F/F (TTT/TTT) | 0 | 59 | 53 | 61 | 51 | 69 |
| C/C (TGT/TGT) | 0 | 4 | 3 | 3 | 0 | 1 |
| F/C (TTT/TGT) | 0 | 29 | 43 | 30 | 47 | 29 |
Frequency of L1014F and L1014C substitutions in response to beta-cypermethrin
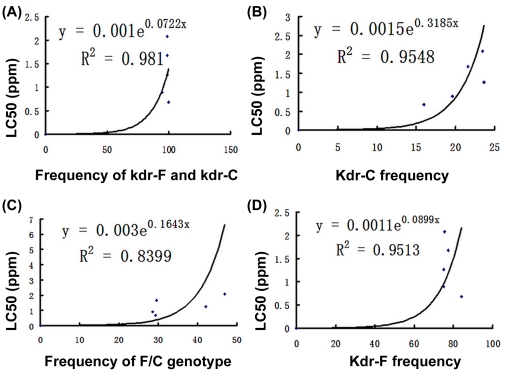
Spearman's rank correlation analysis based on the correlate analysis showed significant correlations between the LC50 and the kdr allele frequencies of kdr-F and kdr-C (p≤0.01). Regression analysis revealed a significant correlation between LC50 estimates and the frequency of kdr-(F+C)(R2 = 0.981), kdr-C (R2 = 0.9548), kdr-F (R2 = 0.9513) and F/C genotype (R2 = 0.8399) (Figure 6). In addition, the frequencies of the RR-F/C (TTT/TGT) genotype in the HY and CS populations (42% and 47%, respectively) were higher than that of the populations from SZ and XZ.
Figure 6. Correlation between LC50 and percentage frequencies for kdr alleles and genotypes.
(A), (B), (C), (D): exponential correlation relationship between frequencies of kdr-F and kdr-C, kdr-C, F/C genotype, kdr-F genotype and the 50% lethal concentration of the five populations against beta-cypermethrin.
Hardy-Weinberg equilibrium test
The Hardy-Weinberg equilibrium test was conducted for analysis of the kdr genotype frequencies in each population with the T-paired test. The unbiased estimates of P-value of the five populations were 1.0, 0.9, 1.0, 1.0, and 0.9 for XZ, HY, NJ, CS and SZ, respectively. The results showed that there were no significant differences between the expected values and the observed values either in genic or in genotypic differentiation. The five populations were therefore all deemed to be present in genetic equilibrium (Table 5).
Table 5. Hardy-Weinberg equilibrium tests on kdr genotype frequencies.
| Genotype Frequency | SS (L/L) | RR (F/F) | RR (C/C) | RS (L/F) | RS (L/C) | RR (F/C) | t | P | |||||||
| population | Sample Size (n) | Exp | Obs | Exp | Obs | Exp | Obs | Exp | Obs | Exp | Obs | Exp | Obs | / | 2-tailed |
| XZ | 84 | 0.003 | 0.01 | 0.6 | 0.6 | 0.04 | 0.04 | 0.084 | 0.05 | 0.02 | 0.04 | 0.3 | 0.3 | −0.009 | 1.0 |
| HY | 38 | 0.0002 | 0.00 | 0.6 | 0.5 | 0.06 | 0.00 | 0.02 | 0.03 | 0.006 | 0.03 | 0.36 | 0.4 | −0.09 | 0.9 |
| NJ | 44 | 0.0001 | 0.00 | 0.6 | 0.6 | 0.05 | 0.03 | 0.02 | 0.02 | 0.005 | 0.00 | 0.3 | 0.3 | 0.01 | 1.0 |
| CS | 49 | 0.0001 | 0.00 | 0.6 | 0.5 | 0.06 | 0.00 | 0.02 | 0.02 | 0.005 | 0.00 | 0.4 | 0.5 | −0.00 | 1.0 |
| SZ | 75 | 0.00 | 0.00 | 0.7 | 0.7 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.3 | 0. 3 | 0.1 | 0.9 |
T-Paired test: P (2-tailed) = 1.0(XZ), 0.9 (HY), 1.0(NJ), 1.0 (CS), 0.900(SZ).
