Abstract
The indication for testosterone therapy in aging hypogonadal men without hypothalamic, pituitary, or testicular disease remains to be elucidated. The aim of this study was to investigate the effect of testosterone therapy on insulin sensitivity, substrate metabolism, body composition, and lipids in aging men with low normal bioavailable testosterone levels using a predefined cutoff level for bioavailable testosterone. A randomized, double-blinded, placebo-controlled study of testosterone treatment (gel) was done on 38 men, aged 60–78 years, with bioavailable testosterone <7.3 nmol/l and a waist circumference >94 cm. Insulin-stimulated glucose disposal (Rd) and substrate oxidation were assessed by euglycemic hyperinsulinemic clamps combined with indirect calorimetry. Lean body mass (LBM) and total fat mass (TFM) were measured by dual x-ray absorptiometry, and serum total testosterone was measured by tandem mass spectrometry. Bioavailable testosterone was calculated. Coefficients (b) represent the placebo-controlled mean effect of intervention. LBM (b = 1.9 kg, p = 0.003) increased while HDL–cholesterol (b = −0.12 mmol/l, p = 0.043) and TFM decreased (b = −1.2 kg, p = 0.038) in the testosterone group compared to placebo. Basal lipid oxidation (b = 5.65 mg/min/m2, p = 0.045) increased and basal glucose oxidation (b = −9.71 mg/min/m2, p = 0.046) decreased in response to testosterone therapy even when corrected for changes in LBM. No significant changes in insulin-stimulated Rd was observed (b = −0.01mg/min/m2, p = 0.92). Testosterone therapy increased muscle mass and lipid oxidation in aging men with low normal bioavailable testosterone levels; however, our data did not support an effect of testosterone on whole-body insulin sensitivity using the euglycemic hyperinsulinemic clamp technique.
Keywords: Testosterone therapy, Insulin sensitivity, Substrate oxidation, Aging men
Introduction
Testosterone replacement therapy is indicated in severe hypogonadism. The indication for testosterone therapy in aging hypogonadal men without hypothalamic, pituitary, or testicular disease is, however, debated. In aging men, low testosterone is associated with abdominal obesity, sarcopenia, and indices of insulin resistance. It has not been clarified if the low levels of testosterone are the cause or the consequence of obesity and insulin resistance. In severe hypogonadism, for example, idiopathic hypogonadotropic hypogonadism, testosterone therapy improved insulin sensitivity (Naharci et al. 2007). Theoretically, testosterone may have the potential to improve or deteriorate insulin sensitivity. On one hand, testosterone may improve insulin sensitivity by increasing lean body mass (LBM) and thus muscle mass (DeFronzo et al. 1985). On the other hand, a testosterone-induced change in lipid metabolism may decrease insulin sensitivity. In animal studies, testosterone increased lipolysis in fat cells (Xu et al. 1990), and in studies of human abdominal subcutaneous adipose tissue, testosterone both inhibited uptake of fatty acids by an inhibition of lipoprotein lipase and increased lipolysis by stimulation of the hormone sensitive lipase (Rebuffe-Scrive et al. 1991).
To our knowledge, no studies have investigated the effect of testosterone therapy on insulin sensitivity in aging males with low normal bioavailable testosterone levels defined by a cutoff level for bioavailable testosterone (Nielsen et al. 2007) using the gold standard method, the euglycemic hyperinsulinemic clamp. The cutoff level of bioavailable testosterone (7.3 nmol/l) was defined from observations in a thoroughly characterized population of men aged 20–30 years (Nielsen et al. 2007).
We conducted a randomized, double-blinded, placebo-controlled study of 6 months duration to assess the effects of testosterone supplementation on insulin sensitivity, substrate metabolism, body composition, lipids, and safety parameters in aging men with bioavailable testosterone below 7.3 nmol/l and a waist circumference above 94 cm.
Methods
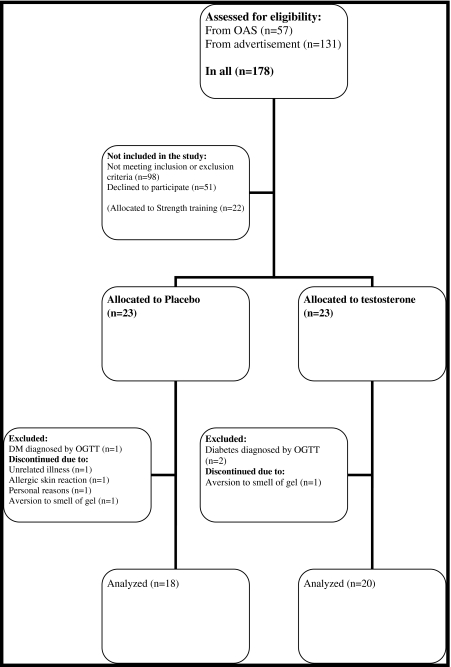
The study was a single-center, randomized, double-blinded, placebo-controlled study to assess the effect of testosterone gel and strength training on body composition, components of the metabolic syndrome, and quality of life in men aged 60–78 years with low normal bioavailable testosterone levels. In this paper, we report the results from the testosterone and the placebo group (Fig. 1).
Fig. 1.
Flowchart of the inclusion procedure
The study was approved by the local Ethics Committee and declared in ClinicalTrials.gov (identifier: NCT00700024). All participants gave written informed consent at screening visit.
Study population
The inclusion criteria for participation in the study were age 60–78 years, a bioavailable testosterone <7.3 nmol/l, and a waist circumference >94 cm. The exclusion criteria were hematocrit >50%, prostate cancer or a prostate specific antigen (PSA) >3 ng/dl, previous or ongoing malignant disease, severe ischemic heart or respiratory disease, disability, diabetes mellitus, alcohol or drug abuse, abnormal routine blood samples (TSH, ionized calcium, hemoglobin, liver and kidney function), and treatment with 5α reductase inhibitors, morphine, or oral glucocorticoid steroids.
