Abstract
Adenosine monophosphate-activated protein kinase (AMPK) is an evolutionary conserved energy sensor sensitive to changes in cellular AMP/ATP ratio which is activated by phosphorylation (pAMPK). pAMPK levels decrease in peripheral tissues with age, but whether this also occurs in the aged brain, and how this contributes to the ability of the aged brain to cope with ischemic stress is unknown. This study investigated the activation of AMPK and the response to AMPK inhibition after induced stroke in both young and aged male mice. Baseline levels of phosphorylated AMPK were higher in aged brains compared to young mice. Stroke-induced a robust activation of AMPK in young mice, yet this response was muted in the aged brain. Young mice had larger infarct volumes compared with aged animals; however, more severe behavioral deficits and higher mortality were seen in aged mice after stroke. Inhibition of AMPK with Compound C decreased infarct size in young animals, but had no effect in aged mice. Compound C administration led to a reduction in brain ATP levels and induced hypothermia, which led to enhanced neuroprotection in young but not aged mice. This work demonstrates that aging increases baseline brain pAMPK levels; aged mice have a muted stroke-induced pAMPK response; and that AMPK inhibition and hypothermia are less efficacious neuroprotective agents in the aged brain. This has important translational relevance for the development of neuroprotective agents in preclinical models and our understanding of the enhanced metabolic stress experienced by the aged brain.
Keywords: Aging, AMPK, Compound C, Hypothermia, Ischemic stroke, Middle cerebral artery occlusion (MCAO)
Introduction
AMPK is an evolutionary conserved energy sensor that regulates cellular metabolism. In general, activation of AMPK acts to maintain cellular energy stores, switching on catabolic pathways that produce ATP while switching off anabolic pathways that consume ATP (Hardie 2007). A rise in AMP levels or an increase in the AMP/ATP ratio signals declining energy stores, activating AMPK (see review by Li and McCullough 2010). Although responsive to ATP depletion (increased AMP/ATP ratio), AMPK is also activated by related stimuli such as exercise, starvation, hypoxia, cellular pH, and redox status (Ronnett et al. 2009). Ischemic brain injury involves a complex sequence of excitotoxic and oxidative events, including cellular energy depletion, disrupted protein synthesis, and apoptosis, etc. (Love 2003), and is a robust stimulus for AMPK activation (McCullough et al. 2005b). Former studies in young animals subjected to middle cerebral artery occlusion (MCAO) confirmed that phosphorylated AMPK (pAMPK), the active form of this kinase, is rapidly increased after MCAO. Administration of an AMPK inhibitor, Compound C, decreased pAMPK and led to a reduction of infarct size (McCullough et al. 2005b; Li et al. 2007).
Age is the most important independent risk factor for stroke (Rosamond et al. 2008) and the aged brain undergoes numerous neurochemical and physiological changes compared with young brains (Anyanwu 2007). Most experimental stroke studies have been performed on young animals, and therefore may not fully replicate the effects of ischemia on neural tissue in aged subjects. Aged male mice have less histological damage after MCAO despite increased functional deficits and mortality, the underlying mechanism of which remains elusive (Liu et al. 2009b). Recent studies have demonstrated that aging is accompanied by an increase in AMP/ATP ratio in multiple tissues (Hardie and Hawley 2001; Petersen et al. 2003; Wang et al. 2003), yet AMPK levels are surprisingly lower in aged muscle (Qiang et al. 2007; Reznick et al. 2007). Hypoxia was unable to induce AMPK activity in aged hepatocytes, suggesting that responsiveness of AMPK signaling decreases with age (Mulligan et al. 2005) and may contribute to the inability of aged animals to effectively cope with stressors. SIRT1 is a member of the Sirtuin family and interacts with AMPK to exert effects on life span, aging, and metabolism (Imai 2007; Fulco and Sartorelli 2008). SIRT1 is widely expressed in mammalian cells and has been studied in many tissues including brain. SIRT1 responds to increases and decreases in nutrient availability (caloric restriction or starvation) and energy expenditure (Nemoto et al. 2004; Suwa et al. 2008). Little is known regarding the response of SIRT1 to ischemic challenge or to manipulations in AMPK signaling. Compound C, also termed dorsomorphin, is a competitive inhibitor of ATP binding to the catalytic α subunit of AMPK (Zhou et al. 2001). Compound C exerts neuroprotective effects in ischemia-induced brain injury via AMPK inhibition, as its effects are ameliorated in mice with deletion of AMPKα (McCullough et al. 2005b; Li et al. 2007). The present study examined the expression of total AMPK, pAMPK and SIRT1 in young and aged mice after stroke. The neuroprotective efficacy of Compound C was also evaluated.
Materials and methods
Animals
C57BL/6 mice were purchased from Charles River Laboratories (National Institute on Aging). All experiments were performed according to NIH guidelines for the care and use of animals in research and under protocols approved by the UCHC Animal Care and Use Committee. Both young male mice (9–12 weeks; 21–25 g) and aged male mice (16–18 months; 30–40 g) were utilized.
Focal cerebral ischemic model
Focal transient cerebral ischemia was induced by reversible MCAO (90 min) under isoflurane anesthesia followed by reperfusion as described previously (Liu et al. 2009b). 6-0 nylon suture filaments were utilized to occlude the MCA in both young and aging mice, but 0.21 mm (dimension) silicon-coated tips were used for young and 0.23-mm tips were used for aged mice to ensure equivalent cerebral blood flow (CBF) reduction. Several cohorts of animals were prepared for infarct size (evaluated at 24 h or at 30 days) and Western analysis (evaluated at 4 and 24 h). Mice evaluated at 30 days were subjected to a 60-min MCAO. In these cohorts, temperature was controlled at 37.5°C during ischemia and 24 h after reperfusion. CBF was measured by laser Doppler flowmetry (LDF, Moor Instruments Ltd, England) in all cohorts. Occlusion was confirmed by a drop in LDF by 85% of baseline in all mice. Neurological deficit scores (NDS) were recorded after stroke. The scoring system was as follows: 0, no deficit; 1, forelimb weakness and torso turning to the ipsilateral side when held by tail; 2, circling to affected side; 3, unable to bear weight on affected side; and 4, no spontaneous locomotor activity or barrel rolling.
