Abstract
We investigated the association between repeat polymorphism in intron 4 of the tyrosine hydroxylase (TH) gene and two personality traits, activity-impulsivity and inattention, in German Shepherd Dogs. The behaviour of 104 dogs was characterized by two instruments: (1) the previously validated Dog-Attention Deficit Hyperactivity Disorder Rating Scale (Dog-ADHD RS) filled in by the dog owners and (2) the newly developed Activity-impulsivity Behavioural Scale (AIBS) containing four subtests, scored by the experimenters. Internal consistency, inter-observer reliability, test-retest reliability and convergent validity were demonstrated for AIBS.
Dogs possessing at least one short allele were proved to be more active-impulsive by both instruments, compared to dogs carrying two copies of the long allele (activity-impulsivity scale of Dog-ADHD RS: p = 0.007; AIBS: p = 0.023). The results have some potential to support human studies; however, further research should reveal the molecular function of the TH gene variants, and look for the effect in more breeds.
Introduction
Canine and human behaviour were shaped by similar evolutionary processes, therefore dogs demonstrate a complex level of similarity with humans in a set of functionally shared behavioural features (reviewed in [1]). Accordingly, due to a mixture of both homologies and analogies at different levels of biological organisations like genes and behaviour traits, dogs could serve as a useful model for studying the genetic background of complex human behavioural diseases [2]–[7]. However, the identification of the behavioural phenotype is often difficult. A widely-used method involves relying on breed stereotypes provided by experts such as dog-trainers [8], [9], but to reveal a valid association between behaviour and genetic factors, direct and precise behavioural phenotyping at the individual level is definitely as important as accurate genotyping.
Earlier Vas et al. [10] successfully adapted a human parental ADHD questionnaire [11] for dogs. The Dog-ADHD Rating Scale (Dog-ADHD RS) showed satisfactory test-retest and inter-observer reliability, internal consistency, and external validity. This finding was recently replicated on a large, predominantly North American sample [12].
Activity refers to self-initiated movement (e.g. [13]). This feature of behaviour is usually determined by measuring the ambulation of a dog in a closed arena with gridline on the floor (“open field”; e.g. [14]). According to Gosling and John [15] level of activity has links with the Extraversion dimension of the most widely accepted map of human personality structure: the Five Factor Model (FFM).
Human impulsivity is considered as the opposite of Conscientiousness, which includes facets as self-discipline, dutifulness and impulse control [15]. In dogs these facets are related to the Responsiveness to Training trait [16], assessed by for example retrieving an object [17] and by examining the dogs' reactions and interest in its environment across a variety of situations [18]. According to our knowledge, impulsivity was not tested directly on dogs by behavioural tests.
In this study we aimed at developing a valid and reliable test battery for measuring activity and impulsivity traits in dogs. Our second goal was to identify one of the underlying genetic factors of these complex traits.
Earlier, by using the Dog-ADHD RS [10] we found that police German Shepherd Dogs (GSDs) possessing at least one 3a allele in dopamine D4 receptor (DRD4) exon 3 showed significantly higher scores on the activity-impulsivity scale than dogs lacking this allele [19]. Subsequently, we found that repeat polymorphisms at exon 3 and intron 2 of the DRD4 gene contributed to the social impulsivity of pet GSDs [20]. Social impulsivity is manifested in approaching and following behaviour while encountering a friendly, unfamiliar experimenter.
Tyrosine hydroxylase (TH) is catalyzing the conversion of the precursor of dopamine (dihydroxyphenylalanine, DOPA). Dopamine is the precursor of the catecholamines norepinephrine (noradrenaline) and epinephrine (adrenaline). Dopamine is involved in the brain's reward system, and has many other functions in cognition, movement control, and attention [21]. Norepinephrine as a neurotransmitter plays a role in attention and focus and together with dopamine, they may be implicated in ADHD [22]. However, TH is found not only in the brain but in all cells containing catecholamines (“fight-or-flight” hormones), too.
The human TH gene is associated with mood disorders [23]–[24], personality factors [25] and may be involved in hypertension [26].
There is a high homology between the dog and human TH gene, and significant variations of the allelic frequencies among dog breeds suggest that the polymorphism of this gene is probably an important marker for the genetic background of behavioural characteristics in dogs [27]. Based on the metabolic pathways of TH, we suppose that it could be involved in the activity-impulsivity trait of dogs.