A total of 68 participants were included in the study. Twenty-one participants were included from a previous population-based study—the Odense Androgen Study (OAS), and 47 participants were included using advertisements in newspapers and magazines in Odense and surroundings. The inclusion procedure was shown in Fig. 1.
Randomization and blinding
Subjects meeting in- and exclusion criteria were randomly assigned to receive testosterone (n = 23) or placebo (n = 23) or engage in strength training (n = 22). Randomization numbers were assigned to the participants in order of enrolment into the study. The randomization list, medicine labeling, and randomization- and code break envelopes were generated by Ipsen Scandinavia (Kista, Sweden) to ensure double blinding.
Reporting of results
A total of 63% of participants were on antihypertensive drugs, 24% were on cholesterol-lowering drugs, 9% were on inhalation steroids, 6% were on antidepressants, and 4% were treated for enlarged prostate. The distribution of concomitant medication in the placebo and the testosterone group was equal. No cholesterol-lowering drugs were introduced during the study. The participants from OAS all had normal serum prolactin levels prior to the study. In the participants recruited from advertisement, prolactin was measured after the study, and one participant in the placebo group was diagnosed with hyperprolactinemia, possibly stalk compression. The data from this participant at inclusion and during placebo treatment were in accordance with the other results in the placebo group.
Intervention
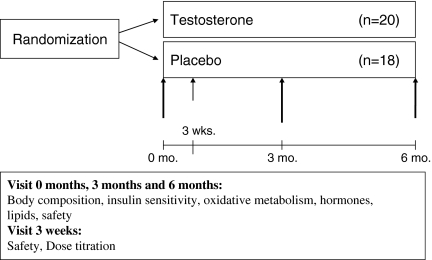
In the testosterone group, participants initially received 5 g gel, containing 50 mg testosterone (Testim®, Ipsen, France), and in the placebo group, the participant received 5 g gel (placebo). After 3 weeks of treatment, safety parameters and testosterone levels were evaluated. If bioavailable testosterone levels were <7.3 nmol/l, the dose was increased to 10 g gel (100 mg testosterone). The dose was increased in all participants in the placebo group and in 8/20 participants in the testosterone group. The study outcomes were evaluated at 0, 3, and 6 months (Fig. 2). Compliance was monitored by participant self-reporting at each visit.
Fig. 2.
Study visits
Safety monitoring
Digital rectal examination of the prostate was performed to record changes at baseline, after 3 months, and after 6 months. Measurements of PSA, hemoglobin, and hematocrit were conducted at baseline, after 3 weeks of treatment, after 3 months, and after 6 months. Safety monitoring was externally handled to ensure continued blinding.
Controlling for exercise and nutritional intake
Subjects were advised to refrain from all self-initiated resistance exercise training and intense endurance training, but were allowed to continue on other habitual activities throughout the study. Subjects were informed not to change their diet.
Hormone assays
Testosterone was measured after an overnight fast between 8 and 9 am. Serum total testosterone (TT) was measured by liquid chromatography tandem mass spectrometry after ether extraction. For testosterone measurements, the intraassay coefficient of variation (CV) was less than ± 10% for TT >0.2 nmol/L and CV was less than ± 30% in the range between 0.1 and 0.2 nmol/l.
Sex hormone-binding globulin (SHBG) was measured by autoDELFIA assay, and free testosterone (FT) and bioavailable testosterone (BT) were calculated. A single measurement of testosterone was performed to determine eligibility. Bioavailable testosterone levels were all below cutoff limits at reevaluation after 3 weeks in the placebo group (n = 18).
Serum levels of luteinizing hormone (LH) were analyzed by time-resolved fluoroimmunoassay using commercial kits (autoDELFIA; PerkinElmer Life Sciences, Oy, Turku, Finland). Intraassay CV was 1.0–9.3%, and interassay CV was 2.3–3.9%.
Serum levels of insulin were analyzed by time-resolved immunofluorometric assay (AutoDELFIA; PerkinElmer Life Sciences, Oy, Turku, Finland). The intraassay CV was 2.1–3.7%, and the interassay CV was 3.4–4.0%.
Dual x-ray absorptiometry
TFM and LBM were measured by dual x-ray absorptiometry using a Hologic Discovery device (Waltham, MA, US). The CV was 8% for TFM and 9% for LBM, respectively.
Euglycemic hyperinsulinemic clamp
At 8 am, two well-functioning intravenous lines were established for blood sampling and for infusion of insulin and glucose. The arm used for collection of blood samples was placed in a heated Plexiglas box for arterialization of venous blood. After a 120-min basal tracer equilibration period, insulin (Actrapid; Novo Nordisk, Bagsvaerd, Denmark) was infused at a rate of 40 mU/m2/min for 180 min. A primed-constant [3-3H]-glucose infusion was used throughout the 300-min study, and [3-3H]-glucose was added to the glucose infusates to maintain plasma specific activity constant at baseline levels during the 180-min clamp period as described (Hother-Nielsen et al. 1996). Using this protocol, physiological hyperinsulinemia of serum insulin at approximately 400 pmol/l was obtained in the insulin-stimulated period. Plasma glucose levels were clamped at approximately 5 mmol/l, using a variable infusion rate of 20% glucose based on bedside plasma glucose measurements (ABL800 Flex, Radiometer, Copenhagen, Denmark) every 10–20 min. Serum insulin, plasma free fatty acids (FFA), and glucose concentrations were measured as described (Hojlund et al. 2006). The basal and insulin-stimulated steady-state periods were defined as the last 20 min of the 120- basal and 180-min insulin infusion periods, respectively. Tritiated glucose-specific activity was determined on samples deproteinized with barium and zinc as described elsewhere (Hother-Nielsen et al. 1996). Steele’s non-steady-state formulas were used to calculate rates of total glucose appearance (Ra) and glucose disposal (Rd), assuming a glucose distribution volume of 200 ml/kg body weight and a pool fraction of 0.65 (Hother-Nielsen et al. 1996). Hepatic glucose production (HGP) was calculated as the difference between Ra and the glucose infusion rates. Insulin-stimulated Rd was taken as an estimate of whole-body insulin sensitivity.
