Abstract
Increased physical activity is associated with successful long-term weight loss maintenance due to mechanisms likely more complex than simply increased energy expenditure. The impact of physical activity on the central regulation of food intake may be an important mechanism of this effect. The objective of this study was to examine the effects of exercise training and acute exercise on the neuronal response to food cues as well as eating behaviors. fMRI was performed in the fasted state at baseline and again after a 6month progressive exercise intervention (supervised, 5 days/wk) both with and without an acute exercise bout in 12 overweight/obese (5 women, 7 men; BMI 33±4 kg/m2) healthy adults. fMRI data were acquired while subjects were presented with visual stimuli of foods of high hedonic value as compared to neutral control objects. Questionnaires on eating behaviors, ratings of appeal and desire for foods, and ratings of appetite (hunger, satiety, prospective intake) using visual analog scales were also performed at baseline and again after the 6-month exercise intervention. While only a trend was observed for a reduction in body weight (102±5 to 99±6 kg, p=0.09), a significant reduction in fat mass was observed (36.4±2.8 to 33.7±3.2 kg, p=0.04), although as expected changes in fat mass were variable (−10.0 to +3.7 kg). Chronic exercise was associated with a reduction in the neuronal response to food, primarily in the posterior attention network and insula. A significant positive correlation between change in fat/body mass and change in insula response to food cues with chronic exercise was observed. An acute exercise bout attenuated the effects of chronic exercise. The exercise intervention, however, did not impact any of the measures of appetitive behavior. In summary, despite no effects on behavioral measures of appetite, chronic exercise training was associated with attenuation in the response to visual food cues in brain regions known to be important in food intake regulation. The insula, in particular, appears to play an important role in potential exercise-induced weight loss and weight loss maintenance.
Keywords: fMRI, neuroimaging, physical activity, food intake, appetite
1. Introduction
The regulation of energy intake is a complex process requiring the integration of physiologic or homeostatic signals and non-homeostatic signals, both internal and external. Much has been learned about the homeostatic regulation of energy balance and the effects of adiposity and gut signals on hunger and satiety [1, 2]. Ultimately, however, the decision to initiate food intake, how much to consume, and when to terminate a meal is affected by not only these homeostatic mechanisms but also by ‘non-homeostatic’ mechanisms such as learned and motivated behaviors, cognitive factors, habits, social context, availability of food, external sensory cues and the integration of these different sensory inputs [3–5].
Exercise and/or physical activity interventions have been shown to have modest (<3%) but highly variable effects on weight loss [6]. Exercise accentuates diet-induced weight loss, but more importantly exercise has been shown to be central to the prevention of weight gain and/or weight regain [7–9]. While the increased energy expenditure associated with exercise is key to its effects on energy balance, a complex interaction with energy intake is likely also important. The impact of exercise or physical activity on eating behaviors, appetite, and energy intake has been studied and reviewed recently elsewhere [10–12]. While high intensity exercise has been shown to acutely reduce hunger ratings [13, 14], other studies of acute exercise have shown no effects on appetite or energy intake [15, 16]. Overall acute exercise does not appear to have a significant impact on appetite or energy intake as might be expected from the exercise-induced negative energy balance [10–12, 15, 16]. There appears to be, though, significant variability in these responses [16] with, for example, men having more of a reduction in hunger than women with acute exercise [17]. More importantly from a body weight regulation perspective are the effects of chronic exercise on ingestive behavior. Long-term exercise interventions have shown to result in only modest to no effects on appetite ratings and energy intake [11, 15, 16, 18]. Again, these effects appear to be highly variable when examined at an individual level [12, 19, 20]. Nevertheless, why exercise, either acute or chronic, does not induce greater compensation in appetite and energy intake is unclear.
With the use of neuroimaging techniques, which are less subjective than behavioral and appetite measures, we have begun to better understand the neural circuitry associated with the processes involved in ingestive behavior. A number of studies have examined the neuronal response to visual food cues, finding involvement of a network of brain regions known to be important in reward, motivation, attention, memory, inhibitory behavior as well as energy homeostasis [21–29]. The salience of the food-related stimulus, nutritional state, and obesity status also appear to modulate these responses, further implicating these networks as central to the regulation of ingestive behavior [22, 26, 28–32]. A recent meta-analysis has found consistent activation in posterior visual cortex, orbitofrontal cortex and insula in response to food cues [33]. Furthermore, the insula response has been shown to be modulated by alterations in acute/chronic energy balance as well as by peptides and hormones known to be important in energy intake regulation suggesting that this brain region plays an important role in these processes [22–24, 26, 27, 34–40]. To our knowledge, however, there are no neuroimaging studies that have examined how exercise or physical activity modulates the neuronal response to visual food cues.
Based on these observations we hypothesized that despite a relative negative energy balance, measures of eating and appetitive behaviors would not be significantly affected by a chronic exercise intervention. Furthermore, we hypothesized that chronic exercise would attenuate the neuronal response to food, potentially explaining the lack of enhanced appetitive and reward-related eating behaviors. The present study was designed to examine these hypotheses.
2. Methods
2.1. Research Participants
Healthy, overweight to moderately obese (mean BMI 33.3 ± 4.3 mg/kg2) adult men and women (mean 38.2 ± 9.5 years) were recruited and screened. The study was approved by the Colorado Multiple Institutional Review Board, and all subjects gave informed consent. Eligible participants were free of metabolic and psychiatric disease and eating disorders and were not actively dieting (purposely restricting food intake for weight control) at the time studies were performed. Twelve participants (5 women and 7 men) were enrolled into the study. All but two participants were right-handed. No differences were observed when data were analyzed without the left-handed participants, so all participants were included in the final analyses.
