Abstract
Background
Nasopharyngeal colonization by Streptococcus pneumoniae precedes pneumococcal disease. Elucidation of procedures to prevent or eradicate nasopharyngeal carriage in a model akin to the human would help to diminish the incidence of both pneumonia and invasive pneumococcal disease.
Methods
1) a survey of the nasopharynx of infant rhesus macaques from our breeding colony, in search of natural carriers of S. pneumoniae, 2) experimental induction of colonization by nasopharyngeal instillation of a human S. pneumoniae strain (19F).
Results
None of 158 colony animals surveyed carried S. pneumoniae in the nasopharynx. Colonization was induced in 8 of 8 infant rhesus by nasopharyngeal instillation and lasted 2 weeks in 100% of the animals and 7 weeks in over 60%.
Conclusion
Rhesus macaques are probably not natural carriers of S. pneumoniae. The high rate and duration of colonization obtained in our experiments indicates that the rhesus macaque will serve as a human-like carriage model.
Introduction
Infections caused by Streptococcus pneumoniae are a major cause of mortality throughout the world. This organism is usually a commensal in the upper respiratory tract of humans. From this colonization site it can descend to the lower tract and cause pneumonia, as well as invasive disease, primarily bacteremia and meningitis, in high-risk individuals. These are children under the age of two years, the elderly, and the immune deficient, including HIV-infected patients and/or malnourished persons [2]. Pneumonia and invasive disease also may occur in normal adults, particularly when homeostasis of S. pneumoniae carriage is perturbed by infections with viruses such as influenza, respiratory syncytial virus, and adenovirus [9]. S. pneumoniae is also the most frequent cause of sinusitis and acute otitis media. Recent evaluations of morbidity and mortality associated with pneumococcal disease among the 91.5 million older adults (≥ 50 years) in the US estimated the yearly occurrence of 29,500 cases of invasive pneumococcal disease, 502,600 cases of nonbacteremic pneumococcal pneumonia, and 25,400 pneumococcal-related deaths [21].
Colonization of the nasopharynx by S. pneumoniae always precedes pneumococcal disease. Colonization also provides the basis for the horizontal spread of the organism [1]. Nasopharyngeal colonization by S. pneumoniae is most prevalent in children who are 3 years old or less (up to 55%) and then declines steadily with increasing age to roughly 8% at age 10 [1]. Prevalence is greatly affected by crowding and can be as high as 82% in infants living in an orphanage or attending day-care centers [1].
Elucidation of procedures to prevent, control, diminish, or eradicate nasopharyngeal carriage of S. pneumoniae would preeminently serve the purpose of diminishing the incidence and associated morbidity of both pneumonia and invasive pneumococcal disease. To achieve this goal we contend that a nonhuman primate model of pneumococcal carriage would be the ideal resource. Such a model would make it possible to: 1) evaluate vaccines that may prevent colonization or therapeutically remove it after the fact; 2) examine the effect on carriage of vaccines developed to prevent pneumonia and invasive disease; and 3) assess topical remedies aimed at weakening or altogether removing attachment of the pneumococcus to the nasopharyngeal epithelium. Even the use of harmless bacteria that could favorably compete with S. pneumoniae for the nasopharyngeal niche could be assessed. With regard to the pathogen, expression of S. pneumoniae virulence factors differentially expressed during colonization, pneumonia, and invasion could easily be evaluated. Rodent models of S. pneumoniae colonization have been utilized [4, 7, 12, 13, 15, 22], but the marked anatomical, and probable physiological, differences that exist between the rodent and the primate respiratory tracts [10] make it unlikely that the information derived from these models may straightforwardly apply to humans. We thus endeavored to develop a rhesus monkey model of S. pneumoniae carriage.
To that end we used a dual approach. First, we surveyed the nasopharynx of infant rhesus macaques (about one year of age) from the Tulane National Primate Research Center (TNPRC) breeding colony, in search of animals that were natural carriers, presumably of autochthonous strains of S. pneumoniae. Second, we attempted to induce carriage experimentally in infant animals, by nasopharyngeal instillation of a human S. pneumoniae strain (19F). This was done both in antibiotic pre-treated and untreated animals. Antibiotic pre-treatment was directed at temporarily curbing the normal nasopharyngeal flora, which in our colony rhesus macaques is composed predominantly by Staphylococcus spp., including S. aureus [5] and non-pneumococcal Streptococcus spp. In humans, there exists nasopharyngeal niche competition between S. aureus and S. pneumoniae [3, 18]. We also searched for pneumonia and disseminated infection secondary to colonization, and for whether these forms of infection could be facilitated by splenectomy. Splenectomy is a recognized risk factor for invasive pneumococcal infection [6]. Here we report the results of these studies.
