Abstract
Objectives
The current study explored the value of visuospatial findings for predicting the occurrence of visual hallucinations (VH) in a sample of patients with Dementia with Lewy bodies (DLB) compared to patients with Alzheimer’s disease (AD).
Participants/Measurements
Retrospective analysis of 55 autopsy-confirmed DLB and 55 demographically-similar, autopsy-confirmed AD cases determined whether severe initial visuospatial deficits on the WISC-R Block Design subtest predicted the development of VH. Visuospatial deficits were considered severe if Block Design z-scores were 2.5 or more standard deviations below the mean of a well-characterized normal control group (Severe-VIS; DLB: n=35, AD: n=26) and otherwise were considered mild (Mild-VIS; DLB: n=20, AD: n=29).
Results
Forty percent of the Severe-VIS DLB group had baseline VH compared to 0% of Mild-VIS DLB patients. Only 8% of the Severe-VIS and 3% Mild-VIS AD patients had baseline VH. During the follow-up period (mean=5.0 years), an additional 61% of the Severe-VIS but only 11% of the Mild-VIS DLB patients developed VH. In that period, 38% of the Severe-VIS and 20% of the Mild-VIS AD patients developed VH. After considering initial MMSE score and rate of decline, logistic regression analyses found that performance on Block Design significantly predicted the presence of VH in the DLB group but not the AD group.
Conclusions
The presence of early, severe deficits on neuropsychological tests of visuospatial skill increases the likelihood that patients with suspected DLB will develop the prototypical DLB syndrome. The presence of such deficits may identify those DLB patients whose syndrome is driven by alpha-synuclein pathology rather than AD pathology and may inform treatment plans as well as future research.
Keywords: Lewy body disease; Hallucinations, visual; Alzheimer’s disease; Visuospatial cognition
Dementia with Lewy bodies (DLB) is a common cause of dementia in the elderly accounting for an estimated 20% of all cases.(1) The disease is clinically characterized by progressive cognitive decline in the presence of visual hallucinations, mild parkinsonism, and fluctuations in alertness.(2) Of these core features, visual hallucinations (VH) are the strongest positive predictor of Lewy body pathology at autopsy, but VH are often not present during the early stages of disease.(3) In contrast, marked impairment on cognitive tests of visuospatial function is frequently apparent early in the course of DLB and a strong negative predictor of the presence of Lewy body pathology (i.e., its absence predicts absence of Lewy body pathology).(3) In addition, severity of visuospatial impairment is correlated with subsequent rate of global cognitive decline in patients with DLB.(4) These findings suggest that visuospatial impairment may be an early characteristic of a particularly strong DLB phenotype that foreshadows the later development of other DLB features such as VH.
Although VH and visuospatial deficits both contribute to the prediction of Lewy body pathology,(3) little is known about the relationship between these two features of DLB. There is some evidence that clinically-diagnosed DLB patients with VH have more severe deficits than those without hallucinations on tests of visuospatial and visuoperceptual abilities but not on tests of other cognitive abilities, but the sequence of onset was not specified.(5–7) A retrospective study of patients with autopsy-confirmed DLB showed that non-hallucinating patients with severe visuospatial impairment at initial evaluation were more likely than those with milder visuospatial deficits to develop VH during the course of their disease.(4) It should be noted, however, that this finding was based on a small sample of DLB patients and did not include non-DLB dementia (e.g., Alzheimer’s disease) patients to determine if the relationship between visuospatial impairment and VH is unique to DLB or a general feature of dementia.
The present study was designed to determine whether performance on a common neuropsychological measure of visuospatial skill was predictive of the emergence of VH in DLB. If early visuospatial impairment is a reliable indicator of an increased risk for developing VH in DLB, its presence can inform more specific treatment plans and more powerful research as well as improve caregiver education. To address this question, a retrospective examination of the development of VH as a function of the severity of visuospatial deficits at initial evaluation was undertaken with autopsy-confirmed DLB or AD patients. We hypothesized that DLB patients who entered a longitudinal study with severe visuospatial deficits would be more likely than those who entered with milder visuospatial deficits (but the same level of global cognitive impairment) to exhibit VH or to develop them over time. We further hypothesized that the relationship between VH and severity of visuospatial impairment would be unique to DLB and not evident in patients with autopsy-confirmed AD.