Drug induced hypothermia
Another cohort of mice underwent 90-min MCAO and were divided into two groups: a body temperature control group and a free regulation non temperature controlled group. In the temperature control group, rectal muscle temperature was monitored with a MONOTHERM system and maintained at approximately 37°C during surgery and ischemia with an automated temperature control feedback system; after surgery, the mouse were returned into a cage with a heating pad system that maintained temperature until sacrifice. In the temperature non-control group, the MONOTHERM system was not applied so that the effects of stroke and Compound C on body temperature could be determined. The rectal muscle temperature of each mouse was monitored every hour for the first 6 h and at 24 h after stroke (right before killing). Compound C ([4-(2-piperidin-1-yl-ethoxy)-phenyl])-3-pyridin-4-yl-pyrrazolo[1,5-a]-pyrimidine; Calbiochem, San Diego, CA) was dissolved in DMSO and further diluted with sterile PBS. Compound C (10 mg/kg, 0.01 cc/g) was injected intraperitoneally at stroke onset (Kim et al. 2004). Control mice were injected with vehicle alone.
Histological assessment
For evaluation of chronic stroke outcomes, the mice were allowed to survive for 30 days and the brains perfused for cresyl violet (CV) staining as previously described (Li et al. 2004). The amount of tissue lost (percent atrophy) was computed by (1 − (ischemic hemisphere-ventricle-cavity) / (contralateral hemisphere-ventricle)) × 100 as in (Li et al. 2004).
For acute measurement of stroke outcomes, both young and aged mice were euthanized 24 h after stroke and the brain removed, cut into five 2-mm slices and stained with 1.5% 2,3,5-triphenyltetrazolium (TTC) and fixed with 4% formalin. Images were digitalized, and the infarct volumes (corrected for edema) were analyzed using Sigmascan Pro5 as previously described (McCullough et al. 2005a).
Whole cell lysate preparation
After 4 and 24 h of MCAO, brain samples were obtained by rapid removal of the brain from the skull, resection of the cerebellum, followed by immediate dissection into the right (R; ischemic) and left (L; non-ischemic) hemispheres. Samples were homogenized in 1.5 ml of ice-cold RIPA buffer containing protease inhibitor tablet (Roche Diagnostics) and 1 mM PMSF using a Dounce homogenizer on ice and briefly sonicated on ice. Extracts were immediately centrifuged at 14,000 ×g and frozen at −80°C.
Western blots
Protein concentration was determined by BCATM Protein Assay Kit (Thermo Fisher Scientific Inc., Rockford, IL) and subjected to Western Blotting as previously described (McCullough et al. 2005a). Sample proteins were resolved on 4% to 20% SDS electrophoresis gels and transferred to a polyvinylidene difluoride membrane. Total AMPK, pAMPK, and SIRT1 were detected using corresponding antibodies from Cell Signaling (AMPK and pAMPK, 1:1,000), Millipore (SIRT1, 1:1,000), and Sigma (β-actin, 1:1,000; loading control). All blots were incubated overnight in primary antibodies at 4°C in TBS containing 4% BSA and 0.1% Tween 20. Secondary antibodies (goat anti-rabbit IgG 1:5,000, goat anti-mouse IgG 1:2000, donkey anti-goat IgG 1:1,000; Santa Cruz) were diluted and ECL detection kit (Amersham Biosciences) was used for signal detection. Densitometry was performed with Scion Image.
Measurement of ATP levels
Brain ATP levels were determined 3 h after Compound C or vehicle injection using an ATP colorimetric/fluorometric assay kit (Biovision, Mountain View, CA, as previously described (Tota et al. 2010). Briefly, tissue was lysed in ATP assay buffer (0.1 mg/μl) and centrifuged at 15,000 ×g for 2 min to pellet insoluble materials. Five microliters of supernatant was brought to a final volume 50 μl/well with ATP assay buffer, and mixed with another 50 μl of ATP reaction mix. After a 30-min incubation, absorbance was measured at 570 nm and ATP concentration was calculated using an ATP standard curve.
Statistics
NDS were expressed as median (range) and analyzed with the Mann–Whitney U test. All other values are expressed as mean ± SEM and analyzed with a t test for two groups, and two-way analysis of variance (infarct volumes and densitometry of Western blotting) with repeated measures (for body temperature analysis) with Turkey post hoc correction, when appropriate. All assessments were performed by a blinded investigator to drug treatment group. Due to the higher body weights and severe behavioral deficits in aged mice, experimenters were aware of the different age groups (aged vs. young). All infarct analysis was performed in a blinded fashion to age and drug effects. The criterion for statistical significance was P < 0.05.