2.2. Study Design and Measurements
Participants first underwent baseline assessments, including 3-day diet diary, body composition measurement by dual-energy x-ray absorptiometry (DPX whole-body scanner, Lunar Radiation Corp., Madison, WI), measurements of resting metabolic rate (RMR) by standard hood indirect calorimetry (TrueOne 2400 metabolic cart, Parvomedics, Sandy, UT), and maximal aerobic capacity measured on a motor-driven treadmill.
Measures of food intake, eating-related behaviors and, neuronal responses to visual food cues as measured by functional magnetic resonance imaging (fMRI) were performed at baseline and then again after a 6-month exercise intervention (described below). Post-intervention fMRI measures were performed twice in random order, once without an acute exercise bout (chronic exercise) to evaluate the chronic effects of exercise training and once within 30 minutes after an acute exercise bout (chronic + acute exercise) to evaluate the acute effects of exercise. Body composition and a 3-day diet diary were also measured after the 6-month exercise intervention.
2.3. Exercise Intervention
Participants were recruited from a larger study designed to evaluate the effects of a 24 week exercise intervention on components of total daily energy expenditure. Individualized exercise prescriptions were designed to target an increase of 2500 kcal per week. Subjects performed a supervised treadmill walking program that gradually increased in intensity (60% to 75%) and duration (~15–20 minutes/day to 40–60 minutes day) to achieve a target workloads (500 kcal/day at 75% of V02max) by week 18. The theoretical weight loss was approximately ~6.8 kg during the 6 month intervention. The exercise prescription was calculated from the maximal aerobic capacity test performed at baseline and updated according to submaximal exercise tests performed every 6 weeks. Subjects were required to attend ≥75% of the scheduled exercise sessions. Body weight was recorded weekly under standardized conditions using a digital scale.
2.4. Non-fMRI Assessments
Participants completed the following measurements before and again after the 6-month exercise intervention after an overnight fast and in the setting of no acute exercise for 24 hours. These assessments were performed on a different day than the fMRI assessments described below. Participants completed the following questionnaires: the Three Factor Eating Inventory, Power of Food Scale, Craving and Mood Questionnaire (CMQ), and Food Craving Inventory (FCQ-S). Fasting blood sampling was performed and analyzed for leptin concentration as determined by radioimmunoassay (Linco Research, Inc., St. Charles, MO). Participants also completed hunger, satiety and appetite ratings by visual analog scale (VAS) before and every 30 minutes for 180 minutes after a test meal breakfast. The meal was served at 7:30 AM and provided 30% of their estimated daily energy intake (55% carbohydrate, 35% fat, 15% protein). Estimates of daily energy needs were made using baseline RMR and lean body mass plus an activity factor of 1.4. The entire meal was required to be consumed. The meal was prepared and provided by the University of Colorado Clinical Translational Research Center (CTRC) kitchen. Hunger was rated by VAS on a line preceded by the question, “How hungry are you right now?” and anchored on the left by “not at all hungry” and by “extremely hungry” on the right. Other questions addressed satiety and prospective food consumption. The questions were downloaded onto a personal data assistant (PDA). The participants were instructed on how to use the PDA and question program.
2.5. Functional Magnetic Resonance Imaging (fMRI)
Within one week of the non-fMRI measurements, participants presented to the Brain Imaging Center at the University of Colorado School of Medicine in the morning after an overnight fast at approximately 8:00 AM. Subjects were asked to not consume any food after 10PM the night before. On one occasion subjects presented without acute exercise for 24 hours and on a separate occasion subjects presented within 30 minutes of an acute exercise bout. The bout was a typical treadmill exercise session for that subject and so varied in intensity (60–75% Vo2max) and duration (40–60 minutes) but was intended to achieve a target workload of 500 kcal.
Prior to scanning, single, fasting VAS measures of appetite were performed. Imaging studies were performed using a GE 3.0 T MR scanner. Prior to functional imaging, a high-resolution, T1-weighted 3D anatomical scan over 10 minutes was acquired for each subject. Functional images were then acquired with an echo-planar gradient-echo T2* blood oxygenation level dependent (BOLD) imaging contrast technique, with TR = 2000 ms, TE = 30 ms, 642 matrix, 240 mm2 FOV, 27 axial slices angled parallel to the planum sphenoidale, 2.6 mm thick, 1.4 mm gap. Additionally, one inversion-recovery echo-planar-image (TI=505ms) volume was acquired to improve coregistration between the echo planar images and gray matter templates used in preprocessing. Head motion was minimized with a VacFix head-conforming vacuum cushion (Par Scientific A/S, Odense, Denmark). Functional imaging was performed while the participants were presented visual stimuli using a projector and screen system. Previously validated visual stimuli consisted of three different categories: neutral nonfood-related objects, foods of high hedonic value, and foods of neutral hedonic value [41]. Because previous studies have shown that comparisons involving neutral food objects to be qualitatively similar but less sensitive [29], the primary analysis examined differences between hedonic foods and nonfood objects. Two runs were performed with each run consisting of a pseudo-randomized block design with 6 blocks of pictures of each category. Seven blocks of a low-level baseline (fixation cross) were also included in each run. Each block consisted of 4 stimuli shown for 4 seconds each for a total of 16 seconds per block. Four additional scans were acquired at the beginning of each run to minimize saturation effects. Subjects were asked lie quietly and to view the images. Total scanning time was no more than 30 minutes.
Following each fMRI session participants were asked to rate the images of foods visualized in the scanner. The images were presented to participants one at a time in a random order. For each image participants were asked to rate by VAS: 1) “how appealing is the image?” 2) “what is your desire to eat this food item?” and 3) “how pleasant is the image?” Desire to eat was specifically defined as the drive to consume some of the presented food at that point in time.
