Abstract
Rationale and Objectives
Drug addiction is not just the repeated administration of drugs, but compulsive drug use maintained despite the accumulation of adverse consequences for the user. In an attempt to introduce adverse consequences of drug seeking to laboratory animals, we have developed the ‘conflict model’ in which the access of rats to a reinforcing lever allowing self-administration requires passing of an electrified grid floor. In this model, the current intensity leading to complete abstinence from drug seeking can be measured individually. The present study was designed to evaluated whether reinstatement of drug or natural reward seeking, despite the presence of the electrical barrier, can be achieved by presentation of discrete cues that were associated with the reward, and whether prolonged home-cage confinement can facilitate such reinstatement in this model.
Methods
The ‘conflict model’ was used to test cue-induced reinstatement in the presence of the electrical barrier, after 1 or 14 days of home-cage confinement, in groups of rats that were previously trained to self-administer cocaine or sucrose.
Results
Although similar shock intensity was required to suppress sucrose or cocaine self-administration, subjects exhibited significantly lower response to sucrose-, as compared to cocaine-, associated cues, during the reinstatement test. Importantly, cue-induced reinstatement of cocaine seeking was attenuated following 14 days of home-cage confinement.
Conclusions
The incorporation of aversive consequence in the self-administration model enable detection of what can be interpreted as a compulsive component unique to drug reinforcers. Moreover, the effect of the aversive consequence seems to increase following home-cage confinement.
Keywords: Addiction, Cocaine, Drug abuse, Abstinence, Relapse, Animal model
Introduction
A devastating feature of drug addiction is chronic relapse (APA 2000; Leshner 1997; Volkow and Li 2005), and cocaine abusers appear to be particularly prone (Washton and Stone-Washton 1990) as 90% of addicted individuals relapse to cocaine taking (DeJong 1994). Yet, addiction is not just the compulsive taking of drugs, but compulsive drug use maintained despite the accumulation of adverse consequences for the user (APA 2000; Deroche-Gamonet et al. 2004; Vanderschuren and Everitt 2004). Indeed, drug abuse in humans is accompanied by a high incidence and wide range of adverse consequences (Petry et al. 1998). For example, cocaine-abusing subjects who called a telephone “helpline” reported on problems with job functioning, interpersonal relationships, and financial status; disturbances of mood and cognitive functioning; and physical symptoms of exhaustion, weight loss, sleep problems, and seizures (Tatarsky 1984). When such adverse consequences come to outweigh the desirable effects of the drug, abstinence attempts tend to occur (Epstein et al. 2006; Pelloux et al. 2007). Accordingly, positive relationships have been consistently observed between help-seeking and psychosocial problems related to substance abuse; escalating negative consequences of substance use were cited most often as important reasons for either getting or staying sober (Laudet et al. 2002); treatment entry was correlated with increased substance-related psychosocial problems and negative events (Marlatt et al. 1997); and patients with the most severe problems are more likely to enter longer-term programs (Simpson et al. 1999). However, after admission for anti-addiction treatments, individuals are often ambivalent about starting treatment, and 29–42% of admitted clients fail to return to begin treatment (Weisner et al. 2001). In that regard, it has been suggested that one’s initial commitment when seeking treatment is usually based on a desire to change the negative consequences of addiction rather than the behavior itself, and that what these patients actually want is to continue drug and alcohol use, but without any adverse consequences (Marlatt et al. 1988; Prochaska 1994).
We postulated that adverse consequences act to counterbalance drug seeking, in an individual manner, by generating an internal conflict within the addict. For that, we and others incorporated adverse consequences in the self-administration (SA) paradigm (Weeks 1962), in which laboratory animals typically lever-press or nose-poke for intravenous drug injections in the presence of distinct cues (e.g. light, tone) (Sanchis-Segura and Spanagel 2006). In the SA model, subjects rarely experience adverse consequences for their drug seeking or drug taking behaviors. Within this model, extinction of SA is most commonly induced by repeated sessions in which lever responding does not result in drug reinforcement or the cue presentations, and cue-induced reinstatement of the extinguished SA behavior is measured in the absence of adverse consequences by acute exposure to the cues (de Wit and Stewart 1981; Kalivas and McFarland 2003; Shaham et al. 2003; Stretch and Gerber 1973). In human drug users, however, the concept of suppression of drug seeking in the face of adverse consequences is one in which drug seeking is refrained even though such seeking would still procure the drug (Pelloux et al. 2007).
Currently, there are several procedures which include adverse consequences of drug SA following training. These procedures include resistance to a Pavlovian conditioned fear stimulus [e.g., (Vanderschuren and Everitt 2004)]; delivery of the drug with an electric shock [e.g., (Deroche-Gamonet et al. 2004)]; and a second order schedule in which pressing a ‘seeking’ lever produce either a foot-shock or insertion of the drug-taking lever (50:50 chance) [e.g., (Economidou et al. 2009)].