Results
Aged mice have less tissue loss than young mice 30 days after stroke
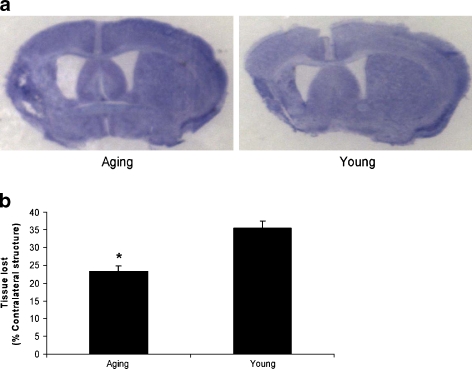
Our previous study found that 24 h after stroke, aged mice have smaller infarct volumes than their young counterparts (Liu et al. 2009b). In order to confirm the persistence of age-related differences in stroke outcomes at chronic endpoints, we measured brain atrophy in mice that were allowed to survive for 30 days after MCAO with CV staining. Consistent with acute endpoints (i.e., 24 h), the amount of tissue lost was significantly less in aged animals compared to young mice (Fig. 1a and b).
Fig. 1.
Chronic stroke outcome in young and aging mice. a Representative CV-stained brain slices from young and aged mice 30 days after MCAO. b Quantitative analysis of tissue lost (% atrophy) expressed as a percentage in young and aged mice. *P < 0.05 versus young mice; n = 6 animals/group
Aged mice had higher levels of pAMPK expression at baseline than young mice
AMPK activity has been reported as a major contributor to stroke outcome (McCullough et al. 2005b; Li et al. 2007). To evaluate baseline of AMPK activation, pAMPK were assessed in brain lysates of young and aged sham mice. Aged sham mice had significantly increased baseline pAMPK levels compared with young sham mice; however, no differences of total AMPK level were seen between groups (n = 4 animals/group; Fig. 2a and b).
Fig. 2.
AMPK expression in young and aged mice after stroke. a Representative western blots of total AMPK and pAMPK protein levels in brain homogenates in sham (Sh) and stroke (St) mice 24 h after stroke onset. β-actin was used as a loading control. b The optical density of samples was expressed as the ratio of the pAMPK bands to control bands (β-actin). Young sham mice had significantly less pAMPK expression than aging shams. Four stroke and two sham brains in biological triplicates were analyzed for statistical analysis. *P < 0.05 versus stroke mice in young group and sham mice in aging group
Stroke-induced activation of AMPK was significantly higher in young mice
Ischemic injury is a potent stimulus to activate AMPK (Li et al. 2007). To compare stroke-induced AMPK activation in young and aged mice, we subjected mice to 90-min MCAO and examined pAMPK levels (Threonine 172) by Western Blot. Young mice had a significant increase in pAMPK levels after stroke compared to sham mice; whereas pAMPK levels were not increased after stroke in aged mice compared to aged shams (n = 6 animals/group; Fig. 2a and b). Although aged sham mice had higher levels of pAMPK than young shams, young mice had more pAMPK levels after stroke than aged animals.
The response to AMPK inhibition differed in aged animals
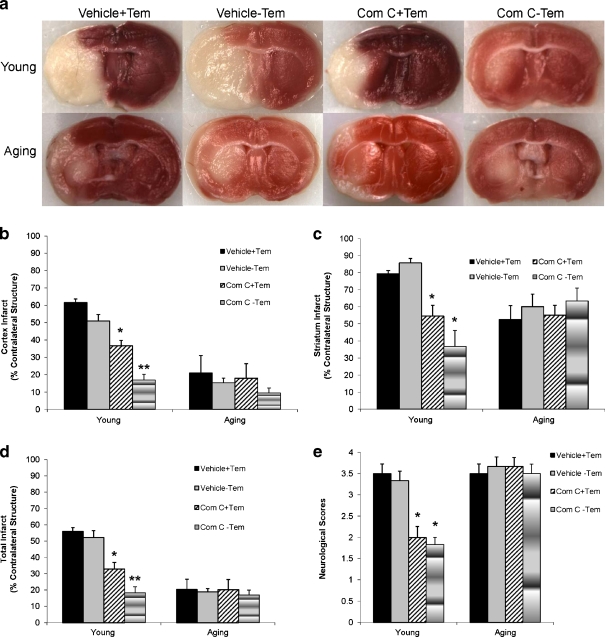
To directly assess the effect of AMPK activation on stroke outcome, we administered the AMPK inhibitor, Compound C to mice at the onset of stroke. All mice had temperature controlled by a biofeedback system to maintain temperature in the physiologic range. Neurological deficits were significantly improved in drug-treated young mice compared to the young vehicle group at 4 h of stroke (vehicle vs. drug, 3.5(1) vs. 2(0); n = 6 animals/group, P < 0.05); however, aged mice had no improvements in the NDS when treated with Compound C (vehicle vs. drug, 3.5(1) vs. 4(0.75); n = 6 animals/group, P > 0.05; Fig. 3e). Histological analysis demonstrated that no differences of infarct size were seen between temperature and non-temperature control vehicle-treated young mice. Administration of Compound C in young mice with temperature control significantly reduced infarct size compared to vehicle-treated mice (total infarct, vehicle vs. drug, 55.9 ± 2.4 vs. 32.8 ± 4.0%, n = 6 animals/group, P < 0.05). Vehicle-treated aged mice had smaller total infarct volumes than their young counterparts (total infarct, aged vs. young, 20.3 ± 6.4 vs. 55.9 ± 2.4%, n = 6 animals/group, P < 0.05), consistent with several previous reports (Shapira et al. 2002; Liu et al. 2009b); however, administration of Compound C had no effect on infarct size in aged mice (Fig. 3a–d). Mortality rates were higher in aged mice (24% in vehicle and 26% in drug-treated group) compared to young mice (12% in vehicle and 8% in drug-treated group).
Fig. 3.