Discussion
Knockdown resistance (kdr) is a type of target-site resistance arising from point mutations in the sodium channel genes of the insect nervous system and is known to confer cross-resistance to DDT and pyrethroids [38]. The resistance of An. sinensis against pyrethroids and DDT has been increasing rapidly in China, but kdr mutations had not previously been detected. To investigate whether this mechanism was general in An. sinensis, the partial sequences of the para-type sodium channel genes from various An. sinensis field populations collected from five sites in Jiangsu province in eastern China were examined. The sequence observed in the lab SS strains of An. sinensis was not consistent with that in the other mosquito species for one codon: the silent replacement of TTA (Leu) by TTG (Leu) at amino acid 1014. After comparing the sequences in the field populations and the SS strain, as reported in An. gambiae, Cx. p. pallens and Cx. p. quinquefasciatus, two types of molecular mutations were found at L1014 alleles in the Xuzhou (XZ) population, in which the resistance level was about 900 fold that of the susceptible SS strain to Beta-cypermethrin. The first point mutation, with TTG (Leu) being replaced with TTT (Phe), accords with prior reports of the kdr mutation in An. gambiae [39], Cx. p. pallens [36], Cx. p. quinquefasciatus [40]. The second, TTG (Leu) being replaced with TGT (Cys), is a new mutation.
The Leu/Phe substitution has been implicated in the development of pyrethroid resistance in several mosquito species, including Cx. p. pallens [41], [42] and Cx. p. quinquefasciatus [43]. Previous electrophysiological studies have shown that the kdr substitution of L1014F on domain II S6 promotes closed-state inactivation so 70–80% of the sodium channels never open [44], thus the Leu/Phe substitution is thought to directly cause the heightening of the insect's resistance based on the results of the bioassay. Since all the tested field populations showed high resistance to beta-cypermethrin, the relationship between L1014F substitution frequency and mosquito survival when challenged with beta-cypermethrin was analyzed in this study. The L1014F substitution not only showed a strong positive correlation with LC50 (R2 = 0.9519) but also a significantly high frequency (75% to 84%), corresponding to the resistance level against beta-cypermethrin in all the field populations (700 to 2100-fold). This finding therefore supports previous studies that suggested that the L1014F substitution is the key mutation responsible of beta-cypermethrin resistance. An alternative substitution of leucine to cysteine (Leu to Cys) in the same location showed a relative low frequency within populations; in spite of its strong positive correlation with LC50, this Leu/Cys substitution is unlikely to play an important role in beta-cypermethrin resistance. Martinez-Torres [22] reported that L1014S substitution in one strain showed a slight increase in resistance to pyrethroids but greatly increased DDT resistance. It seems reasonable to suppose, therefore, that the L1014C substitution may be responsible for resistance to other insecticides within the same family of pyrethroids and DDT. Based on the positive correlation between LC50 and the total frequency of kdr-F and kdr-C (R2 = 0.981), taken together with the results of previous studies on other mosquito species, we consider that kdr mutation screening offers an excellent molecular marker for pyrethroid resistance monitoring in An. sinensis.
