Abstract
Aging is associated with alterations in the intestinal microbiota and with immunosenescence. Probiotics have the potential to modify a selected part of the intestinal microbiota as well as improve immune functions and may, therefore, be particularly beneficial to elderly consumers. In this randomized, controlled cross-over clinical trial, we assessed the effects of a probiotic cheese containing Lactobacillus rhamnosus HN001 and Lactobacillus acidophilus NCFM on the intestinal microbiota and fecal immune markers of 31 elderly volunteers and compared these effects with the administration of the same cheese without probiotics. The probiotic cheese was found to increase the number of L. rhamnosus and L. acidophilus NCFM in the feces, suggesting the survival of the strains during the gastrointestinal transit. Importantly, probiotic cheese administration was associated with a trend towards lower counts of Clostridium difficile in the elderly, as compared with the run-in period with the plain cheese. The effect was statistically significant in the subpopulation of the elderly who harbored C. difficile at the start of the study. The probiotic cheese was not found to significantly alter the levels of the major microbial groups, suggesting that the microbial changes conferred by the probiotic cheese were limited to specific bacterial groups. Despite that the administration of the probiotic cheese to the study population has earlier been shown to significantly improve the innate immunity of the elders, we did not observe measurable changes in the fecal immune IgA concentrations. No increase in fecal calprotectin and β-defensin concentrations suggests that the probiotic treatment did not affect intestinal inflammatory markers. In conclusion, the administration of probiotic cheese containing L. rhamnosus HN001 and L. acidophilus NCFM, was associated with specific changes in the intestinal microbiota, mainly affecting specific subpopulations of intestinal lactobacilli and C. difficile, but did not have significant effects on the major microbial groups or the fecal immune markers.
Keywords: Probiotics, Elderly, Gut microbiota, Cheese, Clostridium difficile
Introduction
Aging is associated with a number of health problems including immunosenescence, more frequent infections, altered gut function and suboptimal nutritional status. In addition, increasing evidence suggests that aging is associated with alterations in the gut microbiota (Bartosch et al. 2004; Biagi et al. 2010; Gavini et al. 2001; Hopkins et al. 2001; Hopkins and Macfarlane 2002; Mueller et al. 2006; Woodmansey et al. 2004). Moreover, elderly subjects living in hospitals and nursing homes are susceptible to increased pathogen challenges. For example, frequent use of antibiotics may increase the prevalence of Clostridium difficile among elderly subjects. In general, the microbiota changes associated with aging are not well defined. Commonly, alterations in intestinal bifidobacteria have been associated with aging (Biagi et al. 2010; Hopkins et al. 2001). A recent large study investigating the gut microbiota of 161 elderly (>65 years) Irish subjects suggested that the gut microbiota of elderly is by average dominated by the phylum Bacteroidetes, more so than in the younger controls, but the variation between the elderly gut microbiota composition is substantial; for example, the proportion of the Bacteroidetes ranged from 3% to 92% within the study population (Claesson et al. 2010). A recent study comparing young adults, 70-year-old seniors, and 100-year-old centenarians showed that gut microbiota at 70 years is still quite similar with healthy adults, with statistically significant differences only in certain bacterial groups such as Collinsella et rel., but significant changes occur as age increases (Biagi et al. 2010). Specifically, very old age was associated with enrichment of facultative anaerobes belonging to Proteobacteria and bacilli as well as an increased Clostridium leptum group and Eubacterium limosum, accompanied with decreased levels of Clostridium cluster XIVa, genus Bifidobacterium and the Faecalibacterium prausnitzii group. Overall, different studies assessing the gut microbiota of the elderly and comparing it to that of younger subjects have yielded varying results. The discrepancies between different studies may be partly explained by differences between the study populations such as genetic background, diet, living environment, and volunteer age and health condition. On the other hand, a major source of variation between the trials is also likely to originate from the differences between the methodologies applied. Alterations in the gut microbiota composition related to aging have provided rationale for therapies aiming at restoration or maintenance of gut microbiota composition associated with healthy adulthood. Probiotics (FAO/WHO 2002) have been explored for this purpose, and indeed, different probiotic treatments have been shown to modify the gut microbiota composition of elderly subjects in a beneficial way, for example, by improving the Bifidobacterium populations (Lahtinen et al. 2009; Ouwehand et al. 2008) or by reducing C. difficile (Plummer et al. 2004).
Immunosenescence, the deterioration of the immune system function with age, has been widely studied both in animals and humans, but the causes and the consequences of this complex phenomenon are still incompletely understood (Panda et al. 2009). Deterioration of immune functions is associated with increased susceptibility to infections and reduced responsiveness to vaccination. Imbalance in the inflammatory and anti-inflammatory functions in aging may result in low-grade chronic inflammation, termed inflammaging, characterized by elevated basal levels of pro-inflammatory immune mediators (Franceschi et al. 2007). Alterations in the mucosal immunity associated with aging have also been identified, including reduction in intestinal antigen-specific IgA antibody responses (Fujihashi and Kiyono 2009). Improvement of innate and mucosal immune functions in the elderly therefore has great potential for improving the health status in the elderly. The emerging health problems associated with aging and the potential for probiotics to improve immune and gut function make the elderly a particularly important target group for probiotic therapies. Several clinical studies have demonstrated the ability of selected probiotic bacteria. For example, probiotic strains Lactobacillus rhamnosus HN001 and Bifidobacterium animalis subsp. lactis HN019 have been shown to improve innate immune functions in elderly subjects (Gill et al. 2001; Gill and Rutherfurd 2001). Moreover, we have recently demonstrated that the innate immune functions such as phagocytic activity and the natural killer cell activity of elderly subjects can be improved in healthy elderly volunteers by the consumption of a probiotic cheese containing L. rhamnosus HN001 and Lactobacillus acidophilus NCFM (Ibrahim et al. 2010).
