Abstract
Hormones are potent mediators of developmental programming and maternal epigenetic effects. In vertebrates, developmental exposure to maternal androgen hormones has been shown to impact multiple behavioral and physiological traits of progeny, but the possible consequences of this early exposure in terms of aging-related changes in mortality and fitness remain largely unexplored. Avian eggs naturally contain variable doses of maternal hormones—in particular, androgens—which have documented effects on embryo growth and differentiation as well as adult behavior and physiology. Here, we report that injections of a physiological dose of testosterone (T) into yolks of freshly laid eggs of a small, seasonally breeding songbird, the house sparrow (Passer domesticus), increased survivorship in a semi-natural aviary environment. In addition, survival effects of developmental T exposure were sex-dependent, with males generally having a higher risk of death. Separate analyses for young birds in their first year of life (from hatching up to the first reproductive period the following calendar year) and in adulthood (after the first breeding season) showed similar effects. For first-year birds, mortality risk was higher during the winter than during the period after fledging; for adults, mortality risk was higher during the reproductive than the non-reproductive phase (post-breeding molt and winter). T treatment did not affect nestling body mass, but resulted in higher body mass at 3–4 months of age; T and body mass at this age interacted to influence mortality risk. Embryonic exposure to maternal testosterone may result in lower adult mortality by modifying intrinsic physiological processes involved in health or aging over the lifespan of adult birds.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-011-9222-8) contains supplementary material, which is available to authorized users.
Keywords: Developmental plasticity, Maternal effect, Non-genomic inheritance, Aging, Prenatal programming, Mortality, Yolk testosterone
Introduction
Conditions experienced during development can exert lifelong influences on adult phenotype and health: this process is known as “developmental programming” (e.g., Hales and Barker 2001; Gluckman and Hanson 2004). Prenatal exposure to maternal hormones, including androgens, can influence multiple traits, including morphology, physiology, behavior, disease susceptibility (Arnold 2002; Lummaa and Clutton-Brock 2002; Seckl 2001; Doblhammer and Vaupel 2001; Monaghan 2008; Gluckman and Hanson 2004; Hales and Barker 2001) and, ultimately, aging and longevity. The eggs of birds serve as a repository for a variety of maternally derived hormones and growth factors (e.g. Schwabl 1998; Gil et al. 1999; Schwabl et al. 2007) that influence development (e.g., Schwabl 1996), as well as the adult phenotype (Schwabl 1993; Strasser and Schwabl 2004; Eising and Groothuis 2002; reviews, Birkhead et al. 2001; Groothuis et al. 2005; Tobler and Sandell 2007; Groothuis and Schwabl 2008; Partecke and Schwabl 2008). Some of these maternal hormonal effects are likely to be obligatory prerequisites for normal development; others may act as mediators of developmental plasticity (e.g., Groothuis and Schwabl 2008).
Prenatal hormonal effects are expected to be adaptive in an evolutionary sense if the resulting phenotypic modifications enhance Darwinian fitness over the course of the reproductive lifespan (Fox and Mousseau 1998; Uller 2008). The adaptive effects of hormones, however, are predicted to carry accompanying physiological costs or trade-offs that can increase risks of disease and death (Folstad and Karter 1992; reviewed in Finch and Rose 1995; Bribiescas and Ellison 2008). Fitness trade-offs mediated via hormones early in life are predicted to have a variety of direct or indirect influences on physiological processes influencing adult survivorship and senescence patterns, including stress responses, immune function, and antioxidant defenses (reviewed in Ricklefs 2008; Monaghan 2008).
In a number of animal species, aging rates, mortality patterns, and disease susceptibility have been shown to be associated with more rapid early-life growth rates and specific cellular metabolic pathways regulated by hormones, including growth hormone, insulin-like growth factors (IGFs), and others (Brown-Borg et al. 1996; Tatar et al. 2003; Harper et al. 2004; Sonntag et al. 2005). The effects of early exposure to maternal sex steroids on mortality risk are generally much less well studied, but there is growing evidence that these are important influences on the health and mortality of adults of both sexes, particularly in mammals (reviewed in Bribiescas and Ellison 2008). In birds, variable concentrations of yolk androgens (i.e., testosterone) previously have been shown to influence offspring development and growth, metabolic rate, behavior, immune function, the ability to combat oxidative damage, and survival of embryos and nestlings. Sometimes these effects are sex linked (e.g., Schwabl 1996; Sockman and Schwabl 2000; Eising et al. 2001; von Engelhardt et al. 2006; Navara and Mendonca 2006; Tschirren et al. 2005; Tobler et al. 2007: Cucco et al. 2008; Tobler and Sandell 2009). In this study, we investigated whether yolk testosterone influences age-specific mortality patterns during the first years of adult life in the house sparrow, a small, seasonally breeding passerine bird. In previous work, both sex-linked and sex-independent long-term effects of embryonic exposure to maternal testosterone have been demonstrated using the house sparrow model (Strasser and Schwabl 2004; Partecke and Schwabl 2008).