In the ‘conflict model’ (Cooper et al. 2007), acquisition of SA is also established with no adverse consequences, but thereafter an ‘Electric Barrier’ (Jenkins et al. 1926) is present during sessions, and rats have to cross this barrier to achieve the reinforcer. This ‘Electric Barrier’ consists of 2/3 of the cage floor, and is constantly active during the session. The electric intensity delivered to the barrier increases daily until shock-induced abstinence is achieved, and confirmed for 3 consecutive days. The final shock required for complete suppression of lever-press is termed Abstinence Threshold (AT). This AT is individual for each rat, and is used during the subsequent reinstatement tests, which are triggered by non-contingent presentation of drug-related cues. During sessions and tests the rat can remain in a ‘safe’ (i.e., no-shock) area, consists of the remaining 1/3 of the cage floor; or to immediately return to this area following lever-press. Therefore, the conflict model is different from the above mention punishment procedures in several aspects, including (1) approaching the lever requires overcoming an “electric barrier”; (2) increasing electric current is applied for individual determination of shock-induced abstinence; (3) complete suppressions of responding is achieved in all rats; (4) non-contingent cues are presented to trigger the crossing of the “electric barrier” and reinstatement of lever-pressing (followed by contingent presentation of the cues), in face of the same individual electrical current which induced abstinence. This procedure is designed to model reinforced drug seeking under adverse consequences, abstinence induced and maintained by adverse consequences, and cue-induced reinstatement despite of adverse consequences.
Previously, we have showed that non-contingent presentation of the light cue, which was paired with cocaine during SA training, can trigger reinstatement of cocaine seeking in a portion of the animals, despite the ‘electric barrier’ set to the individual’s AT (Cooper et al. 2007). Yet, it has been previously demonstrated that drug seeking on one hand, and fear responses on the other hand, as well as their corresponding neural alterations, can increase with time (Conrad et al. 2008; Grimm et al. 2001; Lu et al. 2004a; Lu et al. 2004b; Lu et al. 2005; Neisewander et al. 2000; Pickens et al. 2009; Sorge and Stewart 2005). Accordingly, while the phenomenon of ‘incubation of craving for drugs’ has been well characterized, cue-induced drug craving in two-weeks abstinent cocaine-dependent individuals, was accompanied by increased fear (Fox et al. 2008). To address the long term effect of adverse consequences we utilized the conflict model to compare between rates of reinstatement after 1 and 14 days of home-cage confinement. In addition, we have tested whether cues can induce such reinstatement in rats trained to lever press for a natural reward (sucrose).
Materials and Methods
A few adjustments and improvements were performed in the conflict model, relative to the earlier publication of Cooper et al. (2007). These included the incorporation of additional reinforcement-associated cue (20Hz tone), replacement of shockers (see Apparatus), the gradual change in shock intensity, and a new location for the experiments within a Specific Pathogen Free (SPF) facility. For detailed Materials and Methods see supplementary.
Subjects
Subjects were male Sprague–Dawley rats (Harlan, Israel, 250–350 g) housed in an SPF animal facility (temperature 21–22°C) under a reverse 12-h light–dark cycle (lights off at 7 A.M.). Food and water were freely available in the rats’ home cage. The experimental procedures were approved by the local animal care and use committee and were conducted in accordance with the National Institutes of Health guidelines for the Care and Use of Laboratory Animals.
Intravenous surgery
Surgeries were conducted as described previously (Cooper et al. 2007). In brief, rats were anesthetized and silastic catheters were inserted into the jugular vein. The catheters were passed subcutaneously to the top of the skull and mounted to the rat’s skull with dental cement. Rats were allowed to recover for 4–7 days.
Apparatus
The experiments were performed in standard self-administration (SA) chambers (Med Associates inc., Vermont USA), controlled by a Med Associates (Georgia, VT) computer program. The chambers were equipped with two levers on one side, and a drinking plate on the other side of the chamber. A 7.5-mA white cue light and a 20Hz cue tone were located above the cocaine (or sucrose)-paired lever. The setup of the “electric barrier” included constant current stimulators (Model ENV-410B, Med Associates, Lafayette, Indiana, USA) that were connected to approximately two thirds of the grid floor adjacent to the levers. The rats closed the electric circuit by touching any two grids with opposite charge. The remaining approximately one third of the chamber, to which electricity was not applied, was the rat’s no-shock area.
General Procedures
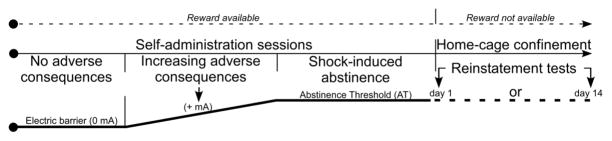
The experimental procedure included four stages as described previously (Cooper et al. 2007) and detailed in figure 1: self-administration training, “Electric Barrier” application resulting in the elimination of the reward-reinforced behavior, verification of AT for 3 consecutive days, and cue-induced reinstatement tests (Fig 1). All phases were conducted during the rats’ dark phase.
