Abstract
Increasing evidence suggests that pro-inflammatory cytokines are at play in lowering peripheral thyroid hormone levels during critical illness. Conversely, thyroid hormones have been suggested to enhance production of inflammatory cytokines. In view of these considerations, we hypothesized a mutual association between triiodothyronine and pro-inflammatory cytokines. Therefore we evaluated the relation between both circulating and induced inflammatory markers and serum thyroid function parameters in the Leiden 85-plus Study. We found that higher circulating levels of inflammatory markers were associated with lower levels of free serum triiodothyronine. In turn, higher serum free triiodothyronine levels were related to higher production capacity of pro-inflammatory cytokines after stimulation with lipopolysaccharide. By combining in vivo and ex vivo data, we were able to demonstrate for the first time the existence of a potential feedback mechanism between thyroid function and immune production capacity. We conclude that maintenance of normal thyroid function might be important for a preserved immune response in elderly human populations.
Keywords: Thyroid, Inflammation, Aging, Humans
Introduction
During critical illness, major changes in serum thyroid hormone metabolism without primary involvement of the thyroid gland can occur. (Wartofsky and Burman 1982; Chopra et al. 1983) This altered thyroid metabolism, referred to as non-thyroidal illness syndrome, is characterized by low serum levels of triiodothyronine and thyroxine, without changes in thyrotropin levels. Although the pathogenesis of non-thyroidal illness syndrome remains poorly understood, the reduction of peripheral thyroid levels is most probably under the influence of pro-inflammatory cytokines, and interleukin-6 in particular. (Davies et al. 1996; Boelen et al. 1996; Papanicolaou 2000)
Conversely, it has been suggested that thyroid hormones induce production of inflammatory cytokines. Earlier studies have demonstrated higher circulating levels of pro-inflammatory cytokines in patients with hyperthyroidism. (Lakatos et al. 1997; Siddiqi et al. 1998) These elevated cytokine levels were independent of autoimmune inflammation usually associated with thyroid disease. Moreover, treatment of hyperthyroidism was associated with lowering of serum pro-inflammatory cytokine levels. (Siddiqi et al. 1999)
In view of these considerations, we postulated a mutual association between triiodothyronine and pro-inflammatory cytokines, in which serum free triiodothyronine has a stimulatory effect on the pro-inflammatory cytokine production capacity, whilst pro-inflammatory cytokines in turn temper this stimulatory effect of triiodothyronine by lowering peripheral thyroid hormone levels. To test this hypothesis, we first assessed the relation between various circulating inflammatory markers and serum thyroid function parameters in the Leiden 85-plus Study, a prospective population-based study among the oldest old. Next, we assessed the relation between serum thyroid function parameters and ex vivo cytokine production capacity. Physical illness was considered a potential confounder in the relation between serum thyroid function parameters and cytokine production capacity.
Materials and methods
The Leiden 85-plus study
We obtained data from the prospective population-based Leiden 85-plus Study involving 85-year-old inhabitants of the city of Leiden, the Netherlands. A total of 599 subjects (397 women and 202 men) participated (with a response rate of 87%). No selection criteria had been imposed for health status or demographic characteristics. A cytokine production capacity assay was performed for 555 subjects. For the present study, subjects using thyroid medication were excluded from analyses (n = 21) as were participants with incomplete thyroid function parameters (n = 22). For further details on enrollment, we refer to previous publications. (van den Biggelaar et al. 2004; Taekema et al. 2007) The Medical Ethical Committee of the Leiden University Medical Center approved the study and informed consent was obtained from all subjects.
Biochemical analyses
All serum measurements were performed with fully automated equipment. For thyrotropin, free thyroxine and free triiodothyronine, the Modular E170 was used and for C-reactive protein (CRP), the Hitachi Modular or the Cobas Integra 800, both from Roche, Almere, the Netherlands were applied. Coefficients of variation (CV) of these measurements were below 5%. In our laboratory, the reference values for thyrotropin were 0.3–4.8 mIU/L; free thyroxine, 13–23 pmol/L; and free triiodothyronine, 2.5–5.5 pmol/L.
