Abstract
Rationale and Objectives
Our knowledge about genes involved in the control of basal motor activity that may contribute to the pathology of the hyperactivity disorders, e.g. attention deficit hyperactivity disorder (ADHD), is limited. Disruption of monoamine neurotransmitter signaling through G protein-coupled receptors (GPCR) is considered to be a major contributing factor to the etiology of the ADHD. Genetic association evidence and functional data, suggest that regulators of G protein signaling proteins of the R7 family (R7 RGS) that form obligatory complexes with type 5 G protein beta subunit (Gβ5) and negatively regulate signaling downstream from monoamine GPCRs, may play a role in controlling hyperactivity.
Methods
To test this hypothesis, we conducted behavioral, pharmacological and neurochemical studies using a genetic mouse model that lacked Gβ5, a subunit essential for the expression of the entire R7 RGS family.
Results
Elimination of Gβ5-RGS complexes led to a striking level of hyperactivity that far exceeds activity levels previously observed in animal models. This hyperactivity was accompanied by motor learning deficits and, paradoxical behavioral sensitization to a novel environment. Neurochemical studies indicated that Gβ5-RGS deficient mice had higher sensitivity of inhibitory GPCR signaling and deficits in basal levels, release and reuptake of dopamine. Surprisingly, pharmacological treatment with monoamine reuptake inhibitors failed to alter hyperactivity. In contrast, blockade of NMDA receptors reversed the expression of hyperactivity in Gβ5-RGS deficient mice.
Conclusions
These findings establish that Gβ5-RGS complexes are critical regulators of monoamine-NMDA receptor signaling cross-talk and link these complexes to disorders that manifest as hyperactivity, impaired learning and motor dysfunctions.
Keywords: Attention deficit hyperactivity disorder (ADHD), basal ganglia, motor control, hyperactivity, synaptic transmission, G protein-coupled receptors, Regulators of G protein signaling (RGS)
Introduction
Attention deficit hyperactivity disorder (ADHD) is a neuropsychatric condition that manifests in restlessness, impulsivity, inattention, and is estimated to affect up to 10% of the world population (Faraone et al. 2003). Although the etiology of ADHD is complex, it is currently well accepted that imbalance in monoamine neurotransmitter signaling is the major contributing factor. Extensive studies investigating ADHD have implicated catecholamine (dopamine, norepinephrine) and serotonin dysfunction in two key brain areas that cooperatively regulate attention and motor functions: the prefrontal cortex (PFC) and striatum (Arnsten 2009; Oades 2008; Prince 2008; Tripp and Wickens 2009).
At the molecular level, all effects of catecholamines, and, with one exception, serotonin are mediated by G protein-coupled receptors (GPCR) (Kristiansen 2004). Activation of GPCR by neurotransmitters promotes GTP binding to the α subunits of downstream G proteins which results in their dissociation into α-GTP and βγ subunits. When dissociated, G protein subunits modulate the activity of effector proteins which produce cellular responses (Gainetdinov et al. 2004; Offermanns 2003). Polymorphisms in GPCRs for dopamine and/or serotonin, as well as the corresponding transporters that affect the volume of transmission, have been genetically associated with ADHD (Faraone et al. 2005; Kirley et al. 2002). Furthermore, inhibitors of dopamine, serotonin and norepinephrine reuptake, which enhance the signaling by these neurotransmitters, ameliorate ADHD symptoms and are among the most frequently prescribed medications for this condition (Heal et al. 2009; Pliszka 2007). Taken together, these observations suggest that changes in the extent and duration of GPCRs signaling in response to monoaminergic stimulation may contribute to the development of ADHD symptoms. Several genetic studies aimed at identifying molecular factors disrupted in ADHD have resulted in the generation of the daunting number of candidates that are potentially linked to the disease (Faraone et al. 2005). While it is generally recognized that understanding ADHD disease mechanisms will ultimately require functional validation of the possible contributions of these molecular players to the etiology of ADHD, relatively little progress has been made in this direction.
Members of the regulator of G protein signaling (RGS) protein family are important intracellular factors that shape GPCR signal transduction. RGS proteins serve as negative regulators of GPCR signaling by stimulating GTP hydrolysis on the Gα subunits to promote their inactivation (Hollinger and Hepler 2002). From the perspective of the monoamine GPCR signaling in the central nervous system, members of the R7 family (R7 RGS): RGS6, RGS7, RGS9 and RGS11, play particularly important roles owning to their enrichment in the striatum and demonstrated roles in the regulation of motor and reward behaviors mediated by dopamine and opioid receptors (Anderson et al. 2009). In the brain, all R7 RGS proteins exist as complexes with two subunits: type 5 G protein β subunit (Gβ5) and R7 binding protein (R7BP) (Anderson et al. 2009). While R7BP regulates the subcellular localization and, in some cases, expression of R7 RGS, Gβ5 is essential for the structural integrity and proteolytic stability of all R7 RGS complexes (Anderson et al. 2009). Indeed, recent crystal structure of the RGS9/Gβ5 indicates that Gβ5 is tightly integrated in the core of the complex (Cheever et al. 2008) and its knockout in mice results in the elimination of R7 RGS expression (Chen et al. 2003). These findings suggest that Gβ5 is a central subunit that is essential for the function of the entire R7 RGS subfamily. Gβ5-R7 RGS proteins regulate Gi/o class of G proteins and their elimination is expected compromise the function of GPCRs that signal via these G proteins.
We recently conducted genetic screening in C. elegans and identified several Gβ5 mutations that are associated with the hyper-locomotor phenotype suggesting that this subunit has a conserved role in the regulation of motor behaviors (Porter et al. 2010). In humans, Gβ5 is located in q21 locus on chromosome 15, and a genetic linkage study has implicated this region (marker D15S659) in ADHD with the LOD score of 1.81 (p=0.002) (Faraone et al. 2008); see also psychiatric genetics evidence database at https://slep.unc.edu/evidence/index.php). Gβ5 was also recently identified as one of the highest scoring genes linked to cognitive ability in a functional group analysis of genetic data derived from a study on ADHD patients (Ruano et al. 2010). Furthermore, our previous studies indicate that Gβ5 complexed with RGS proteins play crucial role in regulating the timing of slow synaptic transmission mediated by GPCRs (Xie et al. 2010). Taken together, this evidence suggests a potential role of Gβ5 in neuropsychiatric disorders including ADHD. To test this hypothesis, we analyzed the behavioral consequences of targeted disruption of the Gβ5 gene in mice (Chen et al. 2003). Our results show that Gβ5 knockout mice develop striking hyperactivity which far exceeds levels of hyperactivity previously observed in animal models (Viggiano 2008). This hyperactivity is accompanied by motor learning deficits and a paradoxical adaptation to novel environment. Concurrent with an increase in the sensitivity of pre- and postsynaptic Gi/o-coupled GPCRs, we found substantial deficits in basal levels, release and reuptake of extracellular dopamine in the striatum. Surprisingly, pharmacological treatment with monoamine reuptake inhibitors aimed at restoring extracellular monoamine levels were ineffective in reducing hyperactivity in Gβ5−/−mice. However, NMDA receptor blockade, completely reversed hyperactivity. These findings establish Gβ5-RGS complexes as major regulators of a number of neuronal GPCRs and link these complexes to disorders that manifest as hyperactivity, impaired learning and motor dysfunctions.