Here, we report on the effects of the consumption of this probiotic cheese containing L. rhamnosus HN001 and L. acidophilus NCFM on the fecal microbiota and fecal immune markers on healthy elderly volunteers (Ibrahim et al. 2010). Fecal microbiota composition was measured with flow cytometry combined with fluorescent in situ hybridization and with quantitative PCR in order to assess whether the beneficial effects on innate immunity are linked with changes in the fecal microbiota. Fecal immune markers such as IgA, calprotectin, and beta-defensin were measured as surrogate markers of the mucosal immune functions of the intestine.
Methods
Study set-up and sample collection
Fecal samples were obtained form 31 healthy elderly (72 to 103 years; median, 86 years) volunteers living in a nursing home in Turku, Finland, and participating in a clinical trial assessing the effects of probiotic cheese consumption on the innate immune functions. The design of this placebo-controlled cross-over trial and the characteristics of the study population have been described in detail elsewhere (Ibrahim et al. 2010). Briefly, the intervention consisted of three phases. During the run-in phase, the volunteers consumed a control cheese without probiotics for 2 weeks; during the intervention phase, the volunteers consumed a probiotic cheese for 4 weeks, and during the wash-out phase, the volunteers consumed the control cheese again for 4 weeks. The control and probiotic products had similar taste and appearance, and were blinded to the volunteers. The daily dose of cheese was 15 g (one slice). Both the control cheese and the probiotic cheese were manufactured by Mills DA (Oslo, Norway) using proprietary starter strains (Choozit 712™, Danisco, Paris); in addition, the probiotic cheese contained approximately 109 CFU of each of the probiotic strains L. rhamnosus HN001 (AGAL NM97/09514; Danisco) and L. acidophilus NCFM (ATCC 700396; Danisco). Fecal samples were collected from the volunteers in the end of each phase. Upon collection, samples were frozen and stored at −80°C until analysis.
Fluorescent in situ hybridization and flow cytometry
The enumeration of major bacterial groups was carried out by fluorescent in situ hybridization combined with flow cytometry as described earlier by Kalliomäki and co-workers (Kalliomäki et al. 2008). Briefly, fecal samples were homogenized in phosphate-buffered saline (PBS), and the cells were fixed with 4% paraformaldehyde at 4°C overnight, centrifuged at 22,000×g for 3 min followed by washing with PBS. Fixed samples were stored in PBS–ethanol (1:1) at −20°C until analysis. The bacteria were labeled with the following group- and genus-specific probes targeting the eubacterial 16S rRNA: Bacteroides–Prevotella group (Bac303, 5′-CCAATGTGGGGGACCTT), the Bifidobacterium genus (Bif164, 5′-CATCCGGCATTACCACCC), the Clostridium histolyticum group (CHis150, 5′-TTATGCGGTATTAATCT(C/T)CCTTT), the Lactobacillus–Enterococcus group (Lab158, 5′-GGTATTAGCA(T/C)GTGTTTCCA) (Kalliomäki et al. 2008) and Akkermansia muciniphila-like bacteria [MUC-1437 (5′-CCTTGCGGTTGGCTTCAGAT-3′] (Derrien et al. 2008). The probes were labeled at the 5′-end with indocyanine (Cy3; MOLBIOL, Berlin, Germany). The EUB 338 probe (Kalliomäki et al. 2008) labeled with fluorescein isothiocyanate (FITC) was used to identify bacterial cells from potential other particles present in the samples. Specific cell enumeration was performed by combining each of the group-specific Cy3 probes with the EUB 338-FICT probe. Fixed cell suspensions were incubated in the presence of each fluorescent probe (50 ng l−1) in hybridization buffer (10 mmol l−1 Tris–HCl, 0.9 mol l−1 NaCl, and 10% sodium dodecyl sulfate). The hybridized cells were collected after washing by centrifugation (22,000×g for 5 min) and resuspended in PBS. Flow cytometric analyses were performed as previously described (Kalliomäki et al. 2008) using a BD LSR II flow cytometer (Becton Dickinson and Co, Franklin Lakes, NJ) equipped with a 488-nm laser at 15 mW and BD FACSDIVA software, version 4.1.1 (Becton Dickinson and Co). Results were expressed as the numbers of cells hybridizing with the specific group-Cy3 probe and total bacteria EUB 338-FITC probe.