In this study, we investigated the effects of embryonic exposure to maternal testosterone on age-specific mortality of offspring of house sparrows in outdoor aviaries that excluded most major extrinsic mortality agents (i.e., contagious pathogens, predators, starvation, and inclement weather).
The house sparrow (Passer domesticus) is an adaptable, non-migratory passerine songbird bird that is abundant in North America. It breeds from April to August in Washington state. House sparrow eggs contain several maternal steroids, including the androgen testosterone (T). T concentrations in yolk vary among clutches of a given female, as well as with the progress of the breeding season and social conditions (Schwabl 1997; Poopatanapong 2002). Previous work has shown that T injections into house sparrow eggs influence sexual and aggressive behavior (Strasser and Schwabl 2004; Partecke and Schwabl 2008), male plumage pigmentation (Strasser and Schwabl 2004; Partecke and Schwabl, unpublished data), endocrine stress responses (Partecke and Schwabl, unpublished data), and adult resting metabolic rate (Partecke and Schwabl, unpublished data). Some of these effects are sex-specific; others are not.
To investigate whether yolk T influences offspring mortality rates, we injected eggs with a high physiological dose of T or sesame oil and monitored survival in semi-natural conditions (outdoor aviaries). Many wild songbirds have exceptional longevities (often >10-year documented species maximum) for their body size (Holmes and Austad 1995; Holmes and Martin 2009), prohibiting lifespan studies in the laboratory. The maximum documented lifespan for house sparrows, derived from thousands of banding records in North America, is 13.3 years (Carey and Judge 2000); few individuals, however, reach more than a few years of age in the wild. The typical lifespan as determined from banding records is 3 years (Klimkiewicz and Futcher 1987). Mortality of free-living house sparrows in their first year of life (up to first reproduction) ranges from 51% to 79%; adult mortality is lower (Summers-Smith 1998; Ringsby et al. 1998; BTO; Farmland Bird Group, Oxford 1998). We monitored age-specific survivorship for 3.5 years, the point at which 100% mortality was reached in the group of control females.
Methods
Study animals and hormone injections
In the spring and summer of 1998 and 1999, nest boxes hung in cattle barns in the vicinity of Pullman, Wash., USA (46°44′ N, 117°10′ W) were checked daily for new eggs. House sparrows lay one egg per day until a complete clutch (usually five eggs) is laid. Within 24 h of the last egg being laid, all the eggs in a clutch were injected with either 200 ng of testosterone in 5 μl of sesame oil or with 5 μl of oil following published protocol (Schwabl 1996). To control for any seasonal change in egg composition (clutches were treated between April 27 and August 1), we alternated testosterone and control injections in successive clutches. Care was taken to use only a single clutch per female. The injected T dose is at the upper limit of the naturally occurring amount of testosterone in the yolks of this species (Poopatanapong 2002). After injections, we returned eggs to their nests for incubation and nestling care for 10 days after hatching; we then removed and weighed nestlings, fitted them with numbered aluminum and colored leg bands and hand-reared them in the laboratory (Strasser and Schwabl 2004). Subjects were housed by clutch in a cage (45 × 22 × 25 cm) with a nest box until they were feeding independently. Birds were then moved to indoor aviaries with a simulated natural photoperiod. In late August/early September, independent birds were transferred to outdoor aviaries (4.7 × 2.3 × 2.3 m) located on the roof of Abelson Hall, WSU (46°44′ N, 117°10′ W), where they were subjected to local climate and photoperiod. At this time mean age of T birds was 113 days (range, 97–128), and mean age of C birds was 104 days (range, 97–130). Food (Vitabird canary seed, Northwood Farm, USA) and water were provided ad libitum. Initially, treatment groups and sexes were held separately, but in early spring birds were transferred to mixed-sex groups with sexes of opposite treatment. Each aviary was furnished with eight wooden nest boxes, perches and a roosting area. To assess female fecundity, we compared time of onset of laying in the breeding season, number of clutches, clutch size, and egg mass in T and control females produced in the aviaries during the 1999 and 2000 breeding season.