Sensitive detection of the mutations associated with knockdown resistance is a prerequisite for resistance management strategies aimed at prolonging insecticide lifetime while maintaining sufficient insect control. A number of methods, for example Allele-specific PCR (AS-PCR), Hot Oligonucleotide Ligation Assay (HOLA), TaqMan probe and PCR-RFLP, have been utilized to detect kdr mutations in several species [45], [46]. In this study, based on the specific mutation types identified in An. sinensis, we developed CPASA and PCR-RFLP methods to detect the two forms of kdr alleles and compared the sensitivity and specificity of the two methods to that obtained using direct sequencing. Although some researchers had reported that the CPASA method could lead to unreliable results [31], [45], our results in the present study indicated that the sensitivity and specificity of this method were relatively high. Considering quick results and low costs, CPASA seems to be a good candidate for automation with microplates and robotic workstations for high throughput. In contrast, PCR-RFLP is less widely used for genotyping kdr alleles because this approach suffers from severe limitations in experiments that lack a digestion pattern and is not suitable for large-scale point mutation screening for low mutation frequency conditions, especially when the relatively high capital expenditure and running costs become a major consideration. However, implementing the PCR-based approach followed by RFLP for allele identification is robust, simple to perform, and easy to interpret, which makes it eminently suitable for use in reference laboratories. In this study, PCR-RFLP was used to further confirm the results obtained from CPASA under strict PCR and agarose gel electrophoresis conditions only when substitutions were used to supply digestion patterns in the primers. The results confirmed that PCR-RFLP was higher in both sensitivity and specificity than CPASA for the alleles genotyped here.
It is important to obtain a baseline level for insecticide sensitivity on both the local and regional scales so that resistance management can be adjusted appropriately to local conditions. Although the results reported here refer to relatively few An. sinensis samples from a very large geographical range, they represent a first effort to analyze the overall distribution of the L1014F and L1014C substitutions in Jiangsu province in East China, where both molecular forms of these species co-occur over most of their range of distribution. The results of the Hardy-Weinberg equilibrium test showed that all the five field populations used for this study were presently in Hardy-Weinberg equilibrium. Our results indicate that there is indeed an increased selective pressure due to the use of pyrethroid insecticide in this region. Since larvae of An. sinensis are most likely living in the rice paddies in the province, insect control activities such as the large-scale use of pyrethroid insecticides for agricultural purposes, and possibly for domestic protection, may be a major factor contributing to the increasing insecticide resistance of An. sinensis. Although beta-cypermethrin is not widely used for agricultural purposes, the high resistance against it exhibited by mosquitoes throughout the region suggests that cross-resistance with other pyrethroids may be occurring in An. sinensis, so rational management of insecticide applications should be carried out in the future in order to minimize the development of resistance and thus better support vector control efforts.
Acknowledgments
We thank the anonymous helpers from Xuzhou Center for Disease Prevention and Control of Jiangsu Province for their assistance in mosquito collection. Anonymous reviewers provided constructive suggestions.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the foundation from the Infective Diseases Prevention and Cure Project of China (NO: 2008zx10004-010). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Su Y, Feng S, He L, Chen J. Current status of malaria prevalence in Henan Province and the strategy for prevention. Henan Journal of Preventive Medicine. 2001;4:195–197. [Google Scholar]
