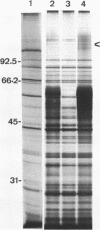
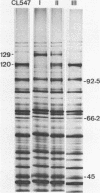
Abstract
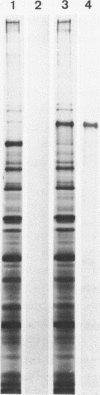
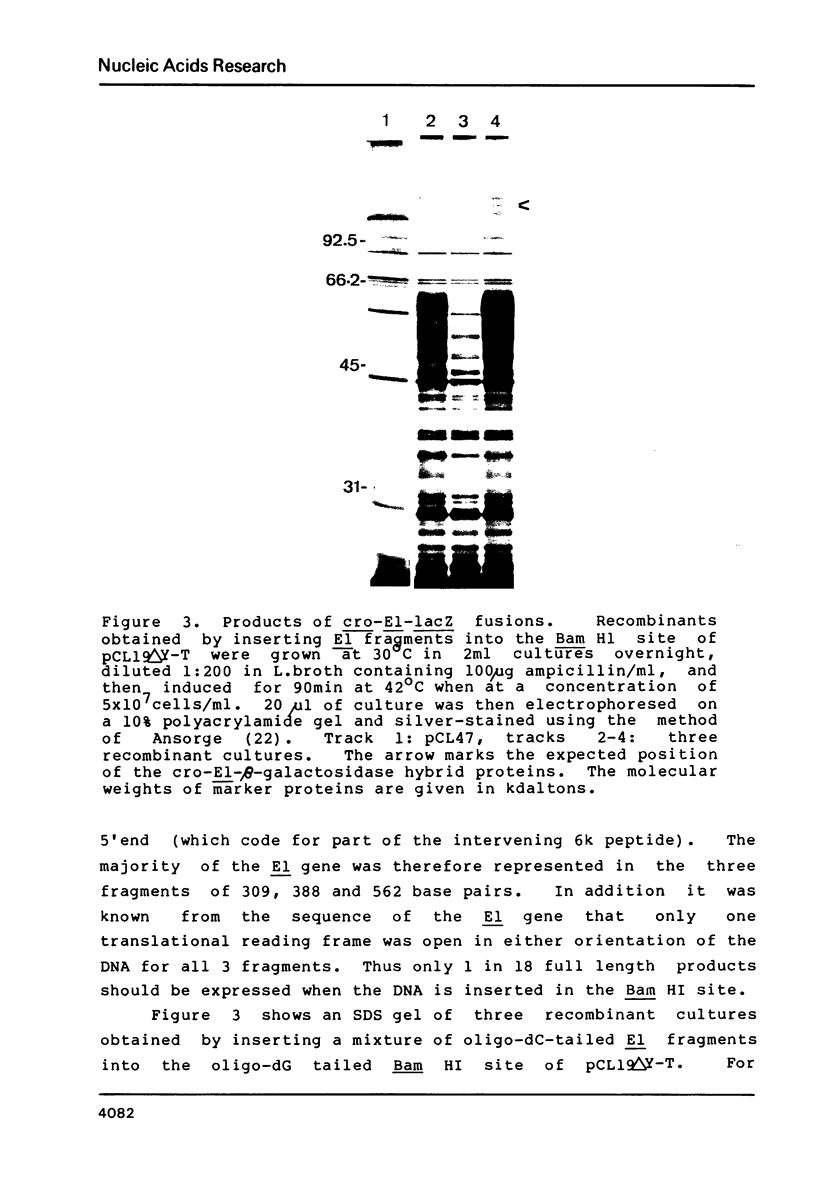
Three DNA fragments representing almost the entire E1 gene of Semliki Forest virus (SFV) were inserted into a cro-lacZ expression vector by oligo dC.oligo dG tailing. Fragments inserted close to the 5' end of the lacZ gene gave rise to hybrid proteins which were rapidly degraded. Insertion of the same fragments at the 3' end, however, resulted in the synthesis of stable hybrid proteins which precipitated in an insoluble form within the bacteria. Insufficient hybrid protein was soluble to allow detection by immunoradiometric assay. Colonies grown on nitrocellulose filters, however, could be solubilized in SDS and subsequently renatured such that antibodies raised against the intact or SDS-denatured E1 protein cross-reacted with the hybrid proteins in a high percentage of colonies. This model system demonstrates a simple procedure for identifying DNA exon fragments by the immunological detection of expressed hybrid proteins.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
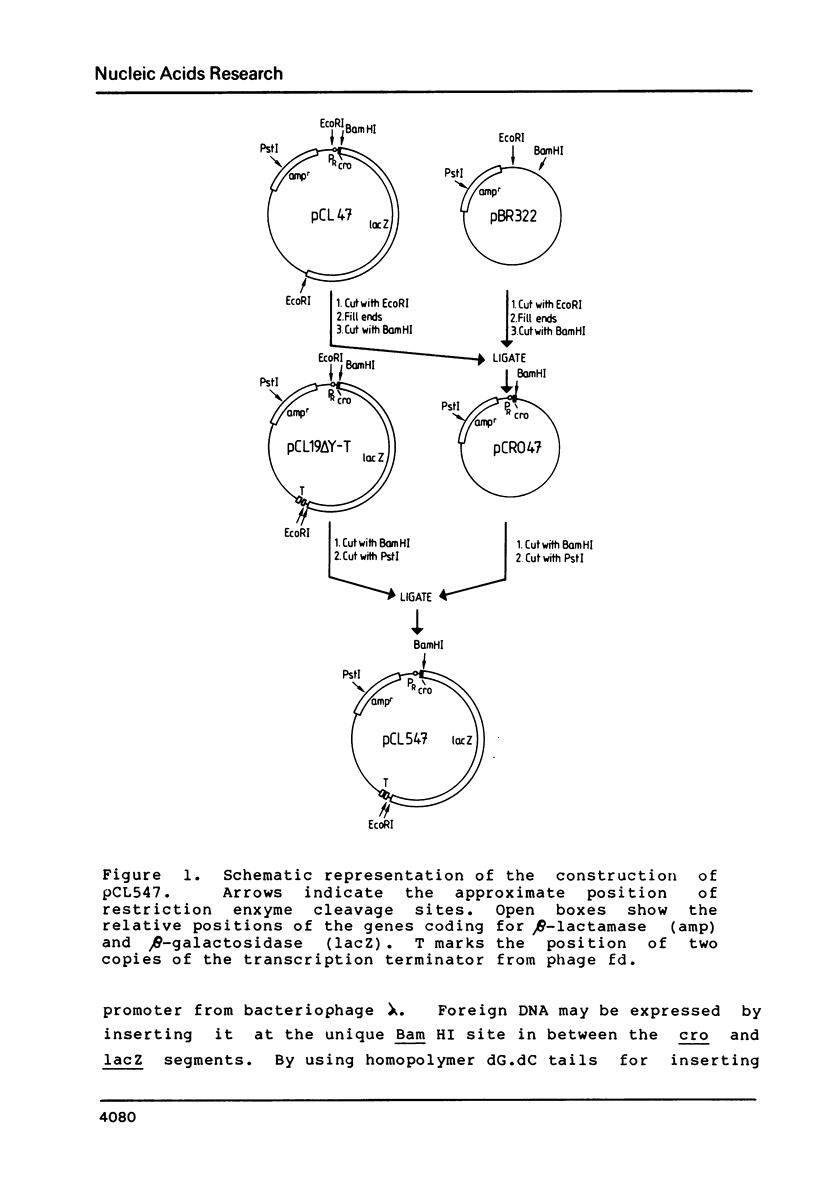
- Bernard H. U., Remaut E., Hershfield M. V., Das H. K., Helinski D. R., Yanofsky C., Franklin N. Construction of plasmid cloning vehicles that promote gene expression from the bacteriophage lambda pL promoter. Gene. 1979 Jan;5(1):59–76. doi: 10.1016/0378-1119(79)90092-1. [DOI] [PubMed] [Google Scholar]
- Broome S., Gilbert W. Immunological screening method to detect specific translation products. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2746–2749. doi: 10.1073/pnas.75.6.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Cheng Y. S., Kwoh D. Y., Kwoh T. J., Soltvedt B. C., Zipser D. Stabilization of a degradable protein by its overexpression in Escherichia coli. Gene. 1981 Jun-Jul;14(1-2):121–130. doi: 10.1016/0378-1119(81)90154-2. [DOI] [PubMed] [Google Scholar]