Materials and Methods
Behavioral Studies
The Gβ5−/−mice were generated previously and were back-crossed with C57/Bl6 mice for six generations. Littermates (2–4 months old) of both sexes were used for behavioral experiments and were derived from heterozygous breeding pairs. All procedures were approved by the Animal Care and Use Committee at the University of Minnesota. Quinpirole (0.03–1.5 mg/kg), LY379268 (0.1–3 mg/kg), amphetamine (1 mg/kg), desipramine (10 mg/kg), atomoxetine (10 mg/kg), citalopram (10 mg/kg), MK-801 (0.2–1 mg/kg) were all dissolved in saline and injected intraperitoneally (i.p.) with a volume of 100 μL per 10 g body weight. Locomotor activities were evaluated in the open field environment using Plexiglas chambers (Med Associates, Inc., St. Albans, VT), as previously described (Anderson et al. 2010). Briefly, a mouse was placed in the center of the open field arena and allowed to freely move for 60 or 180 min while being tracked by an automated tracking system consisting of three 16 beam infrared arrays, the data from which were analyzed by Open Field Activity software (Med Associates). Infrared beam break data were collected in 5 min bins and used to extract ambulatory activity (crossing four beams within 500 ms), and distance traveled. Beam break activity in the absence of consecutive crossing of beams was defined as steretotypic movements. Thigmotaxis (wall hugging) for each subject was determined by dividing the distance traveled in the 7.5-cm wide perimeter of the environment by the total distance traveled during the 180-min session. Motor coordination was measured by performance on an accelerating rotarod device equipped with drums (IITC Life Science) sized for mice (Anderson et al. 2010). Eleven consecutive trials were performed for each animal on the same day. After placing a mouse on the rod, the rod was accelerated from 4 to 27 r.p.m. in 5 min. The endurance of mice on the rotarod was measured by time to fall to the floor of the apparatus, or to turn one full revolution while hanging onto the drum. The hot plate paw-lick test was performed on a platform heated to 55°C with a cutoff of 60 sec. Latency to paw lick or jump was recorded. Baseline responses were determined for each mouse before drug injection. Mice were injected with a volume of 100 μL per 10 g of body weight of either saline or morphine sulfate (s.c.; 1–15 mg/kg), 30 min after acquiring baseline ambulatory activity. The analgesia response was monitored 45 min after injection. The antinociceptive response was calculated as a percentage of maximal possible effect (MPE), where MPE%=(final - baseline)/(60 - baseline)*100%. Evaluation of basal behavior in open field and rotarod assays were done on naïve groups of mice not previously used in other assays. Dose dependence pharmacological studies were done on the same mice starting from morphine administration, and followed by quinpirole and LY379268 always starting from low dose first. At least one week was allowed between different drug applications and different doses were administered in 2-day interval. Administration of amphetamine and MK-801 in each case was done on a separate group of naïve mice and in a week followed by the injection of ADHD medications (citalopram, desipramine, and atomoxetine) with 3-day interval between drugs.
Measurements of dopamine levels by fast-scan cyclic voltammetry (FSCV)
Voltammetry was used as a method of choice for monitoring alterations in kinetic parameters of dopamine dynamics and no information regarding basal dopamine concentration was derived from the results. Mice were decapitated, brains were rapidly removed, and coronal slices (300-μm-thick) containing the striatum were prepared with a microtome. Slices were placed in artificial cerebrospinal fluid (aCSF, 119 mM NaCl, 2.5 mM KCl, 1 mM NaH2PO4, 26.5 mM NaHCO3, 1.3 mM MgSO4, 2.5 mM CaCl2 and 11 mM glucose, pH 7.4) and superfused with 95% O2 and 5% CO2. Carbon-fiber microelectrodes used herein for the fast-scan cyclic voltammetry (FSCV) experiments were fabricated according to a previously published protocol (Hochstetler et al. 2000). All FSCV experiments were conducted at 37±1°C in a temperature-controlled chamber (QE-1 and TCB-324B, Warner Instruments, Hamden, CT). The slice was continuously perfused with pre-warmed oxygenated aCSF solution at 1 mL/min. A carbon-fiber microelectrode was inserted ~75 μm into the caudate-putamen area; a pair of platinum stimulating electrodes (tip separation ~200 μm, #303/8-B, plastics1) was placed on the slice surface and maintained equal distance (~150 μm) to the carbon-fiber microelectrode. The potential on the carbon-fiber microelectrode was scanned from −0.4 to 1.0 V and back to −0.4 V (vs. Ag/AgCl) at 400 V/s with an update frequency of 10 Hz, using an Axopatch 200B potentiostat (Molecular Devices). The electrical stimulation (a single biphasic pulse, 350 μA in amplitude, 2 ms/phase) was delivered by an analog stimulus isolator (model 2200, A-M system Inc), and the stimulated dopamine release was recorded and analyzed by a locally written LabView program. The recorded current signal was converted to dopamine concentration based on a post-calibration using 1 μM dopamine solution in a flow-injection system (Kristensen et al. 1986). Triplicate measurements of dopamine release were performed on a brain slice to account for tissue heterogeneity.
In Vivo microdialysis and measurements of neurotransmitter levels by HPLC
The goal of the microdialysis experiments was to obtain accurate estimate of the extracellular dopamine concentration in the tissues of living mice, an information that FSCV approach does not provide. Microdialysis experiment were Following anesthesia, mice were placed in a stereotaxic frame and dialysis probes (2-mm membrane length, MD-2212 (Bioanalytical Systems) with MD-2255 guide cannulaes) were implanted into the right striatum (antero-posterior 0.0 mm, daiso-ventral -4.4 mm, lateral 2.5 mm relative to bregma) as previsouly described (Zhuang et al. 2001). Twenty four hr after surgery, the dialysis probe was connected to a syringe pump and perfused with Ringer’s Solution (150 mM NaCl, 2.7 mM KCL, 1 mM NaH2PO4, 1.5 mM Na2HPO4, 1.6 mM CaCl2, pH 7.2) at the speed of 1.5 μL/min. Two hr later, perfusates were collected every 20 min. After 100 min of basal dopamine level measurements, mice were then injected with cocaine (20 mg/kg, i.p.) and the post-injection samples were collected for another 80 min. Perfusate samples were assayed for dopamine by using HPLC with electrochemical detection using a previously described method (Farmer et al. 1996).
For analysis of dopamine and the dopamine metabolite, 3,4 dihydroxyphenylacetic acid, in tissue samples, brains were cut frozen in 250 μm serial sections. The sections were thaw mounted on glass slides and refrozen for later microdissection and specific brain regions were microdissected using a 300 μm punch. Monoamine analysis of these regions was performed using high performance liquid chromatography (HPLC) with electrochemical detection as previously described (Renner et al. 1987).
Electron microscopy
Immunohistochemical reactions were carried out using the pre-embedding immunogold method as described earlier (Lujan et al. 1996). Briefly, after blocking with 10% serum for 1 hr at room temperature free-floating sections were incubated for 48 hr with anti-Gβ5 antibodies (1–2 mg/mL). Sections were washed and incubated for 3 hr with goat anti-rabbit IgG coupled to 1.4 nm gold (Nanoprobes Inc) at 1:100 dilution. Sections were washed, postfixed in 1% glutaraldehyde and processed for silver enhancement of the gold particles with an HQ Silver kit (Nanoprobes Inc.). The reacted sections were treated with osmium tetraoxide (1% in 0.1 M PB), block-stained with uranyl acetate, dehydrated in graded series of ethanol and flat-embedded on glass slides in Durcupan (Fluka) resin. Regions of interest were cut at 70–90 nm on an ultramicrotome (Reichert Ultracut E; Leica). Staining was performed on drops of 1% aqueous uranyl acetate followed by Reynolds’s lead citrate. Ultrastructural analyses were performed in a Jeol-1010 electron microscope. Quantification of immunolabelling was performed in the striatum and stratum radiatum of the hippocampus from 60 mm coronal slices as described (Lujan et al. 1996). For each of three animals, three samples of tissue were obtained (nine total blocks).
Statistical Analyses
All data are presented as the means ± and SEMs. Two-way ANOVA analysis was used on the behavioral experiment data with genotype and session number (or dose) as two grouping factors. Tukey’s post hoc tests were conducted for individual pairwise comparisons. Biochemical data were analyzed using Student’s t-test. Statistical significance was considered when p < 0.05.