- 2.Li J, Zhou H, Shen B, Zhu G, Cao J. Experiment observation on residual effect of Anopheles by pyrethroid-impregnated bednet. Chinese Journal of Schistosomiasis Control. 2002;1:32–34. [Google Scholar]
- 3.Li P, Luo D, Li A, Lu D, Li D, et al. Field trial of alphacypermethrin-impregnated bed nets for malaria and mosquito control. Chinese Journal of Parasitology and Parasitic Diseases. 1997;3:223–226. [Google Scholar]
- 4.Lu B, Gao J, Zhang B. Study on the behavior of Anopheles sinensis wiedemann 1828, A jeyporiensis candidiensis Koidzumi, 1924 and An.minimus Theobald, 1901 in DDT and BHC treated huts. ACTA ENTOMOLOGICA SINICA. 1963;2:149–162. [Google Scholar]
- 5.Wu Z, Kan S, Shen Y, Chen F, Lu Z, et al. Studies on DDT residual spraying for controlling Anopheles lesteri anthropohagus and plasmodium falciparum malaria. Chinese Journal of Parasitology and Parasitic Diseases. 1986;4:220–223. [PubMed] [Google Scholar]
- 6.Cai X. Residual spraying of DDT to be an effectively interventional measure in malaria control. Hainan Provincial Institute of Tropical Disease. 2009;10:1957–1960. [Google Scholar]
- 7.Yu P, Zhang H, Zhang S, Xu B. Survey of susceptibility of Anopheline vectors to insecticides in a malaria mesoendemic area, Hubei province. Chinese Journal of Parasitology and Parasitic Diseases. 2000;3:149–151. [PubMed] [Google Scholar]
- 8.Yu P, Zhang H, Huang G, Ming G, Xu B. Surveillance of susceptibility of Anopheles vectors to deltamethrin in anopheles anthropophagous areas. Acta Parasitol Med Entomol Sin. 2003;3:152–156. [Google Scholar]
- 9.Li J, Zhou H, Cao J, Zhu G, Wang W. Sensitivity of Anopheles sinensis to deltamerthrin and cyfluthrin in part areas of Jiangsu Province. Chinese Journal Vector Biology & Control. 2009;5:438–439. [Google Scholar]
- 10.Huang G, Zhang H, Yu P, Liu J, Lan M, et al. Observation of Anopheles anthropophagus density and malaria incidence in the areas of discontinuing impregnating bednets with deltamethrin. Chinese Journal of Vector Biology and Control. 1998;3:11–15. [Google Scholar]
- 11.Shang L, Chen J, Li D, Li P, Su Y, et al. Study on distribution, ecological feature and malaria transmission effect of Anopheles anthropophagus in Henan Province, China. Journal of Pathogen Biology. 2007;4:304–306. [Google Scholar]
- 12.Huang G, Yuan F, Jin X, Zhao C, Su Y, et al. To analyze the epidemic situation and control of malaria in Jiangsu, Shandong, Henan and Hubei provinces. China of Journal Vector Biology & Control. 2007;5:398–401. [Google Scholar]
- 13.Zhang Z, Zhou H, Zhao X, Chang F, Wang H, et al. Epidemiological survery on malaria situation in motuo county of Tibet, China. Chinese Journal of Parasitology and Parasitic Diseases. 2008;5:343–346. [PubMed] [Google Scholar]
- 14.Lund AE, Narahashi T. Kinetics of sodium channel modification as the basis for the variation in the nerve membrane effects of pyrethroids and DDT analogs. Pest Biochem Physiol. 1983;20:203–216. [Google Scholar]
- 15.Narahashi T. Role of various ion channels in insecticide action. Abstr Pap Am Chem Soc. 1988;196:26–AGRO. [Google Scholar]
- 16.Soderlund DM. Sodium channels. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive molecular insect science, Elsevier Pergamon. Vol. 5. New York: 2005. pp. 1–24. [Google Scholar]
- 17.Soderlund DM, Knipple DC. The molecular biology of knockdown resistance to pyrethroid insecticides. Insect biochem.mol.biol. 2003;33:563–577. doi: 10.1016/s0965-1748(03)00023-7. [DOI] [PubMed] [Google Scholar]
- 18.Milani R. Comportamento mendeliano della resistenza alla azione abbattante del DDT: correlazione tran abbattimento emortalia in Musca domestica L. Riv Parasitol. 1954;15:513–542. [PubMed] [Google Scholar]