For the present study we developed a new test battery (Activity-Impulsivity Behavioural Scale, AIBS) for measuring canine activity-impulsivity. We investigated whether the activity-impulsivity trait in dogs measured by owners' report (Dog-ADHD RS [10]) and behaviour tests are affected by a recently reported TH intron 4 repeat polymorphism [28]. In this repeat polymorphism a 36-bp-long sequence in the intron 4 region of the TH gene is reiterated once (duplicated) or not reiterated at all. In case of the reiterated form of the microsatellite it consists of 334 bp (‘allele 2’), the single copy form spans 298 basepairs (‘allele 1’). The frequency of alleles varies among breeds, at least within the four breeds and the gray wolf (Canis lupus) investigated up to now. For example, the frequency of allele 2 is 31% in Groenandaels, 0.89% in German shepherds, and 0.73% in wolves. The polymorphic region is 92 basepairs away from the 5′ end of the intron. The biological function of the repeat variation is supposed to be the modulation of the splicing, as the polymorphism strongly affects the size of the intron. Moreover, based on the localization both the 5′ point and the branch point might be affected, thus the efficiency of the splicing of the two different variants is probably different.
The analysis was carried out within a single breed, the German Shepherd Dog. This breed is popular both as working (guide dog for the blinds, police dog, guarding and protection) and as pet dog. Regarding pet German Shepherds, a recent comparison of Hungarian and American (USA) dog behaviour and dog-keeping practices is also available, suggesting that activity-impulsivity scale of the Dog-ADHD RS is probably not associated with country, therefore the results could be generalized [29]. Age, sex and the level of training was also considered during the analysis as these variables may affect activity-impulsivity [10].
Methods
Ethics statement
No special permission for use of dogs in such non-invasive studies is required in Hungary. The relevant committee that allow to conduct research without special permissions regarding animals is: University Institutional Animal Care and Use Committee. (UIACUC, Eötvös Loránd University, Hungary). Owners attending dog training schools or responding to our advertisement at the department's homepage (http://kutyaetologia.elte.hu) volunteered to participate. Genetic analyses and behavioural testing of the animals were approved by the owners. The buccal smears were collected by the experimenters: Judit Vas, Ildikó Brúder, Borbála Turcsán and Enikő Kubinyi.
The persons shown in the photo gave written consent to the publication of the photo.
Subjects
104 German Shepherd Dogs were involved in the study. Fifty-six dogs were males, 48 females; age range: 1–13 years, mean age: 3.92 years, SD = 2.7. 6% of male and 32% of female dogs were neutered. 58% of owners were women, 42% men; age range: 16–57 years, mean age: 32.7 years, SD = 11.8. 16.3% of the dogs had no formal training, 13.5% had basic training, 52.9% had one specialized training (e.g. rescue, agility), 17.3% had at least two specialized training. None of the subjects were closely related, i.e. littermate and parent-offspring relationships were excluded. Nine owners had two dogs in the sample. Owners attending dog training schools or responding to our advertisement at the department's homepage (http://kutyaetologia.elte.hu) volunteered to participate. Genetic analyses and behavioural testing of the animals were approved by the owners.
DNA sampling and genotyping
Buccal smears were collected, and DNA was isolated with the Gentra purification kit (Valencia, CA). Repeat polymorphisms in the TH intron 4 were analyzed according to the procedure previously described in [28]. Shortly: The PCR reaction mixture contained 1 µM of each primer, approximately 5 ng of DNA template, 200 µM dATP, dCTP, dTTP, 100 µM of dGTP and dITP, 0.025 U HotStarTaq DNA polymerase 1x buffer and 1x Q-solution supplied by the Qiagen HotStarTaq polymerase kit in a 10 µl final volume. Conditions of the PCR cycle and the separation of PCR products by gel electrophoreses were as described before [28].
Phenotyping
The behaviour of dogs were characterised by two instruments: Dog-ADHD Rating Scale [10], and the Activity-Impulsivity Behavioural Scale (AIBS).
I. Dog-ADHD Rating Scale (Dog-ADHD RS)
The Dog-ADHD RS was completed by the owner before the tests, in the presence of the experimenter. The questionnaire consists of two subscales. Seven items compose the activity-impulsivity scale (for example: ‘Your dog fidgets all the time’) and six items make up the inattention scale (for example: ‘It is difficult for your dog to concentrate on a task or play’). The scale scores were calculated for each dog as the mean of the scores given by the owner on a 4 point scale (from 0: never to 3: very often) [10].
