Abstract
Rationale
Long-term smoking can lead to changes in autonomic function, including decreased vagal tone and altered stress responses. One index of the inability to adapt to stress may be blunted vagal reactivity. Stress is a primary mechanism involved in relapse to smoking, but mechanisms leading to stress-precipitated relapse are not well understood.
Objectives
Using an experimental paradigm of stress-precipitated smoking behavior, we examined whether autonomic reactivity mediates the relationship between stress and smoking. High-frequency heart rate variability (HF-HRV), a putative measure of vagal tone and the ratio of low-to-high frequency HRV (LF/HF), a measure of sympathovagal balance were assessed.
Methods
Using a within-subjects design, 32 nicotine dependent 15-hour abstinent smokers (a subgroup from McKee et al. (2011)) were exposed to individualized script-driven imagery of stressful and relaxing scenarios and assessed on the ability to resist smoking and subsequent ad-lib smoking. HRV was monitored throughout each laboratory session (maximum 60 min following imagery).
Results
As expected, stress and ad-lib smoking additively decreased HF-HRV and increased LF/HF. Blunted stress-induced HF-HRV responses reflecting decreased vagal reactivity were associated with less time to initiate smoking and increased craving relief and reinforcement from smoking. These relationships were specific to HF-HRV following stress as neither baseline HF-HRV, HF-HRV following relaxing imagery, or LF/HF predicted smoking behavior.
Conclusions
The current findings are the first to experimentally demonstrate that stress-precipitated decreased vagal reactivity predicts the ability to resist smoking. Findings suggest that strategies that normalize vagal reactivity in early abstinent smokers may lead to improved smoking cessation outcomes.
Keywords: stress, nicotine, abstinence, relapse, vagal tone, heart rate variability, autonomic balance
INTRODUCTION
Cigarette smoking is the single most preventable cause of morbidity and mortality in the United States (CDC, 2002; CDC, 2004) and if global current trends continue, tobacco will kill 7 million people annually by 2020 and more than 8 million people annually by 2030 (Shafey, Eriksen, Ross, & Mackay, 2009). Like other substance use disorders, nicotine dependence is a chronic relapsing condition and fewer than 2% successfully quit on a yearly basis (CDC, 2002). Stress is associated with increased smoking and risk for relapse (al'Absi, 2006; al'Absi, Hatsukami, & Davis, 2005; Shiffman et al., 1996). Smokers often report that acute stress precedes a lapse episode (Shiffman, et al., 1996) and evidence from preclinical and clinical studies suggests stress increases the risk of drug abuse and relapse (Sinha, 2001). Moreover, chronic drug use, including cigarette smoking, can lead to neuroadaptive changes in the systems involved in regulating stress responses, including the hypothalamic-pituitary-adrenal (HPA) axis, brain dopaminergic system, and autonomic nervous system (Sinha, 2007). For example, nicotine, the primary psychoactive agent in cigarette smoke, may influence autonomic function both directly through its effects on nicotinic receptors in the central nervous system and indirectly through its effects on dopamine release in the central nervous system (Lewis, Balaji, Dixon, Syed, & Lewis, 2010; Sjoberg & Saint, 2011). Both acute and chronic elevations in physiological systems involved in the stress response are known to increase allostatic load, characterized by maintaining stress-related responses that are outside the normal homeostatic range (McEwen & Stellar, 1993). Thus, the combined effects of smoking and stress may increase allostatic load in smokers and result in changes in autonomic function (Thayer, Yamamoto, & Brosschot, 2010).
Multiple systems are involved in the stress response and previous research has demonstrated that HPA axis function is associated with stress-related relapse to smoking (al'Absi, 2006; al'Absi, et al., 2005; Cacioppo, 1994; Robinson & Cinciripini, 2006) and cocaine use (Back et al., 2010). Previously, McKee and colleagues (McKee et al., 2011) showed that acute stress reduced the ability to resist smoking and increased HPA axis reactivity (e.g., cortisol and ACTH) among abstinent smokers. Cardiovascular measures (e.g., heart rate and blood pressure) were increased by stress but were not related to the ability to resist smoking (McKee, et al., 2011). Although the HPA axis stress response has been well characterized, it is not the only system impacted by stress. Both the parasympathetic and sympathetic branches of the autonomic nervous system are influenced by stress and are often thought of as indicators of autonomic flexibility (Friedman, 2007; Ulrich-Lai & Herman, 2009). These two branches interact with one another and may provide an additional window into stress-precipitated smoking lapses.
Cardiovascular autonomic activity can be quantified by various metrics including heart rate variability (HRV), which measures fluctuations in the sinus node’s beat-to-beat interval. These measures are thought to reflect vagal activity and autonomic flexibility (Berntson et al., 1997) and typically yield similar results (Allen, Chambers, & Towers, 2007). One measure of HRV is respiratory sinus arrhythmia (RSA), which measures respiratory-linked HRV (Malik et al., 1996). Within the frequency domain, high-frequency (HF) HRV, which is mediated via release of acetylcholine from the vagus nerve, is often used as an index of vagal activity and as long as the individual is breathing in the normal range, is a measure of respiratory sinus arrhythmia (Akselrod et al., 1981). Although the measurement of respiration and the degree to which HRV reflects vagal reactivity are controversial (Egizio, Eddy, Robinson, & Jennings, 2011; Ritz, 2009), it is generally accepted that the parasympathetic and sympathetic nervous systems are in constant flux to maintain autonomic balance. ‘Sympathovagal balance’ is often measured via the ratio of the low-to-high frequency HRV (LF/HF) (Malliani & Montano, 2002). Although the physiological underpinnings of this metric are controversial (Berntson, et al., 1997; Eckberg, 1997), increases in LF/HF may represent a shift towards sympathetic predominance and reduced vagal activity (Malik, et al., 1996).
Altered autonomic function is often described as overactive sympathetic and hypoactive vagal function and is associated with pathological states such as coronary artery disease and increased mortality rates (for a review, see Thayer, et al., 2010) and various chronic stress conditions, including lower socioeconomic status (Lampert, Ickovics, Horwitz, & Lee, 2005), exposure to traumatic events (Lampert, Baron, McPherson, & Lee, 2002), and work stress (Thayer, et al., 2010). Specifically higher HF-HRV reflects healthier physiological states marked increased vagal activity. In contrast, lower HF-HRV and higher LF/HF reflect pathological states indicated by reduced autonomic balance (Berntson, Norman, Hawkley, & Cacioppo, 2008; Friedman, 2007). However, others have suggested higher HF-HRV is associated with pathological states (Egizio, et al., 2011; Ritz, 2009). HRV is also inversely related to HPA-axis activity, such that lower HRV (i.e., decreased vagal activity) is associated with increased cortisol responses following stress (Thayer & Sternberg, 2006). Lower vagal function may also be associated with deficits in self-regulatory behaviors (Porges, Doussard-Roosevelt, & Maiti, 1994).