The production capacity of cytokines (nanograms per milliliter) was assessed by measuring the cytokine production capacity of whole-blood samples upon ex vivo stimulation with lipopolysaccharide (LPS) as described elsewhere. (van der Linden et al. 1998) In short, cytokine production peak levels were assessed with an ex vivo whole-blood assay. All venous blood samples were taken in the morning before 11:00 a.m. to preclude circadian variation. The blood was collected in heparinized tubes and samples were diluted twofold with RPMI-1640 (Sigma, St. Louis, MO) and stored after addition of 10 pg/ml Escherichia coli-derived LPS (Difco Laboratories, Detroit) at 37°C and 5% CO2 for 24 h. After centrifugation, the supernatants were stored at −80°C until assayed using standard ELISA techniques (Central Laboratory of the Blood Transfusion Service, Amsterdam, the Netherlands). The CV for the cytokine assays, influenced also by dilutions for high stimulated values, were below 6% for interleukin (IL)-1β and IL-10; below 8% for TNF-α and IL-6; whereas IL-1 RA ranged up to 12%.
Data on circulating interleukin-6 levels were not available for our study population. Therefore, we used the IL-6 levels in unstimulated whole blood as a surrogate measure for circulating IL-6 levels. (Schram et al. 2007)
Health status
We considered physical illness a potential confounder in the relation between serum thyroid function parameters and cytokine production capacity. Indication of baseline health status was obtained by assessing plasma levels of C-reactive protein, activities of daily living scores, instrumental activities of daily living scores and mini mental state examination. Disability scores in activities of daily living (ADLs) and in instrumental activities of daily living (IADLs) range from nine points, indicating complete independence in all activities, to 36 points indicating complete dependence in all activities. The mini mental state examination (MMSE) scores range from zero points, indicating very severe cognitive impairment, to 30 points indicating optimal cognitive functioning.
Statistical analyses
The association between serum inflammatory markers and thyroid function parameters was tested using a linear regression model adjusted for sex. For the association between thyroid axis parameters and cytokine production capacity, we used a linear regression model adjusted for sex only and additionally adjusted for ADLs, IADLs, MMSE, and CRP to take into account potential confounding by physical illness. Distributions of continuous variables were examined for normality and logarithmically transformed, when appropriate (Thyrotropin, CRP, unstimulated IL-6, and stimulated levels of IL-1β, IL-6, TNF-α, IL-1RA, and IL-10). The Statistical Package for the Social Sciences (SPSS) program for Windows, version 16.0 was used for data analysis. Sixteen subjects had serum thyroid parameters beyond three standard deviations from the geometric mean and were considered outliers. All but two of the 16 outliers were euthyroid.
Results
The baseline characteristics of the 496 participants in the study are presented in Table 1. All study participants were 85 years of age and 327 of 496 participants (65.9%) were female.
Table 1.
Baseline characteristics of the study population
| Study population N = 496 | |
|---|---|
| Demographics | |
| Age (year) | 85 |
| Females (n, %) | 327 (65.9%) |
| MMSE (points) | 26 (22–28) |
| ADLs (points) | 10 (9–15) |
| Instrumental ADLs (points) | 18 (12–26) |
| Serum parameters | |
| Thyrotropin (mU/L) | 1.81 (1.20–2.74) |
| Free thyroxine (pmol/L) | 14.3 (12.7–15.7) |
| Free triiodothyronine (pmol/L) | 3.41 (3.08–3.74) |
| C-reactive protein (mg/L) | 4.0 (1.0–8.0) |
| Unstimulated IL-6 (ng/L)a | 11.0 (1.0–50.5) |
| LPS- induced cytokine production | |
| IL-1β (ng/L) | 3,517 (2,099–6,502) |
| IL-6 (ng/L) | 60,750 (43,406–84,733) |
| TNF-α (ng/L) | 10,325 (7,393–13,418) |
| IL-1RA (ng/L) | 37,297 (28,369–46,016) |
| IL-10 (ng/L) | 764 (490–1,089) |
Data are given as median value with interquartile range, unless stated otherwise
LPS lipopolysaccharide
aIL-6 levels in unstimulated whole blood were used as a surrogate measure for circulating IL-6 levels
Table 2 shows the association between various serum inflammatory markers and different thyroid parameters. Neither CRP nor interleukin-6 were associated with serum thyrotropin or free thyroxine levels. Higher levels of circulating C-reactive protein were significantly related to lower serum levels of free triiodothyronine (p = 0.001). Likewise, higher levels of interleukin-6 were associated with lower levels of triiodothyronine (p = 0.020). We repeated the analyses after excluding persons who on the basis of their CRP levels (higher than 10.0 mg/dL) may be experiencing an acute infection (n = 75). Although after exclusion of subjects with CRP levels above 10 mg/dL the associations between free triiodothyronine levels and CRP and between free triiodothyronine levels and IL-6 lost statistical significance, the effect sizes remained roughly similar, indicating that the observed associations are not completely driven by those with exceptionally high-sensitivity CRP (hsCRP).