Materials and Methods
Practices in the housing and care of animals conformed to the regulations and standards established by the Animal Welfare Act. Animal care facilities were fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care-International. All animal-related protocols were approved by the Institutional Animal Care and Use Committee of the Tulane National Primate Research Center.
Survey of colony rhesus
Samples were collected from subjects under ketamine HCl anesthesia intramuscularly (IM) (10 mg/kg) during semiannual inventory. Each animal was held in an upright position, with its head tilted caudally at a 45° angle. A sterile culture swab was carefully placed into the subject’s nostril and slowly advanced to the nasopharynx before removal. This was repeated in the opposite nostril. Culture swabs were placed in a sterile tube until tested for presence of S. pneumoniae colonies, following Procedure 1, described below.
Swab content culture
Two swabs per animal were received in sterile 15-mL conical tubes. One of the swabs was processed following culture procedure 1 (see below), and the other by procedure 2.
Procedure 1
A volume of 1.0 mL of either Brain-Heart Infusion or Todd Hewitt Broth (Becton Dickinson, BD) was added to each tube, and each swab tip was vortexed briefly. Capped tubes were then left at room temperature for 3–6 hours. After this time, 0.25 mL of the bacterial suspension from each tube was separately plated onto a Trypticase Soy Agar with 5% Sheep Blood (TSA II, BD) and another 0.25 mL was plated onto a Selective strep agar plate (COBA Medium, Hardy Diagnostics), which contains 5% sheep blood, colistin and oxolinic acid, designed to inhibit growth of gram-negative bacilli and staphylococci. Plates were incubated overnight at 37°C in a 5% CO2 incubator and observed next day for alpha hemolytic S. pneumoniae colonies. Identification was confirmed by subculture of putative S. pneumoniae colonies in the presence of an optochin disc (BD) on TSA II plates.
Procedure 2
Swab contents were inoculated onto plates and into fluid media as follows: 1) plate media, a) TSA II, b) MacConkey II Agar (BD # 221261); 2) Fluid medium, Thioglycollate Fluid Medium, 8 mL (BD#221196). Plates and containers with fluid medium were incubated at 37°C in a 5% CO2 environment, the former for 48 h and the latter for 5 days or until growth was observed. If colonies were observable on plates, the bacterial genera were identified by standard procedures and colony numbers were estimated. If no growth was observed on plates but was evident in fluid media, a Gram stain was performed and the bacteria were plated on appropriate solid media, and identified and colony-counted as above. Procedure 1 was used exclusively to quantify S. pneumoniae, whereas Procedure 2 was used to quantify both pneumococcal and normal flora colonization.
Antibiotic-sensitivity testing of rhesus normal flora
Culture Procedure 2 (above) was used to assess antibiotic sensitivity of rhesus normal flora. To this end, once colonies were identified they were individually re-plated and a Kirby Bauer sensitivity test was performed on each organism. The antibiotics used were amikacin (30 µg per disc), ampicillin (10 µg), cefazolin (30 µg), chloramphenicol (30 µg), ciprofloxacin (5 µg), enrofloxacin (5 µg), erythromycin (15 µg), gentamycin (10 µg), penicillin (10 IU), tetracycline (30 µg), and trimethroprim/sulfamethoxazole (1.25 µg/23.75 µg).
Experimentally infected animals, antibiotic pretreatment, S. pneumoniae inoculation, splenectomy, and additional procedures
Eight male rhesus macaques of Indian origin were used in this portion of the study. At the time of inoculation with S. pneumoniae their median age was 1.32 years (range 1.26 – 1.36). Four of the animals were treated for 14 days prior to inoculation with Penicillin G Procaine 30,000 units per Kg IM once a day. Inoculation of all of the animals was with 106 cfu of S. pneumoniae 19F strain into each naris, instilled in a volume of 70 µL of sterile PBS. The nasopharynx of all of the animals was swabbed per both nares once before inoculation and then once every week until the end of the study. Four of the animals were splenectomized at 5–6 weeks after S. pneumoniae instillation, to propitiate the onset of pneumonia and/or invasive disease. Bronchoalveolar lavage (BAL) fluid and blood were collected for bacterial culture by procedures described previously [17], from four of the animals at baseline and at weeks 3 and 5 post-instillation (PI), and from all of the animals on weeks 6, 9, 10, 11, and 12 PI. Thoracic radiographs were taken on weeks 1, 5, 8, 10, and 12 PI, and cerebrospinal fluid (CSF) was collected for culture from all of the animals once per week starting on week 6 PI. Routine serum chemistries and complete blood cell count (CBC) were performed on all animals prior to S. pneumoniae instillation and 5 weeks PI. IgG antibody to the pneumococcal polysaccharide of the 19F S. pneumoniae serotype was determined as described previously [19], in serum samples collected from all of the animals prior to pneumococcal instillation, and at 6 and 12 weeks thereafter.