Effect of Compound C on stroke outcomes in young and aged mice. a Representative TTC stained images of brain coronal sections from mice treated with Compound C or vehicle. b–d Quantification of infarct volumes based on TTC staining in cortex (b), striatum (c), and total hemisphere (d). Compound C-treated young mice with or without temperature control had significantly smaller infarct volumes compared with vehicle-treated mice. The total infarct size in drug-treated young mice without temperature control was significantly smaller than that in drug-treated young mice with temperature control. No differences of infarct were seen between groups in the aged cohort. e Neurological deficit scores (NDS) at 4 h of stroke showed a similar pattern as the infarct size. *P < 0.05 versus vehicle-treated young mice. **P < 0.05 versus vehicle- or drug-treated young mice with temperature control; n = 6 animals/group. Com C ± Tem Compound C-treated mice with or without temperature control
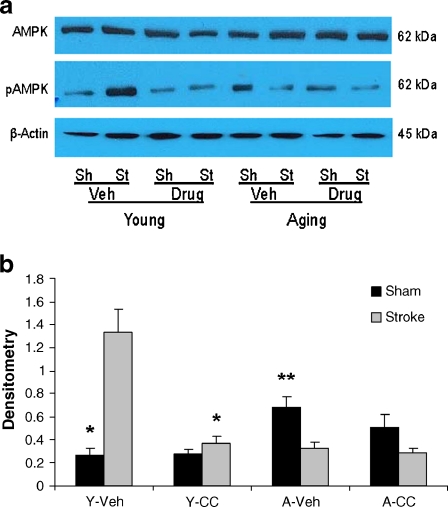
AMPK inhibition with Compound C reduced stroke-induced pAMPK activation in young, but not aged mice; no stroke or age effects were seen in SIRT1 expression
We next examined the effect of administration of Compound C on AMPK and pAMPK expression 24 h after stroke. Total AMPK levels were not significantly different between groups, suggesting no change in protein expression with age. However, levels of pAMPK were significantly higher in vehicle-treated sham aged mice compared to young sham counterparts. Administration of Compound C significantly decreased stroke-induced expression of pAMPK in young mice to levels seen in vehicle-treated sham mice. In aged mice however, although there was a trend towards a reduction in pAMPK activation in Compound C-treated sham mice, no significant difference in pAMPK expression was seen between vehicle- and drug-treated groups (Fig. 4a and b). There were no differences of SIRT1 expression between young and aged, drug- and vehicle-treated groups at either 4 or 24 h of stroke (data not shown).
Fig. 4.
Effect of Compound C on AMPK activation in brains of young and aged mice. a Total AMPK, pAMPK were probed from the brain homogenates of sham (Sh) and stroke (St) animals treated by either Compound C or vehicle. β-actin was used as a loading control. b The optical density of samples was expressed as the ratio of the pAMPK bands to control bands (β-actin). Compound C reduced pAMPK level in young but not aging mice after MCAO. Four pooled stroke brains in biological triplicates were assessed for statistical analysis. *P < 0.05 versus vehicle-treated young stroke mice; **P < 0.05 versus vehicle-treated young sham mice
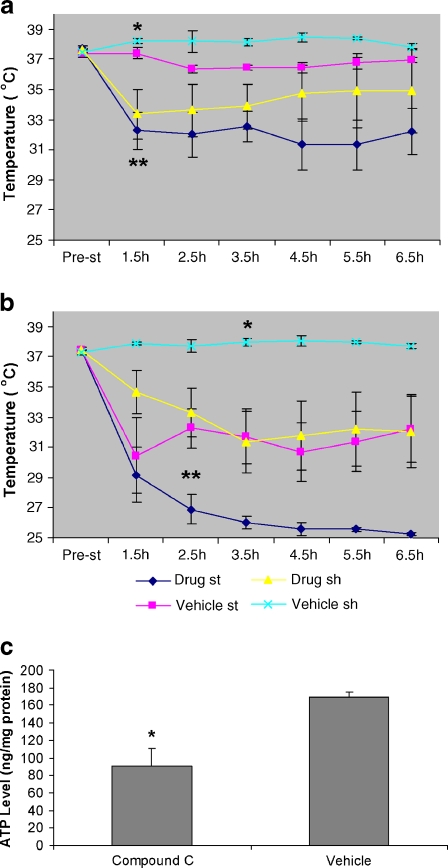
Compound C administration led to hypothermia and decrease in ATP content
Hypothermia is protective in models of both cardiac and brain ischemia (Krieger and Yenari 2004). The beneficial effect of hypothermic preconditioning in cardiac tissue is ameliorated in part by administration of Compound C (Khaliulin et al. 2007). The temperature-controlled group was maintained at 37°C–37.5°C during and after stroke. In the temperature non-control group, the automated temperature control feedback system was discontinued and animals were allowed to autoregulate. Temperatures before stroke were between 37°C–37.5°C and no differences were seen between age or treatment groups. After stroke, the temperatures in the drug-treated stroke mice were significantly decreased compared to vehicle-treated stroke mice every hour for the first 6 h after stroke in young mice and after 2.5 h of stroke in aged mice (n = 6 animals/group, P < 0.05; Fig. 5a and b). Compound C reduced rectal temperatures significantly at 1.5 and 3.5 h of stroke in drug-treated sham young mice compared with vehicle-treated mice, and at 3.5 h of stroke in aged group (n = 6aniamals/group, P < 0.05). At 24 h, the temperature returned to 37°C–37.5°C in each group. TTC staining showed that the infarct size in vehicle-treated young mice without temperature control (Vehicle − Tem) was not significantly different from that in temperature control group (Vehicle + Tem; Fig. 3). Drug-treated young mice without temperature control had significantly smaller infarcts than either drug- or vehicle-treated mice with temperature control; however, there were no differences of infarct volumes between groups of aged mice, suggesting a loss of hypothermia-induced neuroprotection. Compound C significantly reduced brain ATP levels in young mice 3 h after treatment compared to vehicle-treated group (Fig. 5c).