Quantitative PCR
DNA was extracted with the use of the QIAamp DNA stool Mini kit (Qiagen, Hilden, Germany) from the PBS homogenates of the samples (see above) following the manufacturer’s instructions. Quantitative polymerase chain reactions (qPCR) were performed in a total volume of 25 μl containing 1 ng of template DNA as follows: the SYBR Green methodology (Applied Biosystems, Foster City, CA, USA) was used for C. difficile (Cdif_F2: 5′-TTGAGCGATTTACTTCGGTAAAGA-3′, Cdif_R2: 5′-3′CCATCCTGTACTGGCTCACCT-3′, 150 nM of both), clostridial cluster XIV (300 nM), sulfate reducers (300 nM), F. prausnitzii (250 nM), and L. rhamnosus (250 nM), while the TaqMan methodology was used for L. acidophilus NCFM (300 nM of primers and 200 nM of the probe). The PCR primers targeting different groups and species of bacteria are listed in Table 1. Each reaction mixture consisted of 25 μl qPCR Master Mix containing 1 ng of template DNA. The amplification and detection of DNA were performed with the ABI-PRISM sequencing detection system (Applied Biosystems). To obtain standard curves, a tenfold dilution series ranging from 10 pg to 10 ng of DNA from the bacterial standard cultures (Table 1) were included in the PCR assays. For determination of DNA, triplicate samples were used, and the mean quantity per gram wet weight was calculated.
Table 1.
The primers, the probes, the annealing temperatures, and the standard bacterial strains used in the quantitative PCR analysis of the fecal samples
| Target species | Standard strain | Primer | Annealing temp (°C) | Reference |
|---|---|---|---|---|
| C. difficile | C. difficile DSM 1296 | Cdif_F2 | 60 | This study |
| Cdif_R2 | ||||
| Clostridial cluster XIVab | Clostridium bolteae DSM 15670 | CXIV F1 | 52 | (Song et al. 2004) |
| CXIV F2 | ||||
| F. prausnitzii | F. prausnitzii ATCC 27768 | Fpraus_F | 62 | (Rinttilä et al. 2004) |
| Fpraus_R | ||||
| L. rhamnosus | L. rhamnosus HN001 | Lr1 | 55 | (Furet et al. 2004) |
| Lc1 | ||||
| L. acidophilus NCFM | L. acidophilus NCFM | NCFM_F | 60 | (Ouwehand et al. 2009) |
| NCFM_R | ||||
| NCFM_P | ||||
| Sulfate reducers | Desulfovibrio intestinalis DSM 11275 | apsA1F | 62 | (Tiihonen et al. 2008) |
| apsA1R |
Fecal immune markers
Fecal immune markers IgA, calprotectin, and β-defensin were measured from supernatants of the fecal homogenates (see above). Briefly, homogenates were centrifuged (16,000×g, 5 min), and the supernatants were collected. For IgA measurement, the samples were extracted with bovine serum albumin as described previously (Peuranen et al. 2004). Concentrations of IgA, calprotectin, and β-defensin were determined by ELISA with human IgA ELISA Quantitation Kit E80-102 (Bethyl Laboratories Inc., Montgomery, TX, USA), Phical ELISA test (Eurospital S.p.A., Trieste, Italy), and the β-defensin 2 ELISA kit K 6500 (Immundiagnostik AG, Bensheim, Germany), respectively, according to the manufacturer’s instructions, with the following minor modification: in the β-defensin analysis, only 50% of the recommended substrate concentration was used. The results were expressed as micrograms per gram of feces (fresh weight) for IgA and calprotectin, and nanograms per gram for β-defensin.
Statistical analyses
Microbial counts were log-transformed prior to statistical analysis. Paired t test was used to compare the microbial levels between different time points. In the case of C. difficile, an additional post hoc t test was included to investigate the changes in C. difficile levels in those subjects who harbored these bacteria in the first place. Changes in the immune parameters were assessed by two-sided one-sample t test by comparing the changes between the time points to the test value of 0.
Results
Fecal microbiota by quantitative PCR
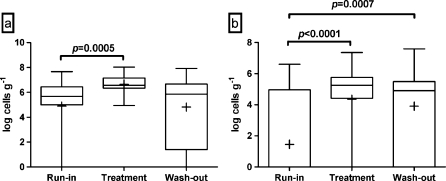
L. rhamnosus species was present in all but five volunteers during the run-in phase, but, in general, the levels of this species were low, and the average for the whole study population was log 4.89 cells g−1 of feces. Following the intervention with probiotic cheese, L. rhamnosus was detected in all samples tested, and the average level of this species was log 6.66 cells g−1 (Fig. 1a). This was significantly (p = 0.0005) higher than during the run-in. Following the wash-out phase, the levels of L. rhamnosus had decreased to log 4.82 cells g−1, which was not significantly different from the run-in.
Fig. 1.
Fecal levels of L. rhamnosus (a) and L. acidophilus NCFM (b) of the elderly volunteers after the run-in, the treatment, and the wash-out periods, as measured by quantitative PCR. The top and the bottom of the box represent upper and lower quartiles, respectively, and the band within the box is the median. The whiskers indicate the minimum and the maximum, and the average of the data is marked with “plus sign”
L. acidophilus NCFM (or other NCFM-like organisms) was present at detectable levels only in seven volunteers during the run-in phase, with the average level of log 1.46 cells g−1 for the whole group of volunteers. Following the intervention, L. acidophilus NCFM was detected in the fecal samples of all but six volunteers. The average level of NCFM was log 4.35 cells g−1, which was significantly higher than before the intervention (p < 0.0001; Fig. 1b). After the 4-week wash-out period, L. acidophilus NCFM was still detectable in all but eight volunteers at the average level of log 3.91 cells g−1, which was not statistically different from the intervention phase, but was significantly (p = 0.0007) higher when compared with the run-in phase.