Table 2.
Association between serum inflammatory markers and thyroid function parameters
| Change per standard deviation increase | ||
|---|---|---|
| All subjects | Restricted to CRP levels ≤ 10 (mg/dL) | |
| Ln C-Reactive protein | ||
| ln thyrotropin (mU/L) | 0.02 (0.03) | 0.04 (0.05) |
| p value | 0.57 | 0.32 |
| Free thyroxine (pmol/L) | 0.07 (0.10) | −0.09 (0.13) |
| p value | 0.47 | 0.52 |
| Free triiodothyronine (pmol/L) | −0.07 (0.02) | −0.05 (0.03) |
| p value | 0.001 | 0.09 |
| Ln unstimulated IL-6 | ||
| ln thyrotropin (mU/L) | 0.02 (0.03) | 0.03 (0.04) |
| p value | 0.57 | 0.40 |
| Free thyroxine (pmol/L) | 0.05 (0.10) | 0.01 (0.11) |
| p value | 0.59 | 0.92 |
| Free triiodothyronine (pmol/L) | −0.05 (0.02) | −0.03 (0.02) |
| p value | 0.020 | 0.25 |
Data are given as mean change (with standard error of the mean) in serum thyroid parameter per standard deviation increase in ln C-Reactive protein and Ln unstimulated IL-6
Analyses were adjusted for sex
LPS lipopolysaccharide
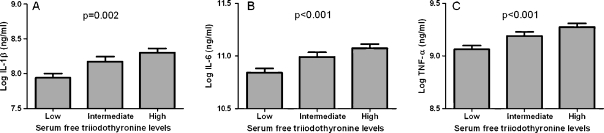
Next, we assessed the association between different thyroid function parameters and whole-blood LPS-stimulated cytokine production capacity. The outcomes are given in Table 3. Serum thyrotropin levels and serum free thyroxine levels were not associated with cytokine production capacity. However, higher levels of serum free triiodothyronine concentrations were consistently associated with a higher production of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) after whole-blood stimulation with LPS. The relation between triiodothyronine levels and the pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) is also illustrated in Fig. 1.
Table 3.
Association between thyroid function parameters and whole-blood LPS-stimulated cytokine production
| Change per standard deviation increase | |||
|---|---|---|---|
| LPS-stimulated cytokine levels | ln Thyrotropin (mU/L) | Free thyroxine (pmol/L) | Free triiodothyronine (pmol/L) |
| ln IL-1β (ng/ml) | −0.06 (0.06) | 0.02 (0.05) | 0.13 (0.04) |
| p value | 0.25 | 0.61 | 0.002 |
| ln IL-6 (ng/ml) | −0.02 (0.04) | 0.00 (0.03) | 0.10 (0.03) |
| p value | 0.56 | 0.99 | <0.001 |
| ln TNF-α (ng/ml) | −0.02 (0.03) | 0.01 (0.03) | 0.09 (0.02) |
| p value | 0.45 | 0.63 | <0.001 |
| ln IL1β RA (ng/ml) | 0.01 (0.03) | 0.02 (0.03) | 0.02 (0.02) |
| p value | 0.96 | 0.36 | 0.39 |
| ln IL-10 (ng/ml) | −0.03 (0.05) | −0.03 (0.04) | 0.07 (0.04) |
| p value | 0.47 | 0.49 | 0.052 |
Data are given as mean change (with standard error of the mean) in LPS stimulated cytokine level per standard deviation increase in serum thyroid parameter
All analyses were adjusted for sex
LPS lipopolysaccharide
Fig. 1.