2.6. Calculations and Statistical Analyses
fMRI data were analyzed using SPM8 (Wellcome Dept. of Imaging Neuroscience, London). Data analyses were blind to treatment group. Data from each subject were realigned to the first echo-planar image, normalized to the Montreal Neurological Institute (MNI) template, using a gray-matter-segmented IR-EPI as an intermediate to improve registration, and smoothed with an 8 mm FWHM Gaussian kernel. The hemodynamic response was modeled with a double gamma function, without temporal derivatives, using the general linear model in SPM8. A 128s high pass filter was applied to remove low-frequency fluctuation in the BOLD signal. To account for both within-group and within-subject variance, a random effects analysis was implemented. Parameter estimates for each individual’s first level analysis (SPM contrast images) contrasting “hedonic food cues” to “nonfood objects” were entered into second-level repeated measures ANOVA. Comparisons across conditions were evaluated with directional contrasts (SPM t-contrasts). Results were considered significant at a whole-brain level if they exceeded a voxel-wise threshold of p < 0.01 and a cluster extent of 67 voxels. This threshold corresponds to a whole-brain cluster corrected level of p < 0.01, based on 10,000 Monte Carlo simulations using AlphaSim in AFNI (http://afni.nimh.nih.gov/afni/). Results in all figures are statistical parametric maps (i.e. colored voxels indicate t-values), thresholded at the above level, overlaid on the group average anatomical image.
Non-imaging analyses were performed using SigmaStat software (Jandel Scientific, San Rafael, CA, USA). The total area under the curve for appetite VAS ratings using all 30 minute time points over three hours post test meal were used. The effects of chronic exercise as compared to baseline were analyzed using a paired t-test. A two-way repeated measures analysis of variance (ANOVA) was used when analyzing acute and chronic effects of exercise. Significance tests were two-sided with significance set at level 0.05. Finally, the Pearson Product Correlation between the fMRI, behavioral, appetite and intake measures was examined.
3. Results
3.1. Body Weight and Leptin
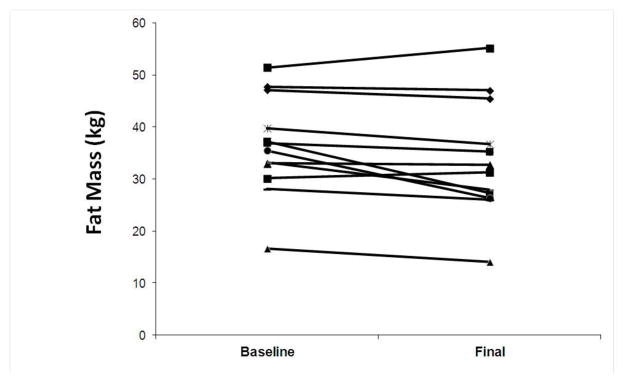
A trend was observed for a reduction in body weight in response to the exercise intervention (101.5±4.9 to 98.7±5.8 kg, p=0.09). Changes in body weight, however, were highly variable, ranging from 12 kg of weight loss to 4 kg of weight gain. Fat mass (36.4±2.8 to 33.7±3.2 kg, p=0.04) as shown in Figure 1 and percent body fat (36.5±1.9 to 34.4±2.0%, p=0.01) were reduced significantly albeit modestly with the exercise intervention and accounted for the total weight loss. Again, the changes in fat mass were highly variable ranging from 10 kg of fat mass loss to almost 4 kg of fat mass gain. There was a significant reduction in leptin concentration after the 6-month exercise intervention (32.1±5.7 to 20.3±4.6 ng/ml, p=0.03). While leptin concentrations correlated with fat mass overall, changes in leptin concentration did not correlate with changes in body weight or fat mass.
Figure 1.
Changes in fat mass in response to a 6-month exercise intervention.
3.2. Appetite and Behavioral Assessments
Behavioral and appetite assessments were performed at baseline and again after the 6-month exercise intervention and are summarized in Table 1. Despite modest weight/fat loss and reductions in leptin, there were no changes in ratings of dietary restraint or disinhibition, food cravings, Power of Food Scale, or food desire and appeal. Ratings of hunger, PFC, and satiety, as measured by the area under the curve in response to a breakfast meal, were also not impacted by the chronic exercise intervention. Fasting ratings of appetite (hunger, PFC, and satiety) and food appeal and desire were also measured in the “chronic + acute exercise” condition but were unchanged as compared to chronic exercise or baseline. Behavioral and appetite measures did not correlate with leptin concentrations, body weight, or fat mass. While changes in disinhibition scores were correlated with changes in body weight (r= 0.52, p=0.04), changes in all other behavioral and appetite measures did not correlate with changes body weight, fat mass or leptin.
Table 1.
Eating Behaviors and Appetite (Mean ± SEM).
| Baseline | Chronic Exercise | |
|---|---|---|
| Restraint | 8.4 ± 1.1 | 7.8 ± 1.4 |
| Disinhibition | 7.9 ± 1.2 | 7.5 ± 1.0 |
| PFSa | 52.7 ± 6.1 | 48.2 ± 4.5 |
| Food Cravings | ||
| CMQb | 37.9 ± 7.4 | 35.2 ± 5.6 |
| FCQ-Sc | 40.5 ± 4.1 | 39.6 ± 4.2 |
| Hunger AUCd | 166.2 ± 35.4 | 187.0 ± 35.7 |
| PFCe AUC | 222.7 ± 37.2 | 226.8 ± 32.8 |
| Satiety AUC | 412.4 ± 48.3 | 390.0 ± 44.4 |
PFS: Power of Food Scale;
CMQ: Craving and Mood Questionnaire;
FCQ-S: Food Craving Inventory;
AUC: area under the curve in response to a meal;
PFC: prospective food consumption.
A 3-day diet diary was also performed at baseline and after the chronic exercise intervention. Overall, mean self-reported total energy intake as assessed by the diet diary was reduced after the exercise intervention (2192 ± 208 to 1980 ± 159 kcal/d, p = 0.049) even when expressed per fat free mass (36.7 ± 2.7 to 31.4 ± 2.0 kcal/kg/d, p = 0.046). These changes in self-reported energy intake, though, did not correlate with changes in any of the other outcomes, including behavioral and appetite measures or neuronal responses to visual food cues. Furthermore, there were no significant changes in self-reported macronutrient composition as assessed by the 3-day diet diary.