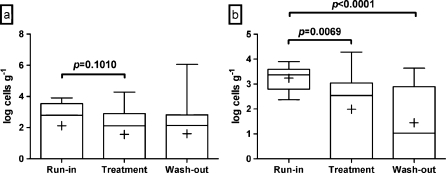
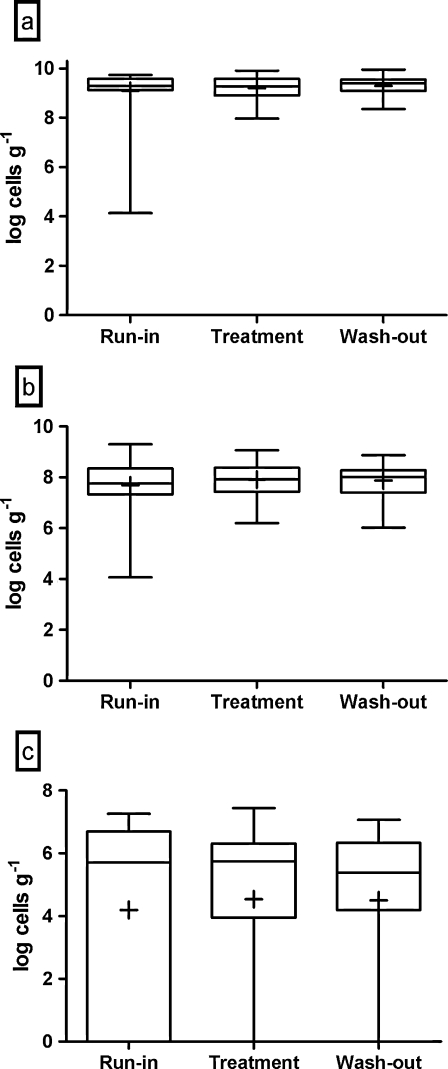
C. difficile was detected in 65% of the run-in samples, but the levels were low; the average level for the whole population was log 2.11 cells g1 per gram of feces, and none of the volunteers had C. difficile levels above log 4 cells g−1. Probiotic intervention tended to reduce the average level of C. difficile; the average level of C. difficile was log 1.51 cells g−1 following the intervention (p = 0.1010; Fig. 2a). Levels of C. difficile after the wash-out phase were not statistically different from the run-in phase. Post hoc subgroup analysis of those volunteers who tested positive for C. difficile at run-in suggested a significant reduction in the average level of C. difficile from log 3.23 cells g−1 at the run-in to log 1.99 cells g−1 following the intervention (p = 0.0069; Fig. 2b). Following the wash-out period the levels of C. difficile remained lower (log 1.45 cells g−1) than during the run-in period (p < 0.0001) in these individuals. After the run-in phase, the levels of the clostridial cluster XIV, F. prausnitzii and sulfate reducers were log 9.10, log 7.68, and log 4.20 cells g−1, respectively, as analyzed by quantitative PCR (Fig. 3). Clostridial cluster XIV and F. prausnitzii were detected from all of the volunteers and sulfate reducers from 69% of the volunteers at the run-in.
Fig. 2.
Fecal levels of C. difficile in all volunteers (a) and in volunteers who were positive for this species at the beginning of the study (b) after the run-in, the treatment, and the wash-out periods, as measured by quantitative PCR. The top and the bottom of the box represent upper and lower quartiles, respectively, and the band within the box is the median. The whiskers indicate the minimum and the maximum, and the average of the data is marked with “plus sign”
Fig. 3.
Fecal levels of clostridial cluster XIV (a), F. prausnitzii (b) and sulfate reducers (c) in the elderly volunteers after the run-in, the treatment, and the wash-out periods, as measured by quantitative PCR. The top and the bottom of the box represent upper and lower quartiles, respectively, and the band within the box is the median. The whiskers indicate the minimum and the maximum, and the average of the data is marked with “plus sign”
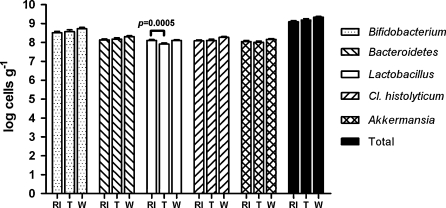
Analysis of microbial groups by fluorescent in situ hybridization and flow cytometry (FISH-FCM) did not reveal major differences in the bacterial populations between the time points. The average levels of the genus Bifidobacterium, the Bacteroides–Prevotella group, the C. histolyticum group, and Akkermansia were log 8.51, log 8.13, log 8.08, and log 8.05 cells g−1 per gram of feces at the end of the run-in, respectively (Fig. 4). The levels of these groups were not significantly changed during the intervention and follow-up phases. A small (from log 8.10 to log 7.91 cells g−1) but statistically significant (p = 0.01) decrease in Lactobacillus–Enterococcus group between run-in and intervention phases was noted. Total microbe numbers remained stable during the trial.
Fig. 4.