Fig 1. Experimental design utilizing the ‘conflict’ model procedure.
A procedure designed to model reinforced drug seeking under adverse consequences, abstinence induced and maintained by adverse consequences (even though seeking would still procure the drug), and cue-induced reinstatement in face of adverse consequences.
Cocaine self-administration
Rats were trained for 3 h/day to lever press for intravenous cocaine HCl (0.5 mg/kg per 0.13 ml sterile saline per infusion, given over 4 s; generously provided by NIDA, USA), as described previously (Cooper et al. 2007; Levy et al. 2007; Shabat-Simon et al. 2008). In brief, for the first 3–5 days, lever responding was reinforced under a fixed ratio (FR) 1, 20-s timeout (TO); followed by 8–10 training sessions under an FR-2, 20-s TO. Sessions began with the illumination of the house-light, the insertion of the active lever, and the activation of the cue light and tone for 20 sec.
Sucrose self-administration
Non-operated rats were trained for 30min/day to lever press for oral sucrose solution (10% in tap water; 0.22 ml infused over 4 s into the drinking plate), as described previously (Levy et al. 2007). In brief, for the first 3–5 days, lever responding was reinforced under a fixed ratio FR1 20-s TO; followed by 8–10 training sessions under an FR-2 20-s TO. The drinking plate was placed in the no-shock area.
“Electric Barrier” application
During this phase, reinforcement was available under an FR-2 20-s TO for 30 min/day. Rats were placed in the no-shock area of the chamber, and the electrical barrier was turned on 2 min prior to initiation of the session. The daily sessions began with the illumination of the house-light, the extension of the active-lever, and the activation of the cue light and tone for 20 sec. The electric current delivered was gradually increased in each daily session according to the following algorithm. For 0, 0.10, 0.12, 0.14, and 0.16 mA barrier intensity, if the subject earned even 1 reinforcement, the current was increased in 0.02 mA on the next session, but the current 30min session was not stopped. For barrier intensity ≥0.18 mA –access to the reinforcement was limited to 5 infusions/session. On the next day, the current intensity was increased by +0.04 mA if the rat earned maximum reinforcements (i.e. 5); +0.02 mA if the rat earned between one and four reinforcements; +0 mA − if the rat failed to achieve a single reinforcement. This procedure was used to minimize rats’ exposure to the barrier, because a pilot study found that following extended exposure rats developed tolerance to the ‘Electric Barrier’ (Supplementary Fig 1). This phase ended for each rat when it did not lever press for three consecutive daily sessions (i.e. when AT was achieved).
Reinstatement tests
Tests for cue-induced reinstatement were conducted 1 or 14 days after the rat completed 3 days of shock-induced abstinence. The experimental conditions present during tests for cue-induced reinstatement of cocaine/sucrose seeking were similar to those present during the last three sessions of SA (during which rats did not approach the levers due to the electrical barrier), with two modifications: 1) active lever responding did not lead to cocaine/sucrose infusions (to avoid reinforcing effects of the unconditioned stimulus); 2) subjects were exposed to non-contingent presentations of the conditioned cues once every 5 minutes. Control groups were not exposed to the non-contingent presentation of the conditioned cues (except for the one signaling session initiation). Electric intensity was set at the shock intensity that led to 3 days of shock-induced abstinence (AT condition), or, in other groups, at 85% of this intensity (0.85AT conditions).
Seeking test
The experimental procedure of seeking tests was similar to reinstatement tests; but the ‘Electric Barrier’ was not activated and all groups were exposed to the non-contingent cues previously paired with reward injections during training.
Statistical analyses
The data from the reinstatement tests were analyzed for active and inactive lever responses; and for the group’s reinstatement rate (percent of rats which resume lever press during tests). Due to lack of normal distribution of the data, Mann-Whitney tests were used to compare the medians of the groups for active and inactive lever-presses; and reinstatement rate of the groups was compared using one-tailed Fisher’s exact test. Comparison of AT was analyzed using unpaired two-tailed t-test.
Results
Self-administration training
All groups of rats showed consistent self-administration behavior at the end of the training phase. The mean ± SEM number of infusions during the last 3 training sessions was 34.7±0.2 for the cocaine, and 40.8±1.6 for the sucrose SA rats. The mean ± SEM number of active vs. inactive lever responses during the last 3 training sessions (under FR2) was 84.9±3.3 vs. 1.9±0.4 and 108.6±6.2 vs. 3.5±0.5 for the cocaine and sucrose SA groups, respectively. The total number of infusions during training sessions was 278.3±8.0 for the cocaine, and 303.5±11.9 for the sucrose SA rats, indicating that the cue-reinforcer pairings was quite similar for both conditions, despite the different durations of the sessions.
