Abstract
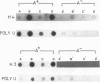
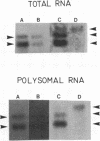
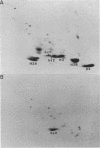
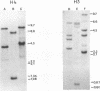
Heterologous probes for yeast H4 and H3 histone genes have been used to study the corresponding histone mRNAs in growing and starved Tetrahymena. Histone mRNAs in both physiological states are polyadenylated. Two types of H4 protein and two types of H3 protein have previously identified in Tetrahymena. Two size classes of H4 messages and three classes of H3 messages have been detected by northern analyses. Southern blot analysis indicate that the number of different kinds of H3 and H4 genes is the same or slightly greater than the number of different messages, suggesting that each message is derived from a different gene. Growing cells have -30 times more histone mRNA than starved cells, even though their total mRNA content is only 4 times greater. The relative abundance of different H4 and H3 messages in growing and starved cells is different, demonstrating that the different messages for a particular type of histone are regulated non-coordinately. In starved cells the presence of a single size class of H3 messages correlates with the preferential synthesis of a previously described macronuclear-specific H3 variant. The fraction of histone messages loaded in growing and starved cells is the same as for bulk mRNAs, and the relative concentrations of the multiple messages for H4 and H3 are the same in polysomal and total RNAs of each cell type. These observations suggest that histone synthesis in Tetrahymena is controlled largely at the level of message abundance, and that very little, if any, control occurs at the translational level.
Full text
PDF






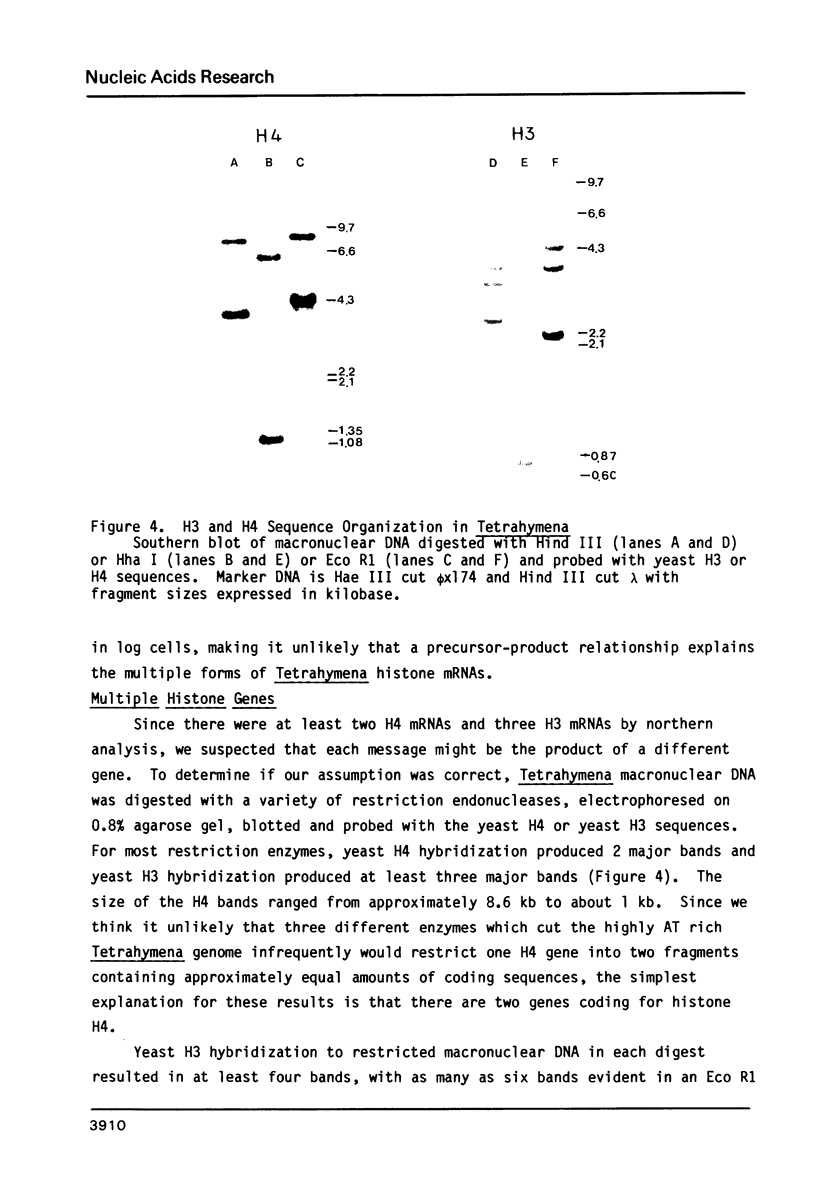

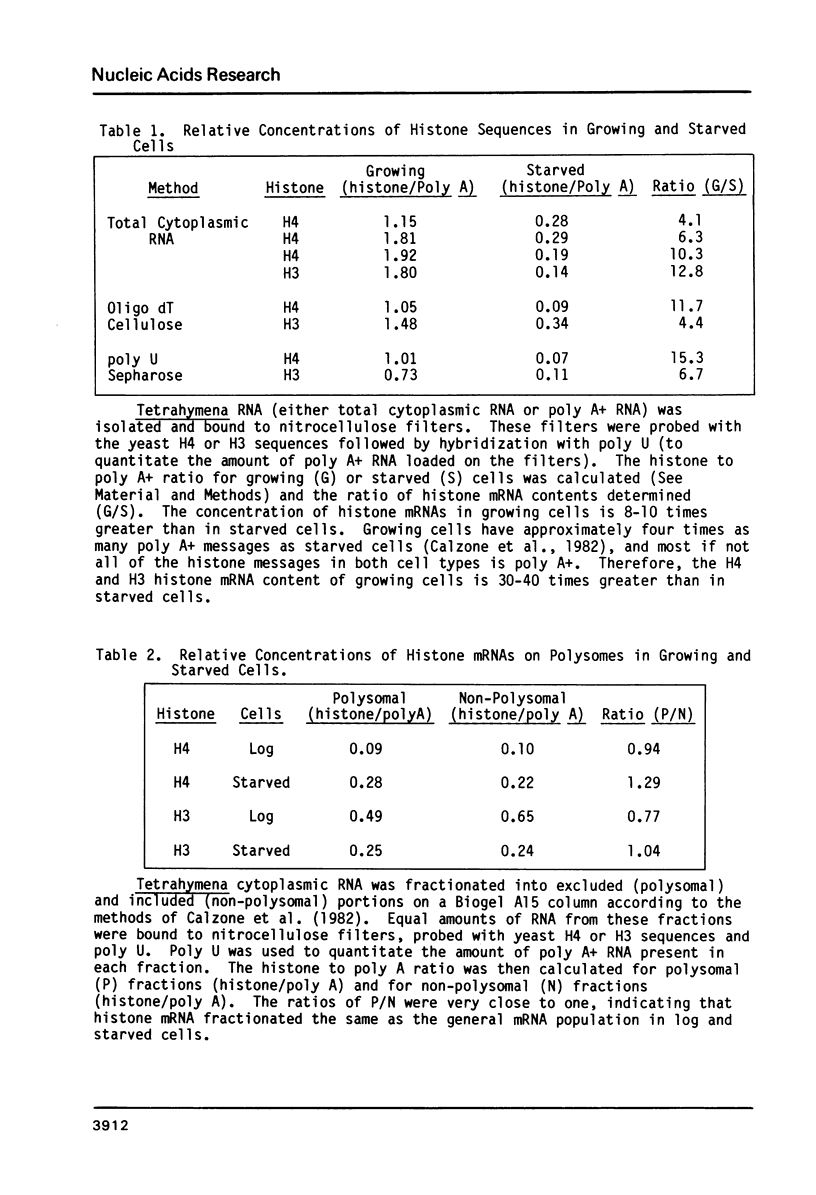





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allis C. D., Glover C. V., Bowen J. K., Gorovsky M. A. Histone variants specific to the transcriptionally active, amitotically dividing macronucleus of the unicellular eucaryote, Tetrahymena thermophila. Cell. 1980 Jul;20(3):609–617. doi: 10.1016/0092-8674(80)90307-4. [DOI] [PubMed] [Google Scholar]
- Allis C. D., Glover C. V., Gorovsky M. A. Micronuclei of Tetrahymena contain two types of histone H3. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4857–4861. doi: 10.1073/pnas.76.10.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allis C. D., Gorovsky M. A. Histone phosphorylation in macro- and micronuclei of Tetrahymena thermophila. Biochemistry. 1981 Jun 23;20(13):3828–3833. doi: 10.1021/bi00516a025. [DOI] [PubMed] [Google Scholar]