METHODS
Participants
Clinical data from 55 autopsy-confirmed patients with DLB and 55 patients with autopsy-confirmed AD (including 38 reported in 4) were retrospectively examined. These individuals were participants in the University of California, San Diego (UCSD) Alzheimer's Disease Research Center (ADRC) longitudinal project between 1985 and 2007. Eligible participants met the following inclusion criteria: 1) autopsy revealed no significant pathological process (e.g., hippocampal sclerosis, metabolic encephalopathy, or infarct with a clinical history of stroke) other than DLB or AD; 2) two senior staff neurologists diagnosed the patient with dementia at the initial ADRC evaluation; 3) baseline Mini-Mental State Exam (MMSE) score was between 18 and 28, inclusive; 4) a comprehensive behavioral, motor, and neuropsychological battery was completed; and 5) a reliable informant was identified and interviewed. Of the 55 DLB cases that met the inclusion criteria, 26 were clinically diagnosed with DLB. Notably, many of the patients who were included in the study were tested before the DLB syndrome was well-conceptualized. The AD comparison group comprised randomly selected AD cases that met the inclusion criteria and were within the DLB group’s range of age, education, and MMSE total score at the baseline visit. A comparable number of DLB and AD patients were prescribed a cholinesterase inhibitor (DLB: 13/55 and AD: 16/55; Χ2 (1) = 0.52; p > 0.6) and/or neuroleptic medications (DLB: 14/55 and AD: 10/55; Χ2 (1) = 0.36; p > 0.4) during the course of participation in the ADRC longitudinal study. The research protocol was reviewed and approved by the human subjects review board at the University of California, San Diego. Informed consent to participate in the present investigation was obtained at the point of entry into the longitudinal study from all patients or their caregivers consistent with California State law. Informed consent for autopsy was obtained at the time of death from the next of kin.
Procedure
Clinical Evaluation
All patients completed yearly neurologic, neuropsychiatric, and neuropsychological evaluations for the duration of their participation in the UCSD ADRC. The details of these evaluation procedures have been reported previously.(8) The neurologic evaluation included a review of history with the informant or patient, calculation of the Hachinski ischemic score,(9) clinical mental status testing, and a physical neurological examination that included the Unified Parkinson’s Disease Rating Scale (UPDRS)(10) or its equivalent (in patients assessed before the UPDRS was formalized). The neuropsychiatric evaluation consisted of interviews of the patient and informant by nurse practitioners using the Diagnostic Interview Schedules (DIS) for psychosis, depression, and substance dependence, and the Neuropsychiatric Inventory (for patients seen after 2000). The results of the interviews were reviewed by a psychiatrist to establish the presence of psychiatric symptoms and any psychiatric diagnosis. The primary focus of the present study was a report that the patient had or had not experienced well-formed visual hallucinations (e.g., seeing people, animals, or objects that were not there) not otherwise explained by acute medical illness, medication interaction, or substance use. A trained psychometrist administered the neuropsychological battery of tests of memory, language, executive function, attention, and visuospatial abilities in a quiet, well-lit room. The primary focus of the present study was baseline (i.e., initial ADRC visit) performance on the Block Design subtest from the Wechsler Intelligence Test for Children-Revised (WISC-R). This widely-used test of visuo-construction ability was used to stage severity of visuospatial disturbance in the DLB and AD patients.(11, 12) In the Block Design subtest the subject is presented with four or nine red and white blocks and asked to construct replicas of 11 designs. The score depends both upon accuracy and speed. The children’s version is used to reduce frustration that might be caused by the more challenging adult version in patients with dementia, and to avoid floor effects for as long as possible in the course of the disease. Raw scores range from 0 to 62 with higher scores indicative of better performance.
Neuropathologic Examination
Autopsy was performed within 12 hours of death using a protocol previously detailed.(14) Briefly, the left hemibrain was fixed by immersion in 10% formalin for 5–7 days. Paraffin-embedded blocks from mid-frontal, rostral superior temporal and inferior parietal neocortex, anterior cingulate gyrus, posterior cingulate gyrus, hippocampus, entorhinal cortex, basal ganglia/substantia innominata, mesencephalon, and pons were cut at 7 µm thickness for hematoxylin-eosin (H & E) and thioflavin-S counts. Total plaques, neuritic plaques, neurofibrillary tangle (NFT), and Lewy body counts were determined by the same examiner (L.A.H) using the same criteria. A modified Braak stage was obtained for each case using previously detailed methods.(15) Briefly, the modified Braak stage involves counting the number of NFT in at least five neuron clusters in layer two of the entorhinal cortex and then averaging the results. Cases with modified Braak stage I to IV have fewer than 18 tangles on average in layer two of the entorhinal cortex and sparse neocortical tangles. Modified Braak stage V cases have moderate numbers of tangles in at least two neocortical sections. In modified Braak stage VI, all neocortical areas assessed have at least moderate numbers of tangles.