‘Electric Barrier’ procedure and Abstinence Threshold
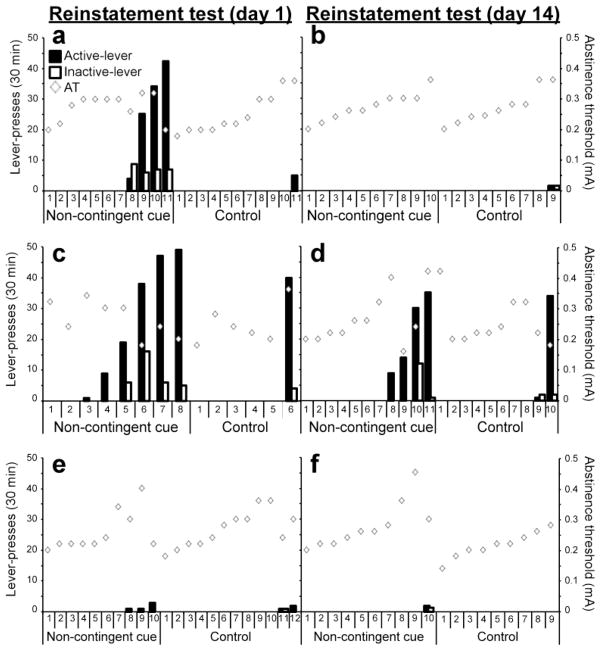
Following the stabilization of SA behavior, the electric intensity delivered to the barrier increased daily until shock-induced abstinence was achieved, and confirmed for 3 consecutive days. AT was found to be almost identical for subjects trained to self-administer sucrose or cocaine (0.26±0.01 mA and 0.25±0.01 mA, respectively; p=0.7, student’s t-test). The individual AT of each rat is presented in Fig 2.
Fig 2. Individual performance of cocaine and sucrose SA groups during the reinstatement test.
The number of previously active (black bars) and inactive (white bars) lever responses during reinstatement tests are presented for each individual rat from each of the indicated groups. The diamond shapes present the individual AT of each rat. Panels a and b present reinstatement to cocaine seeking under the AT condition; panels c and d present reinstatement to cocaine seeking under the 0.85AT condition, and panels e and f present reinstatement to sucrose seeking under the 0.85AT condition.
Reinstatement tests
In the first experiment, cue-induced reinstatement to cocaine seeking was tested under AT conditions in different groups, after 1 or 14 days of home-cage confinement. The presentation of cues induced reinstatement in 4 out of 11 rats when tested after 1 day (Fig 2a), but in none of the 10 rats tested after 14 days of home-cage confinement (Fig 2b).
In the second experiment, we tested whether this phenomenon of reduced reinstatement after home-cage confinement will repeat when subjects are confronted with a lower aversive condition. Therefore, the effect of cues presentation on reinstatement to cocaine seeking was tested under 0.85AT conditions (i.e., 85% of the individual AT). Indeed, in the 0.85AT condition, presentation of cues induced reinstatement in 6 out of 8 rats tested after 1 day (Fig 2c), but only in 4 out of 11 rats tested after 14 days of home-cage confinement (Fig 2d).
In the third experiment, we investigated whether non-contingent cues can also trigger reinstatement to sucrose (a high-incentive natural reinforcer) seeking under 0.85AT conditions, as observed with cocaine. Reinstatement rate and the number of active lever responses were much lower than those of cocaine, and no significant difference was observed between groups tested after 1 day (Fig 2e) and 14 days (Fig 2f) of home-cage confinement.
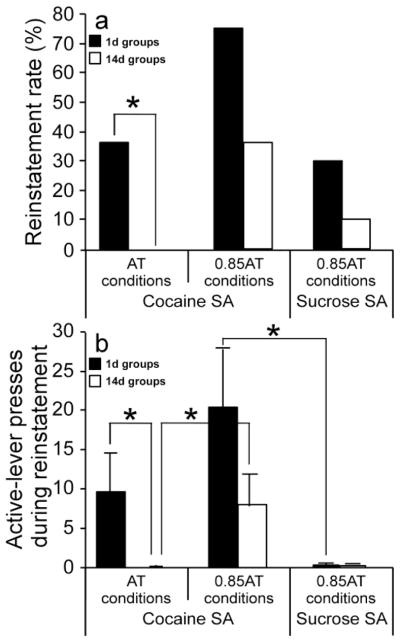
The effect of home-cage confinement period in the first experiment (cocaine seeking under AT conditions) was significant when comparing either reinstatement rates (Fisher’s exact test, p<0.05; Fig 3a) or the corresponding number of active-lever presses (Two samples Mann-Whitney U test, Tied p-value <0.05; Fig 3b). In the second experiment (cocaine seeking under 0.85AT conditions) there was a non-significant trend towards reduction of both reinstatement rate (Fig 3a) and active-lever presses (Fig 3b) following 14 days of home-cage confinement. In the third experiment (sucrose seeking under 0.85AT conditions) there was a non-significant trend towards reduction of reinstatement rate following 14 days of home-cage confinement (Fig 3a).