Survival and statistical analyses
We used Cox proportional hazards regression analysis to model effects of hormone treatment, sex, hatch date, and experimental year on survival, including clutch as a random factor (Collett 2003). In a more refined statistical approach, the data were split into two subsets for juveniles (birds before their first breeding season) and adults. To account for the effects of season (breeding: May through August; feather molt: September through October; or winter: November through April) on age-specific mortality, we included season as a time-dependent covariate. For model selection and inferential conventions, we followed the analytical strategies outlined by Burnham and Anderson (2002). This practice entails constructing only biologically plausible models a priori, tailored to reflect our hypotheses as outlined in the introduction.
We fitted 151 different models; model selection was based on Akaike’s information criterion (AIC) or an appropriate variant (AICc, for small samples after Sugiura 1978). We interpreted model selection results in a weight-of-evidence context based on AIC differences (Δi) and normalized Akaike’s weights (wi) as described by Burnham and Anderson (2002). Briefly, Δi is the difference between the AICc of the top model versus the model considered, and thus reflects the likelihood of a given model relative to the best-supported model which has the lowest AIC. Akaike’s weights derive from this measure and are normalized so that the weights of all models in the set sum to 1. The Akaike’s weights of the top model of each of our three analyses (all birds, juveniles only, and adults only) were below 0.9, suggesting considerable model selection uncertainty. Therefore, we used multi-model inference techniques to judge the relative importance of model variables (Burnham and Anderson 2002). Limiting our models to the models with Akaike’s weights larger than 0.05, we assessed the relative importance of a predictor variables by summing Akaike’s weights for all models in which the predictor was present and is given as w+(predictor) as suggested by Burnham and Anderson (2002).
Results
Hatch rates for eggs of control and T-treated house sparrows were similar (F1, 16 = 0.18; p = 0.681), as were nestling survival rates up to 10 days of age (controls, 82.3%; T-treated, 83.4%). Thus, brood sizes were similar for T and control groups. As we alternated treatment over the breeding season, treatment groups had similar hatch dates (F1, 30 = 0.07; p = 0.795). Clutch sizes (F1, 14 = 2.41; p = 0.148), mean egg mass (F1, 14 = 3.75; p = 0.073), and brood sex ratios (controls, 52.5% males; T, 56.5% males) were similar. Neither clutch size (F1, 15 = 0.047; p = 0.831) nor mean egg mass (F1, 15 = 1.108; p = 0.315) varied with lay date.
Nestling body mass at four days of age was unaffected by T treatment and decreased with hatch date (treatment, F1, 30 = 3.011; p = 0.930; hatch date, F1, 30 = 6.773; p = 0.0142). However, body mass in September, when birds were transferred to outdoor aviaries, was significantly affected by T treatment, hatch date, and the interaction between these factors (treatment, F1, 30 = 16.684; p = 0.0003; hatch date, F1, 30 = 11.813; p = 0.0017; treatment * hatch date: F1, 30 = 14.402; p = 0.0007). At this time, T-treated birds were >4% heavier than control birds (T, 26.4 ± 0.52 g; C, 25.2 ± 0.29 g). At this age (mean age, T birds 113 days and C birds 104 days), house sparrows are fully grown and have achieved adult size; body mass decreased with hatch date in control but not T-treated birds. There was a significant interaction of treatment and body mass at 4 days of age that influenced mass at the age of transfer to aviaries (treatment, F1, 28 = 3.794; p = 0.0615; body mass 4 days, F1, 28 = 3.478; p = 0.0727; treatment * body mass 4 days, F1, 28 = 6.066; p = 0.0202).
Survival
Survival was affected additively by treatment, genetic sex, and clutch as a random factor as shown by the best-supported model (treatment + sex + frailty (clutch)) (Table 1; Fig. 1), as well as the w+(predictor) values (frailty (clutch) = 0.91; treatment = 0.69, sex = 0.69). Neither hatch date (entered as a scalar variable) nor year of the experiment were significant factors in the model.
Table 1.