In line with the notion that substance abuse reflects deficits in self-regulation (Sinha, 2009), there is a strong link between substance use and cardiovascular autonomic function. Alcohol and drug abuse (Sinha et al., 2009) and cigarette smoking have been shown to decrease HF-HRV (Hayano et al., 1990; Tsuji et al., 1996). Likewise, smoking may alter autonomic balance as measured by increases in LF/HF (Kobayashi et al., 2005; Minami, Ishimitsu, & Matsuoka, 1999). Among non-smokers, environmental tobacco smoke (Pope, 2001), ad-lib smoking (Karakaya et al., 2007), and nicotine gum have been linked to decreased HF-HRV (Sjoberg & Saint, 2011). Although this reduction in HF-HRV following short-term smoking may be due, in part, to nicotine’s influence on the cholinergic pathway (Hayano, et al., 1990), chronic smoking also contributes to decreased HF-HRV (Gallagher, Terenzi, & de Meersman, 1992; Kupari, Virolainen, Koskinen, & Tikkanen, 1993; Thayer, et al., 2010). Further, smoking abstinence may lead to increased vagal function and reduced sympathetic stimulation (Lucini, Bertocchi, Malliani, & Pagani, 1998).
Stress-related changes in HF-HRV and LF/HF are hypothesized to reflect vagal reactivity and alterations in autonomic balance, respectively. In laboratory settings, HF-HRV generally decreases and LF/HF increases in response to stressors, including emotional, mental, and social stress tasks (Key, Campbell, Bacon, & Gerin, 2008; Lackschewitz, Hüther, & Kröner-Herwig, 2008; O'Donnell, Brydon, Wright, & Steptoe, 2008; Pagani et al., 1991). Although LF/HF appears less sensitive to acute stressors, to the extent that parasympathetic and sympathetic activity contribute to the regulation of stress responses, dysfunction in these systems may partially explain vulnerability to stress-precipitated relapse (Sinha, 2009).
Several studies have examined autonomic functioning during smoking cessation (Lewis, et al., 2010; Stein, Rottman, & Kleiger, 1996; Yotsukura et al., 1998). However, no study to our knowledge has examined whether stress-related vagal reactivity measured via HF-HRV and sympathovagal balance measured via LF/HF in smokers, predicts the ability to resist smoking and subsequent ad-lib smoking. Using a laboratory paradigm of smoking relapse in a sample of acutely abstinent smokers (McKee, et al., 2011), we assessed whether laboratory-induced stress resulted in altered vagal reactivity and sympathovagal balance. Based on evidence that blunted stress responses may be related to relapse (al'Absi, et al., 2005; Robinson & Cinciripini, 2006), we predict that blunted HF-HRV responses (i.e., reflecting decreased vagal reactivity) and increased LF/HF (i.e., reflecting shifts in sympathovagal balance) following stress will be associated with reduced time to initial lapse and increased ad-lib smoking. However, the evidence regarding the direction of the relationship is mixed (Vanderkaay & Patterson, 2006; Wardle, Munafo, & de Wit, 2011), suggesting that nicotine deprivation may result in a heightened stress response, which may be related to subsequent relapse.
METHODS AND MATERIALS
Participants
Eligible participants were 18– 60 years old, smoked at least 10 cigarettes per day, and had plasma cotinine levels > 50 ng/ml. Exclusion criteria included past 6-month Axis I disorders (except nicotine dependence), illicit drug use (except occasional marijuana use), past 6-month treatment for smoking cessation, and medical conditions that would contraindicate smoking behavior. Of the 37 subjects in the parent study, HRV was collected on 32 subjects1. Thirty-two participants (12 female, 18 Caucasian), aged 37.91 years (SD=10.91), smoked 17.56 cigarettes per day (SD=6.63), with Fagerström Nicotine Dependence scores (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) of 6.16 (SD=2.24; range = 0–10) were included in the study.
Procedures
Intake sessions
All participants were consented using procedures approved by the Yale School of Medicine Human Investigation Committee. The Structured Clinical Interview for DSM-IV (First, Spitzer, Gibbon, & Williams, 1995) was used to assess current Axis I disorders (except nicotine dependence). Smoking history was assessed with a measure developed by the Yale Transdisciplinary Tobacco Use Research Center.
Script development session
A personalized guided imagery procedure was used to create the stress and relaxing conditions (Sinha, 2009). The stress imagery script was developed by having participants describe, in detail, a personal stressful experience that occurred in the last 6 months, experienced as ‘most stressful’. Perceived stress was rated on a 10-point Likert scale where 1=‘not at all stressful’ and 10=‘the most stress they recently felt in their life’. Ratings were made relative to experiences in the past 6 months. Only situations rated as 8 or greater were accepted for script development and all participants were able to describe stressful experiences. Examples of stressful experiences were marital conflict or losing employment. The relaxing script was developed having participants describe a personal relaxing situation, such as sitting at the beach or reading in the park. Scripts were developed by a PhD-level clinician, audiotaped for presentation, and were approximately 5 min in length (see also McKee, et al., 2011).
Laboratory sessions
Participants completed two 6.5-hour laboratory sessions (stress vs. relaxing; order counterbalanced) separated by approximately one week. Participants were compensated $150 per laboratory session and received a $50 bonus for completing all scheduled study appointments (intake session, script development session, and two laboratory sessions). The current laboratory paradigm of smoking relapse has been validated using various relapse precipitants including nicotine withdrawal (Leeman, O'Malley, White, & McKee, 2010), alcohol (McKee, Krishnan-Sarin, Shi, Mase, & O'Malley, 2006), and most recently stress (McKee, et al., 2011). Additional details of the study design and results from physiological and behavioral measures have been presented previously as part of a larger sample which included the 32 participants reported here (McKee, et al., 2011).
Baseline assessment period
Participants were asked to refrain from smoking beginning at 10:00pm the night before their scheduled session. Carbon monoxide (CO) breath samples were used to verify abstinence (i.e., < 50% intake value).
Personalized imagery procedure
At 1:00pm (15 hours after their last cigarette) participants were given the following instructions for guided imagery in each of the stress and relaxing conditions: “You will soon hear a situation being described to you. Your task is to close your eyes and imagine yourself in the situation being described, “as if” it were happening right now. Allow yourself to become completely involved in the situation, by involving your mind and body in actually doing what is being described. Continue imagining until you are asked to stop.” The participant then listened to the script (stress or relaxing).
Delay period
At 1:10pm, participants were told they could smoke at any time over the next 50 minutes, but for each 5-minute block that they ‘resisted’ smoking, they would earn $.50, $1.00, or $1.50, depending on their assigned condition. The monetary condition had no effect on HF-HRV. The time (in minutes) when participants stated they wanted to smoke was recorded (range 0–50 minutes). Following a brief assessment, participants commenced smoking.
