Abstract
Muscles of old laboratory rodents experience exaggerated force losses after eccentric contractile activity. We extended this line of inquiry to humans and investigated the influence of fiber myosin heavy chain (MHC) isoform content on the injury process. Skinned muscle fiber segments, prepared from vastus lateralis biopsies of elderly men and women (78 ± 2 years, N = 8), were subjected to a standardized eccentric contraction (strain, 0.25 fiber length; velocity, 0.50 unloaded shortening velocity). Injury was assessed by evaluating pre- and post-eccentric peak Ca2+-activated force per fiber cross-sectional area (Fmax). Over 90% of the variability in post-eccentric Fmax could be explained by a multiple linear regression model consisting of an MHC-independent slope, where injury was directly related to pre-eccentric Fmax, and MHC-dependent y-intercepts, where the susceptibility to injury could be described as type IIa/IIx fibers > type IIa fibers > type I fibers. We previously reported that fiber type susceptibility to the same standardized eccentric protocol was type IIa/IIx > type IIa = type I for vastus lateralis fibers of 25-year-old adults (Choi and Widrick, Am J Physiol Cell Physiol 299:C1409–C1417, 2010). Modeling combined data sets revealed significant age by fiber type interactions, with post-eccentric Fmax deficits greater for type IIa and type IIa/IIx fibers from elderly vs. young subjects at constant pre-eccentric Fmax. We conclude that the resistance of the myofilament lattice to mechanical strain has deteriorated for type IIa and type IIa/IIx, but not for type I, vastus lateralis fibers of elderly adults.
Keywords: Sarcopenia, Muscle damage, Lengthening contractions, Fiber types, Myosin heavy chain
Introduction
Sarcopenia is characterized by a loss of skeletal muscle mass and an associated deterioration in the functional properties of the remaining muscle tissue (Goodpaster et al. 2006; Larsson et al. 1979). The demographic changes projected for the developed countries and the impact that these changes in muscle function will have on health care costs and quality of life, have made understanding the mechanisms underlying these age-related changes in skeletal muscle contractility of critical importance.
The age-related decline in the ability of skeletal muscles to produce force can be attributed to modified motor unit activation (for recent reviews, see Klass et al. 2007 and Aagaard et al. 2010) and to alterations in the muscle tissue. The latter includes fiber loss and atrophy (Larsson et al. 1979; Sato et al. 1984; Lexell et al. 1988), impairments in the excitation–contraction process (Delbono et al. 1995; Wang et al. 2002), and perturbations to actomyosin cross-bridge function (Larsson et al. 1997; Thompson and Brown 1999; Lowe et al. 2001; Frontera et al. 2000; Hook et al. 2001; Krivickas et al. 2001). These factors are specific to the mode of muscle contraction, impacting force during isometric and shortening contractions but having much less effect on force during lengthening or eccentric, muscular activity (Porter et al. 1997; Poulin et al. 1992; Phillips et al. 1991; Ochala et al. 2006; Hortobagyi et al. 1995).
Despite a disproportionate preservation of eccentric strength in the elderly, the tissue injury that is associated with novel or excessive eccentric muscular activity may be exacerbated by the aging process. This is most evident in animal models where greater immediate post-eccentric force deficits have been observed in lower limb muscles of old vs. young or middle-aged animals (Zerba et al. 1990; Brooks and Faulkner 1996; Lynch et al. 2008). However, findings have been mixed regarding the effect of age on the susceptibility to eccentric-induced muscle injury in human subjects with some reporting an exaggerated response in the elderly (Manfredi et al. 1991), others finding no difference between young and old (Dedrick and Clarkson 1990), and some finding less injury in the elderly (Lavender and Nosaka 2006a).
In addition to the acute effects of eccentric muscular activity, the ability to recover from injury and to adapt to this mode of contractile activity have been reported to be impaired in old animals and elderly human subjects (Cutlip et al. 2006; McBride et al. 1995; Dedrick and Clarkson 1990; Clarkson and Dedrick 1988; Lavender and Nosaka 2006b; Brooks and Faulkner 1990; Rader and Faulkner 2006). In fact, it has been proposed that an increased susceptibility to eccentric exercise and/or an impaired ability to recover and adapt may underlie the force deficits that are characteristic of old age (Brooks and Faulkner 1990). Thus, understanding how muscles of the elderly respond to contraction-induced damage may have important clinical relevance for this population.
In the present study, we used a novel experimental approach to evaluate eccentric muscle injury in elderly adults. The approach utilized Ca2+-activated skinned muscle fiber segments that were subjected to a single, standardized eccentric contraction, with injury assessed from measures of pre-eccentric and post-eccentric force. Because this preparation bypasses the physiological processes responsible for cell activation, injury can be attributed to eccentric contraction-induced alterations at the level of the myofilament lattice. This approach is physiologically relevant because the myofilament lattice is the site where the injury process is thought to originate (Proske and Morgan 2001; Morgan 1990). Following the physiological measurements, each fiber segment was recovered for electrophoretic analysis. This allowed us to differentiate responses of muscle cells based upon their myosin heavy chain (MHC) isoform expression, an important consideration as muscle cells isolated from faster muscles of adult laboratory rodents appear be more sensitive to injury at the level of the myofilament lattice than fibers isolated from slower muscles (Warren et al. 1994; Macpherson et al. 1996).
Methods
Subjects Eight elderly subjects participated in this study (five males and three females; mean age = 78 ± 2 years; range, 73–87 years). Each subject completed a medical history questionnaire and was examined by a physician before entry into the study. Subjects were excluded if they had any acute or chronic illness or injury, had been diagnosed with neuromuscular or cardiovascular disease, had uncontrolled hypertension (>150/90 mmHg), had experienced an upper or lower extremity fracture in the past 6 months, or had any other unstable medical condition. None of the subjects were using drugs that affected neuromuscular function and none of the females were on estrogen replacement therapy. At a minimum, subjects had not participated in any regular endurance or resistance exercise training during the previous 6 months.All subjects were informed of the study purpose, procedures, and risks, and each subject consented to participate in writing. The institutional review boards of Spaulding Rehabilitation Hospital and the Jean Mayer USDA Human Nutrition Research Center on Aging at Tufts University approved the study, including the procedures for recruiting, informing, and enrolling subjects.