II. Activity-impulsivity Behavioural Scale (AIBS)
Dogs were observed in a test battery conducted outdoors, on a remote area of a dog training school or at a quiet location on the campus of the Eötvös Loránd University 50 m away from the nearest building. The owner, a female experimenter and a camera-woman were present. The experimenter assessed the behaviour of the dogs at the testing location by filling in a score-sheet. The test-retest reliability of the AIBS was measured by retesting 14 dogs and comparing the test and retest AIBS scores. Retesting has been applied in a 1 week long dog camp. Interval between test and retest varied between 2 and 6 days. Owners were randomly asked to participate in the retesting. Inter-observer reliability was assessed by comparing the AIBS scores of the experimenter who scored the behaviour at the testing location, and of an independent coder, a trained biology MSc student, assessing 28 dogs from the video-recordings.
Testing protocol (Figure 1)
Figure 1. Illustrations for the subtests.
A) Spontaneous activity: The owner stands still while holding the dog on a leash. B) Separation and play: The dog is tethered to a tree, while the owner is hiding behind a tree. C) Lying on the side: The owner commands the dog to lie down. Then owner crouches, turns the dog on the side and keeps the dog in this position for 30 seconds. D) Approaching the owner: The experimenter holds the dog on the leash, meanwhile the owner hides behind a large tree. After 30 seconds, the experimenter releases the dog.
The order of sub-tests was fixed.
1. Spontaneous activity
The owner (O) stands still without paying special attention to the dog, while holding the dog on a leash (1.5-2 m). The dog is allowed to move freely within the range of the stretched leash and is not corrected or rewarded for any behaviour. This test lasts for 1 minute. Experimenter (E) stays at a distance of at least 3 m from the dog without paying any attention to the dog.
2. Separation and play
The dog is tethered to a tree on a 3 m leash, while the O is hiding behind an object (e.g. a tree) 5-6 m from the dog, which blocks the dog from seeing the O. After 1 min has elapsed the E approaches the dog and initiates play with a tug (tug of war) for 30 s, and at the end she steps back to the camera. After 1 minute elapses E asks the O to come back.
3. Lying on the side
O commands the dog to lie down. Then O crouches (down next to the dog) and turns the dog on the dog's side. O tries to keep the dog in this position for 30 seconds. If the dog gets up before the 30 seconds elapses, then the test restarts. Petting is allowed. If the dog refuses to lie on the side, or gets up again during the second try, the test is terminated after 60 seconds.
4. Approaching the owner
E holds the dog on leash, and O is asked to hide behind a large tree or a house 15-20 m away from the dog. After 30 seconds, E releases the dog and says ”Go!”. If the dog does not start to move at once E gently by touches the rear end. If the dog does not approach the owner within 5 seconds, the E asks O to call the dog.
Behavioural variables
All variables were coded on a 0-3 scale.
Duration of moving the legs during the (1) Spontaneous activity test, (2) before the experimenter plays with the dog during the Separation and play test (1 min), and (3) after the experimenter plays with the dog during the Separation and play test (1 min): (0) no moving, (1) less than half of the time, (2) more than half of the time, (3) continuously.
(4) Latency of lying down in the Lying on the side test: (0) immediate lying down, (1) 1-14 s, (2) 15-30 s, (3) if the owner could not make the dog lie down on the side.
(5) Duration of vocalization in the Approaching the owner test: (0) no vocalization, (1) less than half of the time, (2) more than half of the time, (3) continuously.
(6) Latency to approach the owner in the Approaching the owner test: (0) no approach, (1) approach after calling, (2) approach in less than 5 sec, (3) immediate approach.
Statistical analysis
SPSS for Windows was used for all statistical analyses. Cronbach's alpha was calculated to assess the internal consistency of the AIBS variables. Inter-observer and test-retest reliability was computed using intraclass correlation coefficient (ICC 1,1, one-way random single measures). Pearson correlation test was applied for investigating the links between the Dog-ADHD RS and AIBS. Multivariate General Linear model (MANCOVA) tested the main effects of independent variables (age, sex, training, TH genotype) on the Dog-ADHD RS and AIBS. Age was included as covariate, sex, training and TH genotype were fixed factors. Non-significant effects were removed through backward elimination. ANOVA with SNK post hoc test was used to investigate differences associated with trainings.