The DLB cases met consensus criteria for the pathologic diagnosis of DLB based on hematoxylin-eosin (H & E) staining and antiubiquitin immunostaining,(2) and anti-α-synuclein immunostaining.(1) Cases were only construed as DLB if Lewy bodies were found in the locus coeruleus, substantia nigra, and/or nucleus basalis of Meynert, as well as in the neocortex. Because all cases categorized as DLB had neocortical as well as brainstem Lewy bodies, they would all fall into either the limbic (transitional) or neocortical categories proposed in the 1996 consensus guidelines for the pathologic diagnosis of DLB.(2) Further, all DLB cases were neocortical stage 5 or 6 according to the proposed Lewy-body based staging of brain pathology related to sporadic Parkinson’s disease.(16) Cases were not classified as DLB if Lewy bodies were only found in the amygdala.(1) Lewy bodies were absent in cases of “Pure” AD.
Statistical Analysis
Analyses were completed using SPSSv18. Age- and education-corrected z-scores [(patient score − NC group mean)/NC group standard deviation] were computed for each patient based on WISC-R Block Design data obtained from 103 well-characterized, healthy normal control (NC) subjects who participated in the ADRC longitudinal study.(13) Z-scores below −2.5 on the Block Design test indicated relatively severe visuospatial dysfunction (Severe-VIS) while scores above −2.5 indicated relatively mild dysfunction (Mild-VIS). Group differences in demographic and clinical data were compared using two-way analysis of variance (ANOVA) using Group (DLB and AD) and Visuospatial Performance (Mild-VIS and Severe-VIS) as fixed factors. Differences in the frequency of VH between the groups were tested using Chi-square analysis. The predictive value of Block Design was tested using logistic regression analysis.
RESULTS
Demographic and Clinical Characteristics
The DLB and AD groups were split into those with relatively severe (Severe-VIS) or mild (Mild-VIS) visuospatial dysfunction based on their initial performance on the WISC-R Block Design subtest (as described in the Statistical methods; See Table 1 for raw scores). This procedure resulted in 35 Severe-VIS versus 20 Mild-VIS DLB patients and 26 Severe-VIS versus 29 Mild-VIS AD patients. Demographic and clinical data are detailed in Table 1, which displays the results of the omnibus two-factor ANOVAs of group and visuospatial impairment differences on these variables. As expected, no significant differences were found with respect to age or years of education. Chi-square analysis revealed no difference in the sex distribution of the groups.
Table 1.
Key demographic and clinical characteristics of Dementia with Lewy bodies (DLB) and Alzheimer’s Disease (AD) groups with severe (Severe-VIS) or mild (Mild-VIS) visuospatial deficits at the initial evaluation. Differences were tested with a two-way ANOVA using Group and Visuospatial Impairment as factors. Superscripts annotate significant main or interaction effects.
| DLB | AD | |||||
|---|---|---|---|---|---|---|
| Severe-VIS n = 35 |
Mild-VIS n = 20 |
Severe-VIS n = 26 |
Mild-VIS n = 29 |
|||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Overall Significance of Differences |
Partial Eta squared |
|
| Age | 73.8 (5.4) | 75.2 (6.0) | 74.6 (7.5) | 73.5 (6.1) | F[3,106] = 0.36; p > 0.7 | 0.01 |
| Education | 15.1 (3.0) | 14.0 (2.8) | 15.2 (2.8) | 13.9 (3.2) | F[3,106] = 1.41; p > 0.2 | 0.04 |
| Sex (% men) | 71% | 55% | 73% | 62% | χ2[3] = 2.3; p > 0.5* | |
| Mini-Mental State Exam Score | 21.8 (2.8) | 23.3 (3.1) | 21.7 (2.6) | 24.2 (3.0) | F[3,106] = 5.09; p < 0.01a | 0.13 |
| Pfeffer Outpatient Disability Scale | 13.6 (4.0) | 11.4 (5.1) | 11.9 (4.3) | 10.4 (4.2) | F[3,104] = 3.03; p < 0.05a | 0.08 |
| Duration between initial test and death, y | 4.0 (2.8) | 7.1 (4.1) | 4.1 (2.3) | 6.0 (3.7) | F[3,104] = 5.23; p < 0.01 a | 0.13 |
| WISC-R Block Design | 6.0 (6.6) | 28.9 (9.1) | 8.7 (6.8) | 31.5 (8.4) | F[3,106] = 85.27; p < 0.001a | 0.70 |
| Boston Naming Test | 22.5 (4.6) | 21.2 (5.1) | 20.4 (5.7) | 24.5 (3.5) | F[3,106] = 3.93; p < 0.05b | 0.10 |
| Dementia Rating Scale Memory Subtest | 16.2 (4.7) | 15.1 (4.2) | 14.8 (4.5) | 16.8 (4.2) | F[3,101] = 1.10; p > 0.3 | 0.03 |
| Lewy body count (midfrontal) | 6.2 (8.8) (n = 20) |
4.5 (6.9) (n = 12) |
----- | ----- | t[30] = 0.55; p > 0.5 | 0.01 |
| Modified Braak stage | 2.9 (1.7) | 4.6 (1.4) | 5.3 (0.9) | 5.4 (0.8) | χ2[3] = 46.0; p <0.001 *c | |
Analyzed with nonparametric Kruskal-Wallis test
Significant main effect of Visuospatial Impairment such that Severe-VIS are worse than Mild-VIS patients