Fig 3. Group rates of cue-induced reinstatement behavior.
Panel a presents the total cue-reinstatement rate for the groups tested after 1d (black bars) and 14d (white bars) of home-cage confinement. Panel b present the mean ± SEM number of previously active lever responses during the cue-induced reinstatement test, for the groups tested after 1d (black bars) and 14d (white bars) of home-cage confinement (*p<0.05).
Newsworthy is that the cocaine and sucrose SA groups tested under 0.85AT conditions, though exhibiting very similar AT, demonstrated distinct reinstatement behaviors. The number of active-lever presses during the reinstatement test was significantly higher in the cocaine SA group (Fig 3b; Two samples Mann-Whitney U test, Tied p-value <0.05).
In a seeking tests performed 24h after the reinstatement test, in which the ‘Electric Barrier’ was turned off, all groups resumed lever pressing. Interestingly, the cocaine and sucrose groups (that were previously tested under 0.85AT conditions), which showed very different reinstatement rates, did not differ in the number of lever presses during this free seeking test (Fig 4). Two Way ANOVA (Reinforcer type (cocaine vs. sucrose) X Home-cage confinement period (1d vs. 14d)) with repeated measurement for active vs. inactive lever responses revealed significant effect of lever (F(1,40)=103.829, p<0.001) confirming that subjects responded significantly more on the active lever, but no effect for Reinforcer type, home-cage confinement period, and no significant interactions, indicating that response rate was similar for cocaine and sucrose and after 1 or 14 days of home-cage confinement.
Fig 4. Cocaine or sucrose seeking behavior during the no-shock reinstatement test.
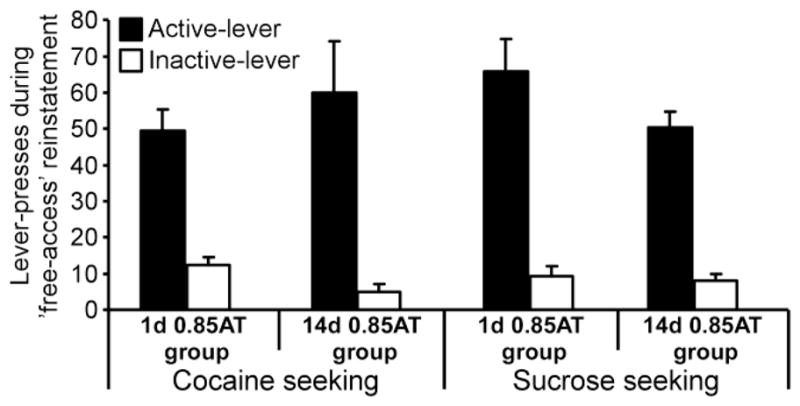
The mean ± SEM number of lever presses on the previously active (black bars) and inactive (white bars) levers during the ‘free access’ (no shock) reinstatement test is presented for the various cocaine or sucrose groups that were previously tested under the 0.85AT condition.
It should be noted that no correlation was observed between the individual AT and the tendency to reinstate responding during tests (see Fig 2). In addition, sensitivity to general pain measured by the hot-plate procedure (see supplementary method) in a different subgroup of rats (not tested for reinstatement) did not change after 14 days of home-cage confinement (without access to cocaine). The latency for the first sign of discomfort in this procedure was 26.0 ± 2.4 and 24.3 ± 3.8 seconds after 1 and 14 days of home-cage confinement, respectively (n=9; P=0.42).
Discussion
In human cocaine addicts, ongoing drug-seeking episodes, as well as abstinence, involve constant conflict between the desire for the drug’s positive effects and the aversive consequences of pursuing it. With continued use, the aversive consequences of drug-use may accumulate, until eventually these aversive consequences outweigh the desire for the drug, and abstinence is imposed. That is, the conflict is diverted towards abstinence; and the drug addict seeks treatment (DeJong 1994; Marlatt et al. 1988; Marlatt et al. 1997; Prochaska 1994; Simpson et al. 1999; Tatarsky 1984; Washton and Stone-Washton 1990; Weisner et al. 2001). However, when abstinent cocaine addicts are exposed to drug-associated cues, they experience increased craving for the drug that may induce relapse [e.g.,(Childress et al. 1999; Kosten et al. 2006; Sinha et al. 2006; Volkow et al. 2010; Volkow et al. 2006)].
In the present study, application of increasing levels of electricity to the grid floor, gradually attenuated and finally completely eliminated cocaine and sucrose self-administration of well-trained rats. As we have shown previously (Cooper et al. 2007), non-contingent presentations of conditioned cues trigger reinstatement to cocaine-seeking behavior in a portion of the rats, despite the electrical barrier (set at AT conditions). However, the presentation of cues failed to induce such reinstatement of cocaine seeking after two weeks of home-cage confinement.