- 19.Ingles PJ, Adams PM, Knipple DC, Soderlund DM. Characterization of voltage-sensitive sodium channel gene coding sequences from insecticide-susceptible and knockdown-resistance house fly strains. Insect biochem.mol.biol. 1996;26:319–326. doi: 10.1016/0965-1748(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki M, Ohyama K, Dunlap DY, Matsumura F. Cloning and sequencing of the para-type sodium channel gene from susceptible and kdr-resistant German cockroaches (Blattella germanica) and house fly (Musca domestica). Mol.gen.genet. 1996;252:61–68. [PubMed] [Google Scholar]
- 21.Williamson MS, Martinez-Torres D, Hick CA, Devonshire AL. Identification of mutations in the house fly para-type sodium channel gene associated with knockdown resistance (kdr) to pyrethroid insecticides. Mol.gen.genet. 1996;252:51–60. doi: 10.1007/BF02173204. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, et al. Molecular characterization of pyrethoid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. . Insect mol.biol. 1998;7:179–184. doi: 10.1046/j.1365-2583.1998.72062.x. [DOI] [PubMed] [Google Scholar]
- 23.Martinez-Torres D, Foster SP, Field LM, Devonshire AL, Williamson MS. A sodium channel point mutation is associated with resistance to DDT and pyrethroid insecticides in the peach-potato aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae). Insect Mol Biol. 1999b;8:339–346. doi: 10.1046/j.1365-2583.1999.83121.x. [DOI] [PubMed] [Google Scholar]
- 24.Dong K. A single amino acid change in the para sodium channel protein is associated with knockdown-resistance (kdr) to pyrethroid insecticides in the German cockroach. Insect biochem.mol.biol. 1997;27:93–100. doi: 10.1016/s0965-1748(96)00082-3. [DOI] [PubMed] [Google Scholar]
- 25.Guerrero FD, Jamroz RC, Kammlah D, Kunz SE. Toxicological and molecular characterization of pyrethroid-resistance horn flies, Haematobia irritans: identification of kdr and super-kdr point mutations. Insect biochem.mol.biol. 1997;27:745–755. doi: 10.1016/s0965-1748(97)00057-x. [DOI] [PubMed] [Google Scholar]
- 26.Schuler TH, Martinez-Torres D, Thompson AJ, Denholm I, Devonshire AL, et al. Toxicological, electrophysiological, and molecular characterization of knock-down resistance to pyrethroid insecticides in the diamondback moth, Plutella xylostella (L). Pestic biochem physiol. 1998;59:169–192. [Google Scholar]
- 27.Lee SH, Smith TJ, Knipple DC, Soderlund DM. Mutations in the house fly Vsscl sodium channel gene associated with super-kdr resistance abolish the pyrethroid sensitivity of Vsscl/tip E sodium channels expressed in Xenopus oocytes. Insect biochem.mol.biol. 1999;29:185–194. doi: 10.1016/s0965-1748(98)00122-2. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Torres D, Foster SP, Devonshire AL, Devonshire AL, Williamson MS. A sodium channel point mutation is associated with resistance to DDT and pyrethroid insecticides in the peach-potato aphid, Myzus persicae (Sulzer) (Hempitera: Aphididae). Insect mol.biol. 1999a;8:339–346. doi: 10.1046/j.1365-2583.1999.83121.x. [DOI] [PubMed] [Google Scholar]
- 29.Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J, et al. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anpheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol Biol. 2000;9:491–497. doi: 10.1046/j.1365-2583.2000.00209.x. [DOI] [PubMed] [Google Scholar]
- 30.Verhaeghen K, Van Bortel W, Roelants P, Backeljau T, Coosemans M. Detection of the East and West African kdr mutation in Anopheles gambiae and Anopheles arabiensis from Uganda using a new assay based on FRET/Melt Curve analysis. Malaria Journal. 2006;5:16. doi: 10.1186/1475-2875-5-16. doi: 10.1186/1475-2875-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, Zhong D, Zhang D, Shi L, Zhou G, et al. Molecular Ecology of Pyrethroid Knockdown Resistance in Culex pipiens pallens Mosquitoes. PloS ONE. 2010;5:1–9. doi: 10.1371/journal.pone.0011681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Y, Qu F, Xu J, Zheng Z. Differentiation of Anopheles sinensis and Anopheles anthropophagus using a ribosomal DNA PCR assay. Academic Journal of Second Military Medical University. 1998;19:237–239. [Google Scholar]