- Clarke L., Hitzeman R., Carbon J. Selection of specific clones from colony banks by screening with radioactive antibody. Methods Enzymol. 1979;68:436–442. doi: 10.1016/0076-6879(79)68033-3. [DOI] [PubMed] [Google Scholar]
- Davis A. R., Nayak D. P., Ueda M., Hiti A. L., Dowbenko D., Kleid D. G. Expression of antigenic determinants of the hemagglutinin gene of a human influenza virus in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5376–5380. doi: 10.1073/pnas.78.9.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlich H. A., Cohen S. N., McDevitt H. O. A sensitive radioimmunoassay for detecting products translated from cloned DNA fragments. Cell. 1978 Apr;13(4):681–689. doi: 10.1016/0092-8674(78)90218-0. [DOI] [PubMed] [Google Scholar]
- Goeddel D. V., Kleid D. G., Bolivar F., Heyneker H. L., Yansura D. G., Crea R., Hirose T., Kraszewski A., Itakura K., Riggs A. D. Expression in Escherichia coli of chemically synthesized genes for human insulin. Proc Natl Acad Sci U S A. 1979 Jan;76(1):106–110. doi: 10.1073/pnas.76.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. R., Colot H. V., Guarente L., Rosbash M. Open reading frame cloning: identification, cloning, and expression of open reading frame DNA. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6598–6602. doi: 10.1073/pnas.79.21.6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J., Griffiths G., Louvard D., Quinn P., Warren G. Passage of viral membrane proteins through the Golgi complex. J Mol Biol. 1981 Nov 15;152(4):663–698. doi: 10.1016/0022-2836(81)90122-4. [DOI] [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Warren G., Stuhlfauth I., Jockusch B. M. The role of clathrin-coated vesicles in acrosome formation. Eur J Cell Biol. 1981 Dec;26(1):52–60. [PubMed] [Google Scholar]