Smoking self-administration period
Once the delay period ended (or reached 50 minutes), participants were given the opportunity to smoke as much or as little as they wished (up to 8 cigarettes) during the 60-minute ad-lib smoking period. Participants were also provided a $4 ‘smoking tab’ and were instructed that each cigarette they lit would cost them $.50. Participants were paid at end of the session for any money earned during the delay period or the ad-lib smoking period.
Timing of assessments
Heart rate variability was recorded continuously beginning at 10:00am until participants were discharged at 3:15pm. Subjective ratings of craving, mood, and nicotine withdrawal were assessed pre-imagery, post-imagery, end of delay period, and +30 and +60 minutes during the self-administration period. Additional measures of mood, physiological reactivity, smoking topography, and HPA-axis reactivity were also collected and are reported elsewhere (McKee et al., 2011).
Measures
Heart rate variability
An ambulatory ECG (Holter) monitor (GE Marquette SEER digital system) was used to record heart rhythm during the session. Recordings were digitally sampled and analyzed using a GE-Marquette system. Tapes were manually reviewed to identify R-R intervals with annotations denoting normal beats, types of ectopics, and noise and then processed and analyzed with customized software as in prior work (Bigger et al., 1992; Lampert, et al., 2005). Recordings with greater than 20% interpolated segments were excluded from further analysis. The RR interval time series was sampled using a boxcar window (Berger, Akselrod, Gordon, & Cohen, 1986) to obtain 1024 samples per 5 minutes (3.41333 Hz). The power spectrum was computed using a fast Fourier transform with a Parzen window on four-minute segments with a one-minute sliding window, corrected for attenuation due to windowing and sampling (Hamming, 1973) and integrated over five standard frequency bands (Bigger et al., 1992) High-frequency power (HF; 0.15 to 0.40 Hz), a marker of parasympathetic activity (Akselrod, et al., 1981; Pagani et al., 1986) was compared between experimental conditions. Following the imagery presentation and during each of the ad-lib cigarettes, we identified the minimum value of the high frequency (HF; 0.15 to 0.40 Hz) power band as an index of parasympathetic activity (Akselrod, et al., 1981). The LF/HF was computed by dividing the LF (LF; .04 to .15 Hz) value by the corresponding HF value. The natural log of the minimum value of HF-HRV and LF/HF were the primary dependent variables. Baseline values were calculated as the average of minimum values of HF-HRV and LF/HF over the 30 minutes prior to the imagery condition (Berntson, et al., 1997).
Subjective measures
The Questionnaire of Smoking Urges—Brief (QSU-Brief; Cox, Tiffany, & Christen, 2001) is a 10-item measure used to evaluate tobacco craving and urges to smoke in response to positive (Factor 1) or negative (Factor 2) reinforcement (VAS scale, range 1–100). The Cigarette Effects Scale is a 10-item measure used to assess satisfaction, psychological reward, nausea/dizziness, craving relief, and enjoyment of airway sensations associated with smoking (VAS scale, range 1–100) (Westman, Levin, & Rose, 1992). The Minnesota Nicotine Withdrawal Scale (MNWS; Hughes & Hatsukami, 1986) is an 8-item measure used to assess DSM-IV symptoms of nicotine withdrawal. Instructions were worded to assess current symptoms of withdrawal (range 0–32).
Statistical Analysis
Paired t-tests were used to examine minimum values of HF-HRV and LF/HF following the imagery condition, and during each of the cigarettes smoked during the ad-lib period. Age (De Meersman & Stein, 2007), income, and monetary condition were evaluated as potential covariates in our primary analyses and were not found to contribute significant variance to the primary outcomes. Secondarily, Pearson correlations examined associations between baseline and post-imagery HF-HRV and LF/HF with latency to commence smoking, number of cigarettes smoked, tobacco craving, smoking–related reward, and physiologic reactivity. Because these analyses were exploratory, no correction for multiple tests was used.
RESULTS
Smoking Behavior
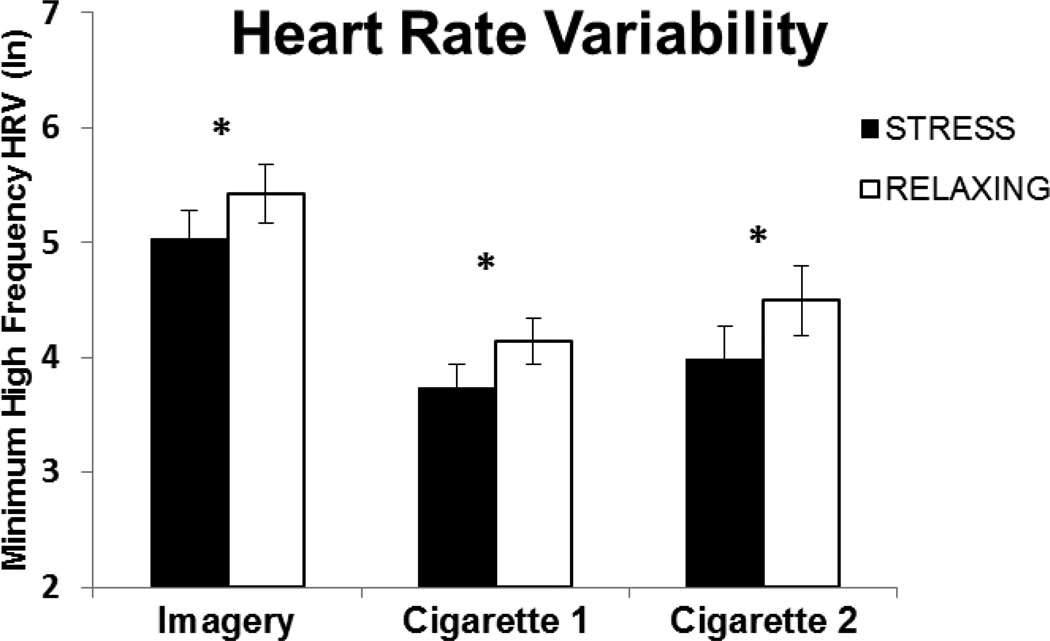
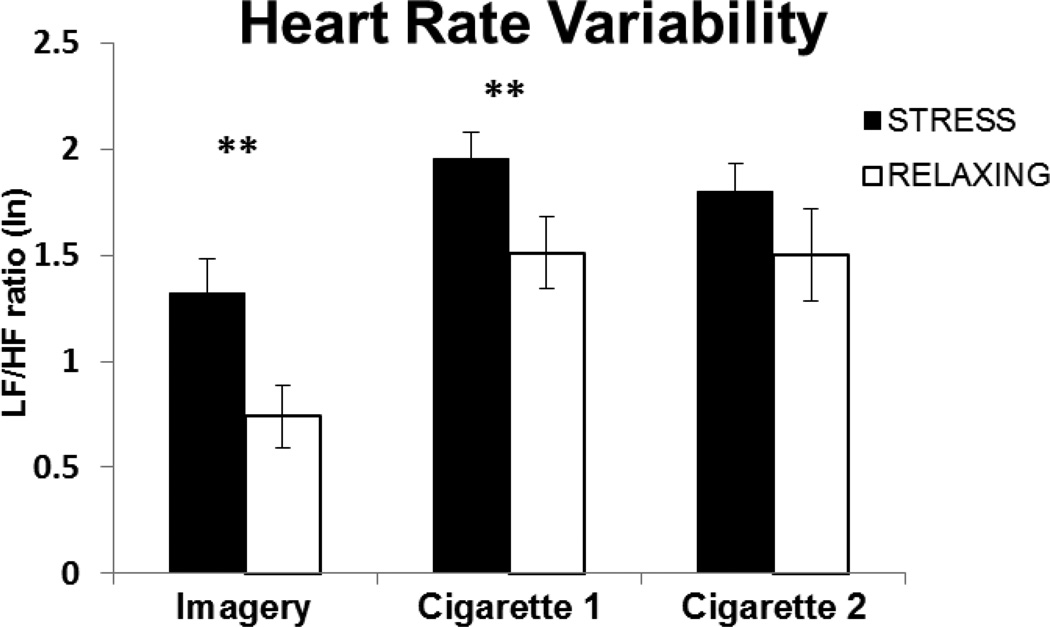
HF-HRV was significantly lower and the LF/HF ratio was higher following the stress imagery compared to the relaxing imagery [t(28) = 2.29, p < 0.04; see Figure 1 and t(27) = 5.22, p < 0.0012; see Figure 2, respectively]. During the ad-lib smoking period, stress and cigarettes additively decreased HF-HRV following both the first [t(20) = 2.65, p < 0.02] and second cigarettes [t(14) = 3.16, p < 0.01]. The pattern was similar for the LF/HF ratio. However, the difference between stress and relaxing imagery was only significant following the first cigarette, [t(20) = 3.0, p < 0.01], but not the second cigarette [t(14) = 1.25, p = 0.23]. While the pattern for subsequent cigarettes was similar, few subjects smoked more than 2 cigarettes precluding analyses for these comparisons.