Statistics
The number of animals to be sampled out of 1020 in the colony survey was determined using the EpiInfo utility (Version 3.5.2, CDC) for calculating a sample size for a population survey using random sampling.
Results
Search for autochthonous strains of S. pneumoniae in breeding-colony rhesus macaques
A total of 158 rhesus macaques (85 males and 73 females) of a median age of 1.23 years (range 0.59 – 1.99) were surveyed for presence of nasopharyngeal S. pneumoniae carriage. No S. pneumoniae colonies were isolated from any of the animals.
Experimental infection
Antibiotic pre-treatment
A total of 8 animals were used in this study. Four of these animals were treated with penicillin for 14 days, with the last dose administered 24 h prior to pneumococcal instillation. Before selecting penicillin as the antibiotic of choice, an antibiotic sensitivity test was performed on the predominant bacterial species isolated from the normal flora. The most abundant bacteria included hemolytic and non-hemolytic, coagulase-positive and coagulase-negative Staphylococci, and gamma-hemolytic and non-pneumococcal alpha-hemolytic Streptococci. Of the 11 drugs tested the most effective was penicillin, as all of the nasopharyngeal bacteria, including less abundant isolates, were sensitive to this antibiotic.
The nasopharynx of all of the animals was swabbed on the day of instillation (day 0), and the swabs were cultured. All of the animals received 106 cfu of S. pneumoniae strain 19F through each naris. The numbers of Staphylococcus spp. colonies from the swab cultures was comparable in treated and untreated animals, but numbers of non-pneumococcal Streptococcus spp. was markedly higher in the untreated animals (Table 1). At seven days post-instillation, the cfu of both Staphylococcus spp. and Streptococcus spp. were higher in untreated animals. Colony numbers of S. pneumoniae were somewhat higher in the untreated animals as compared to the animals that had received antibiotics. By day 14 there was no marked difference in the magnitude of pneumococcal colony carriage between these two groups. Numbers of Staphylococcus spp. colonies were higher in the untreated group by this time.
Table 1.
Normal predominant nasopharyngeal bacterial flora in antibiotic-treated and untreated yearling rhesus macaques before and after instillation with S. pneumoniae.
| Bacteria | CFU per animal* | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Untreated | Treated | ||||||||
| Day/Animal | IH49 | IG74 | IJ55 | IJ99 | IG99 | IH65 | IM44 | IJ92 | |
| Staphylococcus spp. | Day 0 | >200 | 25 | - | - | 1 | >300 | 27 | 3 |
| Day 7 | >400 | >200 | 40 | 6 | 6 | 10 | >50 | 2 | |
| Day 14 | 50 | >225 | 1 | >200 | 25 | 12 | 6 | 5 | |
| Streptococcus spp. | Day 0 | >100 | 54 | 110 | >100 | - | - | - | - |
| Day 7 | >500 | >100 | 40 | 6 | >60 | 4 | >50 | - | |
| Day 14 | >100 | - | - | >50 | >100 | - | - | - | |
| Streptococcus pneumoniae | Day 0 | - | - | - | - | - | - | - | - |
| Day 7 | >100 | >100 | 10 | >100 | >50 | 1 | >50 | 3 | |
| Day 14 | >200 | >50 | >100 | >100 | >200 | >100 | >200 | >200 | |
Only CFU from right naris swabs are tabulated.
Staphylococcus spp.: includes non-hemolytic, coagulase-negative, hemolytic, coagulase-positive and/or negative Staphylococci, and/or S. aureus.
Streptococcus spp.: includes alpha- and/or gamma-hemolytic non-specific Streptococci.
