Abstract
Aims
To evaluate delay discounting and self-reported impulsive behavior in a sample of adolescents experimenting with cigarette smoking compared with adolescents who had never smoked or were daily smokers.
Setting
Columbus, Ohio, a city of approximately 780,000
Participants
A sample of 141 male and female adolescents with a mean age of 15.37 (SD = 1.09) years.
Measurements
Primary measures included a computerized assessment of delay discounting, a self-report assessment of impulsivity (BIS-11-A), and verifications of cigarette smoking status (breath CO and urinary cotinine level).
Findings
Smokers discounted more by delay and had higher impulsivity scores than non-smokers. Experimenters had scores intermediate to those of smokers and non-smokers on both measures. In some analyses the difference between experimenters and non-smokers was significant, with experimenters showing greater delay discounting, but in no case did experimenters differ significantly from the smokers.
Conclusions
Young people who experiment with cigarettes appear to be similar to those who smoke regularly in terms of tendency to discount future gains and report impulsive tendencies, and generally higher in these traits than non-smokers.
INTRODUCTION
Cigarette smoking remains a serious public health concern and is frequently cited as a top preventable cause of death in the United States (e.g., [1]). Initial experimentation with smoking, and also the progression to more regular patterns of smoking, occurs primarily during adolescence [2, 3]. Therefore, research with adolescent populations is necessary to better define risk factors for cigarette smoking, including environmental (e.g., [4]), genetic (e.g., [5, 6]), and behavioral (e.g., [7, 8]) factors.
Delay discounting, which is often defined as a form of impulsive behavior, is one potential behavioral risk for cigarette smoking during adolescence. As a behavioral variable, delay discounting describes the extent to which an individual discounts the value of an outcome because of a delay to its occurrence. Assessments of delay discounting typically require participants to make choices between smaller rewards available immediately (e.g., $4) and more valuable rewards (e.g., $10) available after a specified delay (e.g. [9]). A choice pattern reflecting comparatively more choices for smaller immediate rewards at the expense of larger but delayed rewards indicates impulsive choice. In such cases, the individual may be behaviorally under-controlled by temporally distal events or long-term outcomes and instead be greatly influenced by immediate circumstances and outcomes.
Robust relationships have been identified between delay discounting and many forms of addiction and substance use (see [10, 11], for reviews), with drug users (including cigarette smokers) discounting more by delay than never-addicted control participants. For example, alcoholic samples discount more impulsively on laboratory assessments of delay discounting than demographically matched non-alcoholic samples (e.g., [12, 13]). However, one study [14] found that a group of currently-abstinent alcoholics (abstaining from alcohol use on their own for at least 30 days) were intermediate in their rates of discounting between active- and never-alcoholic participants. Similarly, numerous studies have shown that cigarette smokers discount more by delay than never smokers (e.g., [15, 16]); however, one study [17] found that past smokers who had been abstinent from smoking for at least five years discounted similarly to never smokers. These findings involving abstinent drug users suggest the relationship between delay discounting and drug use may be linked to how recently drugs have been used.
The low rates of delay discounting (i.e., less impulsive discounting) observed in abstinent drug users [14, 17] raise questions about this variable as a behavioral risk factor for drug use. That is, if the high rates of discounting observed in drug-using populations do not predate substantial use of drugs then delay discounting may not be a risk factor for substance use. Alternatively, these findings with abstinent samples may indicate that drug users who discount least by delay (within the variability of delay discounting existing among drug-using populations) are more likely to quit using drugs. Consistent with the latter suggestion are several findings that low rates of delay discounting are associated with better treatment outcomes for adult and adolescent cigarette smokers who are trying to quit smoking [18, 19].
Research specifically exploring delay discounting as a risk for cigarette smoking, or, inversely, nicotine effects on delay discounting, has yielded mixed results. Several studies have demonstrated that adolescent cigarette smokers discount more by delay than adolescent non-smokers [20–22], although the effect size for delay discounting between smokers and non-smokers may be smaller for adolescents than for adults [15]. In one of these studies, delay discounting also was evaluated prospectively as a predictor of initiation and progression of smoking among adolescents [20]. While smokers discounted more than non-smokers at baseline, delay discounting was not a significant predictor of smoking progression over time. However, a more recent finding by this group showed that delay discounting did, in fact, predict initiation of smoking from mid adolescence into early adulthood [23]. Similarly, non-human animal research has not demonstrated consistent nicotine effects on delay discounting, with some studies showing acute nicotine increases in delay discounting [24] but others showing opposite effects [25].