- 33.Collins FH, Mendez MZ, Rasmussen MO, Mehaffey PC, Besansky NJ, et al. A ribosomal RNA gene probe differentiates member species of the Anopheles gambiae complex. Am J Trop Med Hyg. 1987;37:37–41. doi: 10.4269/ajtmh.1987.37.37. [DOI] [PubMed] [Google Scholar]
- 34.Jamroz RC, Guerrero FD, Kammlah DM, Kunz SE. Role of the kdr and super-kdr sodium channel mutations in pyrethroid resistance: correlation of allelic frequency to resistance level in wild and laboratory populations of horn flies (Haematobia irritans). Insect Biochem Mol Biol. 1998;28:1031–1037. doi: 10.1016/s0965-1748(98)00094-0. [DOI] [PubMed] [Google Scholar]
- 35.Zhang AG, Dunn JB, Clark JM. An efficient strategy for validation of a point mutation associated with acetylcholinesterase sensitivity to azinphosmethyl in Colorado potato beetle. Pestic Biochem Physiol. 1999;65:25–35. [Google Scholar]
- 36.Song F, Cao X, Zhao T, Dong Y, Lu B. Pyrethroid resistance and distribution of kdr allele in Culex pipiens pallens in north China. International journal of Pest Management. 2007;53:25–34. [Google Scholar]
- 37.Li Z, Yang D, Ding J, Zhang J, Pan L, et al. Resistance of mosquitoes to insecticide. Chinese Journal of Hygienic Insecticides and Equipments. 2007;13:256–258. [Google Scholar]
- 38.Soderlund DM, Knipple DC. The molecular biology of knockdown resistance to pyrethroid insecticide. Insect biochem.mol.biol. 2003;33:563–577. doi: 10.1016/s0965-1748(03)00023-7. [DOI] [PubMed] [Google Scholar]
- 39.Santolamazza F, Calzetta M, Etang J, Barrese E, Dia I, et al. Distribution of knock-down resistance mutations in Anopheles gambiae molecular forms in west and west-central Afria. Malaria Journal. 2008;7:74. doi: 10.1186/1475-2875-7-74. doi: 10.1186/1475-2875-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wondji CS, Priyanka De Silva WAP, Hemingway J, Ranson H, Parakrama Karunaratne SH, et al. Characterization of knockdown resistance in DDT-and pyrethroid-resistant Culex quinquefasciatus populations from Sri Lanka. Trop Med Int Health. 2008;13:548–555. doi: 10.1111/j.1365-3156.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 41.Martinez-Torres D, Chevillon C, Brun-Barale A, Berge JB, Pasteur N, et al. Voltage-dependent Na+ channels in pyrethroid resistant Culex pipiens L mosquitoes. Pestic Sci. 1999;55:1012–1020. [Google Scholar]
- 42.McAbee RD, Kang KD, Stanich MA, Christiansen JA, Wheelock CE, et al. Pyrethroid tolerance in Culex pipiens pipiens var molestus from Marin County, California. Pest Management Science. 2004;60:359–368. doi: 10.1002/ps.799. [DOI] [PubMed] [Google Scholar]
- 43.Xu Q, Liu H, Zhang L, Liu N. Mechanisms of permethrin resistance in mosquitoes, Culex quinquefasciatus (S.). Pest Management Science. 2005;61:1096–1102. doi: 10.1002/ps.1090. [DOI] [PubMed] [Google Scholar]
- 44.Vais H, Williamson MS, Goodson SJ, Devonshire AL, Warmke JW, et al. Activation of Drosophila sodium channels promotes modification by deltamethrin. Reductions in affinity caused by knock-down resistance mutations. F Gen Physiol. 2000;11:305–318. doi: 10.1085/jgp.115.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lynd A, Ranson H, McCall PJ, Randle NP, Black WC, et al. A simplified high-throughput method for pyrethroid knock-down resistance (kdr) detection in Anopheles gambiae. Malaria J. 2005;4:16. doi: 10.1186/1475-2875-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yewhalaw D, Wassie F, Steurbaut W, Spanoghe P, Van Bortel W, et al. Multiple Insecticide Resistance:An impediment to Insectcide-Based Malaria Vector Control Program. PLoS ONE. 2011;6(1):e16066. doi: 10.1371/journal.pone.0016066. doi: 10.1371/journal.pone.0016066. [DOI] [PMC free article] [PubMed] [Google Scholar]