Fig. 1.
Mean (SE) minimum values of the natural log (ln) of the high frequency heart rate variability (HF-HRV) following stress or relaxing imagery (Imagery), end of the delay period (Cigarette 1) and following ad-lib cigarette smoking (Cigarette 2). Baseline values are the average of the 30 minutes prior to the imagery, stress baseline HF-HRV = 5.92 (SE=.17) and relaxing baseline HF-HRV = 6.01 (SE=.15). *p < .05 for paired comparisons
Fig. 2.
Mean (SE) values of the natural log (ln) of LF/HF following stress or relaxing imagery (Imagery), end of the delay period (Cigarette 1) and following ad-lib cigarette smoking (Cigarette 2). Baseline values are the average of the 30 minutes prior to the imagery, stress baseline HF-HRV = 0.897 (SE=.11) and relaxing baseline HF-HRV = 0.903 (SE=.10). **p < .001 for paired comparisons
Pearson correlations were used to examine the association between smoking behavior and HRV measures. HF-HRV following the stress imagery was negatively associated with the ability to resist smoking following stress (r = −0.37, p < 0.04), but no such association was seen with HF-HRV responses in the relaxing condition (r = −0.18, p > 0.36). In contrast, LF/HF following the stress or relaxing imagery was not significantly associated with the ability to resist smoking following stress (p’s > 0.05). There were no associations between post-imagery HF-HRV or LF/HF and the number of cigarettes smoked (p’s > 0.05).
Based on the significant correlation between HF-HRV and the ability to resist smoking, a supplemental logistic regression analysis was conducted to test whether HF-HRV differed among subjects who were able to resist smoking following the stress imagery for the entire delay period (50 minutes) compared to subjects who gave in and commenced smoking. Specifically, for every one unit increase in HF-HRV, the odds of giving in and smoking increased by an odds of 2.02 [Wald χ2(1) = 4.2, p < 0.05].
Subject response to cigarettes
Pearson correlations were used to examine the relationship between subjective responses to smoking and HRV measures. Blunted stress-induced HF-HRV values (i.e., higher HRV following stress) were associated with greater increases in tobacco craving for withdrawal relief following the stress imagery (r = 0.42, p < 0.05), greater satisfaction ratings from smoking the first cigarette (r = 0.39, p < 0.01), and greater craving relief following the first cigarette (r = 0.51, p < 0.01). Conversely, for the LF/HF ratio, significant associations following stress were negative relationships with satisfaction from smoking (r =−.41, p < .05) and craving relief from smoking (r = −0.40, p < .05). For the relaxing condition, there were no significant associations between post-imagery HF-HRV or LF/HF ratio with craving or smoking-related reward. There were no associations between post-imagery HF-HRV or LF/HF ratio with nicotine withdrawal, or physiologic reactivity (heart rate, blood pressure) for either the stress or relaxing conditions.
DISCUSSION
To our knowledge, this is the first study to examine dynamic changes in vagal function (i.e., HF-HRV) and sympathovagal balance (i.e., LF/HF) among abstinent smokers during stress and to assess whether these changes were related to the ability to resist smoking. Relative to relaxing imagery, stress imagery decreased the ability to resist smoking (McKee, et al., 2011) and consistent with predictions, resulted in greater decreases in HF-HRV and larger increases in LF/HF. Although further replication is necessary, our preliminary results indicated that the ability to resist smoking may be specifically related to blunted vagal reactivity. We found that blunted HF-HRV reactivity during stress (i.e., smaller decreases) was related to reduced time to smoking the first cigarette representing an initial lapse. Supplemental analyses suggested that those who were able to resist smoking during the delay period exhibited a more adaptive stress response (e.g., greater vagal reactivity under stress) compared to those who gave in and commenced smoking. This relationship was not evident for our measure of sympathovagal balance (i.e., LF/HF). Subsequent smoking during the ad-lib period resulted in further decreases in HF-HRV during the stress condition, suggesting that stress and smoking additively reduce HF-HRV. The current HF-HRV findings are consistent with evidence that acute stress (Hjortskov et al., 2004; Keary, Hughes, & Palmieri, 2009; Key, et al., 2008; Lackschewitz, et al., 2008) and smoking (Hayano, et al., 1990; Karakaya, et al., 2007; Kobayashi, et al., 2005) may lead to blunted vagal reactivity.