Indirect calorimetry
Indirect calorimetry was performed during the last 40 min of the basal and insulin-stimulated periods using a ventilated hood system (Parvo Medics TrueOne ® 2400 Metabolic Measurement System). The average gas exchanges recorded during the 30 min of steady state in the basal period and insulin-stimulated period, respectively, were used to calculate glucose oxidation (GOX), lipid oxidation (LOX) rates, and the respiratory quotient (RQ) as described (Frayn 1983).
Safety parameters and lipids
Analysis of prostate specific antigen (PSA) was done by AutoDELFIA® Time-Resolved fluoroimmunoassay. Hemoglobin was measured using a photometric analyzer with a CV of 2.8%. Hematocrit was calculated using standard method. Plasma total cholesterol, high-density lipoprotein cholesterol (HDL), and triglycerides (TG) were analyzed by enzymatic colorimetric reactions (Modular P, Roche), and low-density lipoprotein cholesterol (LDL) was calculated using the Friedewald equation. For fasting lipid parameters, reference intervals were as follows: total cholesterol, 3.6–6.8 mmol/L; LDL, 1.8–4.5 mmol/L; HDL, 0.76–1.68 mmol/L; and TG, 0.47–2.31 mmol/L. Plasma free fatty acids (FFA) were analyzed by enzymatic colonimetric reactions (Modular P; Roche).
Statistical analysis
The sample size of the study was determined by the effect of testosterone on LBM (type 1 error (α) = 0.05, type 2 error (β) = 0.1, SD = 1.3 kg, minimal relevant difference = 1.3 kg, power (1-β) = 90%). This calculation resulted in 15 subjects in each group. We decided to enroll 20 participants in each group to allow for drop-outs. Data were analyzed using Stata version 11.
Differences in baseline values were analyzed using unpaired t test. Not normally distributed data were transformed using natural logarithm (ln) before analysis. The outcomes were compared by multiple linear regression analysis controlled for baseline values equivalent to a generalized t test. Coefficients (b) represent the placebo-controlled mean effect of intervention. The models were checked with residual plots and Box–Cox analysis. Results were considered statistically significant at p < 0.05. Data are given as mean ± SD or median and interquartile range as appropriate.
Results
At baseline, the age of the participants was 68 years (62–72) and 67 years (65–69) in the testosterone and placebo group, respectively; LH levels were 4.6 (3.1–7.6) and 4.1 IE/l (2.9–6.6), respectively; Follicle-stimulating hormone (FSH) levels were 7.6 IE/l (4.6–12.3) and 7.3 IE/l (4.0–11.1), respectively, and prolactin levels were 6 μg/l (5–7) and 4 μg/l (3–6), respectively. One participant had a prolactin of 192 μg/l. Data at baseline, 3 months, and 6 months are reported in Table 1, 2, 3 and 4.
Table 1.
Body composition
| Testosterone group | Placebo group | |||||
|---|---|---|---|---|---|---|
| Start | 3 months | 6 months | Start | 3 months | 6 months | |
| Body weight (kg) | 92.3 ± 12.3 | 94.0 ± 12.6** | 93.5 ± 12.9** | 95.1 ± 15.4 | 95.0 ± 15.6 | 94.7 ± 15.7 |
| BMI (kg/m2) | 30.2 ± 3.6 | 30.8 ± 3.9* | 30.6 ± 4.0* | 30.1 ± 5.0 | 30.0 ± 4.7 | 29.9 ± 4.7 |
| Total lean body mass (kg) | 64.3 ± 7.6 | 66.1 ± 7.9** | 66.0 ± 8.4** | 66.3 ± 8.2 | 66.2 ± 8.3 | 66.2 ± 8.4 |
| Total fat mass (kg) | 25.7 ± 5.9 | 25.5 ± 6.0 | 24.5 ± 6.1* | 25.8 ± 8.3 | 25.8 ± 8.5 | 25.8 ± 8.2 |
| Waist circumference (cm) | 109 ± 8.2 | 108 ± 7.5 | 107 ± 7.3 | 107 ± 10.9 | 108 ± 11.3 | 107 ± 11.3 |
Values are expressed as mean ± SD
*p < 0.05 compared to placebo; **p < 0.01 compared to placebo
Table 2.