Fig. 5.
Effects of Compound C on rectal temperature and ATP level in the brain. a, b Rectal temperatures in young (a) and aged (b) mice. The MONOTHERM system was turned off during MCAO, and rectal temperature was examined in each group. n = 6 animals/group. In a,*P < 0.05 versus drug-treated sham mice at 1.5 and 3.5 h of stroke; **P < 0.05 versus vehicle-treated stroke mice at each post-stroke time point. In b, *P < 0.05 versus drug-treated sham mice at 3.5 h of stroke; **P < 0.05 versus vehicle-treated stroke mice after 2.5 h of stroke. c Brain ATP concentrations in mice treated with Compound C or vehicle. *P < 0.05 versus vehicle-treated group. n = 7 animals/group
Discussion
The present study revealed several important new findings. Firstly, aged male mice have smaller infarct volumes than young mice at 24 h, consistent with previous studies (Shapira et al. 2002, Liu et al. 2009b), and these differences persist at chronic endpoints (30 days). The mechanism is unclear, but may be related to a decline in NMDA receptor-mediated responses in aged, as compared with young animals (Gonzales et al. 1991; Wenk et al. 1991). Infarct size may not be the best indicator of the physiological dysfunction associated with experimental stroke when comparing young to aging animals. Even in animals of the same age, infarct size may not reliably correlate with neurological deficits (Grabowski et al. 1993; Alexis et al. 1996), so behavioral assessments, even in short-term survival studies, should always be performed. Secondly, baseline expression of pAMPK, the activated form of AMPK, was significantly higher in aged mouse brain compared to young mice with no changes in total AMPK levels. Importantly, significant stroke-induced activation of pAMPK only occurred in young mice. This is consistent with previous findings in hepatocytes which also exhibited baseline elevations in pAMPK levels with age, but a muted response to hypoxia (Mulligan et al. 2005). Thirdly, inhibition of AMPK with Compound C decreased both infarct volumes and neurological deficit scores in young mice, but had no effect on infarct size and surprisingly exacerbated behavioral deficits in aged mice. SIRT1 expression was unchanged after stroke and was not influenced by age or Compound C treatment. Finally, Compound C administration decreased ATP production and led to prolonged spontaneous hypothermia in both young and aged mice. The neuroprotection seen with Compound C in young mice was independent of its hypothermic effects, as Compound C retained its neuroprotective effect even when temperature was maintained at 37°C and the effect of hypothermia and AMPK inhibition appeared to be additive. Hypothermia independently reduced infarct in young mice. In contrast, the hypothermia induced by Compound C did not significantly reduce infarct volume in aged mice, suggesting that neither AMPK inhibition nor hypothermia is effective in reducing injury in aged brain.
It is increasingly recognized that AMPK signaling is impaired in aging (Turdi et al. 2010). Contraction-induced AMPK activation in the low-oxidative muscle seen in young rats was attenuated in old animals (Ljubicic and Hood 2009). In humans, aging is accompanied by an increase in mitochondrial dysfunction in muscle (Petersen et al. 2003), which leads to perturbed cellular energy levels. Senescent human fibroblasts have a higher AMP:ATP ratio, a sensitive measure of energy levels, compared to young fibroblasts (Hardie and Hawley 2001; Wang et al. 2003). In aged hepatocytes, AMPK levels rise, yet become less responsive to stressors such as hypoxia (Mulligan et al. 2005). Aging is accompanied by changes in brain structure, function, and metabolism (Baquer et al. 2009), but the effect of aging on AMPK signaling has not been previously evaluated. The present study demonstrates that aging leads to a baseline up-regulation of pAMPK expression in the brain, but similar to peripheral tissues, AMPK signaling becomes less responsive to injury. The AMP/ATP ratio increases with age in Caenorhabditis elegans, leading to activation of AMPK (Apfeld et al. 2004), consistent with our findings. AMPK activity is regulated by the phosphorylation of threonine 172 on the catalytic α subunit (Hawley et al. 1996), and overexpression of aak-2 the gene encoding this subunit in C. elegans, increased lifespan implicating AMPK signaling in longevity (Apfeld et al. 2004). It has been confirmed that limiting energy availability (i.e., caloric restriction) may extend lifespan (Osiewacz 2002; Tissenbaum and Guarente 2002). It is possible that the baseline increase in AMPK activity in the aged brain may reflect a survival mechanism to cope with age-related increases in the AMP/ATP ratio; however it may leave the organism less able to respond to acute stressors such as stroke. Very recent studies have shown abnormal accumulations of p-AMPK in human tauopathies, such as Alzheimer’s disease, and that AMPK can directly phosphorylate tau in vitro (Vingtdeux et al. 2010) implicating AMPK as a key player in other age-related diseases.
The role of AMPK activation in stroke is still somewhat controversial. Several previous studies have suggested that AMPK activation is beneficial, as administration of the AMPK activator AICAR enhanced neuronal survival under conditions of reduced energy availability in vitro (Culmsee et al. 2001; Kuramoto et al. 2007). Interestingly, we, and others, have shown that pAMPK levels are increased after stroke and brain injury (Culmsee et al. 2001; Li et al. 2007). Accumulating evidence has demonstrated a clear relationship between AMPK activation and neuronal death in focal stroke and in vitro models (Jung et al. 2004; McCullough et al. 2005b; Li et al. 2007; Nakatsu et al. 2008). The emerging concept that the duration of AMPK activation is the pivotal factor in determining neuronal death or neuronal survival after ischemia likely explains some of these differing results (Weisová et al. 2010).