Results
Validity and reliability of the AIBS
The Cronbach's alpha was 0.68, satisfactorily high for the six behavioural variables. The mean scores of the Dog-ADHD RS subscales and the AIBS behavioural variables are presented in Table 1 and Table 2. For further analysis a scale score was calculated for each dog as the mean of the six variable scores.
Table 1. Mean scores of the Dog-ADHD RS subscales.
| Subscales | Mean score (SD) |
| Activity-impulsivity | 1.13 (0.59) |
| Inattention | 0.91 (0.46) |
Table 2. Mean scores of the AIBS behavioural variables.
| Variables | Mean score (SD) |
| Duration of moving the legs in ‘Spontaneous activity’ | 1.47 (1.03) |
| Duration of moving the legs before the exp. plays with the dog in ‘Separation and play’ | 1.13 (0.81) |
| Duration of moving the legs after the exp. plays with the dog in ‘Separation and play’ | 0.89 (0.80) |
| Latency of lying down in ‘Lying on the side’ | 0.57 (0.95) |
| Duration of vocalization in ‘Approaching the owner’ | 0.64 (1.02) |
| Latency to approach in ‘Approaching the owner’ | 2.64 (0.75) |
ICCs between two independent coders (inter-observer reliability) was 0.70.
Test-retest reliability was 0.79. Both scales of the Dog ADHD-RS correlated with the AIBS (activity-impulsivity: Pearson r = 0.53, p<0.001; inattention: Pearson r = 0.24, p<0.05), which means they assess the same construct (convergent validity). However, the correlation between AIBS and activity-impulsivity was significantly higher (z = −3.29, p<0.001).
Genotype and allele frequencies
Two alleles were present in the German Shepherd Dogs (as reported previously [28]). In the short allele (allele 1) a 36-bp-long sequence is present as a single copy. In the long allele (allele 2) the sequence is in a duplicated form.
The genotype frequency did not deviate from the Hardy-Weinberg equilibrium (χ2 1 = 1.14, p = 0.29). Two dogs (1.9%) were homozygotes for the short allele (1/1 genotype), 35 (33.7%) dogs were heterozygotes (1/2 genotype) and 67 dogs (64.4%) possessed the longer alleles exclusively (2/2 genotype). As the short allele was rare in the population, homozygotes (1/1) and heterozygotes (1/2) were combined for statistical analysis and defined as the genotype group containing individuals possessing at least one short allele.
Effect of independent variables on the scales
After removing the non-significant effects through backward elimination, we found that TH and training status remained as explanatory variables in the model (TH: F3,97 = 3.002, p = 0.034; training: F3,97 = 2.065, p = 0.033).
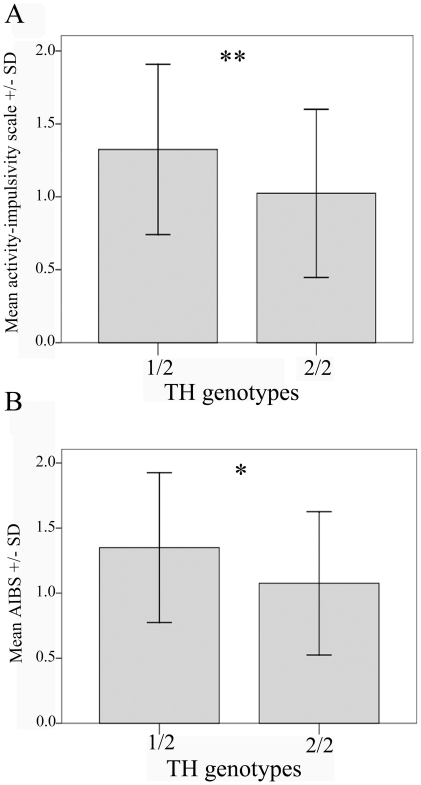
The TH polymorphism was associated with both the Dog-ADHD RS activity-impulsivity scale (F1,99 = 7.489, p = 0.007), and the AIBS (F1,99 = 5.299, p = 0.023). The genotype group containing dogs possessing at least one short allele was reported to be more active-impulsive by the owners and reached higher scores on the behavioural scale than homozygotes possessing the longer alleles exclusively (Figure 2). Omitting the rare 1/1 genotype (two dogs) did not affect the results significantly.