- Calzone F. J., Angerer R. C., Gorovsky M. A. Regulation of protein synthesis in Tetrahymena: isolation and characterization of polysomes by gel filtration and precipitation at pH 5.3. Nucleic Acids Res. 1982 Mar 25;10(6):2145–2161. doi: 10.1093/nar/10.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Fahrner K., Yarger J., Hereford L. Yeast histone mRNA is polyadenylated. Nucleic Acids Res. 1980 Dec 11;8(23):5725–5737. doi: 10.1093/nar/8.23.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovsky M. A., Glover C., Johmann C. A., Keevert J. B., Mathis D. J., Samuelson M. Histones and chromatin structure in Tetrahymena macro- and micronuclei. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):493–503. doi: 10.1101/sqb.1978.042.01.052. [DOI] [PubMed] [Google Scholar]
- Grunstein M. Hatching in the sea urchin Lytechinus pictus is accompanied by a shift in histone H4 gene activity. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4135–4139. doi: 10.1073/pnas.75.9.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Schedl P. Isolation and sequence analysis of sea urchin (Lytechinus pictus) histone H4 messenger RNA. J Mol Biol. 1976 Jun 25;104(2):323–349. doi: 10.1016/0022-2836(76)90275-8. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Schedl P., Kedes L. Sequence analysis and evolution of sea urchin (Lytechinus pictus and Strongylocentrotus purpuratus) histone H4 messenger RNAs. J Mol Biol. 1976 Jun 25;104(2):351–369. doi: 10.1016/0022-2836(76)90276-x. [DOI] [PubMed] [Google Scholar]
- Hereford L. M., Osley M. A., Ludwig T. R., 2nd, McLaughlin C. S. Cell-cycle regulation of yeast histone mRNA. Cell. 1981 May;24(2):367–375. doi: 10.1016/0092-8674(81)90326-3. [DOI] [PubMed] [Google Scholar]
- Hereford L., Bromley S., Osley M. A. Periodic transcription of yeast histone genes. Cell. 1982 Aug;30(1):305–310. doi: 10.1016/0092-8674(82)90036-8. [DOI] [PubMed] [Google Scholar]
- Isenberg I. Histones. Annu Rev Biochem. 1979;48:159–191. doi: 10.1146/annurev.bi.48.070179.001111. [DOI] [PubMed] [Google Scholar]
- Kedes L. H. Histone genes and histone messengers. Annu Rev Biochem. 1979;48:837–870. doi: 10.1146/annurev.bi.48.070179.004201. [DOI] [PubMed] [Google Scholar]
- Lichtler A. C., Detke S., Phillips I. R., Stein G. S., Stein J. L. Multiple forms of H4 histone mRNA in human cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1942–1946. doi: 10.1073/pnas.77.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtler A. C., Sierra F., Clark S., Wells J. R., Stein J. L., Stein G. S. Multiple H4 histone mRNAs of HeLa cells are encoded in different genes. Nature. 1982 Jul 8;298(5870):195–198. doi: 10.1038/298195a0. [DOI] [PubMed] [Google Scholar]