Levels (mean with SEM) of the Bifidobacterium genus, the Bacteroides–Prevotella group, the Lactobacillus–Enterococcus group, the C. histolyticum group, A. muciniphila-like bacteria and the total bacteria analyzed with fluorescent in situ hybridization and flow cytometry after the run-in (RI), the treatment (T), and the wash-out (W) phases. The levels of these groups were not significantly changed during the intervention and follow-up phases apart from small decrease in Lactobacillus–Enterococcus group between the run-in and the intervention phases
Fecal immune markers
Overall, the probiotic treatment was associated with a trend towards reduced average fecal IgA (119 μg g−1 feces) as compared with the run-in period (172 μg g−1 feces; p = 0.058). However, this downward trend was attributed almost exclusively to five volunteers who, at run-in, had high initial levels of fecal IgA (between 798 and 2,655 μg g−1 feces). In post hoc analysis of these subjects, the average fecal IgA was reduced significantly during the probiotic treatment (from 1,641 to 430 μg g−1 feces; p < 0.001) and remained significantly lower than at run-in during the wash-out (352 μg g−1 feces; p < 0.001). In all of the remaining subjects, the IgA levels at run-in were below 500 μg g−1 feces with the average of 142 μg g−1 and did not change significantly during the intervention (average 163 μg g−1 feces; p > 0.05) or wash-out (213 μg g−1 feces; p > 0.05). The average values of calprotectin after run-in, after treatment and after wash-out were 19.2, 19.2, and 16.2 μg g−1 feces, respectively. No differences between the time points were observed. The levels of β-defensin after run-in, after treatment and after wash-out were 31.2, 24.0, and 28.5 μg g−1 feces, respectively, and did not differ between the time points.
Discussion
Aging is associated with alterations in gastrointestinal microbiota as well as immunosenescence, both of which may contribute to various health-related problems such as suboptimal digestive function and increased incidence of infections.
Immunosenescence has been widely studied although the phenomenon is not yet completely understood (Panda et al. 2009). In contrast, age-related changes in intestinal microbiota and their potential role on the health status are only starting to be revealed. Probiotic bacteria have great potential in improving the health of the elderly, as probiotics have the capacity to improve immune functions (Gill and Rutherfurd 2001) and modulate specific components of the intestinal microbiota (Lahtinen et al. 2009) particularly within this age group. Recently, we showed that consumption of a cheese containing probiotic strains L. rhamnosus HN001 and L. acidophilus NCFM by elderly volunteers resulted in significantly enhanced innate immune function, manifested by improved cytotoxicity of natural killer cells and an increase in phagocytic activity (Ibrahim et al. 2010). In this study, we assessed whether the probiotic cheese consumption and the observed systemic immune benefits were associated with changes in the intestinal microbiota and selected fecal immune markers.
The main outcome of this study was the observation that probiotic cheese consumption resulted in modifications of specific components of the intestinal microbiota, but did not significantly affect the major bacterial groups, suggesting that the changes in the microbiota induced by the probiotics are specific to certain groups of intestinal bacteria. Our results demonstrate that probiotics administered in cheese survive the gastrointestinal transit. This result supports our earlier study, in which, using the same probiotic cheese, we demonstrated the survival of these probiotics during simulated upper gastrointestinal digestion followed by simulated colon fermentation in a semi-continuous human colon simulator (Mäkeläinen et al. 2009). The fecal levels of L. rhamnosus increased by approximately two log units during the intervention, but were reduced back to the run-in values following the wash-out period, suggesting that L. rhamnosus HN001 became a member of the gut microbiota of the elderly during the cheese consumption, but the effect was transient and lasted less than 4 weeks. The result is in line with many earlier reports demonstrating the transient nature of the colonization of ingested probiotics, including L. rhamnosus HN001 (Tannock et al. 2000). The average level of fecal L. acidophilus NCFM-like organisms in the study population was initially low, as only seven subjects at the run-in were identified as positive for this popular commercial strain. But, during the intervention, the average level of NCFM increased by roughly three log units, suggesting that NCFM survived during the gastrointestinal transit. Unlike L. rhamnosus, the levels of NCFM remained well above the run-in levels even after the 4-week wash-out period. Earlier studies have shown the survival of NCFM in the human gastrointestinal tract (Gilliland et al. 2010; Sui et al. 2002; Varcoe et al. 2002), but previously the colonization of NCFM has not been reported to last beyond 2 weeks after cessation of probiotic consumption (Sui et al. 2002). The observed increases in L. rhamnosus and L. acidophilus NCFM were limited to these specific lactobacilli and were not linked with the total levels of lactobacilli. In fact, the total levels of the group Lactobacillus–Enterococcus decreased during the intervention, although the magnitude of this change was only 0.19 log units (cells g−1) and therefore the biological significance of this observation remains questionable, and it is not clear whether this decrease was related to Lactobacillus or Enterococcus. In comparison, the levels of L. rhamnosus increased by nearly two log units following the probiotic intervention.