For the present study, we recruited and evaluated delay discounting in a sample of adolescents who reported recently experimenting with cigarette smoking for the first time. These “experimenters” were at increased risk for progression to more regular patterns of smoking by virtue of their experimentation with cigarettes [e.g., [26]]. However, there would not likely be sufficient history of smoking by these adolescents to bring about smoking-related, or nicotine, effects on delay discounting. For comparison purposes, two additional groups of gender-matched adolescents were recruited based on smoking status: (a) non-smokers or experimenters and (b) daily smokers. It was hypothesized that if delay discounting is a risk factor for experimentation with smoking (and therefore a risk factor for progression to more regular smoking) that the group of experimenters would discount similarly by delay to the group of daily smokers and discount more than the group of non-smokers. Alternatively, if delay discounting is not a risk factor for initial use of cigarettes, then the experimenters would discount less by delay than the daily smokers and similarly to the non-smokers. As designed, the present study should provide greater insight into the possible role of discounting as behavioral risk factor for cigarette smoking.
For additional comparisons, a self-report measure of impulsivity appropriate for adolescents (BIS-11-A) was included. Findings with this assessment sometimes parallel delay discounting findings [27] but other times provide unique findings [28]. Including this measure stood to provide additional information concerning impulsive behavior defined more generally as it relates to experimentation with smoking. Of note, the current findings for BIS-11-A between non-smokers and smokers have been published previously [27]; however, the present findings pertaining to experimentation with smoking have not been published.
METHOD
Participants
A community sample of adolescent non-smokers (n = 50), experimenters (n = 41), and smokers (n = 50) was recruited from the central Ohio area through posters, advertisements in a local newspaper, and word-of-mouth referrals. An initial phone screening was conducted to determine eligibility before inviting participants to the laboratory. Those eligible for participation were between 13 and 17 years of age and self-report (a) never smoking or experimenting with cigarettes (for the non-smokers), (b) experimenting with cigarettes (no more than three cigarettes total) for the first time within three months of our screening (experimenters), or (c) smoking one or more cigarettes per day at the time of screening and for at least the preceding three months (smokers). Those being screened were not made aware of these inclusion criteria.
To verify smoking status participants provided both breath and urine samples. Breath samples were analyzed for carbon monoxide (CO) content (Micro 4 Smokerlyzer; Bedford Instruments, Bedford Scientific, Kent, United Kingdom). Non-smokers were required to have CO levels ≤ 5ppm, whereas smokers were required to have CO levels ≥ 9 ppm. Urine samples were used to determine cotinine content (a metabolite of nicotine) by homogeneous enzyme immunoassay at Graham-Massey Analytical Labs in New Haven, CT. Non-smokers were required to have quantitative cotinine values ≤ 50 ng/ml, and smokers to have values ≥ 200 ng/ml. Because of their low reported smoking levels, experimenters were expected to have CO and cotinine levels similar to non-smokers. However, one outlier included in analyses met criteria as an experimenter on self-report and CO assessments but had a cotinine value of 453ng/ml. Before participation, a parent or legal guardian provided informed consent, and the adolescent provided informed assent. This research was approved by the Institute Institutional Review Board of the Research Institute at Nationwide Children’s Hospital.
Materials
Demographics
Participants completed a short demographic questionnaire designed for use in this study. Included were queries for age, gender, race, and smoking patterns. There also were questions pertaining to other drugs used by the adolescent and how often these substances were used over the previous six months. The residential zip code of each participant was recorded and later used to determine median household income for each zip-code region of residence based on 2000 U.S. Census data for Columbus, OH [29]. IQ was assessed using the Kaufman Brief Intelligence Test – Second Edition [30].
Session Environment
Testing sessions were conducted at the Research Institute at Nationwide Children’s Hospital, Department of Pediatrics, The Ohio State University. Each participant completed his or her session individually in a private room equipped with a desk, office-type chair, and a Dell Pentium desktop PC equipped with speakers, mouse, and keyboard.
Dependent Measures
Question Based Delay Discounting Measure (DDQ; [31])
For this measure, participants were presented choices between $10 available after a specified delay (i.e., 1, 2, 30, 180, or 365 days) and a smaller amount available immediately (e.g., ‘would you rather have $10 in 30 days or $2 now?’). This computerized task was an adjusting amount procedure (adjusting the immediate amount in increments of ± $0.50) used to derive indifference points between the delayed-standard and immediate-adjusting options for each of the five delays assessed. An indifference point reflected the smallest amount of money an individual chose to receive immediately instead of the delayed standard amount ($10) at the specific delay. The choice questions were presented using a titration procedure that was determined by participant choices, with each participant making approximately 60 choices total. Indifference points across the different delays were characterized with an area under the curve (AUC) method [32], with smaller area values indicating greater discounting by delay and greater impulsivity. Participants were told that their answers to the questions were important because at the end of the session one question would be selected and honored—resulting in either immediate or delayed money. See [33] for participant instructions for the DDQ.