3.3. fMRI
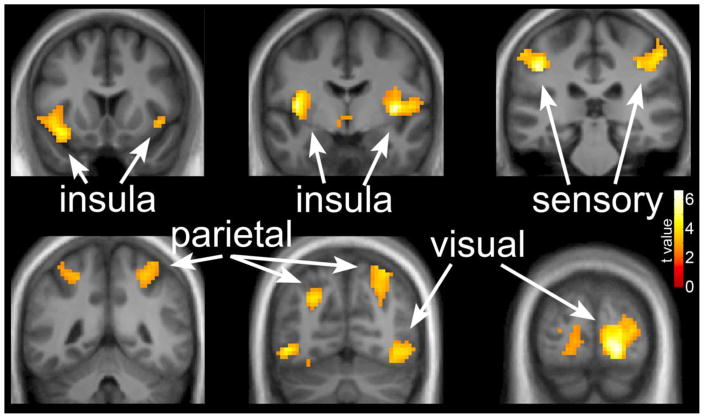
The neuronal response to visual stimuli as measured by fMRI was examined in the fasted state at baseline and again after the 6-month exercise intervention without an acute exercise bout (chronic exercise) and after an acute exercise bout (chronic + acute exercise). In the baseline condition, response to hedonic visual food cues compared to nonfood objects was observed in a network of brain regions, including the bilateral insular cortices, somatosensory cortices, parietal cortices and visual cortex, (Figure 2) similar to our previous reports [29, 34]. MNI coordinates and significance levels are shown in Table 2.
Figure 2.
The neuronal response across all subjects to images of foods of high hedonic value as compared to nonfood objects at baseline before the exercise intervention. Greater activation is observed in a network of brain regions, including the bilateral insular cortices, somatosensory cortices, parietal cortices and visual cortex. Data are shown in the radiological convention (right hemisphere on the left).
Table 2.
Brain regions that responded to visual food cues during the baseline condition.
| Brain Region | MNI Coordinatesa | T Valueb | ||
|---|---|---|---|---|
| x | y | z | ||
| Visual Cortex, primary (L) | −18 | −90 | −6 | 6.64 |
| Visual Cortex, Inferior (R) | 48 | −66 | −6 | 4.54 |
| Somatosensory Cortex (R) | 45 | −33 | 39 | 6.24 |
| Somatosensory Cortex (L) | −48 | −36 | 60 | 4.61 |
| Parietal Cortex (R) | 27 | −69 | 36 | 4.27 |
| Parietal Cortex (L) | −27 | −69 | 54 | 4.09 |
| Insula (L) | −39 | −6 | 6 | 6.1 |
| Insula (R) | 39 | −6 | 6 | 5.76 |
Stereotactic Coordinates in MNI space. T values reported for local maxima within clusters.
Significant at a whole-brain voxel-wise threshold of p < 0.01 with a cluster-corrected level of p < 0.01.
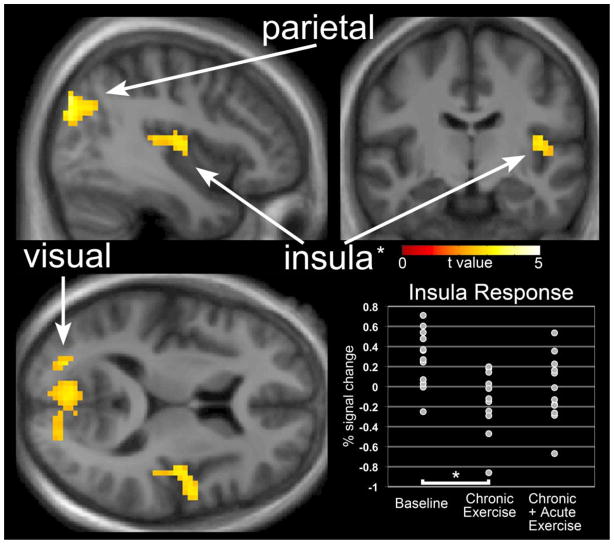
Reduced responses to visual food cues following “chronic exercise” compared to baseline were observed in the bilateral parietal cortices, left insula and visual cortex (Figure 3). MNI coordinates and significance levels are shown in Table 3. No differences between the baseline condition and “chronic + acute exercise” were observed. Responses in the “chronic + acute exercise” condition were intermediate between the baseline and “chronic exercise” conditions (shown for insula in Figure 3 plot). This pattern reached significance in the parietal region such that the response associated with “chronic exercise” was significantly lower (t = 5.02, x = −36, y = 81, z = 36) than the response associated with “chronic + acute exercise.”
Figure 3.
The difference in neuronal response to foods of high hedonic value as compared to nonfood objects following “chronic exercise” as compared to “baseline”. Reduced responses to visual food cues following “chronic exercise” were observed in the bilateral parietal cortices, left insula and visual cortex. No differences between “baseline” and “chronic + acute exercise” were observed. Inset: insula responses in the “chronic + acute exercise” were intermediate between “baseline” and “chronic exercise” conditions.
Table 3.
Brain regions with reduced responses to visual food cues following chronic exercise.
| Brain Region | MNI Coordinatesa | T Valueb | ||
|---|---|---|---|---|
| x | Y | z | ||
| Parietal Cortex (R) | 33 | −81 | 36 | 4.89 |
| 21 | −63 | 36 | 2.97 | |
| 18 | −75 | 30 | 2.86 | |
| Parietal Cortex (L) | −39 | −81 | 33 | 4.42 |
| −45 | −78 | 24 | 3.35 | |
| Insula (L) | −48 | −6 | 6 | 3.35 |
| −42 | −12 | 9 | 3.08 | |
| −54 | −6 | −3 | 2.82 | |
| Visual Cortex | 6 | −78 | 9 | 2.76 |
| 24 | −81 | 12 | 2.74 | |
Stereotactic Coordinates in MNI space. T values reported for local maxima within clusters.