Relation between tertiles of serum free triiodothyronine levels and pro-inflammatory cytokine production capacity for A Log Il-1β, B Log IL-6, and C Log TNF-α. Results were adjusted for sex
We considered physical illness as a potential confounder of the relation between triiodothyronine levels and cytokine production capacity. Therefore we repeated the analyses after inclusion of parameters of physical disability: ADLs score, IADLs score, MMSE and serum CRP. As apparent from Table 4, correction for these parameters did not substantially affect our results. Also, exclusion of subjects with hsCRP levels higher than 10.0 mg/L (n = 75) from the analyses did not materially affect the observed associations between thyroid function parameters and whole-blood LPS-stimulated cytokine production (data not shown): lower free triiodothyronine levels were associated with lower production capacity of pro-inflammatory cytokines after stimulation with LPS (all p values <0.006). No relation was observed between levels of serum thyrotropin and serum free thyroxine and cytokine production capacity.
Table 4.
Association between thyroid axis parameters and whole-blood LPS-stimulated cytokine production in subjects aged 85
| Change per standard deviation increase | |||
|---|---|---|---|
| LPS -stimulated cytokine levels | ln Thyrotropin (mU/L) | Free thyroxine (pmol/L) | Free triiodothyronine (pmol/L) |
| ln IL-1β (ng/ml) | −0.06 (0.05) | 0.03 (0.05) | 0.09 (0.04) |
| p value | 0.24 | 0.47 | 0.047 |
| ln IL-6 (ng/ml) | −0.02 (0.03) | 0.01 (0.03) | 0.06 (0.03) |
| p value | 0.48 | 0.82 | 0.017 |
| ln TNF-α (ng/ml) | −0.03 (0.03) | 0.02 (0.03) | 0.07 (0.03) |
| p value | 0.37 | 0.46 | 0.006 |
| ln IL1β RA (ng/ml) | 0.00 (0.03) | 0.02 (0.03) | 0.01 (0.02) |
| p value | 0.99 | 0.35 | 0.58 |
| ln IL-10 (ng/ml) | −0.03 (0.05) | −0.02 (0.04) | 0.03 (0.04) |
| p value | 0.51 | 0.60 | 0.41 |
Data are given as mean change (with standard error of the mean) in LPS stimulated cytokine level per standard deviation increase in serum thyroid parameter
All analyses were adjusted for sex, ADLs, instrumental ADLs, MMSE, and log serum CRP levels
LPS lipopolysaccharide
Discussion
The purpose of this study was to investigate a mutual association between triiodothyronine and pro-inflammatory cytokines. Here, we show that higher circulating levels of inflammatory markers were associated with lower levels of free serum triiodothyronine. In turn, lower free triiodothyronine levels were related to lower production capacity of pro-inflammatory cytokines after stimulation with LPS. We did not observe such a relation for levels of serum thyrotropin and serum free thyroxine. Our findings were independent of measures of physical and cognitive impairment.
The observed association between higher unstimulated interleukin-6 levels and lower free serum triiodothyronine in our study agrees with earlier reports which show an association between increased inflammatory cytokines and low triiodothyronine syndrome (Davies et al. 1996; Boelen et al. 1996). Moreover, other studies have demonstrated higher circulating levels of pro-inflammatory cytokines in patients with hyperthyroidism, suggesting a stimulatory effect of thyroid hormones on inflammatory cytokine production. (Siddiqi et al. 1999) However, to our knowledge, this is the first study to address a direct relation between serum thyroid hormone levels and inflammatory cytokine production capacity in blood cells.