Figure 2. Association between tyrosine hydroxylase intron 4 genotypes and activity-impulsivity related phenotypes.
(A) Dog-ADHD RS activity-impulsivity scale; (B) Activity-Impulsivity Behavioural Scale (AIBS). 1/2 genotype represents the group of individuals possessing at least one short allele. Subjects in the 2/2 genotype possess two long alleles. Box-plot figures with sample minimum and maximum, lower and upper quartiles and medians. * indicates significant differences (* p<0.05; ** p<0.01).
The training status had effect on the inattention scale only (F3,97 = 5.136, p = 0.002). Dogs with one or two specialized training were reported by the owners to be less inattentive than individuals with basic training or not trained at all.
Discussion
The aim of this study was twofold. First, we demonstrated internal consistency, inter-observer reliability, test-retest reliability, and convergent validity of the Activity-impulsivity Behavioural Scale (AIBS), and investigated its links with the age, sex and training status of dogs.
High scores in the test were characterized by high motor activity, high latency of lying down on the side, high amount of vocalization during separation from the owner and fast approach to the hiding owner. Importantly, test-retest and inter-observer reliability indicated that the behavioural scoring system is reliably applicable at the experimental place. This effectively decreases the time needed for behavioural evaluation (i.e. the experimenter at the spot scored the dogs similarly as an independent observer did so during watching the video-recordings).
The existence of a reliable and valid test battery is crucial for measuring consistent traits [30]. Noteworthy, that the convergent validity, which was assessed by the correlation coefficient between the owner report (Dog-ADHD RS) and behaviour scores (AIBS), was higher (r = 0.54) compared to the mean correlation (r = 0.27) between personality judgements (for the Extraversion, Agreeableness, Neuroticism and Openness dimensions) and behaviour ratings of dogs in a previous study [31].
In previous research assessing a trait, questionnaire rating and behaviour coding or scoring have usually been made by the same individual, thereby compromising the independence of the two measures (but see [32]). In the present study different individuals were used as raters and coders. Coders were completely unacquainted with the subjects, while raters were the owners of the dogs. However, it is known that owners can be biased in their views of their dogs, and their ratings may be vulnerable to appearance-based stereotypes [33]. We tried to avoid this bias by using a single breed. Moreover, recent research has suggested that different levels of experience with dogs are not critical in rating the majority of behaviours ([28], [30], [31]).
Age and sex of the dogs had no effects on the scales of the Dog-ADHD RS in this study. However, training strongly affected the inattention of dogs. Highly trained dogs were reported to be less inattentive. Interestingly, activity-impulsivity trait was not affected by training. Dogs with a specialized knowledge (rescue, agility, guarding, etc) were reported and scored similarly active-impulsive as not or basic trained dogs. This suggests that activity-impulsivity does not counteract with performance during typical tasks in the GSD breed.
Our second aim was to search for associations with a genetic factor, TH polymorphism. We found that TH intron 4 polymorphism was associated with activity-impulsivity trait measured by both the Dog ADHD RS (owner report) and the AIBS (behavioural scoring). Dogs possessing at least one short allele were reported and found to be more active-impulsive, compared to animals possessing the long allele exclusively. This effect was independent of age, sex and training status of the dogs.
According to our knowledge, this is the first report of a TH-behaviour trait association in dogs. The result is in harmony with an earlier assumption that general activity might be related to the TH gene polymorphism in dogs [27]. However, we should note that the former study involved breed comparisons. Although breed comparisons could be thought-provoking, such results do not exclude the possibility that the effect is not linked to the target gene but to other breed-specific genetic or environmental factors. Within-breed phenotype-gene associations always provide stronger evidence because quantitative association studies have small effect sizes, and the characteristic genetic constitutions of the breeds could overshadow the slight effect of candidate genes. Furthermore, in contrast to [27], where four SNPs were identified, our study investigated a repeat polymorphism of the TH gene, which could be analogous with that of humans. The human TH gene contains an informative tetranucleotide repeat in 5-11 copies within intron A, which may function as a transcriptional enhancer, thus it may be directly involved in the transcriptional regulation of the TH gene [25]. This microsatellite was found to be associated with neuroticism and extraversion [27] [36], which are linked with facets of impulsivity in humans [37]. Thus our result (association between repeat polymorphism in intron of the TH gene and the activity-impulsivity trait in dogs) is in accordance with human studies. However, association studies do not provide exclusive evidence, and a replication study is required for any definite conclusion for TH effect on dog activity-impulsivity.