Significant at a whole-brain voxel-wise threshold of p < 0.01 with a cluster-corrected level of p < 0.01
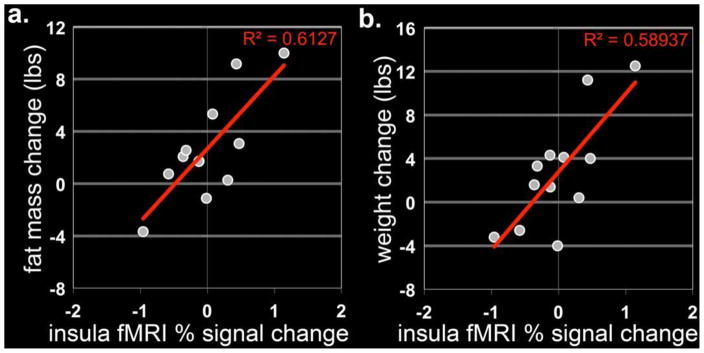
Changes in anterior insula responses between the baseline condition and “chronic exercise” were positively correlated with changes in (r2=0.61, p=0.003) and body weight (r2=0.58, p=0.004) as shown in Figure 4, such that the greater the attenuation in insula response with exercise the greater the fat mass and weight loss or vice versa. The difference in insula and visual cortex responses between the baseline condition and “chronic exercise” were also positively correlated with change in leptin concentrations (r2=0.64, p<0.001 and r2=0.82, p=0.001, respectively). Furthermore, there was an inverse correlation between the change in neuronal response in the hypothalamus and the change in leptin with exercise (r2=0.58, p=0.003).
Figure 4.
Correlation between neuronal response following exercise and the change in fat mass (a) and body weight (b). The difference in anterior insula responses between the baseline condition and “chronic exercise” were positively correlated with change in fat mass and body weight.
4. Discussion
The present study was performed to examine the effects of exercise on the neuronal response to food-related visual cues as well as on various measures of appetite and eating-related behavior. The results of this study demonstrate that a 6-month exercise intervention is associated with attenuation in the response to visual food cues in brain regions known to be important in food intake regulation. The insula, in particular, appears to play an important role in potential exercise-induced weight loss and weight loss maintenance. Measures of eating-related behaviors and appetite, however, were not affected by the exercise intervention even despite modest weight/fat loss, suggesting that exercise may attenuate the changes in ingestive behavior expected with negative energy balance. Alternately, the observation of a neuronal change correlated with body weight and fat mass change in the absence of effects on behavioral measures may suggest that neuronal measures of food intake behaviors are more sensitive than behavioral measures.
As we have consistently shown in previous studies, the neuronal response to food-related visual cues is complex, associated with the activation of a network of brain regions, including the insula, somatosensory cortices, parietal cortices and visual cortex [29, 34]. The activation of a number of these regions is consistent with increased attention to food cues and enhanced motivation to eat and implicates these regions as important in the regulation of food intake.
It has been previously shown that alterations in energy balance impact the neuronal response to food cues. We, for example, have shown that overfeeding-induced positive energy balance results in attenuation of the response to food cues [29, 34]. Similarly, the acutely fed state has also been shown to be associated with reduced neuronal response to food cues [42]. Conversely, prolonged fasting has been shown to result in enhanced response to food cues in brain regions important in motivation, reward and attention [23–25, 43]. We are aware of only one published study examining the effects of weight loss in a longitudinal manner on the neuronal response to food cues. In this study, Rosenbaum et al, showed that the weight-reduced state was associated with changes in the neuronal response to visual food stimuli in brain regions known to be important in homeostatic, emotional and cognitive regulation of energy intake, including increased activity in the limbic system and in brain regions important in executive and decision-making functions and decreased activity in brain regions important in homeostasis, emotional and cognitive control, and motor planning [44]. It might, therefore, be expected that chronic exercise, resulting in weight/fat loss, would result in similar changes in the neuronal response to food cues.
While we show in the present study that relative negative energy balance associated with chronic exercise also impacts similar brain regions, the response appears to be in the opposite direction as compared to diet-related negative energy balance. Specifically, chronic exercise resulted in diminished activation in the insula, parietal cortices, and visual cortex and did not result in increased response in any brain region. The attenuation of these brain regions known to be important in attention, visual processing and motivation, suggest that the salience of the food cues is reduced with exercise. Furthermore, the attenuation of insular response with exercise was associated with weight/fat loss. Other studies have also shown that the insula plays a central role in the regulation of ingestive behavior and may not only be the primary taste cortex but may also relate to the memory of the rewarding aspects of food and eating as well as in representing information about the internal state, ie an integration area [22, 24, 36, 37, 45–49]. Interestingly, though, an acute bout of exercise appears to attenuate the effects of chronic exercise on these responses. While most studies suggest that acute exercise does not significantly impact appetite and energy intake [12], the neuronal response to food may be a more sensitive outcome, suggesting that the acute relative negative energy balance associated with an exercise bout may in fact alter appetitive behavior.
What mechanisms underlie these observed differences in responses to visual food stimuli with chronic exercise? The effects of exercise on leptin action could be important. As has been shown by others [50–52], we found greater reductions in leptin concentrations than would be expected for the degree of fat mass loss with the exercise intervention. In spite of this, we did not see increased hunger or drive to eat and in fact found attenuation of brain regions important in motivation to eat, suggesting potential enhanced leptin sensitivity. Animal studies have shown that exercise improves leptin sensitivity as well as altering the anorexic/orexigenic responses to other mediators such as neuropeptide Y and melanin concentrating hormone [53–55]. Although one might postulate that these central effects would primarily impact homeostatic-related brain regions, fMRI studies examining leptin deficiency and replacement have shown that it can alter higher brain responses such as in the insula to food stimuli [39, 44]. Certainly these exercise effects deserve further investigation.