Significant Group × Visuospatial Impairment interaction such that AD Severe-VIS worse than AD Mild-VIS patients
DLB Severe-VIS < DLB Mild-VIS < AD Mild-VIS = AD Severe-VIS
A two-way ANOVA revealed an overall difference in MMSE total score such that there was a main effect of Visuospatial Impairment with the Severe-VIS groups more impaired than the Mild-VIS groups (F(1, 106) = 12.8; p < 0.01; partial η2 = 0.13) by an average of two points. There was no significant Group or Group × Visuospatial Impairment effect. Sixty percent (21/35) of the DLB Severe-VIS group exhibited extrapyramidal signs (EPS) compared with only 15% (3/20) of the DLB Mild-VIS group (χ2 (1) = 10.5; p < 0.01). Roughly 35% of the AD groups had EPS regardless of the severity of visuospatial impairment (Severe-VIS = 9/26; Mild-VIS = 11/29; χ2 (1) = 0.07; p > 0.7). There were no significant differences in the Mattis Dementia Rating Scale memory subtest score. The groups did, however, differ significantly in their confrontation naming as measured by the Boston Naming Test (see Table 1). There was a significant Group × Visuospatial Impairment interaction (F(1, 106) = 8.9; p < 0.01; partial η2 = 0.08) such that within the AD group, Severe-VIS patients exhibited greater naming deficits than Mild-VIS patients (t(53) = −3.3; p < 0.01), but there was no difference within the DLB group. There was an overall difference in baseline functional disability and the interval between baseline testing and death (see Table 1). ANOVA revealed no main effects of Group or Group × Visuospatial Impairment interactions, but a significant main effect of Visuospatial Impairment indicated that the Severe-VIS groups were more functionally disabled (F(1, 104) = 4.8; p < 0.05; partial η2 = 0.04) and died within a shorter period of time (F(1, 106) = 15.8; p < 0.001; partial η2 = 0.13) than the Mild-VIS groups. With respect to pathology, a nonparametric Kruskal-Wallis test determined a significant group difference in the modified Braak score (χ2 (3) = 46.02; p < 0.001) such that the Severe-VIS DLB group had a lower modified Braak score compared to all other groups and the Mild-VIS DLB group had a lower modified Braak score compared to both of the AD groups. There was no significant difference between the two DLB groups in the number of midfrontal Lewy bodies.
Distribution of VH at Baseline
Despite similar levels of global cognitive dysfunction, a significantly higher percentage of Severe-VIS (40%) than Mild-VIS (0%) DLB patients endorsed well-formed VH at the baseline visit (χ2(1) = 10.7; p < 0.01). In other words, 14/35 DLB patients with severe visuospatial impairment were experiencing or had experienced VH by their initial ADRC visit. In contrast, very few patients with AD were identified as having current or past VH, and the percentage of Severe-VIS (8%) and Mild-VIS (3%) AD patients with VH was not significantly different (χ2(1) = 0.48; p > 0.4). Overall, a significantly higher percentage of DLB patients (26%) than AD patients (6%) had current or past VH at their initial evaluation (χ2(1) = 8.4; p < 0.01). (see Table 2)
Table 2.
The number of Dementia with Lewy Bodies and Alzheimer’s disease patients who presented with or without visual hallucinations at the initial evaluation as a function of severity of visuospatial impairment [severe visuospatial deficits (Severe-VIS) and mild visuospatial deficits (Mild-VIS)].
| Dementia with Lewy Bodies | Alzheimer’s Disease | |||
|---|---|---|---|---|
| Severe-VIS | Mild-VIS | Severe-VIS | Mild-VIS | |
| Visual Hallucinations | 14 | 0 | 2 | 1 |
| No Visual Hallucinations | 21 | 20 | 24 | 28 |
Distribution of VH at Follow-up
Thirty-six of the 41 DLB patients (18 Severe-VIS and 18 Mild-VIS) and 46 of the 52 AD patients (21 Severe-VIS and 25 Mild-VIS) who did not have current or past VH at baseline were reevaluated annually for 1 to 18 additional years (mean years of follow-up; DLB: Severe-VIS = 4.2 ± 3.0 and Mild-VIS = 6.5 ± 4.5, AD: Severe-VIS = 4.3 ± 2.4 and Mild-VIS = 5.8 ± 4.0). A similar percentage of DLB (36%) and AD (28%) patients developed new VH after the initial evaluation (χ2(1) = 0.58; p > 0.4), but emergence of VH only varied by visuospatial impairment in the DLB group. Over the course of follow-up, a significantly higher percentage of Severe-VIS DLB patients (61%) than Mild-VIS DLB patients (11%) developed well-formed visual hallucinations (χ2(1) = 9.8; p < 0.01) (see Table 3). The percentage of Severe-VIS AD patients (38%) and Mild-VIS AD patients (20%) who developed VH was not significantly different (χ2(1) = 1.8; p > 0.1).