Interestingly, metformin, a drug widely used for the treatment of type 2 diabetes mellitus is known to act as an AMPK activator which has been used safely in patients with vascular disease although it would be expected to be detrimental in acute injury (Li and McCullough 2010). We have recently found that acute metformin treatment (3 days prior to injury) exacerbated stroke damage and enhanced post-ischemic lactic acidosis; whereas chronic metformin given daily for 3 weeks prior to stroke was neuroprotective. Chronic metformin ameliorated the stroke-induced activation of AMPK (Li and McCullough 2010), a similar pattern seen in aging mice in the present study. These effects are specific for AMPK, as they are lost in mice with deletion of the catalytic isoform. It is possible that chronic AMPK activation (with aging or chronic metformin treatment) serves as a sub-lethal metabolic stressor leading to an “ischemic preconditioning” response that reduces injury from a subsequent severe insult.
Genetic deletion of AMPK (α2 subunit) or AMPK inhibition reduced injury after in vivo stroke (McCullough et al. 2005b; Li et al. 2007) consistent with Compound C’s effects in young mice in this study. The loss of Compound C’s protective effect in aged mice could be secondary to several factors: (1) AMPK activation is chronically up regulated secondary to enhanced age-related metabolic stress (Apfeld et al. 2004) leaving AMPK unable to respond to acute stressors; (2) strokes were smaller in the aged brain so that a protective effect could not be seen (floor effect); (3) Compound C selectively reduces metabolic demand in the ischemic brain in young mice or (4) PHARMACODYNAMIC differences. The latter is less likely as we did perform dose–response studies at both higher and lower doses and saw similar effects. Interestingly, the lack of stroke-induced activation of AMPK in the aged brain did correlate with smaller infarcts in our aged cohorts, but future studies using genetic models will be required to definitively link outcomes to the lack of stroke-induced activation of AMPK in aging.
SIRT1 belongs to histone deacetylase class III, which reverses protein acetylation and promotes DNA stability. AMPK enhances SIRT1 activity by increasing cellular NAD+ levels (Canto et al. 2009). Although it has been suggested that increased SIRT1 activity leads to extension of life span (Howitz et al. 2003; Wood et al. 2004), what role SIRT1 plays in the response to ischemic brain insults is unknown (Raval et al. 2008; Liu et al. 2009a). In the present study, SIRT1 expression was unaffected by age and ischemic injury in vehicle-treated mice. This is in contrast with a previous report that found elevated cortical SIRT1 protein levels 3 h after stroke (Liu et al. 2009a). This same study found a decrease at 6 h in SIRT1 protein, and differences may reflect the timing of evaluation (at 4 and 24 h in our study). It is possible that other sirtuin analogues, e.g., SIRT3 (Pillai et al. 2010), instead of SIRT1, which were not evaluated in this study, play more important roles interacting with AMPK in response to changes in the energy status. SIRT1 levels may also selectively change in one cellular compartment (i.e., nuclear translocation from the cytoplasm) or in one cell type (i.e., endothelium vs. neuron). As we only examined whole brain homogenates which are comprised of many cell types, and only examined whole cell lysates, we cannot exclude the possibility that aging or stroke influences SIRT1.
Administration of Compound C led to a sustained hypothermic response in both young and aged mice if body temperature was not controlled by using a feed-forward temperature controller. Activation of AMPK enhances ATP-generating, catabolic pathways and inhibits lipid, fatty acid, cholesterol, and protein synthesis through phosphorylation of key regulatory proteins leading to increased ATP availability (Li and McCullough 2010). Compound C reduces ATP-generation by inhibiting AMPK in vitro (Ronnett et al. 2005). This was confirmed in the present study as Compound C significantly decreased ATP production compared with vehicle-treated group (Fig. 5c). Numerous studies have shown that hypothermia is neuroprotective in ischemia-induced brain injury models through multiple mechanisms (see review by Lazzaro and Prabhakaran 2008). Compound C-treated young mice that were allowed to sustain mild hypothermia had significantly smaller infarcts than normothermic animals, indicating Compound C may exert neuroprotection in ischemic stroke through two different pathways, i.e., inhibiting lactic acidosis (Li and McCullough 2010) and inducing hypothermia. However, these two pathways likely overlap as both are triggered by inhibition of AMPK with a subsequent reduction in metabolic demand. Although Compound C administration also caused hypothermia in aged mice, no protective effect was seen on infarct size, possibly due to a “floor effect” secondary to the smaller infarcts in aged mice. However, no effect was seen in the striatum, where the infarcts were relatively large even in the aged cohort. The hypothermia induced by Compound C also occurred more rapidly in young mice (at 1.5 vs. 3.5 h; Fig. 5). This may be due to the larger body weights in the aged mice, but is unlikely to explain the lack of neuroprotection induced by Compound C, as no effect was seen in temperature controlled aged cohorts whereas Compound C remained robustly protective in young mice. Alternations in cell signaling with aging may be responsible for the loss of neuroprotection by AMPK inhibition. Interestingly, neither hypothermic nor ischemic preconditioning has been well studied in aging models of stroke. This is concerning as recent data suggests that ischemic preconditioning is less effective in the aged heart in both experimental and clinical studies (Abete et al. 2010). This may have significant ramifications for trials of induced hypothermia in acute ischemic stroke (Lyden et al. 2005). Our work suggests that the neuroprotection induced by hypothermia may be less robust in the aged. It is important to stress that this study was not directly designed to study hypothermia in aging and further studies are needed. In conclusion, aged and young animals exhibit different profiles of AMPK activation both at baseline and after stroke. AMPK inhibition does not reduce injury in aged mice. Interventional therapies targeting AMPK activation should be assessed in both young and aged subjects.