We did not find significant differences in measures of eating-related behaviors or appetite with acute or chronic exercise. These measures are subjective and have inherent variability, so with our small sample size we may simply not have been powered to see an effect. It may be, though, that chronic exercise attenuates appetitive behavior favoring reduced food intake and resultant weight loss and/or weight maintenance. Perhaps the attenuation in the brain response to food cues seen with chronic exercise is responsible for the less than expected appetitive responses. This may be especially true in those with the greatest suppression in insula activity who also had the greatest reduction in fat mass. Also of note, while there was no exercise-based difference in disinhibition, a correlation was observed between the change in disinhibition and change in body weight in response to the exercise intervention. Does exercise “improve” disinhibited behaviors leading to less energy intake? These effects need to be studied further.
A few limitations must also be discussed. The relatively small sample size limits generalizability. Nevertheless, significant effects of exercise on the neuronal response to food cues were still observed. We were not powered to evaluate sex-based effects. As we and others have reported there are important sex-based differences in the neuronal response to food cues [56, 57]. The effects, though, have been demonstrated in regions not related to those seen impacted by exercise in the present study. The lack of a non-exercise control group raises the concern regarding the potential effects of consecutive testing on the results. Previous fMRI studies suggest that neuronal response to the types of stimuli used in the present study are consistent over time [58]. Additionally, our observation of no significant differences between baseline and “acute exercise” conditions argues against a substantial effect purely from consecutive testing. Finally, we did not measure the effects of exercise on true ad libitum energy intake which may have impacted the lack of correlation seen between energy intake and other measures.
In conclusion, the results of this study demonstrate that chronic exercise training alters the neuronal response to food cues. A 6-month exercise intervention is associated with attenuation in the response to visual food cues in brain regions known to be important in food intake regulation. The insula, in particular, appears to play an important role in potential exercise-induced weight loss and weight loss maintenance. Measures of eating-related behaviors and appetite, however, were not affected by the exercise intervention even despite modest weight/fat loss, suggesting that exercise may attenuate the changes in ingestive behavior expected with negative energy balance. These findings emphasize the important role of external visual cues in the regulation of food intake and suggest that an intervention such as exercise can alter these responses.
Highlights.
Chronic exercise has a variable effect on weight loss.
Despite modest weight loss exercise does not impact appetitive behaviors.
Chronic exercise was associated with a reduction in the neuronal response to food, primarily in the posterior attention network and insula.
Change in BW with chronic exercise correlated with change in insula response.
An acute exercise bout attenuated the effects of chronic exercise.
Acknowledgments
We acknowledge and thank Debra Singel and Yiping Du for their assistance with the fMRI studies. We also thank the dietary services and metabolic kitchen of the University of Colorado Denver CTRC. This publication was supported by NIH/NCRR Colorado CTSI Grant Number UL1 RR025780, NIH/NIDDK Clinical Nutrition Research Unit Grant Number DK48520, NIH/NIDK R01DK07708, NIH/NCRR Grant Number RR016185 and NIH/NIDDK Grant Numbers R01DK089095 and R01DK072174. Its contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baskin DG, Figlewicz Lattemann D, Seeley RJ, Woods SC, Porte D, Jr, Schwartz MW. Insulin leptin: dual adiposity signals to the brain for the regulation of food intake body weight. Brain Res. 1999;848:114–23. doi: 10.1016/s0006-8993(99)01974-5. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz MW. Central nervous system regulation of food intake. Obesity (Silver Spring) 2006;14 (Suppl 1):1S–8S. doi: 10.1038/oby.2006.275. [DOI] [PubMed] [Google Scholar]
- 3.Berthoud HR. Mind versus metabolism in the control of food intake and energy balance. Physiol Behav. 2004;81:781–93. doi: 10.1016/j.physbeh.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 4.Watts AG. Understanding the neural control of ingestive behaviors: helping to separate cause from effect with dehydration-associated anorexia. Horm Behav. 2000;37:261–83. doi: 10.1006/hbeh.2000.1581. [DOI] [PubMed] [Google Scholar]
- 5.Zheng H, Lenard NR, Shin AC, Berthoud HR. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes (Lond) 2009;33 (Suppl 2):S8–13. doi: 10.1038/ijo.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jakicic JM. The Effect of Physical Activity on Body Weight. Obesity. 2009;17:S34–S8. doi: 10.1038/oby.2009.386. [DOI] [PubMed] [Google Scholar]
- 7.Heini AF, Kirk KA, Lara-Castro C, Weinsier RL. Relationship between hunger-satiety feelings and various metabolic parameters in women with obesity during controlled weight loss. Obes Res. 1998;6:225–30. doi: 10.1002/j.1550-8528.1998.tb00341.x. [DOI] [PubMed] [Google Scholar]
- 8.Wing RR, Hill JO. Successful weight loss maintenance. Annu Rev Nutr. 2001;21:323–41. doi: 10.1146/annurev.nutr.21.1.323. [DOI] [PubMed] [Google Scholar]
- 9.Jakicic JM. The role of physical activity in prevention and treatment of body weight gain in adults. J Nutr. 2002;132:3826S–9S. doi: 10.1093/jn/132.12.3826S. [DOI] [PubMed] [Google Scholar]