Barratt Impulsiveness Scale – Adolescent (BIS-11-A; [34])
The BIS-11-A is a 30 item self-report questionnaire designed to measure impulsiveness. Items are on a 4-point scale (1 = rarely/never to 4 = almost always/always). The BIS-11-A is an adaptation of the adult BIS-11 [35]. However, due to high inter-correlations among the sub-factors for adolescents, it has been recommended that total scores are the most appropriate index of impulsivity for this age group. Higher total scores reflect greater impulsivity. Past research has found that the BIS-11-A has good internal consistency in adolescent samples (α = 0.78; [34]). However, alpha for the current data set was lower but still marginally acceptable (α = 0.59).
Procedure
Upon participants’ arrival to the laboratory, consent and assent were obtained. Breath and urine samples were then collected for determination of CO and cotinine levels, respectively. Urine samples were collected in a private bathroom close to the laboratory using specially designed, heat-sensitive specimen containers for determining the temperature of samples immediately after being obtained. Participants then completed all of the self-report assessments, which required ~20 min. Participants then completed a battery of laboratory behavioral tasks (including the Go/Stop task and measures of sustained attention and delay discounting), with a 5-min break following the first two assessments. Task order was counterbalanced across all participants. Only data from the measure of delay discounting are presented here, with much of the data from the other behavioral assessments being published already [27].
Data Analysis
Again, the AUC method [32] was used to characterize data from the DDQ. From the AUC method, smaller values reflect greater discounting and impulsivity. The AUC data were inspected for normality using a Fisher’s skew statistic., To improve normality, these data were log-10 transformed, and after transformation, the AUC data were determined to be adequately normal—with a Fisher statistic > −2 and < 2.
SPSS (Version 17.0) was used for all statistical analyses. One-way analyses of variance (ANOVAs) were used to compare participant demographic data across smoking-status groups. An exception was race, which is a categorical variable and was compared across smoking status groups using Spearman chi-square test. All dependent measures were compared using separate between-subjects two-way ANOVAs. For these analyses, smoking status and gender were the grouping variables. In cases where there were significant group differences on demographic variables that may represent confounds, these variables were entered as covariates in follow-up ANOVA analyses to determine if the observed effects existed above and beyond what might be accounted for based on demographic differences. Partial eta squares (ηp2) were calculated as estimates of effect size. Based on Cohen’s conversion [36], ηp2 values near .0099 reflect a small effect size, .0588 a medium effect size, and .1378 a large effect size. Finally, correlations were calculated using Pearson r coefficients to determine associations between measures.
RESULTS
Participant Characteristics
Participant demographic data are presented in Table 1. The non-smokers and experimenters had significantly lower breath CO and urinary cotinine levels than did smokers, thus verifying smoking status. The non-smokers and experimenters did not differ in their levels of CO or cotinine. Also, the non-smokers had higher IQ scores than the experimenters and smokers, with the experimenters and smokers not differing significantly in IQ scores. The three groups differed on self-reported alcohol and marijuana use. The non-smokers reported the least use and the smokers the greatest use of these substances. Experimenters reported intermediate levels of use between the non-smokers and smokers. The only instance of regular use of these substances was from the smokers, who reported on average consuming marijuana monthly or weekly.
Table 1.
Participant Demographics and Drug Use (N = 141)
| Non-Smokers | Experimenters | Smokers | |
|---|---|---|---|
| Demographics | |||
| Sex (n; male:female) | 17:33 | 15:26 | 17:33 |
| Age [years; M (SD)] | 15.1 (1.10)a | 15.3 (1.06)a | 15.7 (1.06)b |
| Race (n; white:black:other) | 23:22:5 | 18:19:2 | 22:26:2 |
| Annual Household Income ($; Median)a | 58,933.00 | 56,589.00 | 50,766.00 |
| Carbon Monoxide [ppm; M (SD)] | 1.90 (1.33)a | 2.05 (1.22)a | 11.04 (7.49)b |
| Cotinine [ng/ml; M (SD)] | 1.58 (8.81)a | 15.4 (72.43)a | 1278 (833)b |
| KBIT2 [IQ; M (SD)] | 100.4 (14.4)a | 91.2 (14.44)b | 87.8 (14.2)b |
| Drug Useb | |||
| Alcohol [M (SD)] | 0.36 (0.53)a | 1.17 (0.92)b | 1.87 (1.16)c |
| Marijuana [M (SD)] | 0.14 (0.40)a | 1.00 (1.12)b | 2.85 (1.76)c |
Note. Means in the same row that do not share the same subscript differ at p < .05.