Acknowledgment
This work was supported by the National Institute of Neurological Diseases and Stroke (NINDS; NS050505 and NS055215 to LDM)
References
- Abete P, Cacciatore F, Testa G, Della-Morte D, Galizia G, Santis D, Calabrese C, Cioppa A, Ferrara N, Rengo F. Ischemic preconditioning in the aging heart: from bench to bedside. Ageing Res Rev. 2010;9:153–162. doi: 10.1016/j.arr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Alexis NE, Back T, Zhao W, Dietrich WD, Watson BD, Ginsberg MD. Neurobehavioral consequences of induced spreading depression following photothrombotic middle cerebral artery occlusion. Brain Res. 1996;15:273–282. doi: 10.1016/0006-8993(95)01180-3. [DOI] [PubMed] [Google Scholar]
- Anyanwu EC. Neurochemical changes in the aging process: implications in medication in the elderly. Scientific World J. 2007;7:1603–1610. doi: 10.1100/tsw.2007.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfeld J, O’Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004;18:3004–3009. doi: 10.1101/gad.1255404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baquer NZ, Taha A, Kumar P, McLean P, Cowsik SM, Kale RK, Singh R, Sharma D. A metabolic and functional overview of brain aging linked to neurological disorders. Biogerontology. 2009;10:377–413. doi: 10.1007/s10522-009-9226-2. [DOI] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD + metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culmsee C, Monnig J, Kemp BE, Mattson MP. AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci. 2001;17:45–58. doi: 10.1385/JMN:17:1:45. [DOI] [PubMed] [Google Scholar]
- Fulco M, Sartorelli V. Comparing and contrasting the roles of AMPK and SIRT1 in metabolic tissues. Cell Cycle. 2008;7:3669–3679. doi: 10.4161/cc.7.23.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales RA, Brown LM, Jones TW, Trent RD, Westbrook SL, Leslie SW. N-methyl-d-aspartate mediated responses decrease with age in Fischer 344 rat brain. Neurobiol Aging. 1991;12:219–225. doi: 10.1016/0197-4580(91)90100-X. [DOI] [PubMed] [Google Scholar]
- Grabowski M, Brundin P, Johansson BB. Paw-reaching, sensorimotor, and rotational behavior after brain infarction in rats. Stroke. 1993;24:889–895. doi: 10.1161/01.STR.24.6.889. [DOI] [PubMed] [Google Scholar]
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays. 2001;23:1112–1119. doi: 10.1002/bies.10009. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Imai S. Is Sirt1 a miracle bullet for longevity? Aging Cell. 2007;6:735–737. doi: 10.1111/j.1474-9726.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- Jung JE, Lee J, Ha J, Kim SS, Cho YH, Baik HH, Kang I. 5-Aminoimidazole-4-carboxamide-ribonucleoside enhances oxidative stress-induced apoptosis through activation of nuclear factor-kappaB in mouse Neuro 2a neuroblastoma cells. Neurosci Lett. 2004;354:197–200. doi: 10.1016/j.neulet.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Khaliulin I, Clarke SJ, Lin H, Parker J, Suleiman MS, Halestrap AP. Temperature preconditioning of isolated rat hearts—a potent cardioprotective mechanism involving a reduction in oxidative stress and inhibition of the mitochondrial permeability transition pore. J Physiol. 2007;581:1147–1161. doi: 10.1113/jphysiol.2007.130369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EK, Miller I, Aja S, Landree LE, Pinn M, McFadden J, Kuhajda FP, Moran TH, Ronnett GV. C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. J Biol Chem. 2004;279:19970–19976. doi: 10.1074/jbc.M402165200. [DOI] [PubMed] [Google Scholar]
- Krieger DW, Yenari MA. Therapeutic hypothermia for acute ischemic stroke: what do laboratory studies teach us? Stroke. 2004;35:1482–1489. doi: 10.1161/01.STR.0000126118.44249.5c. [DOI] [PubMed] [Google Scholar]
- Kuramoto N, Wilkins ME, Fairfax BP, Revilla-Sanchez R, Terunuma M, Tamaki K, Iemata M, Warren N, Couve A, Calver A, Horvath Z, Freeman K, Carling D, Huang L, Gonzales C, Cooper E, Smart TG, Pangalos MN, Moss SJ. Phospho-dependent functional modulation of GABA(B) receptors by the metabolic sensor AMP-dependent protein kinase. Neuron. 2007;53:233–247. doi: 10.1016/j.neuron.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaro MA, Prabhakaran S. Induced hypothermia in acute ischemic stroke. Expert Opin Investig Drugs. 2008;17:1161–1174. doi: 10.1517/13543784.17.8.1161. [DOI] [PubMed] [Google Scholar]
- Li J, McCullough LD. Effects of AMP-activated protein kinase in cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:480–492. doi: 10.1038/jcbfm.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zeng Z, Viollet B, Ronnett GV, McCullough LD. Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke. 2007;38:2992–2999. doi: 10.1161/STROKEAHA.107.490904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Liu D, Gharavi R, Pitta M, Gleichmann M, Mattson MP. Nicotinamide prevents NAD + depletion and protects neurons against excitotoxicity and cerebral ischemia: NAD + consumption by SIRT1 may endanger energetically compromised neurons. Neuromolecular Med. 2009;11:28–42. doi: 10.1007/s12017-009-8058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab. 2009;29:792–802. doi: 10.1038/jcbfm.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljubicic V, Hood DA. Diminished contraction-induced intracellular signaling towards mitochondrial biogenesis in aged skeletal muscle. Aging Cell. 2009;8:394–404. doi: 10.1111/j.1474-9726.2009.00483.x. [DOI] [PubMed] [Google Scholar]