Preparation of skinned fiber segments Muscle samples were obtained from the vastus lateralis using the percutaneous biopsy technique. As previously described (Choi and Widrick 2010), the muscle sample was immediately placed in cold (4°C) relaxing solution (for composition, see below), divided into small bundles, and the bundles tied with silk surgical suture to glass capillary tubes and chemically skinned for 24 h in a solution containing 50% relaxing solution and 50% glycerol (4°C). Bundles were subsequently stored at −20°C for up to 4 weeks. Skinned muscle bundles were incubated for 30 min in relaxing solution containing 0.5% Brij-58 (polyoxyethylene 20 cetyl ether; Sigma Chemical) prior to experiments to ensure disruption of the sarcoplasmic reticulum.
Preparation of individual segments for study On the day of an experiment, a single fiber segment was carefully isolated from a fiber bundle and secured to connectors extending from an isometric force transducer (Model 400, Aurora Scientific, Aurora, ON, Canada) and a high-speed servomotor (Model 308B, Aurora Scientific) using 10-0 suture as previously detailed (Choi and Widrick 2010). The length of the connector extending from the motor was 3 mm as in a previous study (Choi and Widrick 2010). Sarcomere length (SL) was the average of measurements made along three distinct portions of the fiber using a calibrated eyepiece micrometer. We aimed for an SL of 2.6 μm. Fiber length (FL) was measured using a stage micrometer. Fiber cross-sectional area (CSA) was calculated from fiber width and depth measurements (obtained from a right angle prism orientated next to the fiber) as previously described (Choi and Widrick 2010).
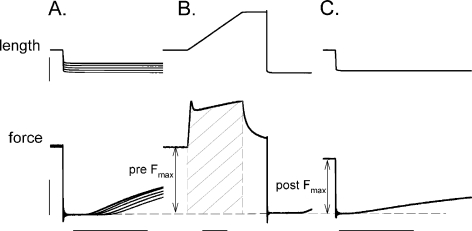
Experimental protocol The experimental design and procedure were identical to previous experiments conducted in our laboratory on skinned fibers from young subjects (Choi and Widrick 2010). The activating and relaxing solutions contained 7.0 mM EGTA, 14.5 mM creatine phosphate, 20.0 mM imidazole, 4 mM Mg2+-ATP, and 1 mM free Mg2+, with either 0.001 μM free Ca2+ for the relaxing solution (pCa, 9.0) or 31.6 μM free Ca2+ for the activating solution (pCa, 4.5). Solution pH and total ionic strength were 7.0 and 180 mmol, respectively. The concentration of metals and ligands required to yield the required free Ca2+ concentrations were determined with an iterative computer program (Fabiato 1988) using stability constants adjusted for pH, temperature, and total ionic strength as previously described (Choi and Widrick 2010). Solution temperature was continuously monitored by a small thermocouple submerged in the solution next to the fiber. All experiments were conducted at a solution temperature of 15°C.The experimental protocol is summarized in Fig. 1. A slack test procedure was used to determine pretreatment unloaded shortening velocity (Vo). Fibers were then maximally activated, force was allowed to plateau, and the fiber was lengthened by 0.25 FL at a velocity of 0.50 Vo. The fiber was held at the final length for 100 ms, and then slacked to ∼80% of initial FL in order to zero the force transducer. The fiber was immediately transferred into relaxing solution and once it had relaxed, re-extended to its original FL. Posttreatment force was measured by activating the fiber, allowing it to attain peak force, and subjecting it to a slack step ≤20% of FL in order to zero the transducer. Figure 1b illustrates how dependent variables of force and work were evaluated. Pre-treatment force was defined as the force immediately prior to the onset of lengthening or, in the case of control fibers (see below), at the conclusion of the fixed-end contraction. Posttreatment force was defined as the maximal force attained by the fiber during four to five posttreatment activations (several posttreatment activations were conducted to ensure that force had stabilized). Maximal eccentric force was defined as the peak force attained during lengthening. The work done on the fiber during the eccentric contraction was calculated as the force–time integral during fiber lengthening. The force transducer zero output was used as a baseline for all force measurements. All forces were normalized to the fiber’s CSA.
Fig. 1.
Experimental protocol. a Shows five superimposed length steps and below those, the corresponding superimposed force responses obtained during a slack test. The time for force redevelopment was plotted against the slack length step and fit by linear regression to yield Vo (per fiber length). b The same fiber was activated and lengthened 0.25 fiber length at a velocity of 0.50 Vo, held at the final length for 100 ms, and then slacked to zero the transducer. The work done on the fiber was calculated as the force–time integral during the eccentric contraction (hatched area). c Shows one posttreatment force evaluation for the same fiber. As in b, a slack step used to establish a consistent force baseline. Pre-treatment and posttreatment force (Po) were evaluated as shown. Control fibers were treated in an identical manner except that the fiber was not lengthened during treatment in b. Vertical and horizontal calibration bars represent 200 μm for length, 0.5 mN for force, and 100 ms for time. Note that force and length calibrations are the same across all panels but that the time scale is compressed in b
Control experiments Some fibers were assigned to a control treatment in order to verify that the changes observed following the eccentric treatment could be attributed to the treatment per se and not to a time or contraction-dependent deterioration of the preparation. Control fibers were treated exactly as described above except that a fixed-end contraction (followed by a slack step to zero the transducer) substituted for the lengthening contraction.
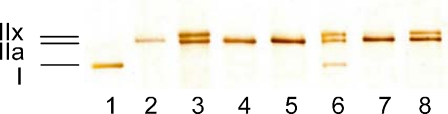
Fiber type identification Fiber segment MHC isoform content was evaluated using gel electrophoresis and a silver staining procedure as previously described (Choi and Widrick 2010). An example of a silver stained gel, illustrating identification of all three adult MHC isoforms present in human skeletal muscle, is presented in Fig. 2.
Fig. 2.
Silver-stained 6% polyacrylamide gel illustrating identification of type I, IIa, and IIx MHC isoforms in skinned segments of human vastus lateralis fibers. Lane 6 contains a human MHC isoform standard. All other lanes were loaded with a single skinned muscle fiber segment
Quality control Fibers were excluded from analysis if they broke or if they showed partial myofibrillar tearing at any observational time point following the experimental treatment. An image of a partially torn fiber has been presented in our previous work (Fig. 4a of reference Choi and Widrick 2010).