Nasopharyngeal colonization rate and duration, invasive infection, and effect of splenectomy
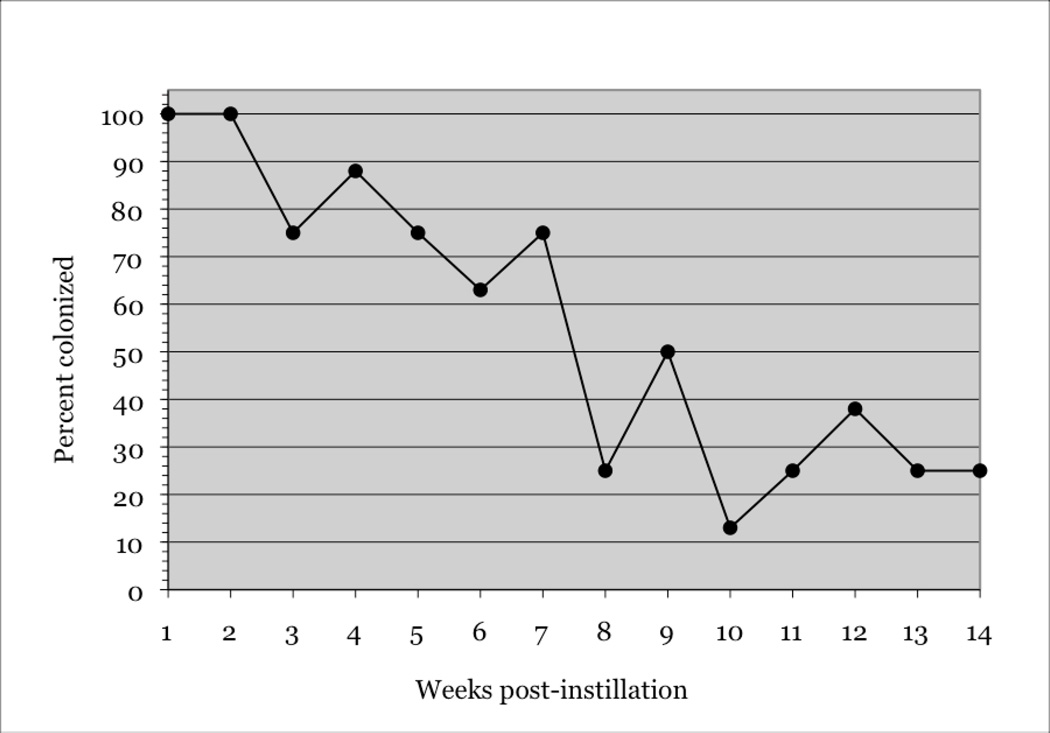
Rate of nasopharyngeal colonization remained at 100% for 2 weeks PI, and above 60% for 7 weeks. Subsequently, it oscillated around 30% until week 14, the time when the study ended (Figure 1).
Figure 1.
Rate of S. pneumoniae colonization (percent) of rhesus macaques as a function of time (in weeks) PI. The total number of animals given an instillation with the 19F serotype of S. pneumoniae was n = 8 (100%).
Four of the animals were splenectomized between weeks 5 and 6 PI (Table 2, shaded column). There was no invasive infection or disease in any of the animals, splenectomized or eusplenic, at any time during the duration of the study. Blood, CSF, and BAL cultures were negative throughout, and thoracic radiographs were normal at all sampling time points. CBC and serum chemistry analyses were normal as well. Splenectomy did not ostensibly affect the rate of colonization or the number of cfu recovered from nasal swabs of each of the animals taking into consideration the values observed prior to splenectomy (Table 2).
Table 2.
S. pneumoniae CFU in cultures from both nares as a function of time post-instillation
| Animal/Weeks PI | S. pneumoniae CFU*** | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| IG74 | >100 | >150 | - | - | - | - | - | - | - | - | - | - | >100 | >500 |
| IH49 | 600 | TMTC | - | >375 | 3 | 250 | >120 | 2 | - | - | - | 2 | - | - |
| IH65 | 2 | >100 | 100 | <10 | - | <10 | - | - | - | - | - | - | - | >100 |
| IJ55 | 42 | >450 | 200 | >220 | >20 | - | 1 | - | ** | - | - | - | - | - |
| IJ92* | >250 | TMTC | TMTC | TMTC | >400 | 1 | >50 | - | 9 | - | - | - | - | - |
| IJ99* | >400 | TMTC | TMTC | >700 | TMTC | 250 | >5 | - | 10 | - | >400 | >100 | - | - |
| IM44* | >200 | TMTC | TMTC | >400 | >200 | - | >100 | - | >200 | - | - | - | - | - |
| IG99* | >250 | >350 | TMTC | >300 | TMTC | >170 | >200 | 25 | >225 | 12 | >260 | 150 | 28 | - |
animals that were splenectomized (at 6 weeks post-instillation, shaded column).
no swab was taken from this animal at this time.