Results
Gβ5 knockout mice (Gβ5−/−) develop severe hyperactivity, motor learning deficits and display paradoxical adaptation to the novel environment
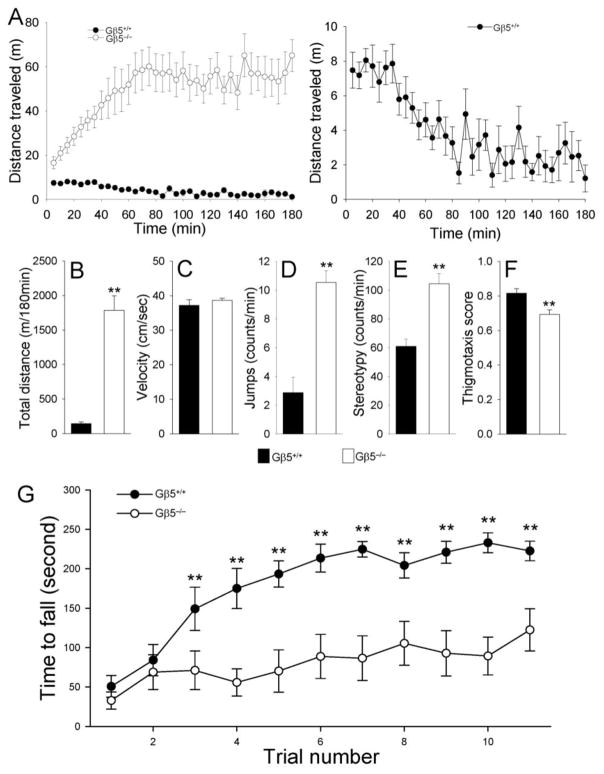
First, we tested the involvement of Gβ5-RGS in the regulation of behavior by evaluating Gβ5−/−mice and wild-type littermates in the open field test. During the first 5 min, Gβ5−/−mice traveled twice as far as their wild-type littermates (Fig 1A; 7.47±1.04 vs. 16.56±2.68 m). By 60 minutes, wild-type mice displayed a robust decrease in locomotion which is typically interpreted as habituation to the novel environment (Fig. 1A). In contrast, Gβ5−/−mice showed a dramatic increase in motor activity that coincided with the timing of developing motor habituation in wild-type animals, with both strains reaching a steady state level of activity in about 60 min. Cumulatively, Gβ5−/−mice ran approximately 12 times further than wild-type subjects over the 180 min test period (144.2 ± 23.8 vs. 1784 ± 210 m, p < 0.001; Fig. 1B). Higher ambulatory activity by Gβ5−/−mice was accompanied by an increased frequency of other movements, such as jumps and stereotypic movements (Fig. 1D, E). These differences did not appear to be the result from differences in speed (Fig. 1C). The overall pattern of ambulatory activity appeared to be similar between wild type and Gβ5−/−mice (Supplemental Fig. 1), however, Gβ5−/−mice spent slightly more time in the center of the open field relative to the periphery as evidenced by a reduced thigmotaxis score (Fig. 1F).
Figure 1. Gβ5−/− mice display dramatic hyperactivity, impaired habituation to novel environment and marked motor learning deficits.
Behavior of Gβ5−/−mice and their wild-type littermates (Gβ5+/+) in the open field chamber (A-F). A, Gβ5−/−mice display paradoxical habituation to the novel environment and severe hyperactivity. ANOVA analysis revealed a significant habituation effect for both wild type (F(10,35) = 129.9, p < 0.001) and Gβ5−/− (F(10,35) = 83.8, p < 0.001). However, the direction of the effect was opposite: Gβ5+/+ mice showed progressive decrease in activity during habituation, whereas the activity of Gβ5−/− mice increased. This difference in the habituation behavior was found significant by two-way ANOVA analysis (genotype x trial interaction, F(35, 720) = 3.14, p < 0.001). Right panel shows habituation behavior of wild-type subjects on magnified scale. B-F, Quantification of typical mouse behavioral traits in the open field. Statistical significance of differences was analyzed using pair-wise comparison by Student’s t-test; **p < 0.01 (n=11/group). G, Gβ5−/−mice exhibit severe deficits in motor learning behavior in rotarod test. A two-way ANOVA analysis revealed significant effect of trial for both groups of mice (F(10,187) = 7.38, p < 0.001), genotype (F(1,187) = 120.25, p < 0.001) and interaction between trial and genotype (F(10,187) = 2.248, p < 0.05). Pairwise comparions by post hoc Tukey’s test revealed that Gβ5−/−mice had a deficit in rotarod performance from the third trial (**, p < 0.01).
We next evaluated coordination and motor learning behavior using the rotarod paradigm as was previously used in characterizing the role of striatal R7BP and RGS9-2 proteins (Anderson et al. 2010; Blundell et al. 2008). Consistent with the motor deficits in mice lacking RGS9-2 or R7BP, Gβ5−/−mice exhibited severe deficits in the acquisition of motor skills (Fig. 1G). While performances of both genotypes were not significantly different during the initial two trials, Gβ5−/−mice exhibited dramatically shorter latencies to fall when compared to their wild-type littermates in subsequent trials. We therefore conclude that despite pronounced hyperactivity, Gβ5−/−mice have relatively normal motor coordination skills but their motor learning ability is severely compromised.
Broad pre- and postsynaptic localization of Gβ5 in central nervous system neurons
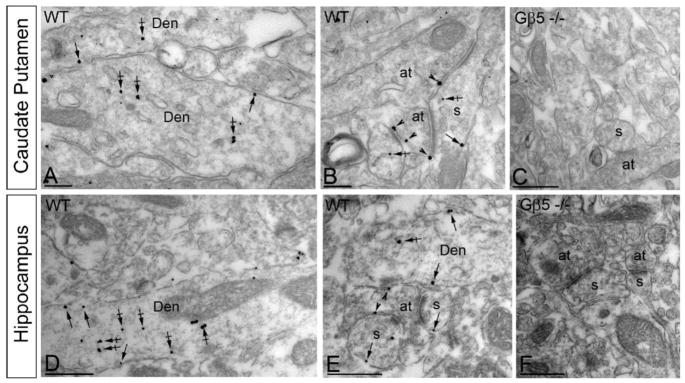
Gβ5 is expressed in multiple regions of the central nervous system (Zhang et al. 2000). However, high resolution information about the subcellular localization of Gβ5 has only been reported for its complexes with RGS9-2 in the striatum (Anderson et al. 2010; Anderson et al. 2007; Mancuso et al. 2010) where it was predominantly postsynaptic. Yet, several evidence suggest that RGS proteins play critical roles in controlling pre-synaptic processes (Chen and Lambert 2000; Han et al. 2006). In order to clarify the mode of Gβ5-RGS complexes action, we examined the distribution of Gβ5 protein at the ultrastructural level by immuno-gold electron microscopy in two representative brain regions: hippocampus and striatum. In both brain regions, most of the Gβ5 immunoreactivity was confined to neurons where it was especially prominent in dendritic shafts and spines. Representative images illustrate that Gβ5 is found both postsynaptically at the plasma membrane and in intracellular parts of the dendritic spines and shafts (Fig. 2A,D). Presynaptically, Gβ5 was detected along the extrasynaptic plasma membrane and the active zone of the axonal terminals in both striatum and hippocampus (Fig. 2B,E). In a quantitative comparison of pre- vs. postsynaptic Gβ5 immunoreactivity in the striatum, 495 out of 1455 immunoparticles (34%) were found at presynaptic sites and 960 out of 1455 (66%) were found at postsynaptic sites; in the hippocampus, 274 out of 1522 immunoparticles (18%) were found at presynaptic sites and 274 out of 1248 (82%) were found at postsynaptic sites. Importantly, no labeling was observed when tissues from Gβ5−/−mice were evaluated, indicating the specificity of the method for Gβ5 detection. These data suggest that in addition to recognized postsynaptic action, Gβ5-RGS complexes are easily detectable at pre-synaptic sites where they are likely to play role in controlling neurotransmitter release.
Figure 2. Subcellular localization of Gβ5 in the striatum and hippocampus.
Electron micrographs show immunolabeling for Gβ5 in different neuronal compartments of WT mice, as detected using a pre-embedding immunogold method. In striatum (A-C), and hippocampus (D-F) immunoparticles for Gβ5 were detected postsynaptically along the somatic plasma membrane (arrows), and intracellular sites (crossed arrows) of dendritic shafts (Den) and dendritic spines (s. In addition, immunoparticles for Gβ5 were detected at presynaptic sites, along the extrasynaptic plasma membrane (arrowheads) of axon terminals (at) establishing excitatory synapses with dendritic shafts or dendritic spines (s). Immunoreactivity for Gβ5 was completely absent in Gβ5−/−samples (C,F). Scale bar: 0.2 μm.