Human studies indicate reduction in relapse rates following an adverse event. For example, spontaneously discontinuations of smoking habits are most likely in individuals who have recently experienced a cardiac health event, and smoking relapse tended to occur in individuals with lesser health impairment (Bigelow et al. 1986). In addition, perceived high risk of arrest was found to increase the probability of staying in rehabilitation treatment in drug addicts (Sung and Richter 2007); and contracts stipulating severe consequences (e.g., notification of one’s employer) reduced the chance of relapse to cocaine during treatment (Anker and Crowley 1982). The effect of abstinence length on the propensity to relapse in humans may be suggested by Simpson et al. (1999) indicating that cocaine-dependent patients that were treated for 90 days or longer showed reduced weekly cocaine use at one year follow-up, relative to short-term patients. Similarly, in former opioid addicts, a longer period of abstinence predicted greater chances of abstinence in a one year follow-up (Simpson and Marsh 1986).
The substantial difference between the conflict model utilized in the present study and the extinction-reinstatement model (i.e. the fact that cocaine seeking and taking is accompanied by adverse consequences and these adverse consequences, rather than operant extinction, lead to complete suppression of drug seeking) might explain the reduction in cue-induced reinstatement observed in this study; while others [e.g., (Di Ciano and Everitt 2002)] using the extinction-reinstatement model observed increased cue-induced reinstatement following increasing abstinence periods. Indeed, as cue-induced craving for cocaine was found to incubate over time (Grimm et al. 2001) so did context-induced fear (Bindra and Cameron 1953; Hendersen 1978; Houston et al. 1999; Pickens et al. 2009; Quirk 2002; Wiltgen and Silva 2007). More specifically, context associated fear (as reflected by duration of freezing) was found to increase for up to 60 days from the last conditioning trial (Hendersen 1978; Houston et al. 1999; Quirk 2002). Therefore, it is reasonable to assume that during the home-cage confinement period in the conflict model both craving and fear underwent ‘incubation’, such that after 14 days fear was stronger than craving, driving subjects to choose not to resume cocaine-seeking behavior. This means that the balance between the positive and the negative consequences of cocaine use tipped more toward the negative, and drug-related cues were less able to divert the conflict towards reinstatement. This change in balance toward the negative, after 14d of home-cage confinement, could not be explained by reduction in craving, as drug-seeking in the absent of the barrier was not affected by the home-cage confinement (Fig 4); nor it can be attributed to increase in general pain thresholds, as indicated from the similar response latencies in the hot-plate procedure following 1 or 14 days of home-cage confinement. Moreover, when the balance was tipped back towards the positive, by reducing the aversive test conditions (to 0.85AT), rats demonstrated a much more vigor reinstatement behavior compared to the AT condition groups. Therefore, it is likely that the fear of the shock, or physical pain induced by the shock, were increased during home-cage confinement; but this increase was not sufficient to entirely prevent reinstatement when confronted with higher accessibility (0.85AT conditions) of the reinforcing lever. In addition, it is possible that the reduced rate of cue-induced reinstatement after home cage confinement results from further consolidation of the association between the cue and the shock during that period. The neurobiological explanation for incubation of fear, also reported by Quirk (2002), could be related to the potentiation of CRF-induced LTP in the amygdala found after 14 days of abstinence from cocaine (Krishnan et al. 2010). Further studies will be required to characterize this phenomenon in the conflict model.
Importantly, despite the fact that the sucrose and cocaine-trained groups were exposed to a very similar number of cue-reinforcer pairings during training, did not differ in their AT, and exhibited similar “free-access” reinstatement, there was a large difference between these groups in the cue-induced reinstatement test. Not only fewer sucrose-trained rats resumed sucrose-seeking, but even the few rats that did reinstate this behavior, made significantly less responses relative to the cocaine-trained rats. In other words, while the reinforcing efficacy of cocaine and sucrose, as reflected by the “price” subjects were willing to “pay” in order to receive the reinforcer was similar (i.e. no difference between the groups in their average AT); exposure to conditioned cues had dramatically different effect on the cocaine relative to the sucrose-trained subjects. While sucrose-trained subjects were able to refrain from sucrose-seeking behavior despite the presentation of reward-associated cues, cocaine-trained subjects tended to resume cocaine seeking in response to the cues, despite the adverse consequence. It seems then that the present model, by utilizing aversive consequences for reward seeking behavior, is uniquely suitable to detect what can be interpreted as “compulsive” nature of cue-induced relapse in cocaine-trained subjects. However, further studies are required for the determination of this feature in the conflict model. “Compulsive” cue-induced relapse to cocaine seeking is consistent with prevalent theories of addiction emphasizing the “abnormal” control over behavior of cocaine associated cues (e.g. (Everitt and Robbins 2005; Jentsch and Taylor 1999; Robinson and Berridge 2000)). In particular, Jentsch & Taylor (1999) argue that cocaine addicts exhibit increased response to cocaine cues which is mediated by the basal ganglia, but possess reduced ability to inhibit such inappropriate urge to seek cocaine due to malfunction of their medial prefrontal cortex (mPFC). Such hypothesis is consistent with the many reports on reduced function of this area in human cocaine addicts (Fein et al. 2002; Franklin et al. 2002; Lim et al. 2002; Matochik et al. 2003; Volkow et al. 1992). It is therefore possible that the reduced ability to inhibit cue-induced resumption of cocaine-seeking behavior reported here resulted from increased responsivity of the nucleus accumbens to such cues, compounded by reduced function of the mPFC. Future studies are needed to support this hypothesis.