Our finding suggests that the dog model might help to understand the underlying genetic factors of complex traits in humans. However, further study is needed to reveal the molecular function of the TH gene variants, look for the effect in more dog breeds, and find out how activity-impulsivity trait mimics human ADHD features.
Conclusions
In this study we have investigated whether there is an association of the tyrosine hydroxylase gene with activity-impulsivity and inattention trait in a pet German Shepherd Dog population, assessed by dog owners filling in a questionnaire, and by experts scoring the behaviour of dogs performing in a test battery. We have found, that the tyrosine hydroxylase intron 4 repeat polymorphism was related to both the questionnaire and the behavioural test scale. To our knowledge, applying multiple instruments to measure a trait for detecting gene-behaviour association in animals is unique in the literature. Previous studies used either rating of traits (e.g. horse: [38], dog [39], [40] or behavioural coding exclusively (e.g. great tit: [41], rhesus macaque: [42], vervet monkey [43]).
Moreover, our study reveals new information about a popular working and pet dog breed, and could provide novel means for diagnosing canine hyperactivity. The present results also have some potential to support human studies; however, further studies should examine other personality traits involved in the activity-impulsivity of dogs, and the links with human ADHD.
Acknowledgments
The authors are grateful to Anita Illés, Bianca Jansen, Zsuzsánna Horváth and Erika Mirkó for their help in the data collection. We thank Michele Wan, Julie Hecht, Barbara Gáspár, József Haller and two anonymous referees for the useful comments on the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the EU FP7 ICT-215554 LIREC, the Hungarian Scientific Research Fund (K 84036) and the Bolyai Foundation of the Hungarian Academy of Sciences. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Topál J, Miklósi Á, Gácsi M, Dóka A, Pongrácz P, et al. The Dog as a Model for Understanding Human Social Behavior. Adv Stud Behav. 2009;39:71–116. [Google Scholar]
- 2.Sutter NB, Ostrander EA. Dog star rising: the canine genetic system. Nat Rev Genet. 2004;5:900–10. doi: 10.1038/nrg1492. [DOI] [PubMed] [Google Scholar]
- 3.Spady TC, Ostrander EA. Canine Behavioral Genetics: Pointing Out the Phenotypes and Herding up the Genes. J Human Genet. 2008;82:10–18. doi: 10.1016/j.ajhg.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karlsson EK, Lindblad-Toh K. Leader of the pack: gene mapping in dogs and other model organisms. Med Biochem. 2008;9:713–725. doi: 10.1038/nrg2382. [DOI] [PubMed] [Google Scholar]
- 5.Overall KL. Natural animal models of human psychiatric conditions: assessment of mechanism and validity. Prog Neuro-Psychopharmacol Biol Psychiat. 2000;24:727–776. doi: 10.1016/s0278-5846(00)00104-4. [DOI] [PubMed] [Google Scholar]
- 6.Ostrander EA, Galibert F, Patterson DF. Canine genetics comes of age. Trends Genet. 2000;16:117–124. doi: 10.1016/s0168-9525(99)01958-7. [DOI] [PubMed] [Google Scholar]
- 7.Parker HG, Shearin AL, Ostrander EA. Man's best friend becomes biology's best in show: genome analyses in the domestic dog. Annu Rev Genet. 2010;44:309–336. doi: 10.1146/annurev-genet-102808-115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones P, Chase K, Martin A, Davern P, Ostrander EA, et al. Single-Nucleotide-Polymorphism-Based Association Mapping of Dog Stereotypes. Genetics. 2008;179:1033–1044. doi: 10.1534/genetics.108.087866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chase K, Jones P, Ostrander EA, Lark KG. Genetic Mapping of Fixed Phenotypes: Disease Frequency as a Breed Characteristic. J Hered. 2009;1-5 doi: 10.1093/jhered/esp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vas J, Topál J, Péch E, Miklósi Á. Measuring attention deficit and activity in dogs: A new application and validation of a human ADHD questionnaire. Appl Anim Behav Sci. 2007;103:105–117. [Google Scholar]