Dynamics of striatal dopamine transmission in Gβ5−/−mice
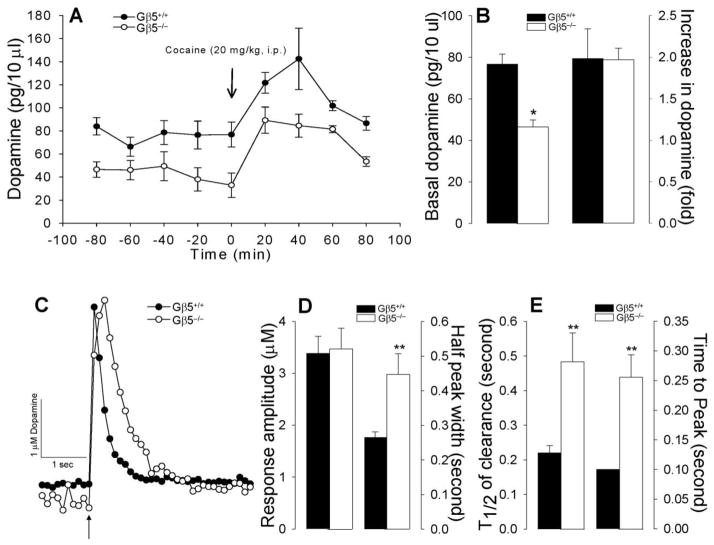
Because striatal dopamine signaling is thought to be a major factor controlling motor activity (Groenewegen 2003), we compared dopamine concentrations in Gβ5−/−and Gβ5+/+ mice. No significant differences in the concentrations of dopamine (or its major metabolite 3,4-dihydroxyphenylacetic acid) were detected in tissue punches from key regions of the mesolimbic dopamine system including the anterior-dorsal striatum (Supplemental Table 1), suggesting normal dopamine biogenesis. However, measurement of extracellular dopamine concentrations in the dorsal striatum by microdialysis in awake, unrestrained mice revealed significantly lower levels in Gβ5−/−mice (Fig. 3A,B). Cocaine administration resulted in robust increases in extracellular dopamine concentrations of similar magnitude in both Gβ5−/−and Gβ5+/+ mice (Fig. 3A,B).
Figure 3. Dynamics of striatal dopamine transmission in Gβ5−/−mice.
A, Measurements of basal extracellular levels of dopamine in the striatum of freely moving mice by microdialysis. B, Measurements of fast changes in dopamine release and re-uptake in coronal slices containing the striatum by cyclic voltammetry. Dopamine release was evoked by a single electrical pulse as indicated by arrow. Extracellular DA was sampled every 100 ms. Asterisk denotes statistically significant difference (*p < 0.05) as revealed by Student’s t-test. C and D, Quantification of kinetic parameters of dopamine concentration changes suggesting slower dopamine release and clearance in response to electrical stimulation in Gβ5−/−striatal slices. Clearance parameters were obtained by fitting recordings to a single exponential decay and analyzed by Student’s t-test (*p < 0.05, **p < 0.01 for pair-wise comparisons between wild-type (Gβ5+/+) and Gβ5−/−mice.
We next evaluated changes in dopamine concentration in striatal slices by fast scan cyclic voltammetry, a method that allows assessment of real-time dopamine release and reuptake kinetics following in situ electrical stimulation. We observed no differences in the amplitudes of striatal dopamine signals in tissue slices from Gβ5−/−and Gβ5+/+ mice, indicating similar changes in dopamine concentration upon stimulation (Fig 3C,D). However, the kinetics of dopamine release and reuptake were significantly slower in Gβ5−/−samples (Fig. 3D,E).
Taken together with the microdialysis data, these observations suggest the presence of increased tonic inhibition of dopamine release in Gβ5−/−mice.
Elimination of Gβ5 increases behavioral sensitivity to stimulation of several GPCRs
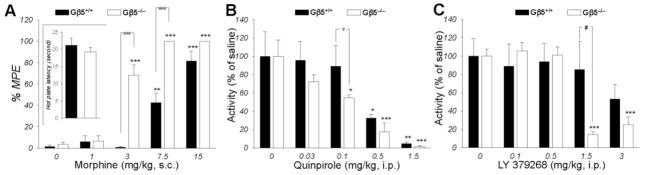
The presence of a significant fraction of Gβ5-RGS proteins at the axonal terminals, in addition to their known postsynaptic localization, and the observed inhibition of dopamine release in the absence of Gβ5, prompted us to evaluate behavioral responses of Gβ5−/−mice to the activation of inhibitory Gi/o-coupled GPCRs with known pre-synaptic effects. We found that stimulation of the μ-opioid receptors by morphine produced dramatically higher anti-nociceptive effects in Gβ5−/−mice (Fig. 4A). Similarly, administration of the D2 selective agonist quinpirole (Fig. 4B) or the mGluR2/3 selective agonist LY379268 (Fig. 4C; Supplemental Fig. 2) had greater motor inhibitory effects in Gβ5−/−animals, possibly through the activation of the respective autoreceptors. These observations are consistent with the idea that Gβ5-RGS complexes integrate signaling downstream from the receptors, at the level of activated Gαi/o proteins, and therefore inhibit responses mediated by several GPCRs in the nervous system.
Figure 4. Enhanced GPCR signaling in Gβ5−/−mice.
A, Gβ5−/−mice exhibit greater morphine-induced analgesia but normal nociceptive thresholds in the hot plate test (n=7 9) when compared with their wild-type littermates. A two-way ANOVA (doses by genotype) revealed a significant effect of dose (F(4,58) = 92.278, p < 0.001) indicating analgesic effect of morphine in both genotypes and significant difference between genotypes (F(1,58) = 61.339, p < 0.001). Gβ5−/−had higher sensitivity to morphine effects as indicated by the interaction between dose and genotype (F(4,58) = 13.804, p < 0.001). Pairwise post hoc Tukey’s test confirmed a significance of morphine effects (*p < 0.05; **p < 0.01, ***p < 0.001 morphine vs saline control) and differences between genotypes (###, p < 0.001). B, Elevated behavioral sensitivity of Gβ5−/−mice to motor depressant effects of the D2 receptor agonist quinpirole. Locomotor activity of wild-type Gβ5+/+ (n=6) and Gβ5−/−(n= 6) mice was measured in the open field chamber for 3 hr following i.p. drug administration and then normalized to the activity of saline-treated controls of the same genotype. ANOVA showed quinpirole had significant inhibitory effect on activity of both Gβ5+/+ (F(4,25)=13.137, p < 0.001) and Gβ5−/−mice (F(4,25)=20.462, p < 0.001). Post hoc Tukey’s test confirmed higher doses of quinpirole inhibited mice activity (*p < 0.05; **p < 0.01, ***p < 0.001 vs saline control). ANOVA analysis followed by Tukuey test showed significant difference at dose of 0.1 mg/kg between genotypes ( #p < 0.05, Gβ5−/−mice vs Gβ5+/+). C, Elevated behavioral sensitivity of Gβ5−/−mice to motor depressant effects of the mGluR2/3 receptor agonist LY379268. Locomotor activity of wild-type Gβ5+/+ (n=6) and Gβ5−/−(n= 6) mice was measured in the open field chamber for 3 hr following i.p. drug administration and then normalized to the activity of saline-treated controls of the same genotype. ANOVA showed LY379268 had significant inhibitory effect on activity of Gβ5−/−mice (F(4,25)=33.596, p < 0.001). Pairwise post hoc Tukey’s test confirmed higher doses of LY379268 inhibited activity of Gβ5−/−mice (***p < 0.001 vs saline control). ANOVA analysis followed by Tukey’s test showed significant difference at dose of 1.5 mg/kg between genotypes ( #p < 0.05, Gβ5−/−mice vs wild-type). Error bars represent SEM values.