An interesting observation in this study was the trend for reduced fecal levels of C. difficile during the probiotic intervention. C. difficile is a major intestinal pathogen and particularly common among the elderly (Simor et al. 2002). C. difficile was detected at low levels in 65% of the volunteers at the run-in. At run-in, all of the volunteers were free of acute gastrointestinal infections, suggesting that C. difficile was present in these volunteers at sub-clinical levels at the time of the study; the average level of C. difficile was log 2.11 cells g1 per gram of feces for the whole study population and log 3.23 cells g1 per gram for those subjects who harbored C. difficile to begin with. Despite the low initial levels of C. difficile, the probiotic cheese consumption showed a trend for reduction of the fecal levels of this pathogen in the whole population and, more importantly, a significant reduction in those subjects who were carrying detectable levels of C. difficile at run-in. While the subjects in this study were not found to be affected by C. difficile-associated diseases, the reduction of the fecal levels of this pathogen even when present at sub-clinical levels does have potentially important clinical benefits. C. difficile is the most common infectious agent in antibiotic associated diarrhea in adults and in elderly. Elderly subjects, particularly those living in nursing homes, are susceptible to recurrent infections and are commonly prescribed antibiotics. Antibiotic treatments offer an opportunity for C. difficile to increase in numbers at the expense of normal intestinal microbiota, which is broadly affected by antibiotics. Considering the fact that the study population (elderly) is particularly prone to antibiotic use, the reduction of C. difficile levels by probiotic cheese consumption even at sub-clinical levels may offer potential benefits. It is possible that such reduction, together with enhanced immune function (Ibrahim et al. 2010), may offer enhanced protection against infections caused by pathogens such as C. difficile.
Apart from the changes in the subpopulations of lactobacilli and the levels of C. difficile, the probiotic treatment did not cause major changes in the main bacterial groups assessed in this study. This result is in line with the fact that the quantity of the administered probiotic bacteria represents only a tiny fraction of the total microbial cell numbers in the intestine, and therefore, it is perhaps unlikely that such administration will change the balance of the major bacterial groups. However, since the administered probiotic strains adhere strongly to human mucus and epithelial cells (Buck et al. 2005; Gopal et al. 2001), administration of these strains may result in changes in the mucosa-adherent microbiota of the intestine. The mucosa-adherent microbiota is different from the luminal and fecal microbiota (Eckburg et al. 2005), and while mucosal microbiota represents only a minor fraction of the total intestinal microbiota, this fraction is likely to have a critically important role in host–microbe interactions including the interactions with the host immune cells, since the microbes adherent to mucus have close proximity to and longer duration of exposure with the host cells than the microbes in the intestinal lumen. Assessment of the potential changes in the mucosal microbiota would have required the analysis of intestinal biopsies instead of fecal samples, which in this study was unfortunately not possible.
Despite the clear effects of the probiotic cheese administration on markers of innate immunity in the study population (Ibrahim et al. 2010), the probiotic cheese was not found to significantly alter the levels of the measured fecal immune markers: total IgA, calprotectin, and β-defensin. The result suggests that the beneficial effects on the immunity in the elderly subjects caused by the probiotic administration may be mainly systemic as none of the measured intestinal immune mediators, which are more related to local mucosal immune responses than systemic responses, were altered by the probiotic administration. However, another possible explanation of the lack of effect on the intestinal immune markers may be the sample type; the probiotic treatment may have induced changes locally at the mucosal level, e.g., in the small intestine, but these changes may not be reflected in fecal samples which at best exhibit the changes in colon. Furthermore, the lack of effect of the probiotic treatment on the total levels of fecal IgA does not exclude the possibility for probiotic effects on the secretion of antigen-specific IgA. Indeed, the current evidence suggests that aging does not markedly affect the levels of total IgA synthesis in mucosa or serum; instead, the quality of secreted IgA in intestinal mucosa may be altered, as a result of alterations in the gut microbiota associated with aging (Fujihashi and Kiyono 2009). Elevated fecal calprotectin and β-defensin concentrations have been associated with local inflammatory diseases (Gaya and Mackenzie 2002; Kapel et al. 2009). Also, aging has been associated with chronic low increase in fecal calprotectin concentrations (Amati et al. 2006). No measurable changes in calprotectin levels were detected in this study indicating that the probiotic treatment did not trigger inflammation in the intestine. While an increase in the systemic immune functions such as increased activity of circulating natural killer cells in elderly subjects suffering from immunosenescence is a desirable effect of probiotics, increase in the intestinal immune parameters such as calprotectin levels may not be desirable since such effect could indicate increased intestinal inflammation.
In conclusion, administration of probiotic cheese to elderly volunteers resulted in changes in specific populations of fecal microbiota, but did not alter the major bacterial groups in the gut. Specifically, the cheese administration significantly increased the fecal levels of L. rhamnosus and L. acidophilus NCFM, indicating the survival of the probiotic bacteria during the gastrointestinal transit and good compliance. Moreover, a trend for reduction in the fecal levels of C. difficile during the cheese administration, with a significant reduction in the subjects harboring this pathogenic species at the start of the study, was recorded. Due to its role in antibiotic associated diarrhea, a reduction of the basal levels of C. difficile may have clinically relevant benefits on the health of the elderly. Lastly, we did not observe changes in intestinal immune markers during the study, suggesting that the beneficial effects of the probiotic cheese administration are mainly on the systemic innate immunity (Ibrahim et al. 2010) and, furthermore, that there are no adverse inflammatory effects in the intestine.