The fact that smoking activates the stress response also fits with previous evidence of the long-term effects of smoking on autonomic functioning (Gallagher, et al., 1992; Hayano, et al., 1990; Thayer, et al., 2010). Cigarette smoking may represent repeated allostatic load leading to a chronic elevation of stress effectors. This chronic state of dysregulation may reflect a decreased ability to respond to stressful situations and result in less physiological flexibility and increased vulnerability to relapse (Koob & Kreek, 2007; Sinha, 2008). Evidence from both animal and human studies support this hypothesis, suggesting that such alterations in physiological capacity to respond to stress predicts relapse (Koob, 2008; Sinha, Garcia, Paliwal, Kreek, & Rounsaville, 2006). Although much of this work has focused on HPA axis activity (e.g., al'Absi, 2006), the stress response involves multiple physiological systems. Although the current findings require replication, they provide preliminary support for the hypothesis that the physiological capacity to adapt to stress, measured as blunted vagal reactivity, is related to the ability to resist smoking following stress.
We also demonstrated that subjective ratings following stress were related to a less adaptive autonomic stress response. Specifically, blunted HF-HRV reactivity following the stress imagery was associated with greater increases in tobacco craving, and also greater self-reported satisfaction from smoking and craving relief following the first cigarette during the ad-lib period. There was also an inverse relationship between sympathovagal balance and satisfaction from smoking and craving relief. These patterns were not evident during the relaxing condition or at baseline, suggesting that stress may play an important role in reinforcement from smoking and craving relief during abstinence (Buchmann et al., 2010; Childs & de Wit, 2010; Erblich, Boyarsky, Spring, Niaura, & Bovbjerg, 2003). Specifically, it appears that the blunting of the immediate stress response may be a critical component in determining an individual’s stress response. These findings require replication in future work given that we did not correct for the number of correlations examined. Nevertheless, this finding fits with the notion that dysregulated stress responses may be associated with negative emotional states (i.e., increased craving) that contribute to relapse (Koob & Le Moal, 2008). Vagal reactivity is hypothesized to reflect the system’s ability to adapt (Berntson, et al., 1997). The finding that decreased physiological flexibility is related to the ability to resist smoking and increased craving and satisfaction from smoking suggest that in order to fully understand the relationship between stress and relapse, we need a better understanding of the role of both the sympathetic and parasympathetic nervous system and how they may interact.
Several limitations of the current study warrant mention. First, in contrast to the findings for time to resist smoking, stress-related HF-HRV reactivity was not related to ad-lib smoking. During the ad-lib procedure, we provided monetary reinforcement for not smoking in order to assess the relative reinforcing effects of stress (Gilbert, Crauthers, Mooney, McClernon, & Jensen, 1999; McKee, et al., 2011; Perkins, Stitzer, & Lerman, 2006). As a consequence, most participants smoked fewer than two cigarettes during the ad-lib period, which may have masked relationships with HF-HRV and LF/HF. Although it is possible that a more sensitive measure of smoking behavior (e.g., smoking topography) would be related to HRV measures, the correlations between smoking topography and HRV were not significant in the current study. In addition, the current sample consisted of non-treatment seeking smokers. It remains to be seen whether ad-lib smoking, the ability to resist, and HRV differ among a group of individuals motivated to quit.
The parent study was designed to examine the effects of stress on the ability to resist smoking within a laboratory paradigm (McKee, et al., 2011). Thus, smoking behavior was the primary outcome of the study and was not controlled during the ad-lib period. Since smoking may reduce vagal tone and increase sympathetic activation through nicotine’s action on the cholinergic system (Hayano, et al., 1990), this may have limited our ability to detect a relationship between HF-HRV and the number of cigarettes smoked. Recent work has examined the separate effects of nicotine deprivation, transdermal nicotine, and smoking on HPA axis activity (e.g., Wardle, et al., 2011) and we recently reported effects on HPA axis reactivity (McKee, et al., 2011). However, the focus of the current paper was on HRV and future work would benefit by examining vagal function a predictor of stress-precipitated relapse in other populations and paradigms.
Another important consideration for the current findings is that without a control group we cannot necessarily conclude that the blunted HF-HRV response in this sample is specific to smokers. However, several studies have demonstrated that laboratory-induced generally results in decreases in HF-HRV (i.e., increased vagal reactivity) among non-smokers (Chida & Hamer, 2008). In addition, recent evidence suggests that conditions designed to induce relaxation may reduce stress during abstinence (Ussher, Cropley, Playle, Mohidin, & West, 2009). The control condition used in the current study may have been experienced as relaxing and may have contributed to the size of the stress effect. Therefore, future work would benefit from exploring the differences between ‘neutral’ and ‘relaxing’ conditions in the context of laboratory-induced stress.
Another potential limitation is that respiration was not controlled. Given that the vagus nerve is responsible for HRV within the respiratory frequency band (Allen, et al., 2007), for subjects breathing slower than 0.15–.4 Hz, HF-HRV will not reflect vagal activity, and respiratory changes can impact HRV (Brown, Beightol, Koh, & Eckberg, 1993; Eckberg, 1983; Grossman, Karemaker, & Wieling, 1991; Grossman & Kollai, 1993). Although we cannot definitively test whether participants’ rate of respiration fell within this frequency band, several aspects of this study make it unlikely that this has impacted our findings. First, a within-subjects design was employed and all procedures were consistent across sessions. The Society for Psychophysiological Research suggests that “potential respiratory influences on RSA must be considered but … may not preclude contrasts if experimental parameters do not alter respiration appreciably” (Berntson, et al., 1997). Participants were relatively sedentary throughout the recording period and were not subjected to postural changes, which may alter respiration (Cacioppo, Uchino, & Berntson, 1994). Second, studies have shown that stress-related HF-HRV does not differ between controlled and spontaneous breathing (Bernardi et al., 2000; De Meersman, Reisman, Daum, & Zorowitz, 1996; Grossman, Brinkman, & de Vries, 1992; Madden & Savard, 1995; Pagani, et al., 1991; Patwardhan, Evans, Bruce, & Knapp, 2001). Third, although respiration rates may differ between smokers and non-smokers (c.f., Hayano, et al., 1990), all participants in the current study were regular, daily smokers who were in a state of nicotine deprivation.