Hyperactivity triggered by the loss of Gβ5-RGS complexes is resistant to treatment with monoamine reuptake blockers
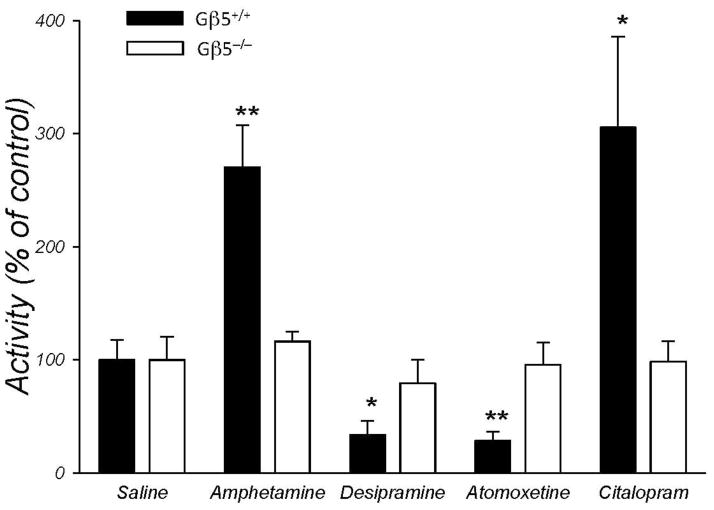
Changes in the concentration of monoamine neurotransmitters are considered to be one of the leading causes of the hyperactivity (Arnsten 2009; David et al. 2005; Tripp and Wickens 2009). Consequently, behavioral hyperactivity is often responsive to pharmacological treatments with monoamine reuptake inhibitors that act to increase synaptic monoamine concentrations (Arnsten 2009; Stahl 2010). Therefore, we next evaluated the effects of several catecholamine reuptake inhibitors commonly used to combat hyperactivity disorders in human patients (Fig. 5). We have found that relatively unselective psychostimulant, amphetamine (Fig. 5 and Supplemental Fig. 3) as well as selective serotonin reuptake inhibitor citalopram caused significant increases in activity in wild-type mice. On the other hand, both selective norepinephrine reuptake inhibitor atomoxetine and the tricyclic antidepressant desipramine that affects norepinephrine and, to a lesser extent, serotonin reuptake, decreased activity of wild-type mice (Fig. 5). In contrast, none of these pharmacological treatments at significantly affected the hyperactivity of mice lacking Gβ5-RGS complexes (Fig. 5), indicating that Gβ5−/− mice are resistant to these treatments at least at concentrations effective in wild-type subjects. These results suggest that the observed deficiencies in the basal concentrations and reuptake kinetics of the catecholamine neurotransmitters are unlikely to be the primary cause underlying the hyperactivity observed in Gβ5−/−mice.
Figure 5. Gβ5−/− mice are resistant to the effects of monoamine reuptake inhibitors.
Mice (n=6/group) were injected i.p. with desipramine (10 mg/kg), atomoxine (10 mg/kg), citalopram (10 mg/kg) or amphetamine (1 mg/kg). Immediately after injection, mice were placed in an open field chamber and their activity was monitored for 2 hrs. Total distances run by animals after drug injection were normalized to those after the injection of saline. Statistically significant differences have been analyzed by Student’s t-test comparing drug-treated subjects and saline injected controls. (*p < 0.05, **p < 0.01 vs saline control). All error bars represent SEMs.
Blocking NMDA receptor completely alleviates hyperactivity in Gβ5−/−mice
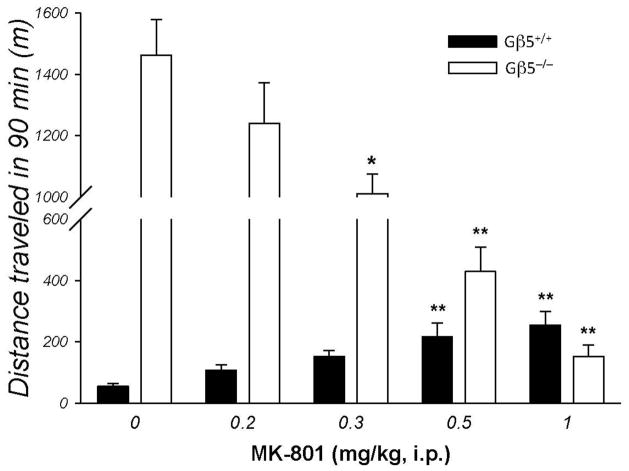
Glutamate neurotransmission is the second major system in the striatum that is critically involved in controlling motor activity. Therefore, we next tested the possibility that hyperactive behavior of Gβ5−/−may be associated with the imbalances in the glutamatergic transmission. Administration of the NMDA receptor blocker MK-801 disrupts catecholamine-glutamate interactions in the striatum and results in marked increases in motor activity (David et al. 2005; Gainetdinov et al. 2001). Consistent with earlier work (Diana and Sagratella 1994; Druhan et al. 1996; Ginski and Witkin 1994; Liljequist et al. 1991; Shen and Phillips 1998), administration of MK-801 caused a robust dose-dependent increase in the motor activity of the wild-type mice (Fig. 6). However, in Gβ5−/−injections of MK-801 resulted in the opposite effect and dose-dependently inhibited locomotor behavior (Fig. 6). The highest dose of MK-801 tested decreased activity in the Gβ5−/−mice to near normal levels (Fig. 6). These findings implicate changes in glutamatergic signaling involving NMDA receptors in the development of the motor hyperactivity.
Figure 6. Paradoxical effects of NMDA receptor blockade in mice lacking Gβ5.
Gβ5−/−mice or their wild-type littermates (n=5) were injected either with saline or various doses of MK-801 and their ambulatory activities were monitored in the open field chamber. The same subjects were used to test the different MK-801doses with 2 days allowed between treatments. ANOVA revealed a significant effect of MK-801 on activity of both Gβ5+/+ (F(4,29) = 6.896, p < 0.001) and Gβ5−/− (F(4,29) = 34.921, p < 0.001) mice. Pairwise post hoc Tukey’s test revealed significant differences between treatments and saline control when indicated (*p < 0.05; **p < 0.01). Error bars represent SEMs.
Discussion
We report a striking behavioral consequence resulting from Gβ5-RGS elimination is the expression of pronounced motor hyperactivity. Combined with concurrent paradoxical sensitization to novel environment, motor learning deficits and lower extracellular dopamine concentration, these findings correspond to several symptomatic characteristics attributed to ADHD. Several lines of evidence point to potential contribution of Gβ5 to ADHD and associated neuropsychiatric disorders. Gβ5 gene is located within q21.2 locus on chromosome 15. Genetic aberrations in the 15q arm contribute to a variety of neuropsychiatric conditions including mental retardation, motor and speech development problems and, most notably, autism spectrum disorders (Allen-Brady et al. 2010; Battaglia 2005; Battaglia et al. 2010; Nayate et al. 2005). Although current diagnosis guidelines prevent co-diagnosis of autism and ADHD, both disorders respond to the same general pharmacological treatments and are increasingly viewed as having common molecular underpinnings (Murray 2010; Nayate et al. 2005; Rommelse et al. 2010). Moreover, recent genome-wide association studies also link 15q region to ADHD (Bakker et al. 2003; Nijmeijer et al. 2010). The Gβ5 gene is located within the D15S659 marker on 15q21.2 linked to ADHD (Faraone et al. 2008) and was recently identified among the genetic traits associated with cognitive abilities (Ruano et al. 2010). Taken together with our behavioral observations, this evidence suggests that disruption in Gβ5 function may be important in the pathophysiology of the ADHD. Clearly, more studies will be needed to affirm this connection as well as to establish the exact mechanisms of Gβ5-RGS contribution to this process. For example, in future studies it would be valuable to test the effects of the loss of Gβ5 on cognitive deficits such as attention, spatial or associative learning and memory.
Regardless of whether disruption in Gβ5 might be linked to the development of ADHD in human patients, we think that this genetic model offers important insights into the neurochemical mechanisms underlying the control of motor behavior. The mesolimbic dopamine system plays a central regulatory role in motor control (Graybiel 2000; Kreitzer and Malenka 2008). The striatum, which is the largest effector nucleus of this system, abundantly expresses several R7 RGS members complexed with Gβ5 (Anderson et al. 2009). Dopamine action in the striatum is mediated by two convergent circuits: a direct pathway that contains neurons expressing D1 receptors and stimulates movement, and an indirect pathway that contains neurons expressing D2 receptors and inhibits movement (Gerfen et al. 1990; Graybiel 2000). It is accepted that dopamine acts to enhance motor activity by stimulating the direct pathway and inhibiting the indirect pathway (Kreitzer and Malenka 2008). Depletion of dopamine from the striatum by either pharmacological (Clark and White 1987; Zigmond and Stricker 1989) or genetic (Zhou and Palmiter 1995) approaches reduces motor activity. In contrast, psychostimulants and many other addictive substances increase dopamine concentrations in the striatum and cause marked increases in locomotion (Wise and Bozarth 1987). Similarly, disruption of dopamine re-uptake by genetic knockout of the dopamine transporter leads to the elevation of extracellular dopamine concentrations and enhances locomotor activity (Giros et al. 1996; Zhuang et al. 2001). Additionally, imbalances in the processing of the dopamine signals in striatal pathways underlie a number of movement and hyperactivity disorders (DeLong and Wichmann 2007).