Acknowledgments
We are grateful to Brita Mäki, Jaana Larsson-Leskelä, Marika Björklund (Danisco Health and Nutrition), and Hilkka Terho (Functional Foods Forum) for their skillful technical assistance in the analysis of the fecal samples. Suvi Rovio (Turku City Hospital) and Fandi Ibrahim (Functional Foods Forum) are thanked for collection and logistics of the fecal samples.
References
- Amati L, Passeri ME, Selicato F, Mastronardi ML, Penna A, Jirillo E, Covelli V. New insights into the biological and clinical significance of fecal calprotectin in inflammatory bowel disease. Immunopharmacol Immunotoxicol. 2006;28:665–681. doi: 10.1080/08923970601067326. [DOI] [PubMed] [Google Scholar]
- Bartosch S, Fite A, Macfarlane GT, McMurdo ME. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl Environ Microbiol. 2004;70:3575–3581. doi: 10.1128/AEM.70.6.3575-3581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, Nikkilä J, Monti D, Satokari R, Franceschi C, Brigidi P, Vos W. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE. 2010;5:e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck BL, Altermann E, Svingerud T, Klaenhammer TR. Functional analysis of putative adhesion factors in Lactobacillus acidophilus NCFM. Appl Environ Microbiol. 2005;71:8344–8351. doi: 10.1128/AEM.71.12.8344-8351.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G, Stanton C, Sinderen D, O’Connor M, Harnedy N, O’Connor K, Henry C, O’Mahony D, Fitzgerald AP, Shanahan F, Twomey C, Hill C, Ross RP, O’Toole PW. Microbes and Health Sackler Colloquium: composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA. 2010 doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien M, Collado MC, Ben Amor K, Salminen S, Vos WM. The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl Environ Microbiol. 2008;74:1646–1648. doi: 10.1128/AEM.01226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/WHO (2002) Guidelines for the evaluation of probiotics in food. Joint FAO/WHO working group report on drafting for the evaluation of probiotics in food. London, Ontario, Canada
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Fujihashi K, Kiyono H. Mucosal immunosenescence: new developments and vaccines to control infectious diseases. Trends Immunol. 2009;30:334–343. doi: 10.1016/j.it.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Furet JP, Quenee P, Tailliez P. Molecular quantification of lactic acid bacteria in fermented milk products using real-time quantitative PCR. Int J Food Microbiol. 2004;97:197–207. doi: 10.1016/j.ijfoodmicro.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Gavini F, Cayuela C, Antoine JM, Lecoq C, Lefebvre B, Membre JM, Neut C. Differences in the distribution of bifidobacterial and enterococcal species in human faecal microflora of three different (children, adults, elderly) age groups. Microb Ecol Health Dis. 2001;13:40–45. doi: 10.1080/089106001750071690. [DOI] [Google Scholar]
- Gaya DR, Mackenzie JF. Faecal calprotectin: a bright future for assessing disease activity in Crohn’s disease. QJM Int J Med. 2002;95:557–558. doi: 10.1093/qjmed/95.9.557. [DOI] [PubMed] [Google Scholar]
- Gill HS, Rutherfurd KJ. Probiotic supplementation to enhance natural immunity in the elderly: effects of a newly characterized immunostimulatory strain Lactobacillus rhamnosus HN001 (DR20™) on leucocyte phagocytosis. Nutr Res. 2001;21:183–189. doi: 10.1016/S0271-5317(00)00294-3. [DOI] [Google Scholar]
- Gill HS, Rutherfurd KJ, Cross ML, Gopal PK. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am J Clin Nutr. 2001;74:833–839. doi: 10.1093/ajcn/74.6.833. [DOI] [PubMed] [Google Scholar]
- Gilliland SE, Speck ML, Nauyok GF, Jr, Giesbrecth FG. Influence of consuming nonfermented milk containing Lactobacillus acidophilus on fecal flora of healthy males. J Dairy Sci. 2010;61:1–10. doi: 10.3168/jds.S0022-0302(78)83543-7. [DOI] [Google Scholar]
- Gopal PK, Prasad J, Smart J, Gill HS. In vitro adherence properties of Lactobacillus rhamnosus DR20 and Bifidobacterium lactis DR10 strains and their antagonistic activity against an enterotoxigenic Escherichia coli. Int J Food Microbiol. 2001;67:207–216. doi: 10.1016/S0168-1605(01)00440-8. [DOI] [PubMed] [Google Scholar]
- Hopkins MJ, Macfarlane GT. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J Med Microbiol. 2002;51:448–454. doi: 10.1099/0022-1317-51-5-448. [DOI] [PubMed] [Google Scholar]
- Hopkins MJ, Sharp R, Macfarlane GT. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16 S rRNA abundance, and community cellular fatty acid profiles. Gut. 2001;48:198–205. doi: 10.1136/gut.48.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim F, Ruvio S, Granlund L, Salminen S, Viitanen M, Ouwehand AC. Probiotics and immunosenescence: cheese as a carrier. FEMS Immunol Med Microbiol. 2010;59:53–59. doi: 10.1111/j.1574-695X.2010.00658.x. [DOI] [PubMed] [Google Scholar]