Statistical analysis Within each identified fiber type, there were no gender differences in SL, FL, pre-treatment-specific force, or Vo. In addition, fibers from males and females showed similar force changes following the experimental treatment. Thus, data were collapsed across gender for subsequent analysis.Multiple linear regression was used to identify the factors contributing to fiber injury. We modeled posttreatment force from fiber MHC isoform content, the pre-treatment force, the maximal force attained during the eccentric contraction, and the work done on the fiber during eccentric treatment as independent or predictor variables. These predictors were chosen because they have been shown in a previous work to be correlated with eccentric damage (Macpherson et al. 1996) or because we had previously shown that they described post-eccentric force of skinned fibers of younger subjects (Choi and Widrick 2010).Because multiple fibers were studied per biopsy, individual fibers are correlated with a particular subject. Therefore, fibers were not considered independent observations. A robust variance estimator was used to adjust standard errors for this clustering of data points within a subject (Hardin and Carroll 2003). All statistical analyses were conducted using Stata 11 (StataCorp LP). The alpha error rate was set at p < 0.05. All data are presented as mean ± SE.
Results
Breakage rate of fibers and final sample sizes One hundred and seventy-one fibers were subjected to control or eccentric treatments. One eccentric treatment fiber was eliminated from analysis because it was the sole fiber co-expressing type I and IIa MHCs. None of the 29 control fibers studied (11 type I, 14 type IIa, and four type IIa/IIx fibers) broke or tore during an experiment. In contrast, 29 out of the 141 fibers subjected to the eccentric treatment showed complete or partial breakage at some point during an experiment and were eliminated from analysis. All broken or partially broken fibers were recovered and subjected to gel electrophoresis for identification of their MHC isoform content. The breakage rate was 20% for type I fibers (12 of 60 eccentric treatment fibers studied), 20% for type IIa fibers (13 of 64 eccentric treatment fibers studied), and 24% for type IIa/IIx fibers (four out of 17 eccentric treatment fibers studied), yielding final sample sizes of 48 type I, 51 type IIa, and 13 type IIa/IIx eccentric treatment fibers, respectively, for analysis. The similar breakage rates across fiber types indicate that our results are unlikely to be confounded by any fiber type-specific breakage bias during the eccentric treatment.
Baseline characteristics of fibers There were no statistical differences in the baseline characteristics of control vs. treatment fibers within any of the three fiber types studied. Sarcomere length (2.61 ± 0.01, 2.62 ± 0.01, and 2.62 ± 0.01 μm for type I, IIa, and IIa/IIx fibers, respectively), FL (1.37 ± 0.03, 1.38 ± 0.02, and 1.32 ± 0.03 mm), and pre-treatment force (115 ± 3, 116 ± 2, and 119 ± 6 kN/m2) did not differ with fiber type for pooled control and eccentric treatment data. As expected, Vo was slower for type I fibers (0.75 ± 0.03 FL per second) compared to type IIa (2.62 ± 0.14 FL per second), and IIa/IIx fibers (2.97 ± 0.30 FL per second). Fiber compliance (defined as the displacement intercept of the slack test plots expressed as a percent of FL), did not vary across fiber types (3.5 ± 0.2, 3.3 ± 0.2, and 3.8 ± 0.4% FL for type I, IIa, and IIa/IIx fibers, respectively).
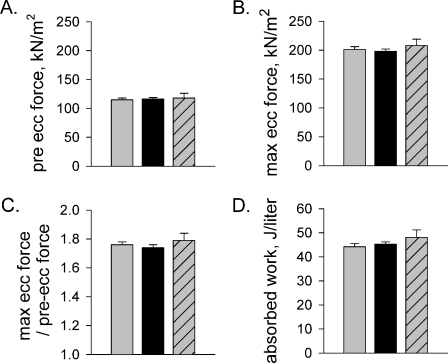
Mechanical characteristics of the eccentric contraction The maximum force attained during the eccentric contraction, the work done on the fiber, and the ratio of pre-eccentric to maximal eccentric force all showed no statistical difference across the three fiber types (Fig. 3). The absence of differences is likely due to our standardization of strain magnitude and velocity to each individual fiber’s FL and Vo, respectively.
Fig. 3.
Mechanical characteristics of the eccentric treatments. a Pre-treatment force. b Maximal force attained during the eccentric contraction. c Ratio of maximal eccentric force and pre-treatment force. d Work done on the fiber during the eccentric contraction. Values are mean ± SE. Gray, black, and gray with hatching represent type I (n = 48), IIa (n = 51), and IIa/IIx (n = 13) fibers, respectively
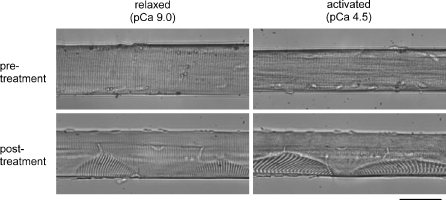
Focal sarcomere disruption following the eccentric contraction Light microscopy revealed regular sarcomere spacing in relaxed and activated fibers before the eccentric treatment (Fig. 4). Disrupted regions of sarcomeres were sometimes apparent after an eccentric contraction in unbroken fibers. These regions were comprised of weaker sarcomeres as evidenced by their extension when the fiber underwent Ca2+ activation. We did not always observe sarcomere disorganization in fibers showing functional deficits. However, we did not routinely examine the entire fiber volume in a systemic manner for signs of damage.
Fig. 4.