The median annual household income was calculated based on average income for postal zip code region of the participant’s residence.
Drug use was assessed with the following question: “Thinking about the past six months, how often have you used the following substances?”: 0 = never tried, 1 = tried, 2 = used 1–2 times per month, 3 = use once a week, 4 = use 2–4 times per week, 5 = use 5 or more times per week.
DDQ
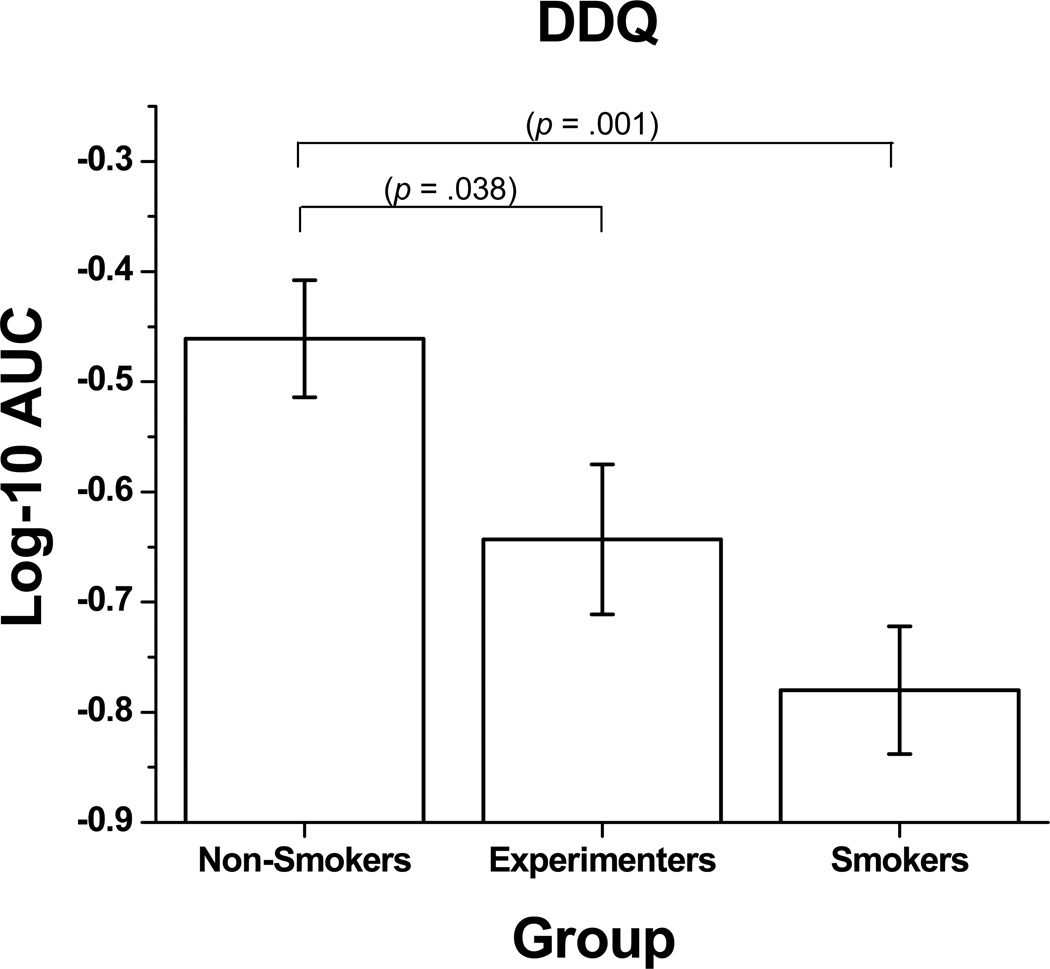
There were no significant interactions between gender and smoking status for the DDQ, nor were there any main effects of gender. However, a main effect was found for smoking status [F = 6.961, p = .001, ηp2 = 0.095], which based on the ηp2 represents a medium effect size. Specifically, the smokers and experimenters discounted significantly more than the non-smokers (see Figure 1). There was no significant difference between experimenters and smokers on rate of discounting.
Figure 1.
Mean (± SEM, denoted by error bars) log-10 transformed total area-under-the-curve (AUC) values between Non-Smokers, Experimenters, and Smokers on a question based delay discounting measure (DDQ). Larger negative values denote greater discounting and impulsivity.