A considerable body of evidence indicates that, in addition to dopamine, related monoamines serotonin and norepinephrine also play critical role in controlling locomotor activities and that the three systems intimately interact in this process (Di Matteo et al. 2008; Oades 2008; Prince 2008). A unifying theory postulates that the levels of basal activity and attention, disrupted in ADHD, are set by the balance between dopamine and serotonin/norepinephrine rather by the changes in dopamine concentrations alone (Arnsten 2009; Stahl 2010). In addition, catecholamine systems interact with the glutamatergic neurotransmission, which provides a second major input to the basal ganglia (David et al. 2005). Most prominently, these interactions occur at the level of the dopamine and NMDA receptors that have been shown to reciprocally modulate one another in neurons (Greengard et al. 1999; Liu et al. 2006; Missale et al. 2006; Wang et al. 2003). An example of how these systems cooperate to control basal motor activity is provided by studies on mice lacking dopamine transporter (DAT), one of the central candidate genes for ADHD (Gainetdinov 2008; Mazei-Robinson and Blakely 2006). DAT knockout leads to the elevation of the dopamine concentrations in the striatum and results in moderate hyperactivity (Gainetdinov et al. 2001; Gainetdinov et al. 1999). This hyperactivity is paradoxically reduced by treatment with psychostimulants that fail to further elevate dopamine concentration in DAT knockout mice (Gainetdinov et al. 2001; Gainetdinov et al. 1999). The paradoxical effects of psychostimulants appear to be mediated by serotonin since treatments that augment extracellular serotonin reduce hyperactivity in DAT knockouts, possibly by normalizing the dopamine/serotonin balance (Gainetdinov et al. 2001; Gainetdinov et al. 1999). Interestingly, dopamine dysregulation and effects of serotonin on motor activity in these mice were found to be dependent on NMDA receptor function. Increase in NMDA receptor activity reduced hyperactivity, whereas its blockade prevented the inhibitory effects of psychostimulants and serotonergic drugs (Gainetdinov et al. 2001).
Our findings with Gβ5 knockout mice provide the converse view of interactions between the same systems in setting basal motor activity. In contrast to DAT knockouts, Gβ5−/−mice have lower extracellular levels of dopamine in the striatum, presumably due to increased inhibition of the presynaptic neurotransmitter release (see Fig. 3B-D). This effect is expected given that Gβ5-RGS proteins act as selective negative regulators for inhibitory Gi/o proteins (Hooks et al. 2003) that mediate pre-synaptic inhibition (Blackmer et al. 2001). Consequently, the animals were found to be resistant to treatments with the monoamine reuptake inhibitors. Furthermore, NMDA receptor blockade completely prevented behavioral hyperactivity, an effect opposite from what was observed in DAT knockout mice (Gainetdinov et al. 2001). Taken together, both studies provide complementary evidence for a model where the level of motor activity is set by the antagonistic interaction between glutamate and catecholamine systems. The hyperactivity caused by the excess dopamine can be compensated by stimulation of NMDA receptors, whereas, when hyperactivity is caused by insufficient dopamine/serotonin signaling, NMDA antagonism is effective. Thus, our results reinforce the central role of glutamate signaling via NMDA receptors in setting the level of motor activity and provide evidence that Gβ5-RGS complexes play important role in dopamine-NMDA receptor cross-talk. This hypothesis is further supported by findings that implicate RGS9, a member of the R7 RGS family that forms complexes with Gβ5 in the striatum, as a possible regulator of NMDA receptor signaling (Bouhamdan et al. 2006; Kovoor et al. 2005). However, it is likely that other Gβ5-binding RGS proteins also contribute to this process since elimination of RGS9 alone is not sufficient to trigger hyperactive behavior (Rahman et al. 2003; Zachariou et al. 2003). Since we found multiple GPCR systems to be affected upon Gβ5-RGS elimination it also seems possible that the hyperactivity phenotype seen in Gβ5−/− mice might be a cumulative result of the general dysregulation in G protein inactivation downstream of several GPCRs, which could act synergistically with the monoaminergic systems.
Finally, our results suggest the possibility that NMDA receptor antagonism may be a useful therapeutic strategy for relieving the hyperactivity symptoms in ADHD, especially in cases that are resistant to treatment with psychostimulants. Indeed, NMDA receptor dysfunction has been suggested as one of the underlying causes of the hyperactivity in the rat model of ADHD (Jensen et al. 2009) and the NMDA receptor blocker memantine has effectively been used to treat pediatric ADHD patients (Findling et al. 2007).
In closing, we would like to mention that, to our knowledge, Gβ5 deficiency leads to the most profound expression of hyperactivity ever reported in mammalian models. We therefore believe that Gβ5-RGS complexes serve as key modulators of signaling pathways that control neuronal excitability and motor activity and thus represent exciting targets for understanding a spectrum of human disorders associated with hyperactivity and motor deficits.
Supplementary Material
Acknowledgments
We would like to thank Dr. Ching-Kang Jason Chen (VCU) for providing Gβ5−/−mouse line and Dr. William Simonds (NIH) for the generous gift of anti-Gβ5 antibodies. This work was supported by NIH grants DA021743 (K.A.M.), DA026405 (K.A.M.) DA019921 (K.J.R.), DA13680 (R.L.M.), McKnight Land-Grant Professorship award (K.A.M.), NSF 0921874 (K.J.R), grants from the Spanish Ministry Science and Innovation (BFU2009-08404/BFI and CONSOLIDER-Ingenio CSD2008-00005; RL), Searle Scholars Award (C.L.H.)
Footnotes
Disclosure
The authors declare that except for the income received from the primary employers and NIH grants listed in the “Acknowledgements” section below no financial compensation has been received from any individual or corporate entity over the past three years of research or professional service that could be perceived as constituting a potential conflict of interest.