Images of a fiber under relaxed and Ca2+-activated conditions, before and after a single eccentric contraction. Note that the two pre-treatment images were not obtained at the same location along the length of the fiber as the two posttreatment images. Also, the images obtained during activation were not necessarily captured when the fiber was at its peak force. Note the reduction in skinned fiber cross-sectional area during Ca2+-activation (Kawai et al. 1993). Not all fibers displayed visible signs of sarcomere disruption as shown here. Scale represents 50 μm
Modeling post-eccentric Ca2+-activated force The average change in force following the eccentric contraction was −5.0 ± 0.6, −18.9 ± 1.2, and −28.2 ± 2.5 kN/m2 for fibers expressing type I, IIa, and IIa/IIx MHC, respectively. Post-force was modeled by multiple linear regression. The best model for predicting post-eccentric force was obtained with pre-eccentric force and fiber type as independent variables (r2 = 0.91, p < 0.001). The model was described as follows:
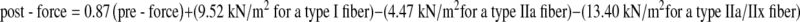 |
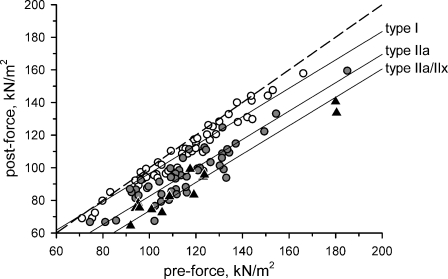
The regression lines for each fiber type, along with the raw data points, are plotted in Fig. 5.
Fig. 5.
Modeling of post-eccentric force. Over 91% of variability in post-eccentric force could be explained by a regression model that used pre-eccentric force and fiber MHC isoform content as independent variables. Shown are the raw data points (type I, open circles; type IIa, filled circles; type IIa/IIx, filled triangles) and the regression lines for each fiber type when pre-eccentric force is held constant. The y-intercept of the regression line describing the response of the type IIa fibers was significantly different from that of the type I fibers (p < 0.001) and the type IIa/IIx fibers (p < 0.05). See text for regression coefficients. The dashed line is the line of identity
The slope of the model indicates that fibers with greater pre-eccentric force per cross-sectional area experienced greater post-eccentric force deficits, independent of fiber type. Significant differences between the type I and IIa y-intercept coefficients of the model (p = 0.001) and between the type IIa and IIa/IIx y-intercept coefficients (p = 0.036) reveal that fiber MHC isoform content also influenced post-eccentric force. The susceptibility of populations of fibers to injury can therefore be described as type IIa/IIx > type IIa > type I (most to least susceptible) at any given pre-eccentric force.
Models were also developed using the mechanical characteristics of the eccentric contraction to describe post-eccentric force. Peak eccentric force (r2 = 0.82) and the work done on the fiber (r2 = 0.67) did not describe post-eccentric force as well as the model presented above.
Responses to the control treatment Force changes after the control treatment averaged −0.64 ± 0.45, −1.36 ± 0.61, and −1.25 ± 0.75 kN/m2 for type I, IIa, and IIa/IIx fibers, respectively. To ensure that our regression model described the response to the eccentric contraction per se, we modeled posttreatment force of all fibers as a function of pre-treatment force, fiber type, and treatment (control treatment, eccentric treatment). This regression analysis (r2 = 0.92, p < 0.0001) revealed significant interactions between the control and eccentric treatment for type I (p = 0.014), type IIa (p = 0.001), and type IIa/IIx (p < 0.001) fibers. For example, at a pre-eccentric force of 115 kN/m2, the model predicted fourfold (for type I fibers), 20-fold (for type IIa fibers), and 30-fold (for type IIa/IIx fibers) greater force deficits following the eccentric treatment compared to the control treatment. The eccentric force deficits are therefore statistically different from, and quantitatively much greater than, any deficits attributable to nonspecific changes in the preparation. This is strong evidence that the model developed in the preceding section represents the effects of the eccentric treatment per se.
Discussion
The present study examined the effects of high mechanical strain on the Ca2+-activated force of single skinned muscle fibers prepared from vastus lateralis muscle biopsies of elderly human subjects. This approach allowed us to apply an eccentric contraction, standardized in terms of stain magnitude and lengthening velocity, to maximally activated fiber segments, functionally assess the extent of any injury, and subsequently identify the MHC isoform content of each individual segment. Multiple linear regression analysis revealed two components, pre-eccentric-specific force and fiber MHC expression, as important predictors of the eccentric-induced damage process. The first of these components indicated that pre-treatment specific force (force/fiber cross-sectional area) is indirectly related to post-eccentric force (or directly related to the force deficit). Quantitatively, the model predicts a 1.3 kN/m2 force reduction for every 10.0 kN/m2 increase in pre-eccentric force. This component is independent of fiber MHC isoform content, i.e., the change in post-eccentric force as pre-eccentric force changes is the same for all fiber types.
The model also reveals that subpopulations of fibers from elderly adults have significantly different responses to the same standardized eccentric contraction. At constant pre-eccentric force, the relationship between the force deficit and fiber MHC isoform could be described as type IIa/IIx > type IIa > type I (from most susceptible to least susceptible). Thus, given a type I, IIa, and IIa/IIx fiber, all with identical pre-eccentric specific force of 115 kN/m2 (an approximate average specific force in this study) the model predicts eccentric force deficits of 5.4 kN/m2 (or 4.7% of pre-eccentric force) for the type I fiber, 19.4 kN/m2 (or 16.9% of pre-force) for the type IIa fiber, and 28.4 kN/m2 (or 24.7% of pre-force) for the type IIa/IIx fiber.
Letting both pre-eccentric force and fiber type vary reveals important features of the model. For instance, there is a fiber type specific threshold for injury by a single standardized eccentric contraction. The model predicts that type I fibers with pre-eccentric force ≤73 kN/m2 will show no force deficit following our protocol. Note that a similar threshold does not exist for type IIa and IIa/IIx fibers because the y-intercept coefficients for these fibers are negative. Thus, at the low end of the pre-eccentric force range studied here, type I fibers show no eccentric force deficit compared to deficits of 14 and 23 kN/m2 for type IIa and IIa/IIx fibers, respectively. This further illustrates the differential susceptibility of slow and fast fibers from elderly adults to injury.
An advantage of our experimental approach is that it allows evaluation of how the intact myofilament lattice responds to mechanical strain under carefully controlled and standardized experimental conditions. Because contraction-induced injury to the muscle tissue has been proposed to originate at the level of the sarcomere (Proske and Morgan 2001; Morgan 1990), the present approach reveals novel information about how the injury process is initiated in different populations of muscle cells from elderly adults. A potential limitation of our chemically skinned fiber preparation is that it bypasses mechanisms of cell activation that are known to be sensitive to eccentric contractile activity (Warren et al. 1993; Balnave and Allen 1995; Ingalls et al. 1998), eliminates the role of extracellular ion influx on the damage process (Zhang et al. 2008), and may remove strain-sensitive membrane-imbedded proteins involved in transmission of force across the sarcolemma to the extracellular matrix (Lovering and De Deyne 2004). Additional caution must be exercised when generalizing the present findings to the responses of fibers in intact organisms. Fiber type recruitment patterns can supersede the sensitivity of individual fibers to eccentric contractile activity (Vijayan et al. 1998, 2001).