Insulin sensitivity
| Testosterone | Placebo | |||||
|---|---|---|---|---|---|---|
| Start | 3 months | 6 months | Start | 3 months | 6 months | |
| Basal Rd (mg/min/m2) |
78.5 (74.0–84.5) | 77.4 (72.7–82.3) | 77.0 (74.5–81.3) | 84.4 (77.4–92.7) | 83.2 (76.9–89.2) | 77.1 (71.6–82.5) |
| Insulin-stimulated Rd (mg/min/m2) |
207.0 (149.5–279.0) | 223.8 (140.1–279.7) | 238.65 (175.4–288.6) | 189.1 (148.0–350.1) | 244.8 (173.4–301.8) | 234.9 (174.2–284.6) |
| Basal HGP (mg/min/m2) | 80.1 ± 10.1 | 76.7 ± 9.0 | 76.0 ± 6.8 | 81.8 ± 13.0 | 79.9 ± 9.2 | 72.8 ± 18.7 |
| Insulin-suppressed HGP (mg/min/m2) |
32.2 (25.8–49.6) | 30.3 (20.8–41.3) | 27.4 (22.9–36.9) | 35.8 (27.9–45.6) | 33.5 (28.8–40.3) | 32.4 (26.3–39.7) |
| FFA fasting (mmol/l) | 0.49 ± 0.22 | 0.53 ± 0.17 | 0.49 ± 0.15 | 0.45 ± 0.14 | 0.45 ± 0.17 | 0.45 ± 0.16 |
| FFA insulin-stimulated (mmol/l) |
0.04 (0.03–0.07) | 0.06 (0.04–0.08)* | 0.04 (0.03–0.06) | 0.06 (0.05–0.08) | 0.04 (0.03–0.06) | 0.03 (0.03–0.04) |
| Insulin fasting (mIU/L) |
52 (36–96) | 56 (42–96) | 48 (39–109) | 56 (36–65) | 44 (37–83) | 49 (33–75) |
| Insulin clamp (mIU/L) |
427 (391–493) | 455 (419–514) | 441 (365–517) | 371 (332–467) | 410 (373–440) | 417 (380–440) |
Values are given as mean ± SD or median (interquartile range) as appropriate
*p < 0.05 compared to placebo; **p < 0.01 compared to placebo
Table 3.
Oxidative metabolism
| Testosterone | Placebo | |||||
|---|---|---|---|---|---|---|
| Start | 3 months | 6 months | Start | 3 months | 6 months | |
| Basal GOX (mg/min/m2) |
50.9 (43.2–65-1) | 45.6 (38.3–51.5)* | 41.3 (33.6–49.7)* | 54.8 (47.1–61.9) | 57.6 (43.7–65.0) | 55.6 (41.7–58.9) |
| Basal LOX (mg/min/m2) | 25.2 ± 11.8 | 31.7 ± 7.6 | 31.8 ± 7.0* | 28.1 ± 8.3 | 28.0 ± 10.2 | 26.7 ± 9.3 |
| Basal RQ | 0.84 ± 0.05 | 0.81 ± 0.02* | 0.80 ± 0.03* | 0.83 ± 0.03 | 0.83 ± 0.04 | 0.83 ± 0.03 |
| Insulin-stimulated GOX (mg/min/m2) |
95.0 (73.6–129.4) | 112.4 (84.7–124.6) | 92.4 (81.5–110.9) | 114.5 (83.8–136.5) | 110.7 (94.0–124.9) | 106.0 (86.9–124.6) |
| Insulin-stimulated LOX (mg/min/m2) |
12.2 (2.1–17.5) | 10.7 (6.4–20.4) | 14.3 (8.3–18.6) | 6.5 (1.6–13.0) | 7.3 (2.4–14.7) | 5.4 (20.1–14.6) |
| Insulin-stimulated RQ (clamp) | 0.92 ± 0.07 | 0.91 ± 0.04 | 0.90 ± 0.05 | 0.93 ± 0.05 | 0.93 ± 0.06 | 0.94 ± 0.06 |
Values are given as mean ± SD or median (interquartile range) as appropriate
LOX lipid oxidation, GOX glucose oxidation, RQ respiratory quotient
*p < 0.05 compared to placebo; **p < 0.01 compared to placebo
Table 4.
Testosterone, SHBG, lipids, and safety monitoring
| Testosterone | Placebo | |||||
|---|---|---|---|---|---|---|
| Start | 3 months | 6 months | Start | 3 months | 6 months | |
| Total testosterone (nmol/l) | 12.5 ± 4.0 | 18.7 ± 8.9** | 22.2 ± 10.8** | 12.7 ± 4.4 | 11.0 ± 3.5 | 10.2 ± 3.2 |
| Bioavailable testosterone (nmol/l) | 5.1 ± 1.1 | 9.3 ± 5.2** | 11.1 ± 6.7** | 4.6 ± 1.4 | 4.1 ± 0.9 | 3.9 ± 1.0 |
| Free testosterone (nmol/l) | 0.27 ± 0.06 | 0.47 ± 0.25** | 0.49 ± 0.23** | 0.24 ± 0.07 | 0.21 ± 0.50 | 0.20 ± 0.05 |
| SHBG (nmol/l) |
36 (25–62) | 31 (27–46) | 33 (25–50) | 44 (39–56) | 40 (30–53) | 39 (35–51) |
| Total Cholesterol (mmol/l) | 5.1 ± 0.9 | 4.9 ± 0.8 | 4.9 ± 0.7 | 5.1 ± 1.0 | 5.1 ± 0.8 | 5.0 ± 0.8 |
| LDL (mmol/l) | 3.0 ± 0.8 | 2.9 ± 0.8 | 2.9 ± 0.7 | 3.2 ± 0.9 | 3.1 ± 0.7 | 3.0 ± 0.8 |
| HDL (mmol/l) | 1.5 ± 0.3 | 1.37 ± 0.3 | 1.34 ± 0.3* | 1.44 ± 0.2 | 1.40 ± 0.2 | 1.39 ± 0.3 |
| Triglyceride s (mmol/l) |
1.44 (1.00–1.74.) | 1.43 (0.80–1.93) | 1.20 (0.91–2.23) | 1.31 (0.94–1.59) | 1.16 (0.83–1.70) | 1.30 (0.88–1.69) |
| Hemoglobin (mmol/l) | 9.1 ± 0.5 | 9.6 ± 0.6** | 9.6 ± 0.7** | 9.0 ± 0.4 | 8.8 ± 0.6 | 8.7 ± 0.6 |
| Hematocrit | 0.43 ± 0.02 | 0.45 ± 0.03** | 0.41 ± 0.13 | 0.42 ± 0.02 | 0.43 ± 0.04 | 0.40 ± 0.1 |
| PSA (μg/l) | 1.3 ± 0.7 | 1.7 ± 0.9 | 1.9 ± 1.2** | 1.1 ± 0.8 | 1.1 ± 0.8 | 1.1 ± 0.9 |
Values are given as mean ± SD or median (interquartile range) as appropriate
*p < 0.05 compared to placebo; **p < 0.01 compared to placebo
There was no significant difference between participants in the placebo group and the testosterone group at baseline regarding age, weight, BMI, waist circumference, lean body mass, fat mass, testosterone levels, LH, FSH, fasting insulin, fasting glucose, safety parameters, lipids, and blood pressure.