All birds: model selection statistics for the confidence set of models (relative likelihood >0.05) and the global model for testosterone-treated male and female house sparrows
| Rank | Model i | K | AICc | Δi | wi |
|---|---|---|---|---|---|
| 1 | Treatment + sex + frailty(family) | 3 | 479.38 | 0.00 | 0.55 |
| 2 | Frailty(family) | 1 | 480.77 | 1.39 | 0.28 |
| 3 | Treatment * sex + frailty(family) | 4 | 482.99 | 3.61 | 0.09 |
| 4a | Treatment + sex * year + frailty(family) | 5 | 484.24 | 4.86 | 0.05 |
Factors in the statistical model were treatment, sex, year of treatment, and family as a random factor. Model notation: “+” denotes additive effect; “*” denotes interaction. For each model, the following parameters are given: the number of estimable parameters (K), Akaike’s information criterion for small samples (AICc), the difference between the minimum AICc of the top model and the model considered (Δi), and Akaike’s weights (wi)
aGlobal model
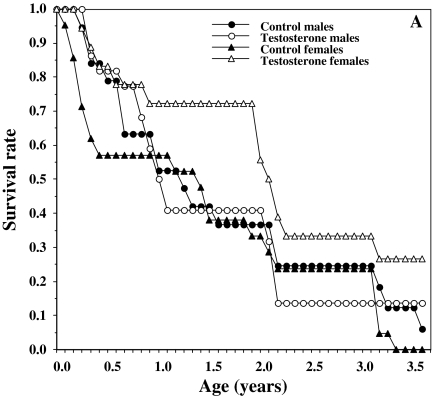
Fig. 1.
Survival and lifespan of house sparrows hatched from control- and testosterone-injected eggs over a study period of 3.5 years
T treatment substantially reduced the overall hazard of death for both sexes by 0.562 (χ2 = 4.91; p = 0.045). This reduction was much higher for females, which had a 0.344-fold lower mortality hazard than treated males (χ2 = 6.21; p = 0.048). Family as a random factor entered the model most significantly with a w+ of 0.91 (χ2 = 28 = 3.58; p = 0.027), suggesting that genetic and phenotypic among-clutch differences were most important in determining survival.
With all birds included in the analysis described above, we were unable to investigate seasonal effects on survivorship without violating proportionality assumptions of the Cox model (Fig. 2) (following Collett 2003). Therefore we performed separate analyses on two subsets of individuals: first-year birds before their first breeding season (birth until April 30th of their second calendar year) and adult birds (after May 1st of their second calendar year).
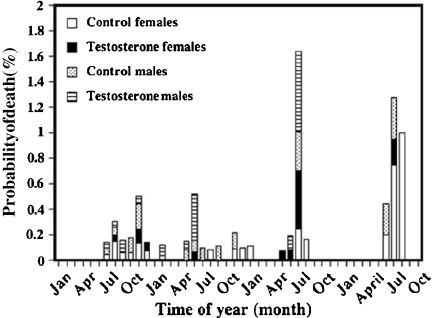
Fig. 2.
Mortality risk of house sparrows housed in predator-proof outdoor aviaries for 3.5 years displayed by month. Birds had hatched between May and July of the first year
First-year birds: separate survival analysis
Model selection for data from first-year birds analyzed separately was not as clear-cut as for the complete dataset (Table 2), but the same factors appeared to be important (w+(frailty (clutch)) = 0.92; w+(treatment) = 0.92; w+(sex) = 0.92; w+(season) = 0.62). The direction of the effects for this subset of the data was similar as for all birds, with T-treated birds having a lower hazard of death of 0.863 (χ2 = 1.95; p = 0.13), males having a slightly increased hazard of 0.226 (χ2 = 1; p = 0.56). Mortality hazard was also lower during the post-juvenile molt in late summer/early fall, but higher in winter compared with the time between hatching and molt. Due to low sample sizes (and the low number of mortality events), no factor reached significance for a specific model for this data subset.
Table 2.
Juveniles only: for explanation of headings and factorial model see Table 1
| Rank | Model i | K | AICc | Δi | wi |
|---|---|---|---|---|---|
| 1 | Treatment + sex * seasonsJMW + frailty(family) | 6 | 254.88 | 0.00 | 0.51 |
| 2 | Treatment + sex * seasons + frailty(family) | 4 | 256.35 | 1.48 | 0.24 |
| 3 | Treatment + sex * seasonsJMW + year + frailty(family) | 7 | 257.93 | 3.06 | 0.11 |
| 4 | Treatment + sex * seasons + year + frailty(family) | 5 | 259.32 | 4.45 | 0.06 |
| 5a | Treatment + sex * seasonsJMW + frailty(family) + year + hatch | 8 | 278.34 | 23.46 |
aGlobal model
Adult birds: separate survival analysis
No particular statistical model was preferred for adult birds. The best-supported model achieved an Akaike’s weight of 0.09 with 20 different models above an Akaike’s weight of 0.02. The factors with the highest w+ values were treatment (w+ = 0.65) and year (w+ = 0.65): T treatment again reduced the mortality hazard by 0.423 (χ2 = 2.317; p = 0.02), and mortality risk in the second year increased by 4.983 (χ2 = 3.386; p = 0.00). Also, season and clutch were important as random factors (season, w+ = 0.47; frailty(family), w+ = 0.33). In all models for this subset of the data, the mortality hazard during the breeding season was 1.553-fold higher than during molt or winter (χ2 = 1.34; p = 0.06). Both sex and hatch date did enter into the models, but were of low importance (sex, w+ = 0.17; hatch date w+ = 0.10).