In previous work, we modeled the susceptibility of single vastus lateralis muscle fibers obtained from young subjects to the same standardized eccentric contraction (Choi and Widrick 2010). There are several similarities between these studies. For instance, in both our previous work and the present study, we found that post-eccentric force deficits for male and female subjects were similar. This suggests that gender does not play a role in the acute responses of the myofilament lattice to high strain lengthening contractions, although our preparation does not replicate some gender-specific factors, such as hormone levels, that may exist under in vivo conditions. In our earlier study on young subjects, we found that for every 10 kN/m2 increase in specific force, the force deficit following a standardized eccentric contraction increased by 1.6 kN/m2 regardless of fiber MHC isoform expression. This is comparable to the MHC-independent force deficit predicted by the present model for elderly subjects. Thus, this stress-dependent component of the injury process appears to vary little with age or is slightly reduced in the elderly.
In contrast, the susceptibility of subpopulations of cells to injury varies greatly between our studies of young and old subjects. The present finding that the relationship between fiber type and susceptibility to injury is type IIa/IIx > type IIa > type I contrasts sharply with our earlier work on fibers from 25-year-old subjects in which the relationship was reported as type IIa/IIx > type IIa = type I (Choi and Widrick 2010). Fibers in our earlier work were studied at the same initial SL as the present fibers and were subjected to a single eccentric contraction standardized to FL and Vo as in the present study. In both studies, injury was assessed with each fiber serving as its own control. Thus, valid inferences regarding age-related differences in the susceptibility of specific fiber types can be drawn by combining data sets and expanding the model to include an age component (young vs. old).
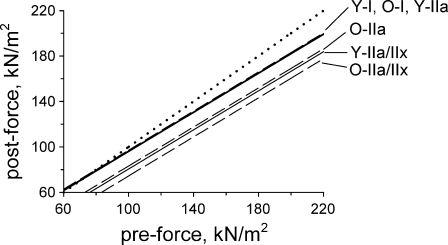
This analysis, graphically summarized in Fig. 6, reveals that with pre-eccentric force held constant, a significant interaction exists between fiber MHC and age for type IIa fibers (p < 0.001) and for type IIa/IIx fibers (p = 0.041), but not for type I fibers (p = 0.689). The primary conclusions are that the resistance of type I fibers to eccentric injury at the level of the myofilament lattice is preserved well into the eighth decade of life, at a time when the myofilament lattice of the type IIa and IIa/IIx fibers have become much more vulnerable to mechanical strain. Quantitatively, the model predicts that a type IIa fiber from an elderly subject will have a 12.7 kN/m2 greater post-eccentric force deficit compared to a type IIa fiber from a younger subject, provided the two fibers had identical pre-eccentric force. For type IIa/IIx fibers of similar pre-eccentric force, the age-related increase in the force deficit is predicted as 6.2 kN/m2.
Fig. 6.
Comparing responses of fibers from young and old subject populations to a single eccentric contraction. Multiple linear regression revealed significant interactions between fiber type and age when post-eccentric force was modeled from predictors of pre-eccentric force, fiber MHC isoform content, and age group (r2 = 0.91, p < 0.0001). Pre-eccentric force had a significant effect on post-eccentric force independent of fiber type (slope = 0.88, p < 0.001). With pre-eccentric force held constant, the predicted post-force of type I fibers from young subjects (designated Y-I) was not different (p = 0.689) from that of type I fibers from old subjects (designated O-I), i.e., the y-intercepts of the regression lines are not statistically different. However, at any given pre-eccentric force, force deficits of type IIa fibers from young subjects (designated Y-IIa) following an eccentric contraction would be significantly greater (p < 0.001) than the deficits predicted for type IIa fibers from old subjects (designated O-IIa), i.e., the y-intercepts of these responses are significantly different. Likewise, type IIa/IIx fibers from old subjects (designated O-IIa/IIx) were predicted to have significantly greater (p = 0.041) force deficits than type IIa/IIx fibers of young subjects (designated Y-IIa/IIx). Note that the responses of the young type I, young type IIa, and old type I fibers virtually overlap and cannot be individually distinguished in the figure. The dotted line is the line of identity. Data for young subjects previously reported by Choi and Widrick (2010); data for old subjects from present study
Our model may have important implications for understanding the muscle weakness that characterizes old age. The heightened susceptibility to damage observed here, coupled with an impaired ability to recover from injury or adapt to repeated exercise reported by others (Dedrick and Clarkson 1990; McBride et al. 1995; Brooks and Faulkner 1990; Rader and Faulkner 2006; Lavender and Nosaka 2006b; Cutlip et al. 2006) could be a contributor to the reduced lower limb muscle strength of elderly adults. Because contraction-induced damage impairs excitation–contraction coupling and cross-bridge function (Balnave and Allen 1995; Warren et al. 1994), a heightened susceptibility to injury could initiate the age-related deterioration in muscle fiber activation and contractility (Delbono et al. 1995; Wang et al. 2002; Larsson et al. 1997; Thompson and Brown 1999; Lowe et al. 2001; Frontera et al. 2000; Hook et al. 2001; Krivickas et al. 2001).
Consistent with our results, studies conducted on skinned fibers from EDL muscles of young and old laboratory rodents have identified age-related deficits at the level of the force-producing or force-transmitting components of the cell (Lynch et al. 2008; Brooks and Faulkner 1996). However, further comparison with the present work is complicated by the fact that fiber MHC isoform content was not determined in these previous studies and that the muscles studied express high levels of an MHC isoform (type IIb) not present in human limb muscles.