These analyses were re-evaluated controlling for group differences on variables other than smoking status. Smoking status main and post hoc effects remained significant after controlling for IQ [F = 5.439, p = .021, ηp2 = 0.051], age [F = 7.401, p = .001, ηp2 = 0.101], and use of alcohol or marijuana [F = 3.528, p = .032, ηp2 = 0.052] as statistical covariates.
BIS-11-A
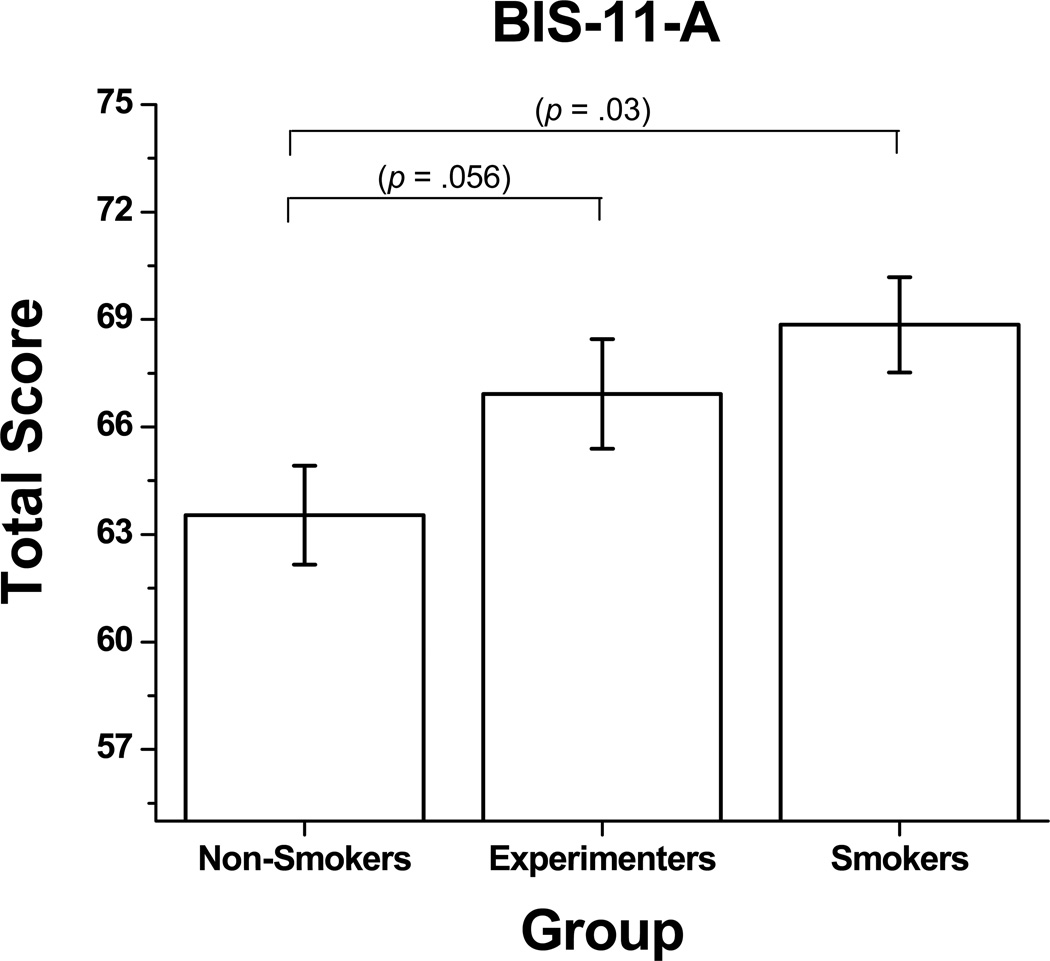
As with the DDQ, there were no significant interactions between gender and smoking status for the BIS-11-A, nor were there any main effects of gender. However, there was a main effect for smoking status [F = 5.624, p = .005, ηp2 = 0.077], representing a medium effect size. Specifically, smokers had significantly higher scores (i.e. more impulsive) on the BIS-11-A than non-smokers (see Figure 2). There was a trend for non-smokers to have lower scores than experimenters on the BIS-11-A. There was no significant difference between experimenters and smokers.
Figure 2.
Mean (± SEM, denoted by error bars) total impulsivity ratings on the Barratt Impulsiveness Scale—Adolescent (BIS-11-A) between Non-Smokers, Experimenters, and Smokers. Larger values denote greater impulsivity.
Covariate analyses revealed that both main and post hoc results for the BIS-11-A remained statistically significant after controlling for IQ [F = 4.110, p = .019, ηp2 = 0.058] and age [F = 6.277, p = .002, ηp2 = 0.086], but not after controlling for alcohol or marijuana use [F = 1.874, p = .158, ηp2 = 0.028].