References
- Allen-Brady K, Robison R, Cannon D, Varvil T, Villalobos M, Pingree C, Leppert MF, Miller J, McMahon WM, Coon H. Genome-wide linkage in Utah autism pedigrees. Mol Psychiatry. 2010;15:1006–15. doi: 10.1038/mp.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GR, Cao Y, Davidson S, Truong HV, Pravetoni M, Thomas MJ, Wickman K, Giesler GJ, Jr, Martemyanov KA. R7BP complexes with RGS9-2 and RGS7 in the striatum differentially control motor learning and locomotor responses to cocaine. Neuropsychopharmacology. 2010;35:1040–50. doi: 10.1038/npp.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GR, Lujan R, Semenov A, Pravetoni M, Posokhova EN, Song JH, Uversky V, Chen CK, Wickman K, Martemyanov KA. Expression and localization of RGS9-2/G 5/R7BP complex in vivo is set by dynamic control of its constitutive degradation by cellular cysteine proteases. J Neurosci. 2007;27:14117–27. doi: 10.1523/JNEUROSCI.3884-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GR, Posokhova E, Martemyanov KA. The R7 RGS protein family: multi-subunit regulators of neuronal G protein signaling. Cell Biochem Biophys. 2009;54:33–46. doi: 10.1007/s12013-009-9052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs. 2009;23(Suppl 1):33–41. doi: 10.2165/00023210-200923000-00005. [DOI] [PubMed] [Google Scholar]
- Bakker SC, van der Meulen EM, Buitelaar JK, Sandkuijl LA, Pauls DL, Monsuur AJ, van 't Slot R, Minderaa RB, Gunning WB, Pearson PL, Sinke RJ. A whole-genome scan in 164 Dutch sib pairs with attention-deficit/hyperactivity disorder: suggestive evidence for linkage on chromosomes 7p and 15q. Am J Hum Genet. 2003;72:1251–60. doi: 10.1086/375143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia A. The inv dup(15) or idic(15) syndrome: a clinically recognisable neurogenetic disorder. Brain Dev. 2005;27:365–9. doi: 10.1016/j.braindev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Battaglia A, Parrini B, Tancredi R. The behavioral phenotype of the idic(15) syndrome. Am J Med Genet C Semin Med Genet. 2010;154C:448–55. doi: 10.1002/ajmg.c.30281. [DOI] [PubMed] [Google Scholar]
- Blackmer T, Larsen EC, Takahashi M, Martin TF, Alford S, Hamm HE. G protein betagamma subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca2+ entry. Science. 2001;292:293–7. doi: 10.1126/science.1058803. [DOI] [PubMed] [Google Scholar]
- Blundell J, Hoang CV, Potts B, Gold SJ, Powell CM. Motor coordination deficits in mice lacking RGS9. Brain Res. 2008;1190:78–85. doi: 10.1016/j.brainres.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhamdan M, Yan HD, Yan XH, Bannon MJ, Andrade R. Brain-specific regulator of G-protein signaling 9-2 selectively interacts with alpha-actinin-2 to regulate calcium-dependent inactivation of NMDA receptors. J Neurosci. 2006;26:2522–30. doi: 10.1523/JNEUROSCI.4083-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheever ML, Snyder JT, Gershburg S, Siderovski DP, Harden TK, Sondek J. Crystal structure of the multifunctional Gbeta5-RGS9 complex. Nat Struct Mol Biol. 2008;15:155–62. doi: 10.1038/nsmb.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK, Eversole-Cire P, Zhang HK, Mancino V, Chen YJ, He W, Wensel TG, Simon MI. Instability of GGL domain-containing RGS proteins in mice lacking the G protein beta-subunit Gb5. Proc Natl Acad Sci USA. 2003;100:6604–6609. doi: 10.1073/pnas.0631825100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Lambert NA. Endogenous regulators of G protein signaling proteins regulate presynaptic inhibition at rat hippocampal synapses. Proc Natl Acad Sci U S A. 2000;97:12810–5. doi: 10.1073/pnas.230260397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D, White FJ. D1 dopamine receptor--the search for a function: a critical evaluation of the D1/D2 dopamine receptor classification and its functional implications. Synapse. 1987;1:347–88. doi: 10.1002/syn.890010408. [DOI] [PubMed] [Google Scholar]
- David HN, Ansseau M, Abraini JH. Dopamine-glutamate reciprocal modulation of release and motor responses in the rat caudate-putamen and nucleus accumbens of "intact" animals. Brain Res Brain Res Rev. 2005;50:336–60. doi: 10.1016/j.brainresrev.2005.09.002. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64:20–4. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Pierucci M, Esposito E, Crescimanno G, Benigno A, Di Giovanni G. Serotonin modulation of the basal ganglia circuitry: therapeutic implication for Parkinson's disease and other motor disorders. Prog Brain Res. 2008;172:423–63. doi: 10.1016/S0079-6123(08)00921-7. [DOI] [PubMed] [Google Scholar]
- Diana G, Sagratella S. Different capability of N-methyl-D-aspartate antagonists to affect locomotor/exploratory activity of mice in a computerized on-line open field test. Pharmacol Biochem Behav. 1994;48:291–5. doi: 10.1016/0091-3057(94)90529-0. [DOI] [PubMed] [Google Scholar]
- Druhan JP, Rajabi H, Stewart J. MK-801 increases locomotor activity without elevating extracellular dopamine levels in the nucleus accumbens. Synapse. 1996;24:135–46. doi: 10.1002/(SICI)1098-2396(199610)24:2<135::AID-SYN5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Doyle AE, Lasky-Su J, Sklar PB, D'Angelo E, Gonzalez-Heydrich J, Kratochvil C, Mick E, Klein K, Rezac AJ, Biederman J. Linkage analysis of attention deficit hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1387–91. doi: 10.1002/ajmg.b.30631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–23. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: is it an American condition? World Psychiatry. 2003;2:104–13. [PMC free article] [PubMed] [Google Scholar]
- Farmer CJ, Isakson TR, Coy DJ, Renner KJ. In vivo evidence for progesterone dependent decreases in serotonin release in the hypothalamus and midbrain central grey: relation to the induction of lordosis. Brain Res. 1996;711:84–92. doi: 10.1016/0006-8993(95)01403-9. [DOI] [PubMed] [Google Scholar]
- Findling RL, McNamara NK, Stansbrey RJ, Maxhimer R, Periclou A, Mann A, Graham SM. A pilot evaluation of the safety, tolerability, pharmacokinetics, and effectiveness of memantine in pediatric patients with attention-deficit/hyperactivity disorder combined type. J Child Adolesc Psychopharmacol. 2007;17:19–33. doi: 10.1089/cap.2006.0044. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR. Dopamine transporter mutant mice in experimental neuropharmacology. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:301–13. doi: 10.1007/s00210-007-0216-0. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Mohn AR, Bohn LM, Caron MG. Glutamatergic modulation of hyperactivity in mice lacking the dopamine transporter. Proc Natl Acad Sci U S A. 2001;98:11047–54. doi: 10.1073/pnas.191353298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–44. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999;283:397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–32. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Ginski MJ, Witkin JM. Sensitive and rapid behavioral differentiation of N-methyl-D-aspartate receptor antagonists. Psychopharmacology (Berl) 1994;114:573–82. doi: 10.1007/BF02244987. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–12. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia. Curr Biol. 2000;10:R509–11. doi: 10.1016/s0960-9822(00)00593-5. [DOI] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–47. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ. The basal ganglia and motor control. Neural Plast. 2003;10:107–20. doi: 10.1155/NP.2003.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Mark MD, Li X, Xie M, Waka S, Rettig J, Herlitze S. RGS2 determines short-term synaptic plasticity in hippocampal neurons by regulating Gi/o-mediated inhibition of presynaptic Ca2+ channels. Neuron. 2006;51:575–86. doi: 10.1016/j.neuron.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Cheetham SC, Smith SL. The neuropharmacology of ADHD drugs in vivo: insights on efficacy and safety. Neuropharmacology. 2009;57:608–18. doi: 10.1016/j.neuropharm.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Hochstetler SE, Puopolo M, Gustincich S, Raviola E, Wightman RM. Real-time amperometric measurements of zeptomole quantities of dopamine released from neurons. Anal Chem. 2000;72:489–96. doi: 10.1021/ac991119x. [DOI] [PubMed] [Google Scholar]
- Hollinger S, Hepler JR. Cellular regulation of RGS proteins: Modulators and integrators of G protein signaling. Pharmacological Reviews. 2002;54:527–559. doi: 10.1124/pr.54.3.527. [DOI] [PubMed] [Google Scholar]