Morgan (Morgan 1990) has proposed that sarcomere length heterogeneity during active stretch results in the extension of some sarcomeres onto the descending limb of their length–tension relationship, making them weaker than sarcomeres that remain on the plateau of their length–tension curve. These weaker sarcomeres are then overextended beyond myofilament overlap, leading to mechanical damage to the cell. Interpreting our results within this framework implies that fast, but not slow, fibers of elderly subjects exhibit greater sarcomere length heterogeneity during stretch and a greater likelihood of overextension and damage than similar fibers from younger subjects.
It is important to note that we did not always observe physically disrupted sarcomeres in every fiber showing a post-eccentric force deficit. We do not feel that this observation invalidates the interpretation discussed above because light microscopy lacks the power and resolution to detect many of the sarcomeres that are disrupted by eccentric contractions (Brown and Hill 1991). Alternatively, functional deficits may not necessarily correspond with histological markers of fiber disruption or degeneration in aged animals, suggesting involvement of more subtle mechanisms. For instance, muscles of old rats chronically exposed to cycles of stretch–shortening contractions produced much less force than muscles from young animals subjected to the same stretch-shortening protocol, yet muscles presented few histological features of fiber degeneration (Cutlip et al. 2006).
While our work has identified an association between fiber MHC isoform content and injury the locus of the problem may or may not reside within MHC per se. Cross-bridge kinetics are altered with aging (Larsson et al. 1997; Thompson and Brown 1999; Lowe et al. 2001; Frontera et al. 2000; Hook et al. 2001; Krivickas et al. 2001) but whether these changes are directly responsible for an age-related increase in damage is unknown. Alternatively, desmin, titin, myosin light chain 2, tropomyosin, and α-actinin covary (either in concentration or isoform expression) with fiber MHC isoform content (Prado et al. 2005; Chopard et al. 2001; Schiaffino and Reggiani 1996) and have all been implicated in contraction-induced muscle injury (Meyer et al. 2010; Lieber et al. 1996; Koh and Escobedo 2004; Zhang et al. 2008; Lehti et al. 2007; Belcastro 1993; Childers and McDonald 2004). Finally, an age-related increase in susceptibility to damage could be due to environmental factors. Elevations in reactive oxygen species (Fielding and Meydani 1997) and altered protein phosphorylation (Gannon et al. 2008) are hallmarks of aging muscle. Acute oxidative stress exacerbates eccentric contraction-induced injury in old laboratory animals (Zerba et al. 1990). Myosin light chain 2 shows an age-related increase in phosphorylation (Gannon et al. 2008), an alteration that has been shown to increase susceptibility to contraction-induced damage in fast fibers from adult rodents (Childers and McDonald 2004). Desmin and tropomyosin, two other strain sensitive proteins, also show increased phosphorylation with aging (Gannon et al. 2008).
In summary, we have found that pre-eccentric fiber force (per CSA) was directly related to the post-eccentric force deficit, independent of fiber type, in vastus lateralis fibers of old adults. In addition, at any given pre-eccentric force, fibers co-expressing type IIa/IIx MHC were most susceptible to injury, those expressing type IIa MHC were moderately susceptible, and those expressing type I MHC were least susceptible. Comparison to earlier data collected on similar fibers from young subjects (Choi and Widrick 2010) reveals that the resistance to mechanical strain, at the level of the myofilament lattice, has deteriorated in type IIa and IIa/IIx, but not type I, vastus lateralis fibers of elderly adults. It has been proposed that an exaggerated susceptibility to contraction-induced injury coupled with an inability to fully recover from injury, contribute to muscle weakness in old age (Brooks and Faulkner 1990). The present results provide evidence supporting this proposal and point to properties intrinsic to the myofilament lattice of fast fibers as one contributing factor.
Acknowledgments
This research was supported by NIA R01-AG-18844 to RA Fielding, the Boston Claude D. Pepper Older Americans Independence Center (1P30AG031679) and by the U.S. Department of Agriculture, under agreement no. 58-1950-7-707. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture.
References
- Aagaard P, Suetta C, Caserotti P, Magnusson SP, Kjaer M. Role of the nervous system in sarcopenia and muscle atrophy with aging: strength training as a countermeasure. Scand J Med Sci Sports. 2010;20(1):49–64. doi: 10.1111/j.1600-0838.2009.01084.x. [DOI] [PubMed] [Google Scholar]
- Balnave CD, Allen DG. Intracellular calcium and force in single mouse muscle fibres following repeated contractions with stretch. J Physiol. 1995;488(Pt 1):25–36. doi: 10.1113/jphysiol.1995.sp020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcastro AN. Skeletal muscle calcium-activated neutral protease (calpain) with exercise. J Appl Physiol. 1993;74(3):1381–1386. doi: 10.1152/jappl.1993.74.3.1381. [DOI] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. Contraction-induced injury: recovery of skeletal muscles in young and old mice. Am J Physiol. 1990;258(3 Pt 1):C436–442. doi: 10.1152/ajpcell.1990.258.3.C436. [DOI] [PubMed] [Google Scholar]
- Brooks SV, Faulkner JA. The magnitude of the initial injury induced by stretches of maximally activated muscle fibres of mice and rats increases in old age. J Physiol. 1996;497(Pt 2):573–580. doi: 10.1113/jphysiol.1996.sp021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LM, Hill L. Some observations on variations in filament overlap in tetanized muscle fibres and fibres stretched during a tetanus, detected in the electron microscope after rapid fixation. J Muscle Res Cell Motil. 1991;12(2):171–182. doi: 10.1007/BF01774036. [DOI] [PubMed] [Google Scholar]