Analyses for the DDQ and BIS-11-A were run again excluding data from the experimenter who had an unusually high cotinine level (described above). All results were the same in terms of statistical significance except the post-hoc difference for the DDQ between non-smokers and experimenters, which approached significance (p = .059).
Correlations
From Table 2, several interesting correlations emerge. The DDQ and BIS-11-A were not correlated; however, the DDQ was correlated with reports of marijuana use (but not alcohol use) and the BIS-11-A with alcohol use (but not marijuana use). In both cases, greater impulsivity with each measure was associated with more use of marijuana or alcohol. The DDQ also was correlated with IQ. Among the smokers (n = 50), cotinine and breath CO levels were significantly correlated (r = .51, p = .001), but neither the DDQ nor BIS-11-A was correlated with cotinine or CO level.
Table 2.
Correlation Matrix
| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| 1. log DDQ | 1.0 | ||||
| 2. BIS-11-A | −0.041 | 1.0 | |||
| 3. IQ | 0.313** | −0.136 | 1.0 | ||
| 4. Alcohol Use | −0.108 | 0.294** | −0.162 | 1.0 | |
| 5. Marijuana Use | −0.268** | 0.120 | −0.329** | 0.512** | 1.0 |
Note:
p < .05,
p < .01
DISCUSSION
The goal of the present study was to evaluate delay discounting and self-reported impulsivity in a sample of adolescents experimenting with cigarette smoking. With both assessments, results generally indicated that adolescents experimenting with smoking were more like daily smokers than non-smokers, though this effect only approached significance with the self-report measure. For both delay discounting and ratings on the BIS-11-A the effect sizes were generally in the medium range as defined by Cohen [36]. Approximately 10% of the variance in smoking status was accounted for by delay discounting, and approximately 8% of the variance in smoking status was accounted for by the BIS-11-A. .
The current finding for delay discounting suggests that discounting is associated with initial experimentation with cigarettes and is therefore likely a risk factor for cigarette smoking. Put another way, this finding does not support the hypothesis that smokers, for example, discount more by delay than non-smokers [e.g., 15 – 17] as a consequence of their smoking. This result contributes to the delay discounting/cigarette smoking literature by highlighting that adolescents experimenting with smoking (who have not been studied before in terms of delay discounting) are more like established smokers than they are like non-smokers. However, it should be noted that while experimenters were more like smokers than non-smokers in their delay discounting they were still intermediate between these two other groups. Therefore, the experimenters were not equivalent to smokers. But, this intermediate pattern of discounting by experimenters maybe should have been expected. For example, while at increased risk of becoming established smokers, these experimenters were not yet smokers. That is, some of these experimenters would become smokers and others would not. It is possible that experimenters who discount most are more likely to move on to more regular patterns of smoking.
Additional analyses revealed that delay discounting was associated with self-reported marijuana use and that the BIS-11-A was associated with frequency of alcohol consumption, but the reverse associations were not significant. Also, smoking status effects for the BIS-11-A were reduced to non-significance when use of marijuana and alcohol were controlled as a covariate. In terms of possible drug effects on delay discounting it is worth noting that the findings for delay discounting based on cigarette smoking status remained statistically significant even after controlling for other substance use. Additionally, there is evidence that use of marijuana and alcohol may have little effect on delay discounting [37, 31]. As such, it is unlikely that there were marijuana or alcohol effects on delay discounting in this sample.
Also, the measures of delay discounting and the BIS-11-A were not significantly correlated, even though both instruments are considered assessments of impulsive behavior. Possible reasons for the lack of correlation between behavioral and self-report measures have been discussed previously (e.g., [38]) and may include (a) the measure of delay discounting assessing a more narrowly defined behavior (i.e., delay-related devaluation) than the BIS-11-A and/or (b) differences in measurement variability based on participant ability to accurately make choices for delayed or immediate money versus ability to accurately rate his or her own behavior on the BIS-11-A (especially for adolescents). However, even with these differences in assessment method, it is notable that the general pattern of findings was consistent across the measures of delay discounting and self-reported impulsivity, thus suggesting robustness of effect based on smoking status.
Also of interest were patterns of association from the measure of IQ. As reported previously [39], IQ scores were significantly correlated with delay discounting. Participants with higher IQ scores discounted less by delay than participants with lower scores. Additionally, experimenters and smokers had lower IQ scores than non-smokers, but did not themselves differ. This pattern of IQ scores across smoking-status groups is strikingly similar to the pattern of findings for delay discounting. Taken together, these results illustrate a linkage between IQ scores and delay discounting at both individual (i.e., correlation) and group levels. However, smoking-status effects remained statistically significant for delay discounting even after controlling for group differences in IQ scores.