Lifespan was positively correlated with body mass at 4 days post-hatch (treatment, F1, 29 = 3.051; p = 0.091; body mass, F1, 29 = 6.936; p = 0.013; treatment * body mass, F1, 29 = 1.587; p = 0.218). Both T treatment and the interaction of T treatment with body mass when birds were moved to aviaries (mean age T birds, 113 days; mean age C birds, 104 days) also had significant effects on lifespan (treatment, F1, 30 = 5.691; p = 0.024; body mass, F1, 30 = 0.202; p = 0.657; treatment * body mass, F1, 30 = 6.169; p = 0.019).
Discussion
Adult lifespan and mortality risk in adulthood have been shown to be influenced by conditions experienced during prenatal development (Lummaa and Clutton-Brock 2002; Gluckman and Hanson 2004; Finch 2007). The fitness consequences of avian maternal hormonal effects have been investigated previously in the context of evolutionary trade-offs (Schwabl 1993; Schwabl et al. 1997; Gil et al. 1999; Badyaev et al. 2002; Müller et al. 2007), with a primary focus on the effects of androgens on early growth and survival. We showed here that early exposure of the avian embryo to maternal testosterone also influences adult survival and mortality rates during the first years of life. Testosterone exposure, clutch and sex all had additive effects on mortality, but effects of genes and early rearing environment could not be distinguished in this study. Testosterone-enhanced survivorship could potentially increase lifetime reproductive success (Daan and Tinbergen 1997; Stearns 1992); this study did not directly address effects of prenatal developmental testosterone on reproduction. Other studies have reported either negative, no, or positive effects of maternal androgens on reproductive performance of female progeny (summarized in Müller et al. 2009).
Maternal testosterone may increase mortality risk from extrinsic factors in house sparrows by altering adult sexual and aggressive behavior, by increasing the risk of exposure to infectious disease, predation, and injury, or by alterations in physiology early in life that could, in turn, affect health, physiological function and survivorship later in life. Since in this study we monitored mortality in the absence of predation, provided birds with food ad libitum, and housed birds in adjacent aviaries, effects on mortality of food supply and differential exposure to predators and pathogens are unlikely. We cannot, however, exclude possible effects of differential susceptibility and responses to pathogens. Seasonal variation in social interactions may also have had an impact on extrinsic mortality patterns.
Intrinsic mortality also could be influenced by developmental effects of hormones or growth factors (e.g., growth hormone; IGF-1 and others) suspected to be responsible for the age-specific failure rates of various physiological systems, cells and macromolecules known to be associated with organismal senescence in a variety of laboratory organisms (reviewed in Kirkwood and Austad 2000; Ricklefs 2008). Since we found similar rates of embryo and nestling mortality in T-treated and control broods, differential mortality pressure before hatching or fledging was not likely to be important, nor did we detect any effect of testosterone exposure on sex-specific viability early in life (Sockman and Schwabl 2000; Sockman et al. 2006).
Possible developmental causes for differential adult survival include differences in early-life body mass, growth rates, and physiological condition (e.g., Hales and Barker 2001; Finch 2007; Monaghan 2008). Small altricial passerine birds, such as the house sparrow, gain mass most rapidly during the nestling stage (lasting about 15 days), and growth rates at this time have been shown to influence survival after fledging (e.g., Gebhardt-Henrich and Richner 1998; Perrins 1965). We found no influence of T on nestling body mass at 4 days of age, but treatment and hatch date as well as their interaction affected body mass when birds were between 97 and 130 days old. In turn, hormone treatment and the interaction of treatment with body mass early in life (2–3 months) influenced adult mortality and lifespan. Thus, it is conceivable that the effect of T on adult survival results from better body condition early in the life course.
Life-history phases associated with high mortality (Fig. 2) often represent times when blood levels of sex steroid hormones are characteristically elevated, as during breeding (Hegner and Wingfield 1986). In other studies, however, we have found no evidence that exposure of house sparrows to testosterone in the egg modifies plasma androgen concentrations during post-juvenile life (Strasser and Schwabl, unpublished data) or the reproductive phase in either sex (Partecke and Schwabl 2008). Moreover, in other studies we have detected no profound effects of developmental T exposure on plasma levels of the stress hormone corticosterone, the acute adrenocortical stress response, or resting metabolic rate during the reproductive phase (Partecke and Schwabl, unpublished data).