We previously reported that cardiovascular measures (e.g., heart rate and blood pressure) increased following stress, but were not related to the ability to resist smoking (McKee, et al., 2011). Regarding cardiac autonomic function, we found that it is specifically stress-related parasympathetic, or vagal, reactivity that may be a useful predictor of relapse vulnerability during a cessation attempt. Conversely, sympathovagal balance (i.e., LF/HF) was increased following stress imagery and subsequent cigarettes, but was not related to the time to resist smoking. Although the physiological underpinnings of the LF/HF ratio are controversial, it is important to note that HRV measures only vagal input to the sinus node and these findings highlight the complexity of the cardiac autonomic system (Porges, 2001). In this study, behavioral changes were associated with changes in cardiac autonomic activity, suggesting an integration of parasympathetic response. Thus, our preliminary findings suggest that HF-HRV may reflect an indicator of vagal reactivity and may inform processes involved in relapse. Although we have shown an association between blunted vagal response and smoking lapse behavior, we cannot confirm a mechanistic role and this finding requires replication. For example, it may be that individuals with lower baseline HF-HRV are more likely to begin smoking and have a harder time quitting. There is some evidence suggesting that individuals with lower baseline HF-HRV exhibit impaired stress responses (Weber et al., 2010). Although we cannot address this question in the current study, future studies incorporating longitudinal designs would be able to address test this hypothesis. For example, in a recent study Hajek and colleagues demonstrated that smokers who successfully quit smoking reported reduced stress levels (Hajek, Taylor, & McRobbie, 2010). Thus, the current findings, combined with previous evidence that vagal tone increases following cessation (Lewis, et al., 2010; Yotsukura, et al., 1998) suggest that “directing intervention techniques toward vagal enhancement” (Friedman, 2007, p. 193) may lead to improved smoking cessation outcomes.
Conclusion
Chronic smoking may represent a constant state of elevated stress, resulting in increased allostatic load leading to dysregulation of adaptive stress responses. Increased allostatic load may reduce an individual’s capacity to adapt to stress and increase vulnerability to relapse (Sinha, 2008). Using a laboratory paradigm of stress-induced relapse, the current study provides support for the hypothesis that loss of physiological flexibility, as measured by blunted vagal reactivity following stress, may be an important predictor of the ability to resist smoking. Those who commenced smoking following stress exhibited less adaptive vagal responses to stress. This study provides insight into the separate roles of parasympathetic and sympathetic autonomic functioning in response to stress among abstinent smokers. To the extent that HF-HRV represents an index of an individual’s physiological capacity to adapt to stress and resist smoking in the face of stress, pharmacological agents that normalize parasympathetic function may lead to improved cessation outcomes among early abstinent smokers.
Acknowledgements
The authors declare that this work was supported by the NIH Roadmap for Medical Research Common Fund through the following grants: RL1DA024857, UL1DE019586, PL1DA024859, and PL1DA024860. Additional NIH grants include R21DA017234, K12DA000167, R25DA020515. This publication was also made possible by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH roadmap for Medical Research.
Footnotes
Financial Disclosures
The authors declare that RS is on the Scientific Advisory Board for Embera Neurotherapeutics and is also a consultant for Glaxo-Smith Kline, Pharmaceuticals. All other authors declare that they have no conflicts of interest.
Results from the parent study were re-analyzed with the current subset of 32 participants. Main findings (i.e., stress reduced the time to resist smoking) remained significant, p = .02; stress mean = 26.4 min (SD = 23.4, relaxing mean = 34.2 min (SD = 20.4).
Due to a data reading error, n = 31 for LF/HF ratio analysis.
References
- Akselrod S, Gordon D, Ubel F, Shannon D, Barger A, Cohen R. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213(4504):220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- al'Absi M. Hypothalamic-pituitary-adrenocortical responses to psychological stress and risk for smoking relapse. Int J Psychophysiol. 2006;59(3):218–227. doi: 10.1016/j.ijpsycho.2005.10.010. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Hatsukami D, Davis G. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology (Berl) 2005;181(1):107–117. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- Allen JJB, Chambers AS, Towers DN. The many metrics of cardiac chronotropy: A pragmatic primer and a brief comparison of metrics. Biological Psychology. 2007;74(2):243–262. doi: 10.1016/j.biopsycho.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Back SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark AL, Price KL, et al. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 2010;106(1):21–27. doi: 10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger RD, Akselrod S, Gordon D, Cohen RJ. An efficient algorithm for spectral analysis of heart rate variability. IEEE T Bio-Med Eng. 1986;33:900–904. doi: 10.1109/TBME.1986.325789. [DOI] [PubMed] [Google Scholar]
- Bernardi L, Wdowczyk-Szulc J, Valenti C, Castoldi S, Passino C, Spadacini G, et al. Effects of controlled breathing, mental activity and mental stress with or without verbalization on heart rate variability. Journal of the American College of Cardiology. 2000;35(6):1462–1469. doi: 10.1016/s0735-1097(00)00595-7. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Norman GJ, Hawkley LC, Cacioppo JT. Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology. 2008;45(4):643–652. doi: 10.1111/j.1469-8986.2008.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigger JT, Fleiss JL, Steinman RC, Rolnitsky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85:164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- Bigger JT, Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85(1):164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- Brown TE, Beightol LA, Koh J, Eckberg DL. Important influence of respiration on human R-R interval power spectra is largely ignored. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology. 1993;75(5):2310–2317. doi: 10.1152/jappl.1993.75.5.2310. [DOI] [PubMed] [Google Scholar]
- Buchmann A, Laucht M, Schmid B, Wiedemann K, Mann K, Zimmermann U. Cigarette craving increases after a psychosocial stress test and is related to cortisol stress response but not to dependence scores in daily smokers. J Psychopharmacol. 2010;24(2):247–255. doi: 10.1177/0269881108095716. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT. Social neuroscience: autonomic, neuroendocrine, and immune responses to stress. Psychophysiology. 1994;31(2):113–128. doi: 10.1111/j.1469-8986.1994.tb01032.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Uchino BN, Berntson GG. Individual differences in the autonomic origins of heart rate reactivity: The psychometrics of respiratory sinus arrhythmia and preejection period. Psychophysiology. 1994;31(4):412–419. doi: 10.1111/j.1469-8986.1994.tb02449.x. [DOI] [PubMed] [Google Scholar]