The current study is not without limitations. For example, the cross-sectional design of this research is limited in terms of conclusions about progression of smoking over time. Also, there is no established range of cotinine or CO levels to verify that an adolescent has recently experimented with cigarettes. In fact, one experimenter had an elevated cotinine level (but not CO level) compared to what we would expect for non-smokers, which drew into question this person’s exposure to nicotine. However, it is worth noting that this individual’s cotinien level (453 ng/ml) was low when compared with established smokers, with an average cotinine level >1200 ng/ml. Removing this participant from analyses had only minimal effects, but it did reduce the difference in delay discounting between non-smokers and experimenters. Not collecting more date on other possible exposures to nicotine (e.g., environmental tobacco smoke, smokeless tobacco) is a limitation of this research. .
Despite limitations, the present findings point to the likelihood that delay discounting is a risk factor for initial use of cigarettes among adolescents. Extending from this work, future prospective research might explore delay discounting in the progression from experimentation with cigarettes to more regular patterns of smoking as one of several recognized risk factors (e.g., stress, parental monitoring, having peer friends who smoke) to determine the relative contributory role of delay discounting in the progression of cigarette smoking among at-risk adolescents.
Acknowledgments
This research was funded by NIDA research grant R21 DA020423
REFERENCE
- 1.Schepis TS, Adinoff B, Rao U. Neurobiological processes in adolescent addictive disorders. The American Journal on Addictions. 2008;17(1):6–23. doi: 10.1080/10550490701756146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jefferis B, et al. Cigarette consumption and socio-economic circumstances in adolescence as predictors of adult smoking. Addiction. 2003;98(12):1765–1772. doi: 10.1111/j.1360-0443.2003.00552.x. [DOI] [PubMed] [Google Scholar]
- 3.Chassin L, et al. The natural history of cigarette smoking from adolescence to adulthood: Demographic predictors of continuity and change. Health Psychology. 1996;15(6):478–484. doi: 10.1037//0278-6133.15.6.478. [DOI] [PubMed] [Google Scholar]
- 4.Pederson LL, et al. The degree and type of relationship between psychosocial variables and smoking status for students in Grade 8: Is there a dose-response relationship? Preventive Medicine: An International Journal Devoted to Practice and Theory. 1998;27(3):337–347. doi: 10.1006/pmed.1998.0305. [DOI] [PubMed] [Google Scholar]
- 5.Maes HH, et al. Genetic and cultural transmission of smoking initiation: An extended twin kinship model. Behavior Genetics. 2006;36(6):795–808. doi: 10.1007/s10519-006-9085-4. [DOI] [PubMed] [Google Scholar]
- 6.Chassin L, et al. Multiple trajectories of cigarette smoking and the intergenerational transmission of smoking: A multigenerational, longitudinal study of a midwestern community sample. Health Psychology. 2008;27(6):819–828. doi: 10.1037/0278-6133.27.6.819. [DOI] [PubMed] [Google Scholar]
- 7.Tercyak KP, Audrain-McGovern J. Personality differences associated with smoking experimentation among adolescents with and without comorbid symptoms of ADHD. Substance Use & Misuse. 2003;38(14):1953–1970. doi: 10.1081/ja-120025121. [DOI] [PubMed] [Google Scholar]
- 8.Kirisci L, et al. Individual differences in childhood neurobehavior disinhibition predict decision to desist substance use during adolescence and substance use disorder in young adulthood: A prospective study. Addictive Behaviors. 2006;31(4):686–696. doi: 10.1016/j.addbeh.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 9.Rachlin H, Brown J, Cross D. Discounting in judgments of delay and probability. Journal of Behavioral Decision Making. 2000;13(2):145–159. [Google Scholar]
- 10.Reynolds B. A review of delay-discounting research with humans: Relations to drug use and gambling. Behavioural Pharmacology. 2006;17(8):651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- 11.Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: Delay discounting processes. Addiction. 2001;96(1):73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- 12.Bjork JM, et al. Impulsivity in abstinent alcohol-dependent patients: Relation to control subjects and type 1-/type 2-like traits. Alcohol. 2004;34(2):133–150. doi: 10.1016/j.alcohol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Experimental and Clinical Psychopharmacology. 1998;6(3):292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- 14.Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154(3):243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds B, et al. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behavioural Processes. 