In summary, the experimental effects on adult mortality of embryonic testosterone exposure we report here could result from developmental effects on adult physiology, fitness trade-offs, or alterations in either extrinsic or intrinsic aging processes. The possible effects of maternal hormones on the immune system, adrenal-hypothalamic stress axis, resistance to the cumulative effects of oxidative stress, and other physiological and cellular processes associated with aging and disease are still underinvestigated and poorly understood. But variation in avian yolk testosterone levels has been shown to be associated with interspecific variation in such life-history variables as maturation times and adult mortality rates in comparative studies of wild birds (Schwabl et al. 2007; Martin and Schwabl 2008), and may also be related to variation in aging rates. Elucidation of the specific mechanisms underlying the developmental effects of sex steroids represents a potentially novel and fruitful research arena in biogerontology.
Electronic supplementary materials
(DOC 74 kb)
Acknowledgments
We thank M. Webster, R.E. Ricklefs, and two anonymous reviewers for comments on previous versions of the manuscript. B. Duskin, M. Leland, and C. Clark helped with raising the sparrows. Research was supported by grants of the Harry Frank Guggenheim Foundation (to H.S.) and of the National Institutes of Mental Health (no. MH4987 to H.S and no. HD F32HD08542 to R.S.).
References
- Arnold AP. Concepts of genetic and hormonal induction of vertebrate sexual differentiation in the twentieth century, with special reference to the brain. In: Pfaff DW, Arnold AOP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, brain and behavior. New York: Academic; 2002. pp. 105–135. [Google Scholar]
- Badyaev AV, Hill GE, Beck ML, Dervan AA, Duckworth RA, McGraw KJ, Nolan PM, Whittingham LA. Sex-biased hatching order and adaptive population divergence in a passerine bird. Science. 2002;295:316–318. doi: 10.1126/science.1066651. [DOI] [PubMed] [Google Scholar]
- Birkhead T, Schwabl H, Burke T. Testosterone and maternal effects—integrating mechanisms and functions. Trends Ecol Evol. 2001;15:86–87. doi: 10.1016/S0169-5347(99)01803-0. [DOI] [PubMed] [Google Scholar]
- Bribiescas R, Ellison P. How hormones mediate trade-offs in human health and disease. In: Stearns SC, editor. Evolution in health and disease. New York: Oxford University Press; 2008. pp. 77–94. [Google Scholar]
- Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. doi: 10.1038/384033a0. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodel inference: a practical information-theoretic approach. New York: Springer; 2002. [Google Scholar]
- Carey J, Judge D. Longevity records: life spans of mammals, birds, amphibians, reptiles, and fish. Odense: Odense University Press; 2000. [Google Scholar]
- Collett D. Modelling survival data in medical research. Boca Raton: Chapman & Hall/CRC; 2003. [Google Scholar]
- Cucco M, Guasco B, Malacarne G, Ottonelli R, Tanvez A. Yolk testosterone levels and dietary carotenoids influence growth and immunity of grey partridge chicks. Gen Comp Endocrinol. 2008;156:418–425. doi: 10.1016/j.ygcen.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Daan S, Tinbergen JM. Adaptation of life histories. In: Krebs JR, Davies NB, editors. Behavioral ecology: an evolutionary approach. 4. New York: Wiley; 1997. pp. 311–333. [Google Scholar]
- Doblhammer G, Vaupel JW. Lifespan depends on month of birth. Proc Natl Acad Sci U S A. 2001;98:2934–2939. doi: 10.1073/pnas.041431898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eising CM, Groothuis TTG (2002) Long-term effects of maternal yolk androgens: an experimental approach. Intern Soc Behavioral Ecology 9th Congr, Abstracts. pp 35–36
- Eising CM, Eikenaar C, Schwabl H, Groothuis TGG. Maternal androgens in black-headed gull eggs: consequences for chick development. Proc R Soc Lond B. 2001;268:839–846. doi: 10.1098/rspb.2001.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE. The biology of human longevity: inflammation, nutrition, and aging in the evolution of lifespans. Amsterdam: Elsevier; 2007. p. 626. [Google Scholar]