An alternative interpretation for the different response to cues in sucrose and cocaine-trained rats may relate to differences in the saliency of the cues in each case. It is possible that the saliency of the cues is less significant in the sucrose-trained rats, as it precedes sucrose availability that requires self-administration, while in the cocaine-trained rats the cues are more directly associated with the cocaine experience per se. However, as lever responses during cue presentations in the free seeking test was similar for cocaine and sucrose groups (in the absence of the electrical barrier; Fig 4), it is not likely that the differences between these groups during the reinstatement tests merely reflect differences in cues’ saliency.
Taken together, the present study indicates that the aversive consequences of drug use play an integral part in the initiation and maintenance of abstinence, and in the prevention of relapse. Moreover, the impact of adverse consequences has the potential to increase with time. Finally, including adverse consequences in a model of cue-induced resumption of drug seeking behavior enable the detection of what can be interpreted as compulsive component of such resumption that is stronger for a drug reinforcer relative to that of a natural reinforcer. Thus, incorporation of adverse consequences when modeling addiction in animals [e.g., (Deroche-Gamonet et al. 2004; Economidou et al. 2009; Vanderschuren and Everitt 2004)] are highly beneficial and should also be used when studying relapse processes and potential therapeutic interventions.
Supplementary Material
Acknowledgments
This research was supported by the by the National Institute on Drug Abuse (NIDA; Grant Number 1R21DA024224-01). We thank D. Goldian for technical assistance. The experimental procedures were approved by the local animal care and use committee and were conducted in accordance with the National Institutes of Health guidelines for the Care and Use of Laboratory Animals.
Footnotes
The authors declare no conflicts of interests.
Bibliography
- Anker AL, Crowley TJ. Use of contingency contracts in specialty clinics for cocaine abuse. NIDA Res Monogr. 1982;41:452–9. [PubMed] [Google Scholar]
- APA. Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association, American Psychiatric Association; 2000. [Google Scholar]
- Bigelow GE, Rand CS, Gross J, Burling TA, Gottlieb SH. Smoking cessation and relapse among cardiac patients. NIDA Res Monogr. 1986;72:167–71. [PubMed] [Google Scholar]
- Bindra D, Cameron L. Changes in experimentally produced anxiety with the passage of time: incubation effect. J Exp Psychol. 1953;45:197–203. doi: 10.1037/h0055125. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–8. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–21. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A, Barnea-Ygael N, Levy D, Shaham Y, Zangen A. A conflict rat model of cue-induced relapse to cocaine seeking. Psychopharmacology (Berl) 2007;194:117–25. doi: 10.1007/s00213-007-0827-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–43. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- DeJong W. Relapse prevention: an emerging technology for promoting long-term drug abstinence. Int J Addict. 1994;29:681–705. doi: 10.3109/10826089409047904. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–7. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Reinstatement and spontaneous recovery of cocaine-seeking following extinction and different durations of withdrawal. Behav Pharmacol. 2002;13:397–405. doi: 10.1097/00008877-200209000-00013. [DOI] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry. 2009;65:851–6. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Hong KI, Siedlarz K, Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology. 2008;33:796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–42. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–2. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendersen RW. Forgetting of conditioned fear inhibition. Learning and Motivation. 1978;9:16–30. [Google Scholar]
- Houston FP, Stevenson GD, McNaughton BL, Barnes CA. Effects of age on the generalization and incubation of memory in the F344 rat. Learn Mem. 1999;6:111–9. [PMC free article] [PubMed] [Google Scholar]
- Jenkins T, Warner L, Warden C. Standard apparatus for the study of animal motivation. J Comp Psychol. 1926;6:361–382. [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–90. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003;168:44–56. doi: 10.1007/s00213-003-1393-2. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Scanley BE, Tucker KA, Oliveto A, Prince C, Sinha R, Potenza MN, Skudlarski P, Wexler BE. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31:644–50. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- Krishnan B, Centeno M, Pollandt S, Fu Y, Genzer K, Liu J, Gallagher JP, Shinnick-Gallagher P. Dopamine receptor mechanisms mediate corticotropin-releasing factor-induced long-term potentiation in the rat amygdala following cocaine withdrawal. Eur J Neurosci. 2010;31:1027–42. doi: 10.1111/j.1460-9568.2010.07148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudet AB, Savage R, Mahmood D. Pathways to long-term recovery: a preliminary investigation. J Psychoactive Drugs. 