2004;65(1):35–42. doi: 10.1016/s0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell SH. Measures of impulsivity in cigarette smokers and nonsmokers. Psychopharmacology. 1999;146(4):455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- 17.Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: Delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146(4):447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- 18.Krishnan-Sarin S, et al. Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug and Alcohol Dependence. 2007;88(1):79–82. doi: 10.1016/j.drugalcdep.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dallery J, Raiff BR. Delay discounting predicts cigarette smoking in a laboratory model of abstinence reinforcement. Psychopharmacology. 2007;190(4):485–496. doi: 10.1007/s00213-006-0627-5. [DOI] [PubMed] [Google Scholar]
- 20.Audrain-McGovern J, et al. Applying a Behavioral Economic Framework to Understanding Adolescent Smoking. Psychology of Addictive Behaviors. 2004;18(1):64–73. doi: 10.1037/0893-164X.18.1.64. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds B, Patak M, Shroff P. Adolescent smokers rate delayed rewards as less certain than adolescent nonsmokers. Drug and Alcohol Dependence. 2007;90(2):301–303. doi: 10.1016/j.drugalcdep.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds B, et al. Laboratory and self-report assessments of impulsive behavior in adolescent daily smokers and nonsmokers. Experimental and Clinical Psychopharmacology. 2007;15(3):264–271. doi: 10.1037/1064-1297.15.3.264. [DOI] [PubMed] [Google Scholar]
- 23.Audrain-McGovern J, et al. Does delay discounting play an etiological role in smoking or is it a consequence of smoking? Drug and Alcohol Dependence. 2009;103(3):99–106. doi: 10.1016/j.drugalcdep.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kolokotroni KZ, Rodgers RJ, Harrison AA. Acute nicotine increases both impulsive choice and behavioural disinhibition in rats. Psychopharmacology. 2011 doi: 10.1007/s00213-011-2296-2. [DOI] [PubMed] [Google Scholar]
- 25.Anderson KG, Diller JW. Effects of acute and repeated nicotine administration on delay discounting in Lewis and Fischer 344 rats. Behavioural Pharmacology. 2010;21(8):754–764. doi: 10.1097/FBP.0b013e328340a050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patton GC, et al. Depression, anxiety, and smoking initiation: a prospective study over 3 years. American journal of public health. 1998;88(10):1518–1522. doi: 10.2105/ajph.88.10.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fields S, et al. Dimensions of impulsive behavior in adolescent smokers and nonsmokers. Experimental and Clinical Psychopharmacology. 2009;17(5):302–311. doi: 10.1037/a0017185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melanko S, et al. Characteristics of psychopathy in adolescent nonsmokers and smokers: Relations to delay discounting and self reported impulsivity. Experimental and Clinical Psychopharmacology. 2009;17(4):258–265. doi: 10.1037/a0016461. [DOI] [PubMed] [Google Scholar]
- 29.Organization WH. Census 2000 datasets: Summary File 3 for Ohio. 2000 October 2; 2006; Available from: http://www2.census_2000/datasets/Summary_File_3/Ohio/ [Google Scholar]
- 30.Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test, Second Edition: Manual. 2nd ed. Circle Pines: AGS; 2004. [Google Scholar]
- 31.Richards JB, et al. Delay or probability discounting in a model of impulsive behavior: Effect of alcohol. Journal of the Experimental Analysis of Behavior. 1999;71(2):121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. Journal of the Experimental Analysis of Behavior. 2001;76(2):235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reynolds B, et al. Delay and probability discounting as related to different stages of adolescent smoking and non-smoking. Behavioural Processes. 2003;64(3):333–344. doi: 10.1016/s0376-6357(03)00168-2. [DOI] [PubMed] [Google Scholar]
- 34.Fossati A, et al. Psychometric properties of an adolescent version of the Barratt Impulsiveness Scale-11 for a sample of Italian high school students. Perceptual and Motor Skills. 2002;95(2):621–635. doi: 10.2466/pms.2002.95.2.621. [DOI] [PubMed] [Google Scholar]
- 35.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 36.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, N.J: L. Erlbaum Associates; 1988. p. 567. xxi. [Google Scholar]
- 37.Johnson MW, et al. Delay discounting in current and former marijuana-dependent individuals. Experimental and Clinical Psychopharmacology. 2010;18(1):99–107. doi: 10.1037/a0018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reynolds B, et al. Dimensions of impulsive behavior: Personality and behavioral measures. Personality and Individual Differences. 2006;40(2):305–315. [Google Scholar]
- 39.de Wit H, et al. IQ and nonplanning impulsivity are independently associated with delay discounting in middle-aged adults. Personality and Individual Differences. 2007;42(1):111–121. [Google Scholar]