- Finch CE, Rose MR. Hormones and the physiological architecture of life history evolution. Q Rev Biol. 1995;70:1–52. doi: 10.1086/418864. [DOI] [PubMed] [Google Scholar]
- Folstad I, Karter AJ. Parasites, bright males and the immunocompetence handicap. Am Nat. 1992;139:603–622. doi: 10.1086/285346. [DOI] [Google Scholar]
- Fox CW, Mousseau TA. Maternal effects as adaptations for transgenerational phenotypic plasticity in insects. In: Mousseau TA, Fox CW, editors. Maternal effects as adaptations. Oxford: Oxford University Press; 1998. [Google Scholar]
- Gebhardt-Henrich S, Richner H. Causes of growth variation and its consequences for fitness. In: Starck JM, Ricklefs RE, editors. Avian growth and development. New York: Oxford University Press; 1998. pp. 324–340. [Google Scholar]
- Gil D, Graves J, Hazon N, Wells A. Male attractiveness and differential testosterone investment in zebra finch eggs. Science. 1999;286:126–128. doi: 10.1126/science.286.5437.126. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Groothuis TGG, Schwabl H. Hormone-mediated maternal effects in birds: mechanisms matter but what do we know of them? Phil Trans R Soc B. 2008;363:1647–1661. doi: 10.1098/rstb.2007.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis TGG, Eising CM, Dijkstra C, Müller W. Balancing between costs and benefits of maternal hormone deposition in avian eggs. Biol Lett. 2005;1:78–81. doi: 10.1098/rsbl.2004.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- Harper JM, Galecki AT, Burke DT, Miller RA. Body weight, hormones and T cell subsets as predictors of life span in genetically heterogeneous mice. Mech Ageing Dev. 2004;125:381–390. doi: 10.1016/j.mad.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Hegner RE, Wingfield JC. Gonadal development during autumn and winter in house sparrows. Condor. 1986;88(269–2):78. [Google Scholar]
- Holmes DJ, Austad SN. Birds as animal models for the comparative biology of aging: a prospectus. J Gerontol Biol Sci. 1995;50A:59–66. doi: 10.1093/gerona/50A.2.B59. [DOI] [PubMed] [Google Scholar]
- Holmes DJ, Martin K. Special reviews in ornithology. A bird’s-eye view of aging: what’s in it for ornithologists? The Auk. 2009;126(1):1–23. doi: 10.1525/auk.2009.1109. [DOI] [Google Scholar]
- Kirkwood TBL, Austad SN. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- Klimkiewicz MK, Futcher AG. Longevity records of North American birds: Coerbinae through Estrildidae. J Field Ornithol. 1987;58:318–333. [Google Scholar]
- Lummaa V, Clutton-Brock T. Early development, survival and reproduction in humans. Trends Ecol Evol. 2002;17:141–147. doi: 10.1016/S0169-5347(01)02414-4. [DOI] [Google Scholar]
- Martin TM, Schwabl H. Variation in maternal effects and embryonic development among passerine bird species. Phil Trans R Soc B. 2008;363:1635–1645. doi: 10.1098/rstb.2007.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan P. Early development, phenotypic development and environmental change. Phil Trans R Soc B. 2008;363:1635–1645. doi: 10.1098/rstb.2007.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W, Lessells C, Kortsen P, Engelhardt N. Manipulative signals in family conflict? On the function of maternal yolk hormones in birds. Am Nat. 2007;169:E84–E94. doi: 10.1086/511962. [DOI] [PubMed] [Google Scholar]
- Müller W, Vergauwen J, Eens M. Long-lasting consequences of elevated testosterone levels on female reproduction. Behav Ecol Sociobiol. 2009;63:809–816. doi: 10.1007/s00265-009-0714-9. [DOI] [Google Scholar]
- Navara KJ, Mendonca MT. Yolk testosterone stimulates growth and immunity in house finches. Physiol Biochem Zool. 2006;79:550–555. doi: 10.1086/501054. [DOI] [PubMed] [Google Scholar]
- Partecke J, Schwabl H. Organizational effects of maternal testosterone on reproductive behavior of adult house sparrows. Dev Neurobiol. 2008;68:1538–1548. doi: 10.1002/dneu.20676. [DOI] [PubMed] [Google Scholar]
- Perrins CM. Population fluctuations and clutch size in the great tit Parus major. J Anim Ecol. 1965;34:601–647. doi: 10.2307/2453. [DOI] [Google Scholar]
- Poopatanapong A (2002) Relationship between male badge size and female reproductive investment and mate choice in House Sparrow. MS thesis, Washington State University, Pullman, Washington
- Ricklefs R. The evolution of senescence from a comparative perspective. Funct Ecol. 2008;22:379–392. doi: 10.1111/j.1365-2435.2008.01420.x. [DOI] [Google Scholar]