2002;34:305–11. doi: 10.1080/02791072.2002.10399968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–7. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- Levy D, Shabat-Simon M, Shalev U, Barnea-Ygael N, Cooper A, Zangen A. Repeated electrical stimulation of reward-related brain regions affects cocaine but not “natural” reinforcement. J Neurosci. 2007;27:14179–89. doi: 10.1523/JNEUROSCI.4477-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry. 2002;51:890–5. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology (Berl) 2004a;176:101–8. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004b;47(Suppl 1):214–26. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–9. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Baer JS, Donovan DM, Kivlahan DR. Addictive behaviors: etiology and treatment. Annu Rev Psychol. 1988;39:223–52. doi: 10.1146/annurev.ps.39.020188.001255. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Tucker JA, Donovan DM, Vuchinich RE. Help-seeking by substance abusers: the role of harm reduction and behavioral-economic approaches to facilitate treatment entry and retention. NIDA Res Monogr. 1997;165:44–84. [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux Y, Everitt BJ, Dickinson A. Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology (Berl) 2007;194:127–37. doi: 10.1007/s00213-007-0805-0. [DOI] [PubMed] [Google Scholar]
- Petry NM, Bickel WK, Arnett M. Shortened time horizons and insensitivity to future consequences in heroin addicts. Addiction. 1998;93:729–38. doi: 10.1046/j.1360-0443.1998.9357298.x. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Golden SA, Adams-Deutsch T, Nair SG, Shaham Y. Long-lasting incubation of conditioned fear in rats. Biol Psychiatry. 2009;65:881–6. doi: 10.1016/j.biopsych.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JO, Norcross JC, DiClemente CC. Changing for good: a revolutionary six-stage program for overcoming bad habits and moving your life positively forward. New York: Avon; 1994. [Google Scholar]
- Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Mem. 2002;9:402–7. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95(Suppl 2):S91–117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Shabat-Simon M, Levy D, Amir A, Rehavi M, Zangen A. Dissociation between rewarding and psychomotor effects of opiates: differential roles for glutamate receptors within anterior and posterior portions of the ventral tegmental area. J Neurosci. 2008;28:8406–16. doi: 10.1523/JNEUROSCI.1958-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Simpson DD, Joe GW, Fletcher BW, Hubbard RL, Anglin MD. A national evaluation of treatment outcomes for cocaine dependence. Arch Gen Psychiatry. 1999;56:507–14. doi: 10.1001/archpsyc.56.6.507. [DOI] [PubMed] [Google Scholar]
- Simpson DD, Marsh KL. Relapse and recovery among opioid addicts 12 years after treatment. NIDA Res Monogr. 1986;72:86–103. [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–31. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sorge RE, Stewart J. The contribution of drug history and time since termination of drug taking to footshock stress-induced cocaine seeking in rats. Psychopharmacology (Berl) 2005;183:210–7. doi: 10.1007/s00213-005-0160-y. [DOI] [PubMed] [Google Scholar]
- Stretch R, Gerber GJ. Drug-induced reinstatement of amphetamine self-administration behaviour in monkeys. Can J Psychol. 1973;27:168–77. doi: 10.1037/h0082466. [DOI] [PubMed] [Google Scholar]
- Sung HE, Richter L. Rational choice and environmental deterrence in the retention of mandated drug abuse treatment clients. Int J Offender Ther Comp Criminol. 2007;51:686–702. doi: 10.1177/0306624X07299226. [DOI] [PubMed] [Google Scholar]
- Tatarsky AMWaA. Adverse effects of cocaine abuse. Med Lett Drugs Ther. 1984;26:51–2. [PubMed] [Google Scholar]
- Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–9. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Jayne M, Ma Y, Pradhan K, Wong C, Swanson JM. Cognitive control of drug craving inhibits brain reward regions in cocaine abusers. Neuroimage. 2010;49:2536–43. doi: 10.1016/j.neuroimage.2009.10.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11:184–90. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Li TK. Drugs and alcohol: treating and preventing abuse, addiction and their medical consequences. Pharmacol Ther. 2005;108:3–17. doi: 10.1016/j.pharmthera.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–8. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washton AM, Stone-Washton N. Abstinence and relapse in outpatient cocaine addicts. J Psychoactive Drugs. 1990;22:135–47. doi: 10.1080/02791072.1990.10472539. [DOI] [PubMed] [Google Scholar]
- Weeks JR. Experimental morphine addiction: method for automatic intravenous injections in unrestrained rats. Science. 1962;138:143–4. doi: 10.1126/science.138.3537.143. [DOI] [PubMed] [Google Scholar]
- Weisner C, Mertens J, Tam T, Moore C. Factors affecting the initiation of substance abuse treatment in managed care. Addiction. 2001;96:705–16. doi: 10.1046/j.1360-0443.2001.9657056.x. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Silva AJ. Memory for context becomes less specific with time. Learn Mem. 2007;14:313–7. doi: 10.1101/lm.430907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.