Body composition
Body weight (b = 1.8 kg, p = 0.001), BMI (b = 0.7, p = 0.04), and LBM (b = 1.9 kg, p < 0.001) increased in the testosterone group compared to placebo after 3 months. There was no change in TFM (b = −0.2 kg, p = 0.56) or waist circumference WC (b = −1.5 cm, p = 0.07) after 3 months (Table 1).
Body weight (b = 1.9 kg, p = 0.003), BMI (b = 0.6, p = 0.047), and LBM (b = 1.9 kg, p = 0.003) increased in the testosterone group compared to placebo after 6 months whereas there was a decrease in TFM (b = −1.2 kg, p = 0.038). WC (b = −0.6 cm, p = 0.57) did not change during treatment (Table 1).
Insulin sensitivity
Basal Rd was unchanged after 3 (b = −10.6 mg/min/m2, p = 0.48) and 6 months (b = 4.7 mg/min/m2, p = 0.07) in the testosterone group compared to placebo. Testosterone therapy on insulin-stimulated Rd was not significantly changed after 3 (b = −18.1 mg/min/m2, p = 0.40) or 6 months (b = −0.01 mg/min/m2, p = 0.92) compared with placebo. No changes were seen in either basal HGP after 3 (b = −2.1 mg/min/m2, p = 0.46) and 6 months (b = 5.9 mg/min/m2, p = 0.13) or insulin-suppressed HGP after 3 (b = −7.7 mg/min/m2, p = 0.19) and 6 months (b = −13.2 mg/min/m2, p = 0.20) in the testosterone group compared to placebo (Table 2).
FFA levels during basal conditions were unchanged in the testosterone group compared to placebo after 3 (b = 0.06 nmol/l, p = 0.19) and 6 months (b = 0.03 nmol/l, p = 0.53). Compared with placebo, FFA levels in the insulin-stimulated state increased (b = 0.03 nmol/l, p = 0.04) in the testosterone group after 3 months, but not after 6 months (b = 0.008 nmol/l, p = 0.13). Neither basal insulin nor clamp insulin levels were changed after 3 (b = −4.2 mIU/l, p = 0.68) or 6 months (b = −1.4 mIU/l, p = 0.84) (Table 2).
Oxidative metabolism
Compared with placebo, testosterone therapy decreased basal glucose oxidation (GOX) after 3 months (b = −12.5 mg/min/m2, p = 0.03) and 6 months (b = −9.71 mg/min/m2, p = 0.046), and basal RQ after 3 months (b = −0.02, p = 0.04) and 6 months (b = −0.02, p = 0.022). No effect of testosterone treatment on basal lipid oxidation (LOX) was observed after 3 months (b = 4.42 mg/min/m2, p = 0.12) whereas an increase was demonstrated after 6 months (b = 5.65 mg/min/m2, p = 0.045) compared to placebo. The increase in basal LOX (b = 0.18 mg/min/kg, p = 0.044) and the decrease in basal GOX (b = −0.35 mg/min/kg, p = 0.022) after 6 months remained significant when calculated as substrate oxidation rates per kilogram LBM. During insulin-stimulated conditions at 3 and 6 months, respectively, GOX (b = −3.90 mg/min/m2, p = 0.66), (b = −14.7 mg/min/m2, p = 0.13), LOX (b = 2.70 mg/min/m2, p = 0.43), (b = 5.02 mg/min/m2, p = 0.14), and RQ (b = −0.01, p = 0.40), (b = −0.03, p = 0.06) were not changed in response to testosterone therapy. Moreover, there was no change in delta-RQ (RQ-clamp minus RQ-basal) after 3 (b = 0.015, p = 0.35) and 6 months (b = −0.002, p = 0.90), respectively (Table 3).
Testosterone and SHBG levels
TT (b = 7.7 nmol/l, p = 0.001), BT (b = 5.3 nmol/l, p < 0.001), and FT (b = 0.26 nmol/l, p < 0.001) increased in the testosterone group compared to placebo after 3 months (Table 4).
TT (b = 12.0 nmol/l, p < 0.001), BT (b = 7.4 nmol/l, p < 0.001), and FT (b = 0.24 nmol/l, p < 0.001) increased in the testosterone group compared to placebo after 6 months.
There was no change in SHBG after 3 (b = −2 nmol/l, p = 0.91) or 6 months (b = −1 nmol/l, p = 0.56). Serum BT levels in the placebo group did not exceed the inclusion criterion of 7.3 nmol/l after 3 weeks, 3 months, or 6 months. The increase in LBM was not associated with the testosterone level at inclusion (data not shown) (Table 4).