Table 3.
The number of Dementia with Lewy Bodies and Alzheimer’s disease patients without visual hallucinations at their initial evaluation who developed visual hallucinations during the course of follow-up. The numbers are presented as a function of severity of visuospatial impairment at the initial evaluation [severe visuospatial deficits (Severe-VIS) and mild visuospatial deficits (Mild-VIS)].
| Dementia with Lewy Bodies | Alzheimer’s Disease | |||
|---|---|---|---|---|
| Severe-VIS | Mild-VIS | Severe-VIS | Mild-VIS | |
| Visual Hallucinations | 11 | 2 | 8 | 5 |
| No Visual Hallucinations | 7 | 16 | 13 | 20 |
| No Follow-up | 3 | 2 | 3 | 3 |
Overall Distribution of VH
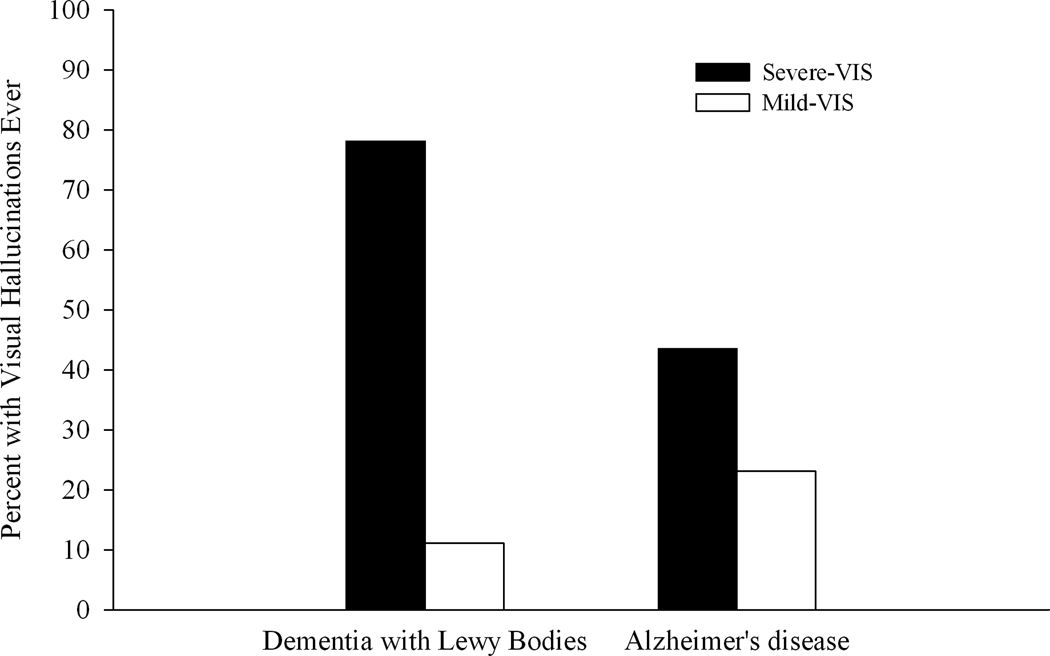
Excluding those patients without follow-up, a higher percentage of DLB patients (27/50 or 54%) than AD patients (16/49 or 33%) were identified as having well-formed VH at some point during the course of their disease (χ2(1) = 4.6; p < 0.05). In addition, the groups differed in the nature of the relationship between VH and severity of visuospatial impairment (see Figure 1). VH occurred in nearly 80% of the DLB patients with severe baseline visuospatial deficits but only 11% of those DLB patients with mild baseline visuospatial deficits (χ2(1) = 20.8; p < 0.001). The percentage of Severe-VIS and Mild-VIS AD patients endorsing VH was not significantly different (χ2(1) = 2.3; p > 0.1). VH emerged significantly earlier in the course of the disease in the DLB group compared to the AD group (mean years of follow-up; DLB = 1.7 ± 1.0 years: AD = 4.63 ± 3.2 years; (t (41) = 4.4; p < 0.001). Furthermore, the severity of dementia at onset of VH was milder in the DLB group than the AD group (mean MMSE total at VH onset; DLB = 19.7 ± 5.7 years: AD = 11.1 ± 10.5 years; (t (40) = −3.4; p < 0.01).
Figure 1.
The percentage of patients with Dementia with Lewy Bodies or Alzheimer’s disease who ever exhibited visual hallucinations (i.e., at the initial evaluation or during follow-up) as a function of severity of visuospatial impairment (severe visuospatial deficits: Severe-VIS:: mild visuospatial deficits: Mild-VIS).