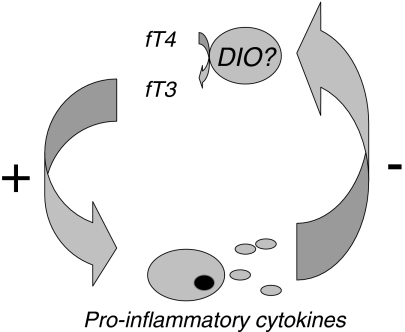
Our findings support the hypothesis of a mutual association between triiodothyronine and pro-inflammatory cytokines. As depicted schematically in Fig. 2, in this proposed feedback system, serum free triiodothyronine stimulates the pro-inflammatory cytokine production capacity, while pro-inflammatory cytokines in turn blunt the stimulatory effect of triiodothyronine by lowering peripheral thyroid hormone levels. Lowering of peripheral triiodothyronine levels under the influence of cytokines possibly occurs through regulation of peripheral deiodinase activity, although this putative mechanism has been disputed. The stimulatory effect of triiodothyronine on the cytokine production capacity is likely mediated via nuclear receptors regulating genes involved in the cell-mediated immune response. In humans, high affinity nuclear thyroid hormone receptors have been identified in mononuclear cells (Buergi and Larsen 1982; Burman et al. 1980). Although these observations provide a tentative explanation for the mutual association between thyroid hormones and cytokines, the epidemiological nature of our study does not allow us to identify the exact underlying mechanisms.
Fig. 2.
Schematic representation of the proposed mutual association between free triiodothyronine and pro-inflammatory cytokines. DIO deiodinase
Another possible limitation of our investigation is the advanced age of our study population. We have however no reason to assume that our findings do not apply to younger age categories. Moreover, the fact that the prevalence of thyroid disorders increases with age (Mariotti et al. 1995) makes our observations done in a population of the oldest old all the more clinically relevant. The age-related increase in the prevalence of thyroid disorders might be involved in changes in immune function with age. Maintenance of normal thyroid function could therefore contribute to a preserved immune response in elderly human populations.
Our study has several strong points, particularly the large size of the study population and the variety of studied inflammatory markers, comprising both pro-inflammatory markers as well as anti-inflammatory markers. In addition, our study is unique in that we were able to combine both in vivo en ex vivo information on our study population. Finally, the current study has been done in the general population composed of healthy to moderately healthy subjects, in contrast to most previous studies on the relation between thyroid hormones and immune response which have been done in clinical settings.
In summary, by combining in vivo and ex vivo data, we are the first to demonstrate a potential feedback mechanism between thyroid function and immune production capacity. These observations suggest that maintenance of normal thyroid function contributes to a preserved immune response in elderly human populations. Therefore our findings could have important implications in the care for a growing elderly population.
Acknowledgments
The Leiden 85-plus Study was in part supported by an unrestricted grant from the Dutch Ministry of Health, Welfare and Sports. This current study was funded by the Netherlands Genomics Initiative/Netherlands Organization for scientific research (NGI/NWO; 05040202 and 050-060-810. NCHA) and the EU funded Network of Excellence Lifespan (FP6 036894). We thank Margo van Schie-Troost and Marja Kersbergen-van Oostrom for their work on the cytokine assays.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Boelen A, Maas MA, Lowik CW, Platvoet MC, Wiersinga WM. Induced illness in interleukin-6 (IL-6) knock-out mice: a causal role of IL-6 in the development of the low 3, 5, 3′-triiodothyronine syndrome. Endocrinology. 1996;137:5250–5254. doi: 10.1210/en.137.12.5250. [DOI] [PubMed] [Google Scholar]