- 11.DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. New York: Guilford Press; 1998. ADHD Rating Scale-IV: Checklist, Norms and Clinical Interpretation. [Google Scholar]
- 12.Lit L, Schweitzer JB, Iosif AM, Oberbauer AM. Owner reports of attention, activity, and impulsivity in dogs: a replication study. Behav Brain Func. 2010;6:1. doi: 10.1186/1744-9081-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hennessy MB, Voith VL, Mazzei SJ, Buttram J, Miller DD, et al. Behavior and cortisol levels of dogs in a public animal shelter, and an exploration of the ability of these measures to predict problem behavior after adoption. Appl Anim Behav Sci. 2001;73:217–233. doi: 10.1016/s0168-1591(01)00139-3. [DOI] [PubMed] [Google Scholar]
- 14.Wilsson E, Sundgren P-E. The use of a behaviour test for the selection of dogs for service and breeding, I: Method of testing and evaluating test results in the adult dog, demands on different kinds of service dogs, sex and breed differences. Appl. Anim. Behav Sci. 1997;53:279–295. [Google Scholar]
- 15.Gosling SD, John JP. Personality Dimensions in Nonhuman Animals: A Cross-Species Review. Curr Dir Psychol Sci. 1999;8:69–75. [Google Scholar]
- 16.Jones AC, Gosling SD. Temperament and personality in dogs (Canis familiaris): A review and evaluation of past research. Appl Anim Behav Sci. 2005;95:1–53. [Google Scholar]
- 17.Slabbert J, Odendaal JSD. Early prediction of adult police dog efficiency—a longitudinal study. ApplAnim Behav Sci. 1999;64:269–288. [Google Scholar]
- 18.Ruefenacht S. A behaviour test on German Shepherd dogs: heritability of seven different traits. Appl Anim Behav Sci. 2002;79:113–132. [Google Scholar]
- 19.Hejjas K, Vas J, Topal J, Szantai E, Ronai Z, et al. Association of polymorphisms in the dopamine D4 receptor gene and the activity-impulsivity endophenotype in dogs. Anim Genet. 2007;38:629–633. doi: 10.1111/j.1365-2052.2007.01657.x. [DOI] [PubMed] [Google Scholar]
- 20.Hejjas K, Kubinyi E, Ronai Z, Szekely A, Vas J, et al. Molecular and behavioral analysis of the intron 2 repeat polymorphism in the canine dopamine D4 receptor gene. Genes Brain Behav. 2009;8:330–336. doi: 10.1111/j.1601-183X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 21.Nieoullon A. Dopamine and the regulation of cognition and attention. Progress in Neurobiology. 2002;67:53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 22.Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Human Genet. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- 23.Persson ML, Wasserman D, Geijer T, Jönsson EG, Terenius L. Tyrosine hydroxylase allelic distribution in suicide attempters. Psychiat Res. 1997;72:73–80. doi: 10.1016/s0165-1781(97)00068-1. [DOI] [PubMed] [Google Scholar]
- 24.Serretti A, Macciardi F, Verga M, Cusin C, Pedrini S, et al. Tyrosine hydroxylase gene associated with depressive symptomatology in mood disorder. Am J of Med Genet. 1998;81:127–130. [PubMed] [Google Scholar]
- 25.Persson ML, Wasserman D, Jönsson EG, Bergman H, Terenius L, et al. Search for the influence of the tyrosine hydroxylase (TCAT)(n) repeat polymorphism on personality traits. Psychiat Res. 2000;95:1–8. doi: 10.1016/s0165-1781(00)00160-8. [DOI] [PubMed] [Google Scholar]
- 26.Sharma P, Hingorani A, Jia H, Ashby M, Hopper R, et al. Positive association of tyrosine hydroxylase microsatellite marker to essential hypertension. Hypertension. 1998;32:676–682. doi: 10.1161/01.hyp.32.4.676. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi Y, Hashizume C, Ha CEM, Momozawa Y, Masuda K, et al. Canine Tyrosine Hydroxylase (TH) Gene and Dopamine β -Hydroxylase (DBH) Gene: Their Sequences, Genetic Polymorphisms, and Diversities among Five Different Dog Breeds. J Vet Med Sci. 2005;67:861–867. doi: 10.1292/jvms.67.861. [DOI] [PubMed] [Google Scholar]