Predictive Value of Visuospatial Impairment
To determine whether the severity of visuospatial deficits as measured by Block Design could usefully predict the development of visual hallucinations in DLB, a logistic regression analysis with backward elimination was performed. The initial model included baseline MMSE score, the linear slope of longitudinal change in MMSE score, Group, baseline Block Design z-score, and Group × Block Design z-score. In each iteration of the backward elimination procedure, variables conferring no predictive advantage based on the likelihood ratio were subsequently removed from the model. The MMSE total score and the linear slope of change in MMSE score were removed from the model because they were not predictive of the development of hallucinations. The final model included Group, Block Design z-score, and the interaction of Group × Block Design z-score (χ2(3) = 23.7; p < 0.001). The interaction was the only variable left in the equation with a significant β value (Wald(1) = 3.9; p < 0.05). To characterize the interaction, the models were rerun in each group separately using the Block design z-score as the sole predictor of the presence of visual hallucinations so that a simplified prediction model could be obtained. This model provided 85% (23/27) sensitivity and 74% (17/23) specificity for identifying DLB patients who developed well-formed VH (χ2 (1) = 18.4, p < 0.001).1 Block design had no predictive value in the AD group (χ2 (1) = 1.2, p > 0.2).
CONCLUSIONS
The results of this study confirm preliminary findings that early, severe visuospatial deficits in patients with DLB predict the development of VH.(4) Specifically, in autopsy-confirmed patients with DLB, visual hallucinations were significantly more prevalent in patients who exhibited severe visuospatial dysfunction despite mild global cognitive deficits (78%) compared to those who did not (11%). Importantly, the absence of early, severe visuospatial deficits in patients who had cortical Lewy bodies at autopsy was associated with the rare occurrence of both visual hallucinations (2/18) and extrapyramidal motor signs (3/20). These data extend those reported by Tiraboschi et al. (3) who concluded that the absence of early visuospatial impairment was a strong negative predictor of Lewy bodies at death. The current results indicated that the core features of DLB are rarely present in patients with autopsy-confirmed cortical Lewy bodies who do not have severe visuospatial deficits at baseline.
The relationship between visuospatial impairment and VH was not a function of dementia severity as the DLB groups were comparable with respect to language, memory, and global cognitive functioning. It was also not a function of dementia in general. Early, severe deficits in visuospatial skills did not portend the occurrence of VH in pathology-confirmed AD patients. Patients with AD developed VH at a similar rate regardless of the severity of their visuospatial dysfunction. Onset of VH was not uncommon in the AD group, but occurred at a more advanced stage of illness when global cognition was more impaired than in the DLB group. Overall, these results indicate that assessment of visuospatial abilities is clinically useful for predicting which patients suspected of having DLB will develop VH.
The ability to identify people who are more likely to develop the prototypical DLB syndrome has important treatment implications because the development of viable therapeutics is hindered by poor diagnostic sensitivity for identifying DLB in the living.(17) As these data demonstrate, the presence of Lewy bodies at autopsy does not necessarily correspond to the strength of the DLB clinical phenotype in the living patient. McKeith and colleagues have pointed out that the development of ever more sensitive mechanisms for identifying Lewy bodies has resulted in their detection in as many as 60% of AD cases.(1) Yet, AD pathology will overshadow Lewy body pathology and result in an AD clinical syndrome in most cases. The majority of our DLB participants had some level of concomitant AD pathology (n = 48), but those DLB cases with severe visuospatial dysfunction possessed a significantly lower tangle burden (indexed by Braak stage) than those with mild visuospatial dysfunction. These data suggest that the presence of early, prominent visuospatial dysfunction delineates the DLB patients whose clinical presentation is driven by alpha-synuclein pathology.
The present data do not address the mechanism underlying the relationship between visuospatial dysfunction and the development of VH in DLB. It is unlikely that visuospatial dysfunction per se is sufficient to produce VH because AD patients with equally poor visuoperceptual deficits do not readily develop them. Some investigators have hypothesized that a combination of disturbed visual perception and fluctuations in consciousness mediated by a highly deficient cholinergic system increases the prevalence of VH in DLB.(18–20) According to this model, early pathology in the basal forebrain results in severe reductions in cholinergic projections to visual cortical association areas and the thalamic reticular nucleus.(20) This system serves a core role in visual selective attention by modulating cortical processing of sensory and associative input to adjust cortical responsiveness (e.g., signal-noise ratio) on the basis of stimulus relevance and novelty.(20–22) Another possible mechanism of VH in DLB is suggested by recent animal models that propose a role of the cholinergic system in initial visual feature binding (i.e., the process by which visual information processed separately by the dorsal and ventral visual streams is conjoined to form a unified percept). Antagonizing the cholinergic muscarinic system resulted in disturbed initial feature binding in rats.(23–25) Visuospatial processing could be degraded in patients with DLB (and more so than in AD) by cholinergic deafferentation of visual association areas if such reductions in input result in the inability to either pull the salient features from the visual scene or to successfully reintegrate those features.