- Hooks SB, Waldo GL, Corbitt J, Bodor ET, Krumins AM, Harden TK. RGS6, RGS7, RGS9, and RGS11 stimulate GTPase activity of Gi family G-proteins with differential selectivity and maximal activity. J Biol Chem. 2003;278:10087–93. doi: 10.1074/jbc.M211382200. [DOI] [PubMed] [Google Scholar]
- Jensen V, Rinholm JE, Johansen TJ, Medin T, Storm-Mathisen J, Sagvolden T, Hvalby O, Bergersen LH. N-methyl-D-aspartate receptor subunit dysfunction at hippocampal glutamatergic synapses in an animal model of attention-deficit/hyperactivity disorder. Neuroscience. 2009;158:353–64. doi: 10.1016/j.neuroscience.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Kirley A, Hawi Z, Daly G, McCarron M, Mullins C, Millar N, Waldman I, Fitzgerald M, Gill M. Dopaminergic system genes in ADHD: toward a biological hypothesis. Neuropsychopharmacology. 2002;27:607–19. doi: 10.1016/S0893-133X(02)00315-9. [DOI] [PubMed] [Google Scholar]
- Kovoor A, Seyffarth P, Ebert J, Barghshoon S, Chen CK, Schwarz S, Axelrod JD, Cheyette BN, Simon MI, Lester HA, Schwarz J. D2 dopamine receptors colocalize regulator of G-protein signaling 9-2 (RGS9-2) via the RGS9 DEP domain, and RGS9 knock-out mice develop dyskinesias associated with dopamine pathways. J Neurosci. 2005;25:2157–65. doi: 10.1523/JNEUROSCI.2840-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–54. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen EW, Wilson RL, Wightman RM. Dispersion in flow injection analysis measured with microvoltammetric electrodes. Anal Chem. 1986;58:986–988. [Google Scholar]
- Kristiansen K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol Ther. 2004;103:21–80. doi: 10.1016/j.pharmthera.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Liljequist S, Ossowska K, Grabowska-Anden M, Anden NE. Effect of the NMDA receptor antagonist, MK-801, on locomotor activity and on the metabolism of dopamine in various brain areas of mice. Eur J Pharmacol. 1991;195:55–61. doi: 10.1016/0014-2999(91)90381-y. [DOI] [PubMed] [Google Scholar]
- Liu XY, Chu XP, Mao LM, Wang M, Lan HX, Li MH, Zhang GC, Parelkar NK, Fibuch EE, Haines M, Neve KA, Liu F, Xiong ZG, Wang JQ. Modulation of D2R-NR2B interactions in response to cocaine. Neuron. 2006;52:897–909. doi: 10.1016/j.neuron.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Mancuso JJ, Qian Y, Long C, Wu GY, Wensel TG. Distribution of RGS9-2 in neurons of the mouse striatum. J Neurochem. 2010;112:651–61. doi: 10.1111/j.1471-4159.2009.06488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazei-Robinson MS, Blakely RD. ADHD and the dopamine transporter: are there reasons to pay attention? Handb Exp Pharmacol. 2006:373–415. doi: 10.1007/3-540-29784-7_17. [DOI] [PubMed] [Google Scholar]
- Missale C, Fiorentini C, Busi C, Collo G, Spano PF. The NMDA/D1 receptor complex as a new target in drug development. Curr Top Med Chem. 2006;6:801–8. doi: 10.2174/156802606777057562. [DOI] [PubMed] [Google Scholar]
- Murray MJ. Attention-deficit/Hyperactivity Disorder in the context of Autism spectrum disorders. Curr Psychiatry Rep. 2010;12:382–8. doi: 10.1007/s11920-010-0145-3. [DOI] [PubMed] [Google Scholar]
- Nayate A, Bradshaw JL, Rinehart NJ. Autism and Asperger’s disorder: are they movement disorders involving the cerebellum and/or basal ganglia? Brain Res Bull. 2005;67:327–34. doi: 10.1016/j.brainresbull.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Nijmeijer JS, Arias-Vasquez A, Rommelse NN, Altink ME, Anney RJ, Asherson P, Banaschewski T, Buschgens CJ, Fliers EA, Gill M, Minderaa RB, Poustka L, Sergeant JA, Buitelaar JK, Franke B, Ebstein RP, Miranda A, Mulas F, Oades RD, Roeyers H, Rothenberger A, Sonuga-Barke EJ, Steinhausen HC, Faraone SV, Hartman CA, Hoekstra PJ. Identifying loci for the overlap between attention-deficit/hyperactivity disorder and autism spectrum disorder using a genome-wide QTL linkage approach. J Am Acad Child Adolesc Psychiatry. 2010;49:675–85. doi: 10.1016/j.jaac.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oades RD. Dopamine-serotonin interactions in attention-deficit hyperactivity disorder (ADHD) Prog Brain Res. 2008;172:543–65. doi: 10.1016/S0079-6123(08)00926-6. [DOI] [PubMed] [Google Scholar]
- Offermanns S. G-proteins as transducers in transmembrane signalling. Prog Biophys Mol Biol. 2003;83:101–30. doi: 10.1016/s0079-6107(03)00052-x. [DOI] [PubMed] [Google Scholar]
- Pliszka SR. Pharmacologic treatment of attention-deficit/hyperactivity disorder: efficacy, safety and mechanisms of action. Neuropsychol Rev. 2007;17:61–72. doi: 10.1007/s11065-006-9017-3. [DOI] [PubMed] [Google Scholar]
- Porter MY, Xie K, Pozharski E, Koelle MR, Martemyanov KA. A conserved protein interaction interface on the type 5 G protein beta subunit controls proteolytic stability and activity of R7 family regulator of G protein signaling proteins. J Biol Chem. 2010;285:41100–12. doi: 10.1074/jbc.M110.163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince J. Catecholamine dysfunction in attention-deficit/hyperactivity disorder: an update. J Clin Psychopharmacol. 2008;28:S39–45. doi: 10.1097/JCP.0b013e318174f92a. [DOI] [PubMed] [Google Scholar]
- Rahman Z, Schwarz J, Gold SJ, Zachariou V, Wein MN, Choi KH, Kovoor A, Chen CK, DiLeone RJ, Schwarz SC, Selley DE, Sim-Selley LJ, Barrot M, Luedtke RR, Self D, Neve RL, Lester HA, Simon MI, Nestler EJ. RGS9 modulates dopamine signaling in the basal ganglia. 2003;38:941–952. doi: 10.1016/s0896-6273(03)00321-0. [DOI] [PubMed] [Google Scholar]
- Renner KJ, Krey LC, Luine VN. Effect of progesterone on monoamine turnover in the brain of the estrogen-primed rat. Brain Res Bull. 1987;19:195–202. doi: 10.1016/0361-9230(87)90085-2. [DOI] [PubMed] [Google Scholar]
- Rommelse NN, Franke B, Geurts HM, Hartman CA, Buitelaar JK. Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder. Eur Child Adolesc Psychiatry. 2010;19:281–95. doi: 10.1007/s00787-010-0092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruano D, Abecasis GR, Glaser B, Lips ES, Cornelisse LN, de Jong AP, Evans DM, Davey Smith G, Timpson NJ, Smit AB, Heutink P, Verhage M, Posthuma D. Functional gene group analysis reveals a role of synaptic heterotrimeric G proteins in cognitive ability. Am J Hum Genet. 2010;86:113–25. doi: 10.1016/j.ajhg.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen EH, Phillips TJ. MK-801 potentiates ethanol’s effects on locomotor activity in mice. Pharmacol Biochem Behav. 1998;59:135–43. doi: 10.1016/s0091-3057(97)00389-4. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Mechanism of action of stimulants in attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2010;71:12–3. doi: 10.4088/JCP.09bs05890pur. [DOI] [PubMed] [Google Scholar]
- Tripp G, Wickens JR. Neurobiology of ADHD. Neuropharmacology. 2009;57:579–89. doi: 10.1016/j.neuropharm.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Viggiano D. The hyperactive syndrome: metanalysis of genetic alterations, pharmacological treatments and brain lesions which increase locomotor activity. Behav Brain Res. 2008;194:1–14. doi: 10.1016/j.bbr.2008.06.033. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhong P, Gu Z, Yan Z. Regulation of NMDA receptors by dopamine D4 signaling in prefrontal cortex. J Neurosci. 2003;23:9852–61. doi: 10.1523/JNEUROSCI.23-30-09852.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–92. [PubMed] [Google Scholar]
- Xie K, Allen KL, Kourrich S, Colon-Saez J, Thomas MJ, Wickman K, Martemyanov KA. Gbeta5 recruits R7 RGS proteins to GIRK channels to regulate the timing of neuronal inhibitory signaling. Nat Neurosci. 2010;13:661–3. doi: 10.1038/nn.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Georgescu D, Sanchez N, Rahman Z, DiLeone R, Berton O, Neve RL, Sim-Selley LJ, Selley DE, Gold SJ, Nestler EJ. Essential role for RGS9 in opiate action. 2003;100:13656–13661. doi: 10.1073/pnas.2232594100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JH, Lai ZN, Simonds WF. Differential expression of the G protein *5 gene: Analysis of mouse brain, peripheral tissues, and cultured cell lines. Journal of Neurochemistry. 2000;75:393–403. doi: 10.1046/j.1471-4159.2000.0750393.x. [DOI] [PubMed] [Google Scholar]
- Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83:1197–209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98:1982–7. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond MJ, Stricker EM. Animal models of parkinsonism using selective neurotoxins: clinical and basic implications. Int Rev Neurobiol. 1989;31:1–79. doi: 10.1016/s0074-7742(08)60277-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.