- Lichtler A. C., stein G. S., Stein J. L. Isolation and characterization of two mRNAs from HeLa S3 cells coding for histone H4. Biochem Biophys Res Commun. 1977 Aug 8;77(3):845–853. doi: 10.1016/s0006-291x(77)80055-7. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marashi F., Baumbach L., Rickles R., Sierra F., Stein J. L., Stein G. S. Histone proteins in HeLa S3 cells are synthesized in a cell cycle stage specific manner. Science. 1982 Feb 5;215(4533):683–685. doi: 10.1126/science.7058333. [DOI] [PubMed] [Google Scholar]
- Newrock K. M., Alfageme C. R., Nardi R. V., Cohen L. H. Histone changes during chromatin remodeling in embryogenesis. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):421–431. doi: 10.1101/sqb.1978.042.01.045. [DOI] [PubMed] [Google Scholar]
- Raff R. A., Brandis J. W., Huffman C. J., Koch A. L., Leister D. E. Protein synthesis as an early response to fertilization of the sea urchin egg: a model. Dev Biol. 1981 Sep;86(2):265–271. doi: 10.1016/0012-1606(81)90184-6. [DOI] [PubMed] [Google Scholar]
- Rickles R., Marashi F., Sierra F., Clark S., Wells J., Stein J., Stein G. Analysis of histone gene expression during the cell cycle in HeLa cells by using cloned human histone genes. Proc Natl Acad Sci U S A. 1982 Feb;79(3):749–753. doi: 10.1073/pnas.79.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruderman J. V., Woodland H. R., Sturgess E. A. Modulations of histone messenger RNA during the early development of Xenopus laevis. Dev Biol. 1979 Jul;71(1):71–82. doi: 10.1016/0012-1606(79)90083-6. [DOI] [PubMed] [Google Scholar]
- Sanders M. M. Identification of histone H2b as a heat-shock protein in Drosophila. J Cell Biol. 1981 Nov;91(2 Pt 1):579–583. doi: 10.1083/jcb.91.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Digan M. E., Mahowald A. P., Scott M., Craig E. A. Two clusters of genes for major chorion proteins of Drosophila melanogaster. Cell. 1980 Apr;19(4):905–914. doi: 10.1016/0092-8674(80)90082-3. [DOI] [PubMed] [Google Scholar]
- Vavra K. J., Allis C. D., Gorovsky M. A. Regulation of histone acetylation in Tetrahymena macro- and micronuclei. J Biol Chem. 1982 Mar 10;257(5):2591–2598. [PubMed] [Google Scholar]
- Wells D. E., Showman R. M., Klein W. H., Raff R. A. Delayed recruitment of maternal histone H3 mRNA in sea urchin embryos. Nature. 1981 Jul 30;292(5822):477–478. doi: 10.1038/292477a0. [DOI] [PubMed] [Google Scholar]
- Woodard J., Kaneshiro E., Gorovsky M. A. Cytochemical studies on the problem of macronuclear subnuclei in tetrahymena. Genetics. 1972 Feb;70(2):251–260. doi: 10.1093/genetics/70.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R. S., Bonner W. M. Separation of basal histone synthesis from S-phase histone synthesis in dividing cells. Cell. 1981 Dec;27(2 Pt 1):321–330. doi: 10.1016/0092-8674(81)90415-3. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Tsai S., Bonner W. M. Patterns of histone variant synthesis can distinguish G0 from G1 cells. Cell. 1982 Dec;31(2 Pt 1):367–374. doi: 10.1016/0092-8674(82)90130-1. [DOI] [PubMed] [Google Scholar]