A recent fMRI study suggests another mechanism by which pathology in the visual association areas might result in VH by demonstrating that visual processing can be redistributed in Lewy body diseases from posterior to anterior brain regions. Stebbins and colleagues performed fMRI while nondemented Parkinson’s disease (PD) patients viewed visual stimuli (e.g., flashing lights or apparent motion).(26) The results showed that these visual stimuli activated anterior rather than posterior brain regions in hallucinating PD patients, but activated the expected posterior brain regions in non-hallucinating PD patients. The investigators speculated that decreased sensitivity to external stimuli in the posterior regions resulted in increased aberrant “top-down” frontal activation.(26) According to the model, such disinhibition of “top-down” processing could result in the release of internally-stored visual representations, thus predisposing patients to visual hallucinations. A similar process could be at play in DLB with reduced cholinergic input resulting in disruption of the quality of visual information that is relayed to limbic and frontal brain regions. This may allow an internally-stored representation to be misinterpreted as a “real” representation of the visual scene. Thus, deficits on tests of visuospatial skills, such as Block Design, may serve as a marker for the integrity of downstream visual association areas and signal an increased risk for developing visual hallucinations.
Future studies are necessary to determine how early in the DLB disease process visuospatial impairment begins and whether visuospatial deficits are indeed related to a shift in visual processing from posterior regions to anterior regions as Stebbins and colleagues reported in Parkinson’s disease.(26) Additionally, while the use of autopsy-confirmed cases confers a degree of diagnostic certainty, the retrospective nature of the study is a limitation because of the reliance on informants’ recall of VH that may have occurred prior to entering the study. Finally, the use of the Block Design subtest as a measure of visuospatial skills raises concern that processing speed, psychomotor speed, or executive dysfunction can also be driving this relationship. Additional research will be required to determine if the relationship between the most basic tests of visuoperception and VH exists. However, ancillary analyses (not reported here) indicate that a simple drawing test (Clock Copy) that was not heavily reliant on speed or executive functions was predictive of VH in the DLB group but not the AD group.
In sum, these data demonstrate that performance on a relatively non-invasive neuropsychological test of visuospatial functioning is helpful in identifying those patients with suspected Lewy body disease who will develop the prototypical features of DLB. Lack of significant early visuospatial deficits was rarely associated with the development of VH whereas severe visuospatial dysfunction predicted the presence of VH. Realizing the relationship between early, severe visuospatial deficits and occurrence of VH may allow clinicians to more actively monitor those patients who are prone to developing VH especially given some patients’ reluctance to admit their occurrence. Educating the patient and caregivers about the nature and frequency of VH in Lewy body diseases may improve quality of life by decreasing the stigma of their experiences and eventually allow for proactive treatment. Finally, if replicated, these findings may permit more powerful therapeutic trials by increasing the homogeneity of treatment groups who have syndromes driven by pathology related to alpha-synuclein.
Acknowledgments
The study was supported by National Institutes of Health grants NS049298 and AG05131. The results of this study were presented by J.M.H. at the March, 2009, meeting of the AD/PD Conference in Prague, Czech Republic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No disclosures to report.