Lipids
Total cholesterol (b = −0.1 mmol/l, p = 0.47), LDL cholesterol (b = −0.06 mmol/l, p = 0.64), HDL cholesterol (b = −0.08 mmol/l, p = 0.14), and TG (b = 0.06 mmol/l, p = 0.71) did not change after 3 months. Compared with placebo, total cholesterol (b = −0.1 mmol/l, p = 0.34), LDL cholesterol (b = −0.01 mmol/l, p = 0.90), and TG (b = −0.11 mmol/l, p = 0.34) remained unchanged after 6 months in the testosterone group, but a decrease in HDL cholesterol levels (b = −0.12 mmol/l, p = 0.043) was observed (Table 4).
Adverse events
Adverse events and serious adverse events were recorded throughout the study.
There were 49 adverse events and two serious adverse events (SAE). Two SAE occurred in two participants and included a single possibly treatment-related event (venous thrombosis in the leg, hematocrit within the normal range) and a single non-related event (car accident). One participant had elevated PSA ( >4 μg/l) at the last visit. After planned discontinuation of therapy at 6 months, he was referred for urological assessment. There was no pathological finding during urological work-up.
Discussion
We conducted a randomized double-blinded placebo-controlled study on aging men with low normal bioavailable testosterone levels, assessed using the gold standard testosterone analysis (Taieb et al. 2003), and increased waist circumference. Testosterone therapy significantly increased LBM in men with bioavailable testosterone <7.3 nmol/l (Nielsen et al. 2007). The increase in LBM was not associated with the testosterone level at inclusion. The changes in body composition were in accordance with the results previously described during testosterone therapy (Isidori et al. 2005) also in aging males (Emmelot-Vonk et al. 2008). Skeletal muscle accounts for approximately 80% of the glucose uptake in the insulin-stimulated state (Shulman et al. 1990). We therefore assumed that the increase in muscle mass as reflected by LBM was accompanied by an increase in whole-body insulin sensitivity. Although caution should be exerted in the interpretation of nonsignificant results, our data suggested that in aging men with predefined low normal bioavailable testosterone levels, insulin sensitivity did not change during testosterone treatment. Indeed, observational studies supported a link between low testosterone and insulin resistance, i.e., low testosterone predisposed to the metabolic syndrome and type 2 diabetes in older men (Oh et al. 2002). Such studies have emphasized the need for interventional studies to clarify cause and effect. In accordance with our findings, four placebo-controlled studies of testosterone therapy in aging men found no effect on insulin sensitivity using surrogate markers of insulin resistance such as the minimal model (Basu et al. 2007; Nair et al. 2006) and HOMA-IR (Svartberg et al. 2008; Emmelot-Vonk et al. 2008). Correspondingly, a placebo-controlled, double-blinded study of aging men treated with HCG therapy to increase endogenous testosterone serum levels showed no increase in insulin sensitivity measured by the euglycemic hyperinsulinemic clamp despite an increase in LBM (Liu et al. 2003). On the other hand, two placebo-controlled, double-blinded studies have reported an increase in insulin sensitivity during testosterone therapy assessed by the euglycemic hyperinsulinemic clamp in obese middle-aged men, in whom no predefined cutoff level for testosterone was used (Marin et al. 1992; Marin et al. 1993). In both studies, no significant increase in LBM was found. These findings suggested that the testosterone dose applied in these studies was insufficient to obtain the expected increase in LBM during testosterone treatment (Isidori et al. 2005). In one of these studies, an oral formulation of testosterone may be the explanation for the lack of increase in testosterone (Marin et al. 1992). Hence, the positive effect of testosterone treatment on insulin sensitivity may be related to other changes during the experiment. The power calculation of our study was based on the well-described effect of testosterone on LBM. We did not know the initial values and standard deviations of the insulin-mediated glucose disposal rates (Rd) in aging men with low testosterone levels a priori using our clamp design. Therefore, it was not possible to obtain reliable power estimates for the changes in insulin sensitivity. We assumed that a significant increase in LBM would be followed by a significant improvement of insulin-mediated glucose disposal (Rd) if testosterone did cause a marked positive effect on insulin sensitivity and other markers investigated. A small effect of testosterone on Rd (decrease or increase) cannot, however, be excluded.
It has been reported that insulin sensitivity may be decreased due to increased lipolysis during testosterone therapy (Rebuffe-Scrive et al. 1991) and increased circulating levels of FFA (Kapoor and Jones 2008). In our study, we did not observe any significant changes in fasting FFA or TG in response to testosterone therapy. We did, however, observe a small significant decrease in the ability of insulin to suppress FFA levels during the clamp after 3 months, but not after 6 months therapy, indicating at least a transient negative effect on the inhibitory action of insulin on lipolysis.
In insulin-resistant conditions such as obesity and type 2 diabetes, measures of the respiratory quotient (RQ) across the tissue bed of the leg have demonstrated an increased glucose oxidation, a reduced lipid oxidation during fasting conditions, and a reduced insulin effect on the stimulation of glucose oxidation and on the suppression of lipid oxidation (Kelley and Mandarino 2000). The impaired ability to switch between lipid oxidation and glucose oxidation in response to insulin and fasting has been termed “metabolic inflexibility” (Kelley and Mandarino 2000). Moreover, a reduced lipid oxidation during fasting has been suggested as a key mechanism underlying the accumulation of intramuscular triglyceride and lipid metabolites, which has been hypothesized to impair insulin signaling in skeletal muscle of insulin-resistant individuals (Hojlund et al. 2008). In the present study, our results suggested a beneficial role of testosterone therapy in aging men with low normal bioavailable testosterone levels on one component of metabolic flexibility as indicated by the increase in lipid oxidation and decrease in glucose oxidation during fasting. This is in agreement with reports of decreased lipid oxidation in men with very low levels of testosterone, i.e., hypogonadism due to pituitary disease (Birzniece et al. 2009). In human skeletal muscle, lipid is the predominate oxidative substrate during fasting, accounting for approximately 80% of oxygen consumption (Dagenais et al. 1976). Therefore, the observed effect of testosterone therapy on fuel selection (decrease in RQ) could, in part, be explained by the increase in muscle mass (LBM). However, the testosterone-induced changes in glucose and lipid oxidation remained significant even when determined as milligrams per minute per kilogram muscle mass (LBM). Intriguingly, despite a decrease in RQ in the basal, fasting state, we observed no effect of testosterone therapy on either insulin-stimulated values of RQ or the incremental increase in RQ in response to insulin. However, our results were limited by the fact that the estimates of substrate oxidation using whole-body indirect calorimetry were based on a number of assumptions (Frayn 1983), and we have used the whole body rather than local indirect calorimetry across the tissue bed of skeletal muscle (Kelley and Mandarino 2000). Thus, further studies using, e.g., the leg-balance technique are warranted to confirm our findings.
