Abstract
Heteroduplex DNA molecules which contain both the wild-type and mutant sequences of a deletion nonhomology possess a characteristic electrophoretic mobility in agarose and can be readily separated from both the wild-type and deletion-containing parental homoduplex fragments. Because of the partial single stranded character of these deletion-containing heteroduplex molecules, they are selectively bound to nitrocellulose filters, and once bound, can be selectively detected by hybridization with radioactively labeled single-stranded DNA which is homologous to the sequences absent in the deletion mutation.
Full text
PDF



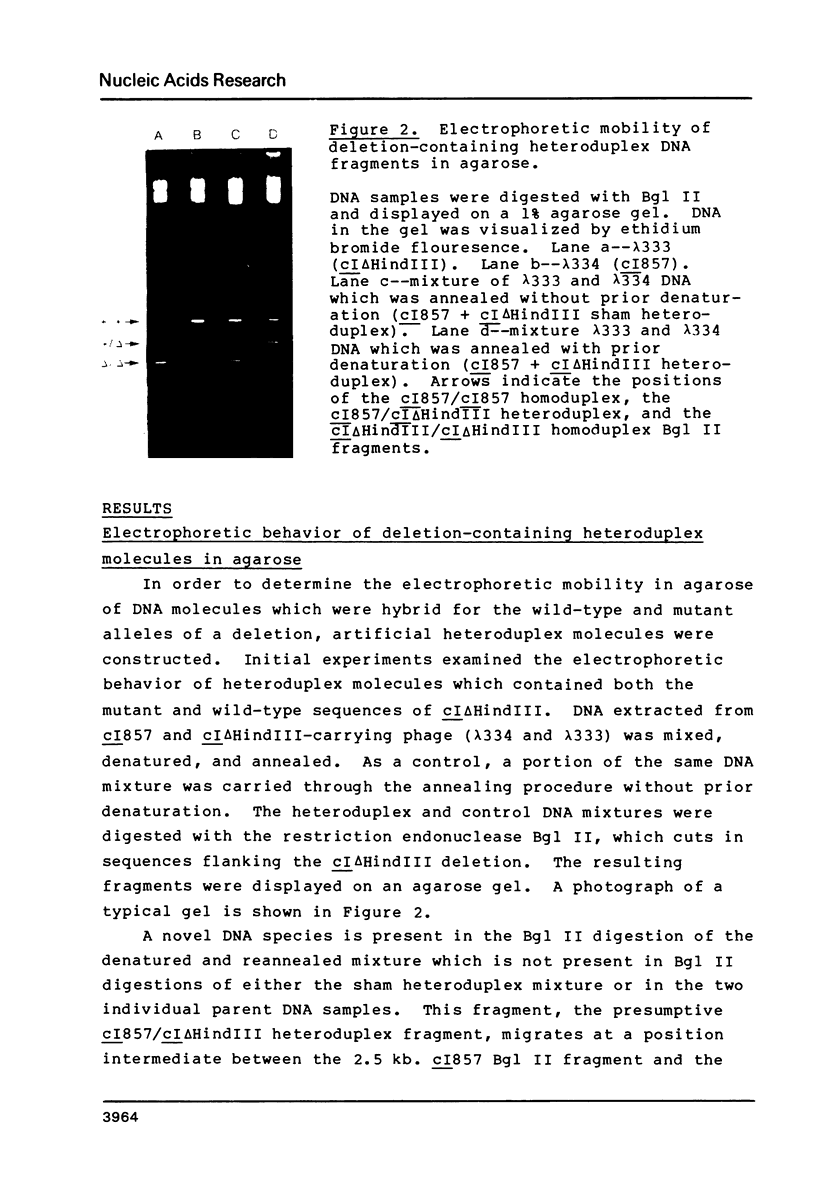






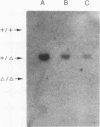
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaij C., Borst P. The gel electrophoresis of DNA. Biochim Biophys Acta. 1972 May 10;269(2):192–200. doi: 10.1016/0005-2787(72)90426-1. [DOI] [PubMed] [Google Scholar]
- Backman K., Ptashne M. Maximizing gene expression on a plasmid using recombination in vitro. Cell. 1978 Jan;13(1):65–71. doi: 10.1016/0092-8674(78)90138-1. [DOI] [PubMed] [Google Scholar]
- Berger H., Warren A. J. Effects of deletion mutations on high negative interference in T4D bacteriophage. Genetics. 1969 Sep;63(1):1–5. doi: 10.1093/genetics/63.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Claverys J. P., Roger M., Sicard A. M. Excision and repair of mismatched base pairs in transformation of Streptococcus pneumoniae. Mol Gen Genet. 1980 Apr;178(1):191–201. doi: 10.1007/BF00267229. [DOI] [PubMed] [Google Scholar]
- Doermann A. H., Parma D. H. Recombination in bacteriophage T4. J Cell Physiol. 1967 Oct;70(2 Suppl):147–164. doi: 10.1002/jcp.1040700411. [DOI] [PubMed] [Google Scholar]
- Fink G. R., Styles C. A. Gene conversion of deletions in the his4 region of yeast. Genetics. 1974 Jun;77(2):231–244. doi: 10.1093/genetics/77.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. P., Dingman C. W. Role of molecular conformation in determining the electrophoretic properties of polynucleotides in agarose-acrylamide composite gels. Biochemistry. 1971 May 11;10(10):1895–1899. doi: 10.1021/bi00786a026. [DOI] [PubMed] [Google Scholar]
- Lataste H., Claverys J. P., Sicard A. M. Physical and genetic characterization of deletions in Streptococcus pneumoniae. J Bacteriol. 1980 Oct;144(1):422–424. doi: 10.1128/jb.144.1.422-424.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. W., Sherman F., Jackson M., Gilmore R. A. Mapping and gene conversion studies with the structural gene for iso-1-cytochrome C in yeast. Genetics. 1975 Dec;81(4):615–629. doi: 10.1093/genetics/81.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichten M., Fox M. S. Effects of nonhomology on bacteriophage lambda recombination. Genetics. 1983 Jan;103(1):5–22. doi: 10.1093/genetics/103.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin G. J., Szybalski W., Blattner F. R. Asymmetric effects of deletions and substitutions on high negative interference in coliphage lambda. Genetics. 1982 Nov;102(3):299–317. doi: 10.1093/genetics/102.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy M. H., Fiandt M., Szybalski W. Electron microscopy of polar insertions in the lac operon of Escherichia coli. Mol Gen Genet. 1972;119(3):207–222. doi: 10.1007/BF00333859. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- St John T. P., Davis R. W. The organization and transcription of the galactose gene cluster of Saccharomyces. J Mol Biol. 1981 Oct 25;152(2):285–315. doi: 10.1016/0022-2836(81)90244-8. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. L., Fox M. S. On the molecular basis of high negative interference. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1544–1548. doi: 10.1073/pnas.71.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]