- 28.Hejjas K, Vas J, Kubinyi E, Sasvari-Szekely M, Miklósi A, et al. Novel repeat polymorphisms of the dopaminergic neurotransmitter genes among dogs and wolves. Mamm Genome. 2007;18:871–879. doi: 10.1007/s00335-007-9070-0. [DOI] [PubMed] [Google Scholar]
- 29.Wan M, Kubinyi E, Miklósi Á, Champagne F. A cross-cultural comparison of reports by German Shepherd owners in Hungary and the United States of America. Appl Anim Behav Sci. 2009;121:206–213. [Google Scholar]
- 30.Diederich C, Giffroy J. Behavioural testing in dogs: A review of methodology in search for standardisation. Appl Anim Behav Sci. 2006;97:51–72. [Google Scholar]
- 31.Gosling SD, Kwan VSY, John OP. A dog's got personality: a cross-species comparative approach to personality judgments in dogs and humans. J Pers Soc Psychol. 2003;85:1161–1169. doi: 10.1037/0022-3514.85.6.1161. [DOI] [PubMed] [Google Scholar]
- 32.Rooney N, Gaines S, Bradshaw J, Penman S. Validation of a method for assessing the ability of trainee specialist search dogs. Appl Anim Behav Sci. 2007;103:90–104. [Google Scholar]
- 33.Kwan VSY, Gosling SD, John OP. Anthropomorphism as a special case of social perception: A cross-species social relations model analysis of humans and dogs. Soc Cogn. 2008;26:129–142. [Google Scholar]
- 34.Tami G, Gallagher A. Description of the behaviour of domestic dog (Canis familiaris) by experienced and inexperienced people. Appl Anim Behav Sci. 2009;120:159–169. [Google Scholar]
- 35.Pongrácz P, Molnár C, Miklósi A, Csányi V. Human listeners are able to classify dog (Canis familiaris) barks recorded in different situations. J Comp Psychol. 2005;119:136–144. doi: 10.1037/0735-7036.119.2.136. [DOI] [PubMed] [Google Scholar]
- 36.Tochigi M, Otowa T, Hibino H, Kato C, Otani T, et al. Combined analysis of association between personality traits and three functional polymorphisms in the tyrosine hydroxylase, monoamine oxidase A, and catechol-O-methyltransferase genes. Neurosci Res. 2006;54:180–185. doi: 10.1016/j.neures.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Whiteside S, Lynam D. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30:669–689. [Google Scholar]
- 38.Momozawa Y, Takeuchi Y, Kusunose R, Kikusui T, Mori Y. Association between equine temperament and polymorphisms in dopamine D4 receptor gene. Mamm Genome. 2005;16:538–544. doi: 10.1007/s00335-005-0021-3. [DOI] [PubMed] [Google Scholar]
- 39.Maejima M, Inoue-murayama M, Tonosaki K, Matsuura N, Kato S, et al. Traits and genotypes may predict the successful training of drug detection dogs. Appl Anim Behav Sci. 2007;107:287–298. [Google Scholar]
- 40.Konno A, Inoue-Murayama M, Hasegawa T. Biol Letters Published online 30 March 2011; 2011. Androgen receptor gene polymorphisms are associated with aggression in Japanese Akita Inu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korsten P, Mueller JC, Hermannstadter C, Bouwman KM, Dingemanse NJ, et al. Association between DRD4 gene polymorphism and personality variation in great tits : a test across four wild populations. Mol Ecol. 2010;19:832–843. doi: 10.1111/j.1365-294X.2009.04518.x. [DOI] [PubMed] [Google Scholar]
- 42.Schwandt ML, Lindell SG, Sjöberg RL, Chisholm KL, Higley JD, et al. Gene-Environment Interactions and Response to Social Intrusion in Male and Female Rhesus Macaques. Biol Psychiat. 2010;67:323–330. doi: 10.1016/j.biopsych.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bailey JN, Breidenthal SE, Jorgensen MJ, McCracken JT, Fairbanks LA. The association of DRD4 and novelty seeking is found in a nonhuman primate model. 2007;17:23–27. doi: 10.1097/YPG.0b013e32801140f2. [DOI] [PubMed] [Google Scholar]