We found a significant decrease in HDL cholesterol in the testosterone group compared to placebo while total cholesterol, LDL cholesterol, and TG were not significantly changed. The reduction in HDL levels may be explained by a reported testosterone-induced decrease in lipoprotein lipase activity in humans (Rebuffe-Scrive et al. 1991), but the mechanism underlying testosterone-induced decrease in HDL levels has not been clarified. Our data were consistent with a meta-analysis (Whitsel et al. 2001) and a recent systematic review (Fernandez-Balsells et al. 2010) that reported a significant small decrease in HDL cholesterol levels in men treated with testosterone; however, a meta-analysis found no change in HDL cholesterol levels in response to testosterone treatment (Isidori et al. 2005). A low HDL is linked to an increased morbidity and mortality of cardiovascular disease, and in a recent study on old men with limitations in mobility, cardiovascular and respiratory events were significantly increased during testosterone therapy compared to placebo (Basaria et al. 2010).
In our study, the testosterone dose was titrated according to bioavailable testosterone levels using the recommended doses (Bhasin et al. 2010). We chose bioavailable testosterone as SHBG is known to increase with age (Wu et al. 2008).We aimed at obtaining bioavailable testosterone within the normal range of bioavailable testosterone in young men (Nielsen et al. 2007), hence, the serum total testosterone levels post treatment would be relatively high. The long-term effects and safety of treatment with testosterone in men with low normal bioavailable testosterone are not known, and further long-term studies are warranted to address this concern. Due to the relative short duration of the study, we cannot draw conclusions on long-term effectiveness on the changes in body composition due to adaptive mechanisms. A few studies have, however, reported lasting increases in lean body mass after 36 months (Snyder et al. 1999) and 48 months (Basu et al. 2007) of testosterone treatment, respectively.
To our knowledge, no studies have investigated the effect of testosterone therapy on aging males, assessing insulin sensitivity using the gold standard method, i.e., the euglycemic hyperinsulinemic clamp and substrate oxidation using indirect calorimetry, in a population with low normal bioavailable testosterone levels using a predefined cutoff level for bioavailable testosterone (Nielsen et al. 2007).
In summary, we reported the results of a randomized, double-blinded, placebo-controlled study of a population of aging men characterized by a bioavailable testosterone below the cutoff for young men and increased body fat. We demonstrated that testosterone therapy for 6 months significantly increased muscle mass (LBM) and whole-body lipid oxidation, while fat mass, HDL cholesterol levels, and glucose oxidation were reduced. However, despite the significant changes in body composition, our data did not support an effect of 6 months testosterone therapy on whole-body insulin sensitivity using the euglycemic hyperinsulinemic clamp technique.
Acknowledgements
We thank the Novo Nordisk Foundation (scholarship 2010), Ipsen Scandinavia for kindly providing Testim ® and placebo, and the Clinical Institute–University of Southern Denmark.
Disclosure summary The authors have nothing to disclose.
References
- Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman KM, Mazer NA, Miciek R, Krasnoff J, Elmi A, Knapp PE, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede-Brierley L, Bhatia A, Collins L, LeBrasseur N, Fiore LD, Bhasin S. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Dalla MC, Campioni M, Basu A, Nair KS, Jensen MD, Khosla S, Klee G, Toffolo G, Cobelli C, Rizza RA. Effect of 2 years of testosterone replacement on insulin secretion, insulin action, glucose effectiveness, hepatic insulin clearance, and postprandial glucose turnover in elderly men. Diab Care. 2007;30:1972–1978. doi: 10.2337/dc07-0359. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- Birzniece V, Meinhardt UJ, Handelsman DJ, Ho KK. Testosterone stimulates extra-hepatic but not hepatic fat oxidation (Fox): comparison of oral and transdermal testosterone administration in hypopituitary men. Clin Endocrinol (Oxf) 2009;71(5):715–721. doi: 10.1111/j.1365-2265.2009.03524.x. [DOI] [PubMed] [Google Scholar]
- Dagenais GR, Tancredi RG, Zierler KL. Free fatty acid oxidation by forearm muscle at rest, and evidence for an intramuscular lipid pool in the human forearm. J Clin Invest. 1976;58:421–431. doi: 10.1172/JCI108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA, Gunnarsson R, Bjorkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J Clin Invest. 1985;76:149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour HR, Aleman A, Lock TM, Bosch JL, Grobbee DE, Schouw YT. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA. 2008;299:39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- Fernandez-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, Agrwal N, Elamin MB, Gallegos-Orozco JF, Wang AT, Erwin PJ, Bhasin S, Montori VM. Clinical review 1: adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:2560–2575. doi: 10.1210/jc.2009-2575. [DOI] [PubMed] [Google Scholar]
- Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–634. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- Hojlund K, Frystyk J, Levin K, Flyvbjerg A, Wojtaszewski JF, Beck-Nielsen H. Reduced plasma adiponectin concentrations may contribute to impaired insulin activation of glycogen synthase in skeletal muscle of patients with type 2 diabetes. Diabetologia. 2006;49:1283–1291. doi: 10.1007/s00125-006-0240-5. [DOI] [PubMed] [Google Scholar]
- Hojlund K, Mogensen M, Sahlin K, Beck-Nielsen H. Mitochondrial dysfunction in type 2 diabetes and obesity. Endocrinol Metab Clin North Am. 2008;37:713–731. doi: 10.1016/j.ecl.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Hother-Nielsen O, Henriksen JE, Holst JJ, Beck-Nielsen H. Effects of insulin on glucose turnover rates in vivo: isotope dilution versus constant specific activity technique. Metabolism. 1996;45:82–91. doi: 10.1016/S0026-0495(96)90204-8. [DOI] [PubMed] [Google Scholar]
- Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, Isidori A, Lenzi A, Fabbri A. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol Oxf. 2005;63:280–293. doi: 10.1111/j.1365-2265.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- Kapoor D, Jones TH. Androgen deficiency as a predictor of metabolic syndrome in aging men: an opportunity for intervention? Drugs Aging. 2008;25:357–369. doi: 10.2165/00002512-200825050-00001. [DOI] [PubMed] [Google Scholar]
- Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- Liu PY, Wishart SM, Celermajer DS, Jimenez M, Pierro ID, Conway AJ, Handelsman DJ. Do reproductive hormones modify insulin sensitivity and metabolism in older men? A randomized, placebo-controlled clinical trial of recombinant human chorionic gonadotropin. Eur J Endocrinol. 2003;148:55–66. doi: 10.1530/eje.0.1480055. [DOI] [PubMed] [Google Scholar]
- Marin P, Holmang S, Jonsson L, Sjostrom L, Kvist H, Holm G, Lindstedt G, Bjorntorp P. The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int J Obes Relat Metab Disord. 1992;16:991–997. [PubMed] [Google Scholar]
- Marin P, Holmang S, Gustafsson C, Jonsson L, Kvist H, Elander A, Eldh J, Sjostrom L, Holm G, Bjorntorp P. Androgen treatment of abdominally obese men. Obes Res. 1993;1:245–251. doi: 10.1002/j.1550-8528.1993.tb00618.x. [DOI] [PubMed] [Google Scholar]
- Naharci MI, Pinar M, Bolu E, Olgun A. Effect of testosterone on insulin sensitivity in men with idiopathic hypogonadotropic hypogonadism. Endocr Pract. 2007;13:629–635. doi: 10.4158/EP.13.6.629. [DOI] [PubMed] [Google Scholar]
- Nair KS, Rizza RA, O'Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla MC, Tindall DJ, Melton LJ, III, Smith GE, Khosla S, Jensen MD. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355:1647–1659. doi: 10.1056/NEJMoa054629. [DOI] [PubMed] [Google Scholar]
- Nielsen TL, Hagen C, Wraae K, Brixen K, Petersen PH, Haug E, Larsen R, Andersen M. Visceral and subcutaneous adipose tissue assessed by magnetic resonance imaging in relation to circulating androgens, sex hormone-binding globulin, and luteinizing hormone in young men. J Clin Endocrinol Metab. 2007;92:2696–2705. doi: 10.1210/jc.2006-1847. [DOI] [PubMed] [Google Scholar]
- Oh JY, Barrett-Connor E, Wedick NM, Wingard DL. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo study. Diab Care. 2002;25:55–60. doi: 10.2337/diacare.25.1.55. [DOI] [PubMed] [Google Scholar]
- Rebuffe-Scrive M, Marin P, Bjorntorp P. Effect of testosterone on abdominal adipose tissue in men. Int J Obes. 1991;15:791–795. [PubMed] [Google Scholar]
- Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990;322:223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, Holmes JH, Dlewati A, Santanna J, Rosen CJ, Strom BL. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647–2653. doi: 10.1210/jc.84.8.2647. [DOI] [PubMed] [Google Scholar]
- Svartberg J, Agledahl I, Figenschau Y, Sildnes T, Waterloo K, Jorde R. Testosterone treatment in elderly men with subnormal testosterone levels improves body composition and BMD in the hip. Int J Impot Res. 2008;20:378–387. doi: 10.1038/ijir.2008.19. [DOI] [PubMed] [Google Scholar]
- Taieb J, Mathian B, Millot F, Patricot MC, Mathieu E, Queyrel N, Lacroix I, Somma-Delpero C, Boudou P. Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography-mass spectrometry in sera from 116 men, women, and children. Clin Chem. 2003;49:1381–1395. doi: 10.1373/49.8.1381. [DOI] [PubMed] [Google Scholar]
- Whitsel EA, Boyko EJ, Matsumoto AM, Anawalt BD, Siscovick DS. Intramuscular testosterone esters and plasma lipids in hypogonadal men: a meta-analysis. Am J Med. 2001;111:261–269. doi: 10.1016/S0002-9343(01)00833-6. [DOI] [PubMed] [Google Scholar]
- Wu FC, Tajar A, Pye SR, Silman AJ, Finn JD, O'Neill TW, Bartfai G, Casanueva F, Forti G, Giwercman A, Huhtaniemi IT, Kula K, Punab M, Boonen S, Vanderschueren D. Hypothalamic–pituitary–testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J Clin Endocrinol Metab. 2008;93:2737–2745. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- Xu X, De PG, Bjorntorp P. The effects of androgens on the regulation of lipolysis in adipose precursor cells. Endocrinology. 1990;126:1229–1234. doi: 10.1210/endo-126-2-1229. [DOI] [PubMed] [Google Scholar]