- Ringsby TH, Saether B-E, Solberg EJ. Factors affecting juvenile survival in House Sparrow. Passer domesticus. J Avian Biol. 1998;29:241–247. doi: 10.2307/3677106. [DOI] [Google Scholar]
- Schwabl H. Yolk is a source of maternal testosterone for developing birds. Proc Natl Acad Sci U S A. 1993;90:11446–11450. doi: 10.1073/pnas.90.24.11446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabl H. Maternal testosterone in the avian egg enhances postnatal growth. Comp Biochem Physiol. 1996;114A:271–276. doi: 10.1016/0300-9629(96)00009-6. [DOI] [PubMed] [Google Scholar]
- Schwabl H. The contents of maternal testosterone in House Sparrow Passer domesticus eggs vary with breeding conditions. Naturwissenschaften. 1997;84:406–408. doi: 10.1007/s001140050418. [DOI] [PubMed] [Google Scholar]
- Schwabl H (1998) Maternal hormonal effects on postnatal development. In: Adams NJ, Slotow RH (eds) Proc. 22. Intl. Ornithol. Congr. Durban, South Africa, CD-ROM
- Schwabl H, Mock DW, Gieg JA.A hormonal mechanism for parental favouritism Nature 1997386231. 10.1038/386231a09069278 [DOI] [Google Scholar]
- Schwabl HM, Palacios G, Martin TE. Selection for rapid embryo development correlates with embryo exposure to maternal androgens among passerine birds. Am Nat. 2007;170:196–206. doi: 10.1086/519397. [DOI] [PubMed] [Google Scholar]
- Seckl JC. Glucocorticoids, feto-placental 11β-hydroxysteroid dehydrogenase type 2 and early life origins of adult disease. Steroids. 2001;62:89–94. doi: 10.1016/S0039-128X(96)00165-1. [DOI] [PubMed] [Google Scholar]
- Sockman KW, Schwabl H. Yolk androgens reduce offspring survival. Proc R Soc Lond B. 2000;267:1451–1456. doi: 10.1098/rspb.2000.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockman KW, Sharp PJ, Schwabl H. Orchestration of avian reproductive effort: an integration of the ultimate and proximate bases for flexibility in clutch size, incubation behavior, and yolk androgen deposition. Biol Rev. 2006;81:629–666. doi: 10.1017/S1464793106007147. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Carter CS, Ikeno Y, Ekenstedt K, Carlson CS, et al. Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology. 2005;146:2920–2932. doi: 10.1210/en.2005-0058. [DOI] [PubMed] [Google Scholar]
- Stearns SC. The evolution of life histories. Oxford: Oxford University Press; 1992. [Google Scholar]
- Strasser R, Schwabl H. Yolk testosterone organizes behavior and male plumage coloration in house sparrows (Passer domesticus) Horm Behav. 2004;47:503–512. [Google Scholar]
- Sugiura N. Further analysis of the data by Akaike’s information criterion and the finite corrections. Commun Stat, Theory Methods. 1978;A7:13–26. [Google Scholar]
- Summers-Smith JD. The sparrows: a study of the genus Passer. Calton: A.D. Poyser Ltd; 1998. [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Tobler M, Sandell MI. Yolk testosterone modulates persistence of neophobic responses in adult zebra finches, Taeniopygia guttata. Horm Behav. 2007;52:640–645. doi: 10.1016/j.yhbeh.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Tobler M, Sandell MI. Sex-specific effects of prenatal testosterone on nestling plasma antioxidant capacity in the zebra finch. J Exp Biol. 2009;212:89–94. doi: 10.1242/jeb.020826. [DOI] [PubMed] [Google Scholar]
- Tobler M, Nilsson J-Å, Nilsson JF. Costly steroids: egg testosterone modulates nestling metabolic rate in the zebra finch. Biol Lett. 2007;3:408–410. doi: 10.1098/rsbl.2007.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschirren B, Saladin V, Fitze PS, Schwabl H, Richner H. Maternal yolk testosterone does not modulate parasite susceptibility in great tit nestlings. J Anim Ecol. 2005;74:675–682. doi: 10.1111/j.1365-2656.2005.00963.x. [DOI] [Google Scholar]
- Uller T. Developmental plasticity and the evolution of parental effects. Trends Ecol Evol. 2008;23:432–438. doi: 10.1016/j.tree.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Engelhardt N, Carere C, Dijkstra C, Groothuis TGG. Sex specific effects of yolk testosterone on survival, begging, and growth of zebra finches. Proc R Soc B. 2006;271:65–70. doi: 10.1098/rspb.2005.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 74 kb)