- Center for Disease, C. Annual smoking-attributable mortality, years of potential life lost, and economic costs United States, 1995–1999. MMWR Morb Mortal Wkly. 2002;Vol. 51:300–303. [PubMed] [Google Scholar]
- Center for Disease, C. Health Effects of Cigarette Smoking-Fact Sheet, Tobacco Information and Prevention Source (TIPS) 2004 [Google Scholar]
- Chida Y, Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: a quantitative review of 30 years of investigations. Psychol Bull. 2008;134(6):829–885. doi: 10.1037/a0013342. [DOI] [PubMed] [Google Scholar]
- Childs E, de Wit H. Effects of acute psychosocial stress on cigarette craving and smoking. Nicotine Tob Res. 2010;12(4):449–453. doi: 10.1093/ntr/ntp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- De Meersman R, Reisman S, Daum M, Zorowitz R. Vagal withdrawal as a function of audience. American Journal of Physiology-Heart and Circulatory Physiology. 1996;270(4):H1381. doi: 10.1152/ajpheart.1996.270.4.H1381. [DOI] [PubMed] [Google Scholar]
- De Meersman RE, Stein PK. Vagal modulation and aging. Biological Psychology. 2007;74(2):165–173. doi: 10.1016/j.biopsycho.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. Human sinus arrhythmia as an index of vagal cardiac outflow. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology. 1983;54(4):961–966. doi: 10.1152/jappl.1983.54.4.961. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. Sympathovagal balance: a critical appraisal. Circulation. 1997;96(9):3224–3232. doi: 10.1161/01.cir.96.9.3224. [DOI] [PubMed] [Google Scholar]
- Egizio VB, Eddy M, Robinson M, Jennings JR. Efficient and cost-effective estimation of the influence of respiratory variables on respiratory sinus arrhythmia. Psychophysiology. 2011;48(4):488–494. doi: 10.1111/j.1469-8986.2010.01086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich J, Boyarsky Y, Spring B, Niaura R, Bovbjerg DH. A family history of smoking predicts heightened levels of stress-induced cigarette craving. Addiction. 2003;98(5):657–664. doi: 10.1046/j.1360-0443.2003.00351.x. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Biometric Research. New York: New York State Psychiatric Institute; 1995. Structured clinical interview for DSM-IV axis I disorders-patient edition (SCID-I/P, Version 2.0) [Google Scholar]
- Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology. 2007;74(2):185–199. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Gallagher D, Terenzi T, de Meersman R. Heart rate variability in smokers, sedentary and aerobically fit individuals. Clinical Autonomic Research. 1992;2(6):383–387. doi: 10.1007/BF01831395. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Crauthers DM, Mooney DK, McClernon FJ, Jensen RA. Effects of monetary contingencies on smoking relapse: influences of trait depression, personality, and habitual nicotine intake. Exp Clin Psychopharmacol. 1999;7(2):174–181. doi: 10.1037//1064-1297.7.2.174. [DOI] [PubMed] [Google Scholar]
- Grossman P, Brinkman A, de Vries J. Cardiac autonomic mechanisms associated with borderline hypertension under varying behavioral demands: evidence for attenuated parasympathetic tone but not for enhanced beta-adrenergic activity. Psychophysiology. 1992;29(6):698–711. doi: 10.1111/j.1469-8986.1992.tb02048.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, Karemaker J, Wieling W. Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: the need for respiratory control. Psychophysiology. 1991;28(2):201–216. doi: 10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, Kollai M. Respiratory sinus arrhythmia, cardiac vagal tone, and respiration: within- and between-individual relations. Psychophysiology. 1993;30(5):486–495. doi: 10.1111/j.1469-8986.1993.tb02072.x. [DOI] [PubMed] [Google Scholar]
- Hajek P, Taylor T, McRobbie H. The effect of stopping smoking on perceived stress levels. Addiction. 2010;105(8):1466–1471. doi: 10.1111/j.1360-0443.2010.02979.x. [DOI] [PubMed] [Google Scholar]
- Hamming R. Numerical Methods for Scientists and Engineers. 2nd ed. NY: Dover Publications, Inc; 1973. [Google Scholar]
- Hayano J, Yamada M, Sakakibara Y, Fujinami T, Yokoyama K, Watanabe Y, et al. Short-and long-term effects of cigarette smoking on heart rate variability. The American journal of cardiology. 1990;65(1):84–88. doi: 10.1016/0002-9149(90)90030-5. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom tolerance questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hjortskov N, Rissen D, Blangsted A, Fallentin N, Lundberg U, Sogaard K. The effect of mental stress on heart rate variability and blood pressure during computer work. European journal of applied physiology. 2004;92(1):84–89. doi: 10.1007/s00421-004-1055-z. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Karakaya O, Barutcu I, Kaya D, Esen A, Saglam M, Melek M, et al. Acute effect of cigarette smoking on heart rate variability. Angiology. 2007;58(5):620. doi: 10.1177/0003319706294555. [DOI] [PubMed] [Google Scholar]
- Keary T, Hughes J, Palmieri P. Women with posttraumatic stress disorder have larger decreases in heart rate variability during stress tasks. Int J Psychophysiol. 2009;73(3):257–264. doi: 10.1016/j.ijpsycho.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Key B, Campbell T, Bacon S, Gerin W. The influence of trait and state rumination on cardiovascular recovery from a negative emotional stressor. J Behav Med. 2008;31(3):237–248. doi: 10.1007/s10865-008-9152-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi F, Watanabe T, Akamatsu Y, Furui H, Tomita T, Ohashi R, et al. Acute effects of cigarette smoking on the heart rate variability of taxi drivers during work. Scandinavian Journal of Work Environment and Health. 2005;31(5):360. doi: 10.5271/sjweh.919. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. American Journal of Psychiatry. 2007;164(8):1149. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59(1):11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annual Review of Psychology. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Kupari M, Virolainen J, Koskinen P, Tikkanen M. Short-term heart rate variability and factors modifying the risk of coronary artery disease in a population sample. The American journal of cardiology. 1993;72(12):897–903. doi: 10.1016/0002-9149(93)91103-o. [DOI] [PubMed] [Google Scholar]
- Lackschewitz H, Hüther G, Kröner-Herwig B. Physiological and psychological stress responses in adults with attention-deficit/hyperactivity disorder (ADHD) Psychoneuroendocrinology. 2008;33(5):612–624. doi: 10.1016/j.psyneuen.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Lampert R, Baron SJ, McPherson CA, Lee FA. Heart rate variability during the week of September 11, 2001. JAMA. 2002;288(5):575. doi: 10.1001/jama.288.5.575. [DOI] [PubMed] [Google Scholar]
- Lampert R, Ickovics J, Horwitz R, Lee F. Depressed autonomic nervous system function in African Americans and individuals of lower social class: a potential mechanism of race- and class-related disparities in health outcomes. Am Heart J. 2005;150(1):153–160. doi: 10.1016/j.ahj.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Leeman RF, O'Malley SS, White MA, McKee SA. Nicotine and food deprivation decrease the ability to resist smoking. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-010-1902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MJ, Balaji G, Dixon H, Syed Y, Lewis KE. Influence of smoking abstinence and nicotine replacement therapy on heart rate and QT time-series. Clinical Physiology and Functional Imaging. 2010;30(1):43–50. doi: 10.1111/j.1475-097X.2009.00902.x. [DOI] [PubMed] [Google Scholar]