REFERENCES
- 1.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 2.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 3.Tiraboschi P, Salmon DP, Hansen LA, Hofstetter RC, Thal LJ, Corey-Bloom J. What best differentiates Lewy body from Alzheimer's disease in early-stage dementia? Brain. 2006;129:729–735. doi: 10.1093/brain/awh725. [DOI] [PubMed] [Google Scholar]
- 4.Hamilton JM, Salmon DP, Galasko D, et al. Visuospatial deficits predict rate of cognitive decline in autopsy-verified dementia with Lewy bodies. Neuropsychology. 2008;22:729–737. doi: 10.1037/a0012949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simard M, van Reekum R, Myran D. Visuospatial impairment in dementia with Lewy bodies and Alzheimer's disease: a process analysis approach. Int J Geriatr Psychiatry. 2003;18:387–391. doi: 10.1002/gps.839. [DOI] [PubMed] [Google Scholar]
- 6.Mori E, Shimomura T, Fujimori M, et al. Visuoperceptual impairment in dementia with Lewy bodies. Arch Neurol. 2000;57:489–493. doi: 10.1001/archneur.57.4.489. [DOI] [PubMed] [Google Scholar]
- 7.Mosimann UP, Mather G, Wesnes KA, O'Brien JT, Burn DJ, McKeith IG. Visual perception in Parkinson disease dementia and dementia with Lewy bodies. Neurology. 2004;63:2091–2096. doi: 10.1212/01.wnl.0000145764.70698.4e. [DOI] [PubMed] [Google Scholar]
- 8.Salmon D, Butters N. Principles of geriatric neurology. New York: FA Davis; 1992. Neuropsychologic assessment of dementia in the elderly; pp. 144–163. [Google Scholar]
- 9.Hachinski VC, Iliff LD, Zilhka E, et al. Cerebral blood flow in dementia. Arch Neurol. 1975;32:632–637. doi: 10.1001/archneur.1975.00490510088009. [DOI] [PubMed] [Google Scholar]
- 10.Fahn SER. Unified Parkinson’s disease rating scale. In: Fahn SMC, Goldstein MCD, editors. Recent developments in Parkinson’s disease. Florham Park: Macmillan Healthcare Information; 1987. pp. 153–163.pp. 293–304. [Google Scholar]
- 11.Capruso D, Hamsher K, Benton A. Clinical evaluation of visual perception and constructional ability. In: Snyder P, Robins D, editors. Clinical neuropsychology: A pocket handbook for assessment. 2 ed. Washington, DC: American Psychological Association; 2006. pp. 547–571. [Google Scholar]
- 12.Lezak M, Howieson D, Loring D, Hannay H, Fischer J. Neuropsychological assessment. New York: Oxford University Press; 2004. [Google Scholar]
- 13.Salmon DP, Thomas RG, Pay MM, et al. Alzheimer's disease can be accurately diagnosed in very mildly impaired individuals. Neurology. 2002;59:1022–1028. doi: 10.1212/wnl.59.7.1022. [DOI] [PubMed] [Google Scholar]
- 14.Terry RD, Peck A, DeTeresa R, Schechter R, Horoupian DS. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann Neurol. 1981;10:184–192. doi: 10.1002/ana.410100209. [DOI] [PubMed] [Google Scholar]
- 15.Hansen LA, Terry RD. Position paper on diagnostic criteria for Alzheimer disease. Neurobiol Aging. 1997;18:S71–S73. doi: 10.1016/s0197-4580(97)00073-0. [DOI] [PubMed] [Google Scholar]
- 16.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 17.Nelson PT, Jicha GA, Kryscio RJ, et al. Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol. 2010;257:359–366. doi: 10.1007/s00415-009-5324-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calderon J, Perry RJ, Erzinclioglu SW, Berrios GE, Dening TR, Hodges JR. Perception, attention, and working memory are disproportionately impaired in dementia with Lewy bodies compared with Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2001;70:157–164. doi: 10.1136/jnnp.70.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collerton D, Perry E, McKeith I. Why people see things that are not there: a novel Perception and Attention Deficit model for recurrent complex visual hallucinations. Behav Brain Sci. 2005;28:737–757. doi: 10.1017/S0140525X05000130. [DOI] [PubMed] [Google Scholar]
- 20.Perry E, Walker M, Grace J, Perry R. Acetylcholine in mind: a neurotransmitter correlate of consciousness? Trends Neurosci. 1999;22:273–280. doi: 10.1016/s0166-2236(98)01361-7. [DOI] [PubMed] [Google Scholar]
- 21.Voytko ML. Cognitive functions of the basal forebrain cholinergic system in monkeys: memory or attention? Behav Brain Res. 1996;75:13–25. doi: 10.1016/0166-4328(95)00143-3. [DOI] [PubMed] [Google Scholar]
- 22.Muir JL, Page KJ, Sirinathsinghji DJ, Robbins TW, Everitt BJ. Excitotoxic lesions of basal forebrain cholinergic neurons: effects on learning, memory and attention. Behav Brain Res. 1993;57:123–131. doi: 10.1016/0166-4328(93)90128-d. [DOI] [PubMed] [Google Scholar]
- 23.Botly LC, De Rosa E. Cholinergic deafferentation of the neocortex using 192 IgG-saporin impairs feature binding in rats. J Neurosci. 2009;29:4120–4130. doi: 10.1523/JNEUROSCI.0654-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Botly LC, De Rosa E. A cross-species investigation of acetylcholine, attention, and feature binding. Psychol Sci. 2008;19:1185–1193. doi: 10.1111/j.1467-9280.2008.02221.x. [DOI] [PubMed] [Google Scholar]
- 25.Botly LC, De Rosa E. Cholinergic influences on feature binding. Behav Neurosci. 2007;121:264–276. doi: 10.1037/0735-7044.121.2.264. [DOI] [PubMed] [Google Scholar]
- 26.Stebbins GT, Goetz CG, Carrillo MC, et al. Altered cortical visual processing in PD with hallucinations: an fMRI study. Neurology. 2004;63:1409–1416. doi: 10.1212/01.wnl.0000141853.27081.bd. [DOI] [PubMed] [Google Scholar]