- Childers MK, McDonald KS. Regulatory light chain phosphorylation increases eccentric contraction-induced injury in skinned fast-twitch fibers. Muscle Nerve. 2004;29(2):313–317. doi: 10.1002/mus.10517. [DOI] [PubMed] [Google Scholar]
- Choi S, Widrick JJ. Calcium-activated force of human muscle fibers following a standardized eccentric contraction. Am J Physiol Cell Physiol. 2010;299:C1409–C1417. doi: 10.1152/ajpcell.00226.2010. [DOI] [PubMed] [Google Scholar]
- Chopard A, Pons F, Marini JF. Cytoskeletal protein contents before and after hindlimb suspension in a fast and slow rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2001;280(2):R323–330. doi: 10.1152/ajpregu.2001.280.2.R323. [DOI] [PubMed] [Google Scholar]
- Clarkson PM, Dedrick ME. Exercise-induced muscle damage, repair, and adaptation in old and young subjects. J Gerontol. 1988;43(4):M91–96. doi: 10.1093/geronj/43.4.m91. [DOI] [PubMed] [Google Scholar]
- Cutlip RG, Baker BA, Geronilla KB, Mercer RR, Kashon ML, Miller GR, Murlasits Z, Alway SE. Chronic exposure to stretch-shortening contractions results in skeletal muscle adaptation in young rats and maladaptation in old rats. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2006;31(5):573–587. doi: 10.1139/h06-033. [DOI] [PubMed] [Google Scholar]
- Dedrick ME, Clarkson PM. The effects of eccentric exercise on motor performance in young and older women. Eur J Appl Physiol Occup Physiol. 1990;60(3):183–186. doi: 10.1007/BF00839156. [DOI] [PubMed] [Google Scholar]
- Delbono O, O’Rourke KS, Ettinger WH. Excitation-calcium release uncoupling in aged single human skeletal muscle fibers. J Membr Biol. 1995;148(3):211–222. doi: 10.1007/BF00235039. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Fielding RA, Meydani M. Exercise, free radical generation, and aging. Aging (Milano) 1997;9(1–2):12–18. doi: 10.1007/BF03340124. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Suh D, Krivickas LS, Hughes VA, Goldstein R, Roubenoff R. Skeletal muscle fiber quality in older men and women. Am J Physiol Cell Physiol. 2000;279(3):C611–618. doi: 10.1152/ajpcell.2000.279.3.C611. [DOI] [PubMed] [Google Scholar]
- Gannon J, Staunton L, O’Connell K, Doran P, Ohlendieck K. Phosphoproteomic analysis of aged skeletal muscle. Int J Mol Med. 2008;22(1):33–42. [PubMed] [Google Scholar]
- Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- Hardin J, Carroll R. Variance estimation for the instrumental variables approach to measurement error in generalized linear models. Stata Journal. 2003;3(4):342–350. [Google Scholar]
- Hook P, Sriramoju V, Larsson L. Effects of aging on actin sliding speed on myosin from single skeletal muscle cells of mice, rats, and humans. Am J Physiol Cell Physiol. 2001;280(4):C782–788. doi: 10.1152/ajpcell.2001.280.4.C782. [DOI] [PubMed] [Google Scholar]
- Hortobagyi T, Zheng D, Weidner M, Lambert NJ, Westbrook S, Houmard JA. The influence of aging on muscle strength and muscle fiber characteristics with special reference to eccentric strength. J Gerontol A Biol Sci Med Sci. 1995;50(6):B399–406. doi: 10.1093/gerona/50A.6.B399. [DOI] [PubMed] [Google Scholar]
- Ingalls CP, Warren GL, Williams JH, Ward CW, Armstrong RB. E-C coupling failure in mouse EDL muscle after in vivo eccentric contractions. J Appl Physiol. 1998;85(1):58–67. doi: 10.1152/jappl.1998.85.1.58. [DOI] [PubMed] [Google Scholar]
- Kawai M, Wray JS, Zhao Y. The effect of lattice spacing change on cross-bridge kinetics in chemically skinned rabbit psoas muscle fibers. I. Proportionality between the lattice spacing and the fiber width. Biophys J. 1993;64(1):187–196. doi: 10.1016/S0006-3495(93)81356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klass M, Baudry S, Duchateau J. Voluntary activation during maximal contraction with advancing age: a brief review. Eur J Appl Physiol. 2007;100(5):543–551. doi: 10.1007/s00421-006-0205-x. [DOI] [PubMed] [Google Scholar]
- Koh TJ, Escobedo J. Cytoskeletal disruption and small heat shock protein translocation immediately after lengthening contractions. Am J Physiol Cell Physiol. 2004;286(3):C713–722. doi: 10.1152/ajpcell.00341.2003. [DOI] [PubMed] [Google Scholar]
- Krivickas LS, Suh D, Wilkins J, Hughes VA, Roubenoff R, Frontera WR. Age- and gender-related differences in maximum shortening velocity of skeletal muscle fibers. Am J Phys Med Rehabil. 2001;80(6):447–455. doi: 10.1097/00002060-200106000-00012. [DOI] [PubMed] [Google Scholar]
- Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol. 1979;46(3):451–456. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- Larsson L, Li X, Frontera WR. Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol. 1997;272(2 Pt 1):C638–649. doi: 10.1152/ajpcell.1997.272.2.C638. [DOI] [PubMed] [Google Scholar]
- Lavender AP, Nosaka K. Comparison between old and young men for changes in makers of muscle damage following voluntary eccentric exercise of the elbow flexors. Appl Physiol Nutr Metab. 2006;31(3):218–225. doi: 10.1139/h05-028. [DOI] [PubMed] [Google Scholar]
- Lavender AP, Nosaka K. Responses of old men to repeated bouts of eccentric exercise of the elbow flexors in comparison with young men. Eur J Appl Physiol. 2006;97(5):619–626. doi: 10.1007/s00421-006-0224-7. [DOI] [PubMed] [Google Scholar]
- Lehti TM, Kalliokoski R, Komulainen J. Repeated bout effect on the cytoskeletal proteins titin, desmin, and dystrophin in rat skeletal muscle. J Muscle Res Cell Motil. 2007;28(1):39–47. doi: 10.1007/s10974-007-9102-0. [DOI] [PubMed] [Google Scholar]
- Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84(2–3):275–294. doi: 10.1016/0022-510X(88)90132-3. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Thornell LE, Friden J. Muscle cytoskeletal disruption occurs within the first 15 min of cyclic eccentric contraction. J Appl Physiol. 1996;80(1):278–284. doi: 10.1063/1.362816. [DOI] [PubMed] [Google Scholar]