- Love S. Apoptosis and brain ischaemia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:267–282. doi: 10.1016/S0278-5846(03)00022-8. [DOI] [PubMed] [Google Scholar]
- Lyden PD, Allgren RL, Ng K, Akins P, Meyer B, Al-Sanani F, Lutsep H, Dobak J, Matsubara BS, Zivin J. Intravascular cooling in the treatment of stroke (ICTuS): early clinical experience. J Stroke Cerebrovasc Dis. 2005;14:107–114. doi: 10.1016/j.jstrokecerebrovasdis.2005.01.001. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Blizzard KK, Debchoudhury I, Hurn PD. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab. 2005;25:502–512. doi: 10.1038/sj.jcbfm.9600059. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280:20493–20502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- Mulligan JD, Gonzalez AA, Kumar R, Davis AJ, Saupe KW. Aging elevates basal adenosine monophosphate-activated protein kinase (AMPK) activity and eliminates hypoxic activation of AMPK in mouse liver. J Gerontol A Biol Sci Med Sci. 2005;60:21–27. doi: 10.1093/gerona/60.1.21. [DOI] [PubMed] [Google Scholar]
- Nakatsu Y, Kotake Y, Hino A, Ohta S. Activation of AMP-activated protein kinase by tributyltin induces neuronal cell death. Toxicol Appl Pharmacol. 2008;230:358–363. doi: 10.1016/j.taap.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306:2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- Osiewacz HD. Genes, mitochondria and aging in filamentous fungi. Ageing Res Rev. 2002;1:425–442. doi: 10.1016/S1568-1637(02)00010-7. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai VB, Sundaresan NR, Kim G, Gupta M, Rajamohan SB, Pillai JB, Samant S, Ravindra PV, Isbatan A, Gupta MP. Exogenous NAD blocks cardiac hypertrophic response via activation of the SIRT3-LKB1-AMP-activated kinase pathway. J Biol Chem. 2010;285:3133–3144. doi: 10.1074/jbc.M109.077271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang W, Weiqiang K, Qing Z, Pengju Z, Yi L. Aging impairs insulin-stimulated glucose uptake in rat skeletal muscle via suppressing AMPKalpha. Exp Mol Med. 2007;39:535–543. doi: 10.1038/emm.2007.59. [DOI] [PubMed] [Google Scholar]
- Raval AP, Lin HW, Dave KR, Defazio RA, Della Morte D, Kim EJ, Perez-Pinzon MA. Resveratrol and ischemic preconditioning in the brain. Curr Med Chem. 2008;15:1545–1551. doi: 10.2174/092986708784638861. [DOI] [PubMed] [Google Scholar]
- Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, Befroy D, Pypaert M, Hardie DG, Young LH, Shulman GI. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007;5:151–156. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnett GV, Kim EK, Landree LE, Tu Y. Fatty acid metabolism as a target for obesity treatment. Physiol Behav. 2005;85:25–35. doi: 10.1016/j.physbeh.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Ronnett GV, Ramamurthy S, Kleman AM, Landree LE, Aja S. AMPK in the brain: its roles in energy balance and neuroprotection. J Neurochem. 2009;109(Suppl 1):17–23. doi: 10.1111/j.1471-4159.2009.05916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- Shapira S, Sapir M, Wengier A, Grauer E, Kadar T. Aging has a complex effect on a rat model of ischemic stroke. Brain Res. 2002;925:148–158. doi: 10.1016/S0006-8993(01)03270-X. [DOI] [PubMed] [Google Scholar]
- Suwa M, Nakano H, Radak Z, Kumagai S. Endurance exercise increases the SIRT1 and peroxisome proliferator-activated receptor gamma coactivator-1alpha protein expressions in rat skeletal muscle. Metabolism. 2008;57:986–998. doi: 10.1016/j.metabol.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L. Model organisms as a guide to mammalian aging. Dev Cell. 2002;2:9–19. doi: 10.1016/S1534-5807(01)00098-3. [DOI] [PubMed] [Google Scholar]
- Tota S, Awasthi H, Kamat PK, Nath C, Hanif K. Protective effect of quercetin against intracerebral streptozotocin induced reduction in cerebral blood flow and impairment of memory in mice. Behav Brain Res. 2010;209:73–79. doi: 10.1016/j.bbr.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Turdi S, Fan X, Li J, Zhao J, Huff AF, Du M, Ren J. AMP-activated protein kinase deficiency exacerbates aging-induced myocardial contractile dysfunction. Aging Cell. 2010;9:592–606. doi: 10.1111/j.1474-9726.2010.00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingtdeux V, Davies P, Dickson DW, Marambaud P (2010) AMPK is abnormally activated in tangle- and pre-tangle-bearing neurons in Alzheimer’s disease and other tauopathies. Acta Neuropathol. doi:10.1007/s00401-010-0759-x [DOI] [PMC free article] [PubMed]
- Wang W, Yang X, Lopez de Silanes I, Carling D, Gorospe M. Increased AMP:ATP ratio and AMP-activated protein kinase activity during cellular senescence linked to reduced HuR function. J Biol Chem. 2003;278:27016–27023. doi: 10.1074/jbc.M300318200. [DOI] [PubMed] [Google Scholar]
- Weisova P, Davila D, Tuffy LP, Ward MW, Concannon CG, Prehn JH (2010) Role of AMP-activated protein kinase (AMPK) in cell survival and death responses in neurons. Antioxid Redox Signal. doi:10.1089/ars.2010.3544 [DOI] [PubMed]
- Wenk GL, Walker LC, Price DL, Cork LC. Loss of NMDA, but not GABA-A, binding in the brains of aged rats and monkeys. Neurobiol Aging. 1991;12:93–98. doi: 10.1016/0197-4580(91)90047-N. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]