CFU from both nares were counted and added for each of the time points.
Serum antibody determination
concentrations of IgG antibody to the pneumococcal polysaccharide of the 19F S. pneumoniae serotype were determined in duplicate at baseline and on weeks 6 and 12 PI. Values for all of the animals were very low at baseline, with a median of 0.0495 µg/mL (range 0.032–0.091 µg/mL, Table 3). At week 6 PI the antibody concentration increased slightly in some animals, with two animals with values above 0.2 µg/mL and a median of 0.091 µg/mL (range 0.037–0.238 µg/mL, Table 4). No meaningful change was observed by week 12 PI, as the median value at this time was of only 0.098 µg/mL, with a range of 0.054–0.180 (Table 3).
Table 3.
Concentration of IgG serum-antibody to the pneumococcal polysaccharide of the 19F S. pneumoniae serotype, as a function of time post-instillation.
| Animal | Concentration (µg/mL) | ||
|---|---|---|---|
| Baseline | Week 6 | Week 12 | |
| IG74 | 0.064 | 0.219 | 0.18 |
| IH49 | 0.045 | 0.095 | 0.107 |
| IH65 | 0.091 | 0.142 | 0.098 |
| IJ55 | 0.055 | 0.238 | 0.098 |
| IJ92 | 0.042 | 0.069 | 0.063 |
| IJ99 | 0.032 | 0.037 | 0.054 |
| IM44 | 0.045 | 0.087 | 0.169 |
| IG99 | 0.054 | 0.074 | 0.084 |
Discussion
Natural carriage of S. pneumoniae by nonhuman primates has never been reported as such. However, there is evidence to suggest prior pneumococcal colonization in chimpanzees, as indicated by a report of an outbreak of invasive pneumococcal disease in animals from a primate rehabilitation unit [11]. The authors identified factors that are known to facilitate the transition from carriage to invasive disease, such as evidence of viral upper respiratory tract infection and splenectomy, in a high proportion of animals with invasive disease. Invasive disease was marginally more common in splenectomized animals, and those with upper respiratory tract infection were 5.7 times as likely to develop invasive disease than those without [11]. More recently, two new clones of S. pneumoniae were identified in wild chimpanzees in a national park, indicating that S. pneumoniae can occur in populations of wild animals [8].
A spontaneous outbreak of invasive pneumococcal disease occurred in a rhesus breeding colony in China’s Sichuan province [23]. The outbreak involved over 1200 animals, and suggests, but does not prove, prior colonization with S. pneumoniae. Similarly, in a survey of causes of death of infant rhesus that was performed at the Delta Regional Primate Research Center, an early incarnation of the TNPRC, 12 out of 18 deaths due to meningitis and 7 out of 16 deaths due to septicemia were attributed to infection with S. pneumoniae [16].
In our study we surveyed 158 infant rhesus macaques. On average, the TNPRC infant-rhesus breeding colony has about 1020 animals of that age. We had estimated that for a study group of that size, and assuming a worst acceptable colonization rate of 1% and an expected rate of 4%, swabbing up to 141 animals would yield a positive outcome with a confidence of 90 – 95%. As we found no evidence of natural pneumococcal carriage we conclude that rhesus macaques are likely not natural carriers of S. pneumoniae, or at the very least, not in the TNPRC breeding colony. It should be noted, however, that in separate studies [17], we found levels of circulating polysaccharide IgG antibody to pneumococcal serotype 19F of up to 3.06 µg/ml in adult rhesus. Such levels would suggest that these animals were either exposed to, or carriers of, this serotype [14]. Alternatively, these may have been cross-reactive antibodies elicited by other organisms.