- 10.King NA, Caudwell P, Hopkins M, Byrne NM, Colley R, Hills AP, et al. Metabolic and behavioral compensatory responses to exercise interventions: barriers to weight loss. Obesity (Silver Spring) 2007;15:1373–83. doi: 10.1038/oby.2007.164. [DOI] [PubMed] [Google Scholar]
- 11.Martins C, Morgan L, Truby H. A review of the effects of exercise on appetite regulation: an obesity perspective. Int J Obes. 2008;32:1337–47. doi: 10.1038/ijo.2008.98. [DOI] [PubMed] [Google Scholar]
- 12.King NA, Horner K, Hills AP, Byrne NM, Wood RE, Bryant E, et al. Exercise, appetite and weight management: understanding the compensatory responses in eating behaviour and how they contribute to variability in exercise-induced weight loss. British Journal of Sports Medicine. 2011 doi: 10.1136/bjsm.2010.082495. [DOI] [PubMed] [Google Scholar]
- 13.King NA, Burley VJ, Blundell JE. Exercise-induced suppression of appetite: effects on food intake and implications for energy balance. Eur J Clin Nutr. 1994;48:715–24. [PubMed] [Google Scholar]
- 14.Broom DR, Stensel DJ, Bishop NC, Burns SF, Miyashita M. Exercise-induced suppression of acylated ghrelin in humans. J Appl Physiol. 2007;102:2165–71. doi: 10.1152/japplphysiol.00759.2006. [DOI] [PubMed] [Google Scholar]
- 15.King JA, Wasse LK, Ewens J, Crystallis K, Emmanuel J, Batterham RL, et al. Differential Acylated Ghrelin, Peptide YY3–36, Appetite, and Food Intake Responses to Equivalent Energy Deficits Created by Exercise and Food Restriction. J Clin Endocrinol Metab. 2011;96:1114–21. doi: 10.1210/jc.2010-2735. [DOI] [PubMed] [Google Scholar]
- 16.Unick JL, Otto AD, Goodpaster BH, Helsel DL, Pellegrini CA, Jakicic JM. Acute effect of walking on energy intake in overweight/obese women. Appetite. 2010;55:413–9. doi: 10.1016/j.appet.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagobian TA, Sharoff CG, Stephens BR, Wade GN, Silva JE, Chipkin SR, et al. Effects of exercise on energy-regulating hormones and appetite in men and women. Am J Physiol Regul Integr Comp Physiol. 2009;296:R233–42. doi: 10.1152/ajpregu.90671.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blundell JE, Stubbs RJ, Hughes DA, Whybrow S, King NA. Cross talk between physical activity and appetite control: does physical activity stimulate appetite? Proc Nutr Soc. 2003;62:651–61. doi: 10.1079/PNS2003286. [DOI] [PubMed] [Google Scholar]
- 19.King NA, Caudwell PP, Hopkins M, Stubbs JR, Naslund E, Blundell JE. Dual-process action of exercise on appetite control: increase in orexigenic drive but improvement in meal-induced satiety. Am J Clin Nutr. 2009;90:921–7. doi: 10.3945/ajcn.2009.27706. [DOI] [PubMed] [Google Scholar]
- 20.Jakicic JM, Otto AD, Lang W, Semler L, Winters C, Polzien K, et al. The Effect of Physical Activity on 18-Month Weight Change in Overweight Adults. Obesity. 2011;19:100–9. doi: 10.1038/oby.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porubska K, Veit R, Preissl H, Fritsche A, Birbaumer N. Subjective feeling of appetite modulates brain activity: an fMRI study. NeuroImage. 2006;32:1273–80. doi: 10.1016/j.neuroimage.2006.04.216. [DOI] [PubMed] [Google Scholar]
- 22.Simmons WK, Martin A, Barsalou LW. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cereb Cortex. 2005;15:1602–8. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- 23.Uher R, Treasure J, Heining M, Brammer MJ, Campbell IC. Cerebral processing of food-related stimuli: Effects of fasting and gender. Behavioural Brain Research. 2006;169:111–9. doi: 10.1016/j.bbr.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Fuhrer D, Zysset S, Stumvoll M. Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity (Silver Spring) 2008;16:945–50. doi: 10.1038/oby.2008.33. [DOI] [PubMed] [Google Scholar]
- 25.LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behav Neurosci. 2001;115:493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- 26.Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. Neuroimage. 2003;19:1381–94. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- 27.St-Onge MP, Sy M, Heymsfield SB, Hirsch J. Human cortical specialization for food: a functional magnetic resonance imaging investigation. J Nutr. 2005;135:1014–8. doi: 10.1093/jn/135.5.1014. [DOI] [PubMed] [Google Scholar]
- 28.Beaver JD, Lawrence AD, van Ditzhuijzen J, Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. J Neurosci. 2006;26:5160–6. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cornier MA, Von Kaenel SS, Bessesen DH, Tregellas JR. Effects of overfeeding on the neuronal response to visual food cues. Am J Clin Nutr. 2007;86:965–71. doi: 10.1093/ajcn/86.4.965. [DOI] [PubMed] [Google Scholar]
- 30.Schur EA, Kleinhans NM, Goldberg J, Buchwald D, Schwartz MW, Maravilla K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. Int J Obes. 2009;33:653–61. doi: 10.1038/ijo.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carnell S, Wardle J. Appetite and adiposity in children: evidence for a behavioral susceptibility theory of obesity. Am J Clin Nutr. 2008;88:22–9. doi: 10.1093/ajcn/88.1.22. [DOI] [PubMed] [Google Scholar]
- 32.Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav. 2009;97:537–50. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Laan LN, de Ridder DTD, Viergever MA, Smeets PAM. The first taste is always with the eyes: A meta-analysis on the neural correlates of processing visual food cues. NeuroImage. 2010;55:296–303. doi: 10.1016/j.neuroimage.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 34.Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Rojas DC, Tregellas JR. The Effects of Overfeeding on the Neuronal Response to Visual Food Cues in Thin and Reduced-Obese Individuals. PLoS ONE. 2009;4:e6310. doi: 10.1371/journal.pone.0006310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang GJ, Volkow ND, Telang F, Jayne M, Ma J, Rao M, et al. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage. 2004;21:1790–7. doi: 10.1016/j.neuroimage.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 36.Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, et al. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc Natl Acad Sci U S A. 1999;96:4569–74. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hinton EC, Parkinson JA, Holland AJ, Arana FS, Roberts AC, Owen AM. Neural contributions to the motivational control of appetite in humans. Eur J Neurosci. 2004;20:1411–8. doi: 10.1111/j.1460-9568.2004.03589.x. [DOI] [PubMed] [Google Scholar]