- Buergi U, Larsen PR. Nuclear triiodothyronine binding in mononuclear leukocytes in normal subjects and obese patients before and after fasting. J Clin Endocrinol Metab. 1982;54:1199–1205. doi: 10.1210/jcem-54-6-1199. [DOI] [PubMed] [Google Scholar]
- Burman KD, Latham KR, Djuh YY, Smallridge RC, Tseng YC, Lukes YG, Maunder R, Wartofsky L. Solubilized nuclear thyroid hormone receptors in circulating human mononuclear cells. J Clin Endocrinol Metab. 1980;51:106–116. doi: 10.1210/jcem-51-1-106. [DOI] [PubMed] [Google Scholar]
- Chopra IJ, Hershman JM, Pardridge WM, Nicoloff JT. Thyroid function in nonthyroidal illnesses. Ann Intern Med. 1983;98:946–957. doi: 10.7326/0003-4819-98-6-946. [DOI] [PubMed] [Google Scholar]
- Davies PH, Black EG, Sheppard MC, Franklyn JA. Relation between serum interleukin-6 and thyroid hormone concentrations in 270 hospital in-patients with non-thyroidal illness. Clin Endocrinol (Oxf) 1996;44:199–205. doi: 10.1046/j.1365-2265.1996.668489.x. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Foldes J, Horvath C, Kiss L, Tatrai A, Takacs I, Tarjan G, Stern PH. Serum interleukin-6 and bone metabolism in patients with thyroid function disorders. J Clin Endocrinol Metab. 1997;82:78–81. doi: 10.1210/jc.82.1.78. [DOI] [PubMed] [Google Scholar]
- Mariotti S, Franceschi C, Cossarizza A, Pinchera A. The aging thyroid. Endocr Rev. 1995;16:686–715. doi: 10.1210/edrv-16-6-686. [DOI] [PubMed] [Google Scholar]
- Papanicolaou DA. Euthyroid sick syndrome and the role of cytokines. Rev Endocr Metab Disord. 2000;1:43–48. doi: 10.1023/A:1010060303031. [DOI] [PubMed] [Google Scholar]
- Schram MT, Euser SM, de Craen AJ, Witteman JC, Frolich M, Hofman A, Jolles J, Breteler MM, Westendorp RG. Systemic markers of inflammation and cognitive decline in old age. J Am Geriatr Soc. 2007;55:708–716. doi: 10.1111/j.1532-5415.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- Siddiqi A, Burrin JM, Wood DF, Monson JP. Tri-iodothyronine regulates the production of interleukin-6 and interleukin-8 in human bone marrow stromal and osteoblast-like cells. J Endocrinol. 1998;157:453–461. doi: 10.1677/joe.0.1570453. [DOI] [PubMed] [Google Scholar]
- Siddiqi A, Monson JP, Wood DF, Besser GM, Burrin JM. Serum cytokines in thyrotoxicosis. J Clin Endocrinol Metab. 1999;84:435–439. doi: 10.1210/jc.84.2.435. [DOI] [PubMed] [Google Scholar]
- Taekema DG, Westendorp RG, Frolich M, Gussekloo J. High innate production capacity of tumor necrosis factor-alpha and decline of handgrip strength in old age. Mech Ageing Dev. 2007;128:517–521. doi: 10.1016/j.mad.2007.07.001. [DOI] [PubMed] [Google Scholar]
- van den Biggelaar AH, Huizinga TW, de Craen AJ, Gussekloo J, Heijmans BT, Frolich M, Westendorp RG. Impaired innate immunity predicts frailty in old age. The Leiden 85-plus study. Exp Gerontol. 2004;39:1407–1414. doi: 10.1016/j.exger.2004.06.009. [DOI] [PubMed] [Google Scholar]
- van der Linden MW, Huizinga TW, Stoeken DJ, Sturk A, Westendorp RG. Determination of tumour necrosis factor-alpha and interleukin-10 production in a whole blood stimulation system: assessment of laboratory error and individual variation. J Immunol Methods. 1998;218:63–71. doi: 10.1016/S0022-1759(98)00108-2. [DOI] [PubMed] [Google Scholar]
- Wartofsky L, Burman KD. Alterations in thyroid function in patients with systemic illness: the “euthyroid sick syndrome”. Endocr Rev. 1982;3:164–217. doi: 10.1210/edrv-3-2-164. [DOI] [PubMed] [Google Scholar]