- Lovering RM, Deyne PG. Contractile function, sarcolemma integrity, and the loss of dystrophin after skeletal muscle eccentric contraction-induced injury. Am J Physiol Cell Physiol. 2004;286(2):C230–238. doi: 10.1152/ajpcell.00199.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe DA, Surek JT, Thomas DD, Thompson LV. Electron paramagnetic resonance reveals age-related myosin structural changes in rat skeletal muscle fibers. Am J Physiol Cell Physiol. 2001;280(3):C540–547. doi: 10.1152/ajpcell.2001.280.3.C540. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Faulkner JA, Brooks SV. Force deficits and breakage rates after single lengthening contractions of single fast fibers from unconditioned and conditioned muscles of young and old rats. Am J Physiol Cell Physiol. 2008;295(1):C249–256. doi: 10.1152/ajpcell.90640.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson PC, Schork MA, Faulkner JA. Contraction-induced injury to single fiber segments from fast and slow muscles of rats by single stretches. Am J Physiol. 1996;271(5 Pt 1):C1438–1446. doi: 10.1152/ajpcell.1996.271.5.C1438. [DOI] [PubMed] [Google Scholar]
- Manfredi TG, Fielding RA, O’Reilly KP, Meredith CN, Lee HY, Evans WJ. Plasma creatine kinase activity and exercise-induced muscle damage in older men. Med Sci Sports Exerc. 1991;23(9):1028–1034. [PubMed] [Google Scholar]
- McBride TA, Gorin FA, Carlsen RC. Prolonged recovery and reduced adaptation in aged rat muscle following eccentric exercise. Mech Ageing Dev. 1995;83(3):185–200. doi: 10.1016/0047-6374(95)01629-E. [DOI] [PubMed] [Google Scholar]
- Meyer GA, Kiss B, Ward SR, Morgan DL, Kellermayer MS, Lieber RL. Theoretical predictions of the effects of force transmission by desmin on intersarcomere dynamics. Biophys J. 2010;98(2):258–266. doi: 10.1016/j.bpj.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DL. New insights into the behavior of muscle during active lengthening. Biophys J. 1990;57(2):209–221. doi: 10.1016/S0006-3495(90)82524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochala J, Dorer DJ, Frontera WR, Krivickas LS. Single skeletal muscle fiber behavior after a quick stretch in young and older men: a possible explanation of the relative preservation of eccentric force in old age. Pflugers Arch. 2006;452(4):464–470. doi: 10.1007/s00424-006-0065-6. [DOI] [PubMed] [Google Scholar]
- Phillips SK, Bruce SA, Woledge RC. In mice, the muscle weakness due to age is absent during stretching. J Physiol. 1991;437:63–70. doi: 10.1113/jphysiol.1991.sp018583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter MM, Vandervoort AA, Kramer JF. Eccentric peak torque of the plantar and dorsiflexors is maintained in older women. J Gerontol A Biol Sci Med Sci. 1997;52(2):B125–131. doi: 10.1093/gerona/52A.2.B125. [DOI] [PubMed] [Google Scholar]
- Poulin MJ, Vandervoort AA, Paterson DH, Kramer JF, Cunningham DA. Eccentric and concentric torques of knee and elbow extension in young and older men. Can J Sport Sci. 1992;17(1):3–7. [PubMed] [Google Scholar]
- Prado LG, Makarenko I, Andresen C, Kruger M, Opitz CA, Linke WA. Isoform diversity of giant proteins in relation to passive and active contractile properties of rabbit skeletal muscles. J Gen Physiol. 2005;126(5):461–480. doi: 10.1085/jgp.200509364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Morgan DL. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol. 2001;537(Pt 2):333–345. doi: 10.1111/j.1469-7793.2001.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rader EP, Faulkner JA. Effect of aging on the recovery following contraction-induced injury in muscles of female mice. J Appl Physiol. 2006;101(3):887–892. doi: 10.1152/japplphysiol.00380.2006. [DOI] [PubMed] [Google Scholar]
- Sato T, Akatsuka H, Kito K, Tokoro Y, Tauchi H, Kato K. Age changes in size and number of muscle fibers in human minor pectoral muscle. Mech Ageing Dev. 1984;28(1):99–109. doi: 10.1016/0047-6374(84)90156-8. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76(2):371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Thompson LV, Brown M. Age-related changes in contractile properties of single skeletal fibers from the soleus muscle. J Appl Physiol. 1999;86(3):881–886. doi: 10.1152/jappl.1999.86.3.881. [DOI] [PubMed] [Google Scholar]
- Vijayan K, Thompson JL, Riley DA. Sarcomere lesion damage occurs mainly in slow fibers of reloaded rat adductor longus muscles. J Appl Physiol. 1998;85(3):1017–1023. doi: 10.1152/jappl.1998.85.3.1017. [DOI] [PubMed] [Google Scholar]
- Vijayan K, Thompson JL, Norenberg KM, Fitts RH, Riley DA. Fiber-type susceptibility to eccentric contraction-induced damage of hindlimb-unloaded rat AL muscles. J Appl Physiol. 2001;90(3):770–776. doi: 10.1152/jappl.2001.90.3.770. [DOI] [PubMed] [Google Scholar]
- Wang ZM, Messi ML, Delbono O. Sustained overexpression of IGF-1 prevents age-dependent decrease in charge movement and intracellular Ca(2+) in mouse skeletal muscle. Biophys J. 2002;82(3):1338–1344. doi: 10.1016/S0006-3495(02)75489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren GL, Lowe DA, Hayes DA, Karwoski CJ, Prior BM, Armstrong RB. Excitation failure in eccentric contraction-induced injury of mouse soleus muscle. J Physiol. 1993;468:487–499. doi: 10.1113/jphysiol.1993.sp019783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren GL, Hayes DA, Lowe DA, Williams JH, Armstrong RB. Eccentric contraction-induced injury in normal and hindlimb-suspended mouse soleus and EDL muscles. J Appl Physiol. 1994;77(3):1421–1430. doi: 10.1152/jappl.1994.77.3.1421. [DOI] [PubMed] [Google Scholar]
- Zerba E, Komorowski TE, Faulkner JA. Free radical injury to skeletal muscles of young, adult, and old mice. Am J Physiol. 1990;258(3 Pt 1):C429–435. doi: 10.1152/ajpcell.1990.258.3.C429. [DOI] [PubMed] [Google Scholar]
- Zhang BT, Yeung SS, Allen DG, Qin L, Yeung EW. Role of the calcium–calpain pathway in cytoskeletal damage after eccentric contractions. J Appl Physiol. 2008;105(1):352–357. doi: 10.1152/japplphysiol.90320.2008. [DOI] [PubMed] [Google Scholar]