- 38.Gautier JF, Del Parigi A, Chen K, Salbe AD, Bandy D, Pratley RE, et al. Effect of satiation on brain activity in obese and lean women. Obes Res. 2001;9:676–84. doi: 10.1038/oby.2001.92. [DOI] [PubMed] [Google Scholar]
- 39.Baicy K, London ED, Monterosso J, Wong ML, Delibasi T, Sharma A, et al. Leptin replacement alters brain response to food cues in genetically leptin-deficient adults. Proc Natl Acad Sci U S A. 2007;104:18276–9. doi: 10.1073/pnas.0706481104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malik S, McGlone F, Bedrossian D, Dagher A. Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab. 2008;7:400–9. doi: 10.1016/j.cmet.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 41.Burger KS, Cornier MA, Ingebrigsten J, Johnson SL. Assessing food appeal and desire to eat: Effects of portion size and energy density. Int J Behav Nutr Phys Act. 2011 doi: 10.1186/1479-5868-8-101. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. Hunger is the best spice: An fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behavioural Brain Research. 2009;198:149–58. doi: 10.1016/j.bbr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 43.Leidy HJ, Lepping RJ, Savage CR, Harris CT. Neural Responses to Visual Food Stimuli After a Normal vs. Higher Protein Breakfast in Breakfast-Skipping Teens: A Pilot fMRI Study. Obesity. 2011;19:2019–25. doi: 10.1038/oby.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenbaum M, Sy M, Pavlovich K, Leibel RL, Hirsch J. Leptin reverses weight loss-induced changes in regional neural activity responses to visual food stimuli. J Clin Invest. 2008;118:2583–91. doi: 10.1172/JCI35055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smeets PA, de Graaf C, Stafleu A, van Osch MJ, Nievelstein RA, van der Grond J. Effect of satiety on brain activation during chocolate tasting in men and women. Am J Clin Nutr. 2006;83:1297–305. doi: 10.1093/ajcn/83.6.1297. [DOI] [PubMed] [Google Scholar]
- 46.Small DM, Zatorre RJ, Dagher A, Evans AC, Jones-Gotman M. Changes in brain activity related to eating chocolate: from pleasure to aversion. Brain. 2001;124:1720–33. doi: 10.1093/brain/124.9.1720. [DOI] [PubMed] [Google Scholar]
- 47.Gordon CM, Dougherty DD, Rauch SL, Emans SJ, Grace E, Lamm R, et al. Neuroanatomy of human appetitive function: A positron emission tomography investigation. Int J Eat Disord. 2000;27:163–71. doi: 10.1002/(sici)1098-108x(200003)27:2<163::aid-eat4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 48.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. Neuroimage. 2004;23:1486–93. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 49.Levy LM, Henkin RI, Lin CS, Finley A, Schellinger D. Taste memory induces brain activation as revealed by functional MRI. J Comput Assist Tomogr. 1999;23:499–505. doi: 10.1097/00004728-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Reseland JE, Anderssen SA, Solvoll K, Hjermann I, Urdal P, Holme I, et al. Effect of long-term changes in diet and exercise on plasma leptin concentrations. Am J Clin Nutr. 2001;73:240–5. doi: 10.1093/ajcn/73.2.240. [DOI] [PubMed] [Google Scholar]
- 51.Polak J, Klimcakova E, Moro C, Viguerie N, Berlan M, Hejnova J, et al. Effect of aerobic training on plasma levels and subcutaneous abdominal adipose tissue gene expression of adiponectin, leptin, interleukin 6, and tumor necrosis factor alpha in obese women. Metabolism. 2006;55:1375–81. doi: 10.1016/j.metabol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 52.Pasman WJ, Westerterp-Plantenga MS, Saris WH. The effect of exercise training on leptin levels in obese males. Am J Physiol. 1998;274:E280–6. doi: 10.1152/ajpendo.1998.274.2.E280. [DOI] [PubMed] [Google Scholar]
- 53.Patterson CM, Bouret SG, Dunn-Meynell AA, Levin BE. Three-weeks of post-weaning exercise in DIO rats produces prolonged increases in central leptin sensitivity and signaling. Am J Physiol Regul Integr Comp Physiol. 2009 doi: 10.1152/ajpregu.90859.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flores MB, Fernandes MF, Ropelle ER, Faria MC, Ueno M, Velloso LA, et al. Exercise improves insulin and leptin sensitivity in hypothalamus of Wistar rats. Diabetes. 2006;55:2554–61. doi: 10.2337/db05-1622. [DOI] [PubMed] [Google Scholar]
- 55.Ropelle ER, Fernandes MF, Flores MB, Ueno M, Rocco S, Marin R, et al. Central exercise action increases the AMPK and mTOR response to leptin. PLoS ONE. 2008;3:e3856. doi: 10.1371/journal.pone.0003856. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR. Sex-based differences in the behavioral and neuronal responses to food. Physiol Behav. 2010;99:538–43. doi: 10.1016/j.physbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Killgore WD, Yurgelun-Todd DA. Sex differences in cerebral responses to images of high vs low calorie food. Neuroreport. 2010;21:354–8. doi: 10.1097/WNR.0b013e32833774f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Manoach DS, Halpern EF, Kramer TS, Chang Y, Goff DC, Rauch SL, et al. Test-Retest Reliability of a Functional MRI Working Memory Paradigm in Normal and Schizophrenic Subjects. Am J Psychiatry. 2001;158:955–8. doi: 10.1176/appi.ajp.158.6.955. [DOI] [PubMed] [Google Scholar]