- Lucini D, Bertocchi F, Malliani A, Pagani M. Autonomic effects of nicotine patch administration in habitual cigarette smokers: a double-blind, placebo-controlled study using spectral analysis of RR interval and systolic arterial pressure variabilities. Journal of Cardiovascular Pharmacology. 1998;31(5):714–720. doi: 10.1097/00005344-199805000-00010. [DOI] [PubMed] [Google Scholar]
- Madden K, Savard GK. Effects of mental state on heart rate and blood pressure variability in men and women. Clinical Physiology. 1995;15(6):557–569. doi: 10.1111/j.1475-097x.1995.tb00544.x. [DOI] [PubMed] [Google Scholar]
- Malik M, Bigger J, Camm A, Kleiger R, Malliani A, Moss A, et al. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93(5):1043. [PubMed] [Google Scholar]
- Malliani A, Montano N. Heart rate variability as a clinical tool. Ital Heart J. 2002;3(8):439–445. [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153(18):2093–2101. [PubMed] [Google Scholar]
- McKee S, Krishnan-Sarin S, Shi J, Mase T, O'Malley S. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology. 2006;189(2):201–210. doi: 10.1007/s00213-006-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee S, Sinha R, Weinberger A, Sofuoglu M, Harrison E, Lavery M, et al. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. Journal of psychopharmacology. 2011;25(4):490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami J, Ishimitsu T, Matsuoka H. Effects of smoking cessation on blood pressure and heart rate variability in habitual smokers. Hypertension. 1999;33(1 Pt 2):586–590. doi: 10.1161/01.hyp.33.1.586. [DOI] [PubMed] [Google Scholar]
- O'Donnell K, Brydon L, Wright C, Steptoe A. Self-esteem levels and cardiovascular and inflammatory responses to acute stress. Brain, Behavior, and Immunity. 2008;22(8):1241–1247. doi: 10.1016/j.bbi.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, et al. Power spectral analysis of heart rate and arterial pressure variability as a marker of sympathovagal interaction in man and conscious dog. Circ Res. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- Pagani M, Mazzuero G, Ferrari A, Liberati D, Cerutti S, Vaitl D, et al. Sympathovagal interaction during mental stress. A study using spectral analysis of heart rate variability in healthy control subjects and patients with a prior myocardial infarction. Circulation. 1991;83(4 Suppl):II43–II51. [PubMed] [Google Scholar]
- Patwardhan A, Evans J, Bruce E, Knapp C. Heart rate variability during sympatho-excitatory challenges: comparison between spontaneous and metronomic breathing. Integrative Psychological and Behavioral Science. 2001;36(2):109–120. doi: 10.1007/BF02734045. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Stitzer M, Lerman C. Medication screening for smoking cessation: a proposal for new methodologies. Psychopharmacology (Berl) 2006;184:628–636. doi: 10.1007/s00213-005-0105-5. [DOI] [PubMed] [Google Scholar]
- Pope C. Acute exposure to environmental tobacco smoke and heart rate variability. Environmental health perspectives. 2001;109(7):711. doi: 10.1289/ehp.01109711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. International Journal of Psychophysiology. 2001;42(2):123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Maiti AK. Vagal tone and the physiological regulation of emotion. Monographs of the Society for Research in Child Development. 1994;59(2–3):167–186. [PubMed] [Google Scholar]
- Ritz T. Studying noninvasive indices of vagal control: The need for respiratory control and the problem of target specificity. Biological Psychology. 2009;80(2):158–168. doi: 10.1016/j.biopsycho.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Robinson J, Cinciripini P. The effects of stress and smoking on catecholaminergic and cardiovascular response. Behav Med. 2006;32(1):13–18. doi: 10.3200/BMED.32.1.13-18. [DOI] [PubMed] [Google Scholar]
- Shafey O, Eriksen M, Ross H, Mackay J. The Tobacco Atlas. 3rd ed. American Cancer Society; 2009. [Google Scholar]
- Shiffman S, Hickcox M, Paty JA, Gnys M, Kassel JD, Richards TJ. Progression from a smoking lapse to relapse: prediction from abstinence violation effects, nicotine dependence, and lapse characteristics. J Consult Clin Psychol. 1996;64(5):993–1002. doi: 10.1037//0022-006x.64.5.993. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158(4):343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Current psychiatry reports. 2007;9(5):388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences. 2008;1141(1):105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol. 2009;14(1):84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34(5):1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Archives of General Psychiatry. 2006;63(3):324. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- Sjoberg N, Saint D. A Single 4 mg Dose of Nicotine Decreases Heart Rate Variability in Healthy Nonsmokers: Implications for Smoking Cessation Programs. Nicotine & tobacco research. 2011 doi: 10.1093/ntr/ntr004. [DOI] [PubMed] [Google Scholar]
- Stein PK, Rottman JN, Kleiger RE. Effect of 21 mg transdermal nicotine patches and smoking cessation on heart rate variability. Am J Cardiol. 1996;77(9):701–705. doi: 10.1016/s0002-9149(97)89203-x. [DOI] [PubMed] [Google Scholar]
- Thayer J, Sternberg E. Beyond Heart Rate Variability. Annals of the New York Academy of Sciences. 2006;1088(1):361–372. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International Journal of Cardiology. 2010;141(2):122–131. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- Tsuji H, Venditti FJ, Jr, Manders ES, Evans JC, Larson MG, Feldman CL, et al. Determinants of heart rate variability. J Am Coll Cardiol. 1996;28(6):1539–1546. doi: 10.1016/s0735-1097(96)00342-7. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10(6):397–409. doi: 10.1038/nrn2647. [10.1038/nrn2647]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussher M, Cropley M, Playle S, Mohidin R, West R. Effect of isometric exercise and body scanning on cigarette cravings and withdrawal symptoms. Addiction. 2009;104(7):1251–1257. doi: 10.1111/j.1360-0443.2009.02605.x. [DOI] [PubMed] [Google Scholar]
- Vanderkaay MM, Patterson SM. Nicotine and acute stress: effects of nicotine versus nicotine withdrawal on stress-induced hemoconcentration and cardiovascular reactivity. Biol Psychol. 2006;71(2):191–201. doi: 10.1016/j.biopsycho.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Wardle MC, Munafo MR, de Wit H. Effect of social stress during acute nicotine abstinence. Psychopharmacology. 2011:1–10. doi: 10.1007/s00213-010-2150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Thayer J, Rudat M, Wirtz P, Zimmermann-Viehoff F, Thomas A, et al. Low vagal tone is associated with impaired post stress recovery of cardiovascular, endocrine, and immune markers. European journal of applied physiology. 2010;109(2):201–211. doi: 10.1007/s00421-009-1341-x. [DOI] [PubMed] [Google Scholar]
- Westman E, Levin E, Rose J. Smoking while wearing the nicotine patch: Is smoking satisfying or harmful. Clinical Research. 1992;40:871A. [Google Scholar]
- Yotsukura M, Koide Y, Fujii K, Tomono Y, Katayama A, Ando H, et al. Heart rate variability during the first month of smoking cessation. Am Heart J. 1998;135(6 Pt 1):1004–1009. doi: 10.1016/s0002-8703(98)70065-1. [DOI] [PubMed] [Google Scholar]