- Kalliomäki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–538. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- Kapel N, Benahmed N, Morali A, Svahn J, Canioni D, Goulet O, Ruemmele FM. Fecal beta-defensin-2 in children with inflammatory bowel diseases. J Pediatr Gastroenterol Nutr. 2009;48:117–120. doi: 10.1097/MPG.0b013e318174e872. [DOI] [PubMed] [Google Scholar]
- Lahtinen SJ, Tammela L, Korpela J, Parhiala R, Ahokoski H, Mykkanen H, Salminen SJ. Probiotics modulate the Bifidobacterium microbiota of elderly nursing home residents. Age (Dordr) 2009;31:59–66. doi: 10.1007/s11357-008-9081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkeläinen H, Forssten S, Olli K, Granlund L, Rautonen N, Ouwehand AC. Probiotic lactobacilli in a semi-soft cheese survive in the simulated human gastrointestinal tract. Int Dairy J. 2009;19:675–683. doi: 10.1016/j.idairyj.2009.06.005. [DOI] [Google Scholar]
- Mueller S, Saunier K, Hanisch C, Norin E, Alm L, Midtvedt T, Cresci A, Silvi S, Orpianesi C, Verdenelli MC, Clavel T, Koebnick C, Zunft HJ, Dore J, Blaut M. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl Environ Microbiol. 2006;72:1027–1033. doi: 10.1128/AEM.72.2.1027-1033.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouwehand AC, Bergsma N, Parhiala R, Lahtinen S, Gueimonde M, Finne-Soveri H, Strandberg T, Pitkälä K, Salminen S. Bifidobacterium microbiota and parameters of immune function in elderly subjects. FEMS Immunol Med Microbiol. 2008;53:18–25. doi: 10.1111/j.1574-695X.2008.00392.x. [DOI] [PubMed] [Google Scholar]
- Ouwehand AC, Tiihonen K, Saarinen M, Putaala H, Rautonen N. Influence of a combination of Lactobacillus acidophilus NCFM and lactitol on healthy elderly: intestinal and immune parameters. Br J Nutr. 2009;101:375. doi: 10.1017/S0007114508003097. [DOI] [PubMed] [Google Scholar]
- Panda A, Arjona A, Sapey E, Bai F, Fikrig E, Montgomery RR, Lord JM, Shaw AC. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol. 2009;30:325–333. doi: 10.1016/j.it.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuranen S, Tiihonen K, Apajalahti J, Kettunen A, Saarinen M, Rautonen N. Combination of polydextrose and lactitol affects microbial ecosystem and immune responses in rat gastrointestinal tract. Br J Nutr. 2004;91:905–914. doi: 10.1079/BJN20041114. [DOI] [PubMed] [Google Scholar]
- Plummer S, Weaver MA, Harris JC, Dee P, Hunter J. Clostridium difficile pilot study: effects of probiotic supplementation on the incidence of C. difficile diarrhoea. Int Microbiol. 2004;7:59–62. [PubMed] [Google Scholar]
- Rinttilä T, Kassinen A, Malinen E, Krogius L, Palva A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J Appl Microbiol. 2004;97:1166–1177. doi: 10.1111/j.1365-2672.2004.02409.x. [DOI] [PubMed] [Google Scholar]
- Simor AE, Bradley SF, Strausbaugh LJ, Crossley K, Nicolle LE. Clostridium difficile in long-term-care facilities for the elderly. Infect Control Hosp Epidemiol. 2002;23:696–703. doi: 10.1086/501997. [DOI] [PubMed] [Google Scholar]
- Song Y, Liu C, Finegold SM. Real-time PCR quantitation of clostridia in feces of autistic children. Appl Environ Microbiol. 2004;70:6459–6465. doi: 10.1128/AEM.70.11.6459-6465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J, Leighton S, Busta F, Brady L. 16 S ribosomal DNA analysis of the faecal lactobacilli composition of human subjects consuming a probiotic strain Lactobacillus acidophilus NCFM. J Appl Microbiol. 2002;93:907–912. doi: 10.1046/j.1365-2672.2002.01767.x. [DOI] [PubMed] [Google Scholar]
- Tannock GW, Munro K, Harmsen HJ, Welling GW, Smart J, Gopal PK. Analysis of the fecal microflora of human subjects consuming a probiotic product containing Lactobacillus rhamnosus DR20. Appl Environ Microbiol. 2000;66:2578–2588. doi: 10.1128/AEM.66.6.2578-2588.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen K, Tynkkynen S, Ouwehand A, Ahlroos T, Rautonen N. The effect of ageing with and without non-steroidal anti-inflammatory drugs on gastrointestinal microbiology and immunology. Br J Nutr. 2008;100:130–137. doi: 10.1017/S000711450888871X. [DOI] [PubMed] [Google Scholar]
- Varcoe J, Zook C, Sui J, Leighton S, Busta F, Brady L. Variable response to exogenous Lactobacillus acidophilus NCFM consumed in different delivery vehicles. J Appl Microbiol. 2002;93:900–906. doi: 10.1046/j.1365-2672.2002.01764.x. [DOI] [PubMed] [Google Scholar]
- Woodmansey EJ, McMurdo ME, Macfarlane GT, Macfarlane S. Comparison of compositions and metabolic activities of fecal microbiotas in young adults and in antibiotic-treated and non-antibiotic-treated elderly subjects. Appl Environ Microbiol. 2004;70:6113–6122. doi: 10.1128/AEM.70.10.6113-6122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]