- Kemp D. J., Cowman A. F. Direct immunoassay for detecting Escherichia coli colonies that contain polypeptides encoded by cloned DNA segments. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4520–4524. doi: 10.1073/pnas.78.7.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen M., Rüther U., Müller-Hill B. Immunoenzymatic detection of expressed gene fragments cloned in the lac Z gene of E. coli. EMBO J. 1982;1(4):509–512. doi: 10.1002/j.1460-2075.1982.tb01199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Sutcliffe J. G., Shinnick T. M. Antibodies to chemically synthesized peptides predicted from DNA sequences as probes of gene expression. Cell. 1981 Feb;23(2):309–310. doi: 10.1016/0092-8674(81)90126-4. [DOI] [PubMed] [Google Scholar]
- Müller-Hill B., Kania J. Lac repressor can be fused to beta-galactosidase. Nature. 1974 Jun 7;249(457):561–563. doi: 10.1038/249561a0. [DOI] [PubMed] [Google Scholar]
- Shine J., Fettes I., Lan N. C., Roberts J. L., Baxter J. D. Expression of cloned beta-endorphin gene sequences by Escherichia coli. Nature. 1980 Jun 12;285(5765):456–463. doi: 10.1038/285456a0. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. A technique for ultracryotomy of cell suspensions and tissues. J Cell Biol. 1973 May;57(2):551–565. doi: 10.1083/jcb.57.2.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabeau M., Stanley K. K. Enhanced expression of cro-beta-galactosidase fusion proteins under the control of the PR promoter of bacteriophage lambda. EMBO J. 1982;1(10):1217–1224. doi: 10.1002/j.1460-2075.1982.tb00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]