Our second approach to establishing a model of pneumococcal carriage involved the nasopharyngeal instillation of 8 infant rhesus with bacteria of the 19F strain of S. pneumoniae. Evidence of nasopharyngeal niche competition between Staphylococcus aureus and S. pneumoniae had been put forward, whereby S. pneumoniae carriage rate in unvaccinated children was negatively associated with S. aureus nasal carriage [3, 18]. Since we had found that the nasopharyngeal bacterial flora of rhesus macaques from our colony was chiefly composed of Staphylococcus and non-pneumococcal Streptococcus species [5], both of which could compete for the nasopharyngeal niche with S. pneumoniae, half of the animals (n = 4) were treated with penicillin for 2 weeks prior to pneumococcal instillation. While the antibiotic treatment reduced the normal flora colony numbers cultured from the animals’ nasopharynx, this partial clearance did not ostensibly affect the numbers of S. pneumoniae cfu recovered. If anything, the latter were marginally lower in the treated animals at seven days PI, perhaps reflecting the effect of residual penicillin. The results do not support the notion that the normal staphylococcal and streptococcal flora of rhesus macaques competes with pneumococci for the nasopharyngeal niche.
As with humans, nasopharyngeal colonization, here induced experimentally, was self-limited in the macaque model. However, it was detectable in all of the animals in the study for 2 weeks PI, and in more than 60% of the animals up to 7 weeks PI. This long-lasting carriage period indicates stable colonization rather than simple contamination of the nasopharynx. It would provide ample time to evaluate vaccines that may prevent colonization, and assess the effect on carriage, of vaccines developed to prevent pneumonia and invasive disease. It will also be possible to examine the role of S. pneumoniae virulence factors that are putatively involved in nasopharyngeal attachment, or that are differentially expressed during colonization and pneumonia, using the present model together with the S. pneumoniae pneumonia rhesus model we previously developed [17].
The inoculum dose used, 2 × 106 cfu (1 × 106 cfu per naris) is small compared to doses regularly used in mice, and yielded nonetheless 100% colonization. In immune deficient adult CBA/N mice, a strain that has a defective antibody response to certain T-cell independent antigens, including bacterial capsular polysaccharide, inoculum doses of at least 2 × 107 cfu were required to colonize 100% of the animals [22]. With doses of 2 × 106 only half of the mice were colonized [22]. Considering that the average weight of an adult laboratory mouse is 25 g, and the average weight of the 8 infant rhesus used in our study was 2.5 kg, the dose in cfu per kg body weight necessary to achieve 100% colonization in CBA/N mice is a thousand-fold higher than it is in rhesus monkeys. These are data to suggest that the nasopharyngeal environment of rhesus macaques may be more favorable to pneumococcal colonization than that of mice. This could be a reflection of the marked anatomical, and likely also physiological, differences that exist between the rodent and the primate respiratory tracts [10], especially considering the high levels of pneumococcal colonization found in infant humans.
At weeks 6 PI half of the animals (n = 4) were splenectomized. Splenectomy is recognized as a trigger for pneumonia and invasive infection by S. pneumoniae [6]. Neither of these signs was detected, as both splenectomized and eusplenic animals continued to yield negative S. pneumoniae cultures of blood, BAL and CSF. Thoracic radiographs also were normal throughout. While it appeared as though the splenectomized animals had higher cfu in their nasopharyngeal swabs (Table 2), it is more likely that this was simply the consequence of the higher cfu levels these animals showed prior to splenectomy. We were unable to apply proper statistics to compare rates of colonization in both groups of animals, as the number of animals was too small for us to be able to apply logistic regression for repeated measures, the appropriate statistical algorithm, to the carriage data. It is conceivable that splenectomy may have been more effective in facilitating invasive infection if it had been performed several weeks before S. pneumoniae instillation, as this would have impeded the induction of S. pneumoniae-specific adaptive immune functions associated with the spleen and perhaps still residually available in other immune organs after spleen removal.
The concentration of IgG antibody to the 19F pneumococcal polysaccharide remained low throughout the study, although it did increase somewhat at 6 weeks PI in most of the animals. Nonetheless, it remained below a maximum of 0.238 µg/mL at all the time points when it was determined, most probably a consequence of the young age of the animals. Anti-pneumococcal polysaccharide antibody is therefore unlikely to have played a role in causing cessation of colonization in this model. In fact, a recent study in mice concluded that Toll-like receptor (TLR)2-dependent mechanisms as well as CD4 T-cell mediated immunity and not a humoral adaptive immune response were important for clearance of S. pneumoniae from the murine nasopharynx [20].
In conclusion, the high rate and duration of colonization obtained in our experiments indicates that infant rhesus macaques and perhaps even adult animals will serve as an adequate model for S. pneumoniae carriage.
Acknowledgments
Secretarial help by Avery MacLean is gratefully acknowledged.
Acknowledgements of funding
This work was supported by a grant from the Tulane Research Enhancement Fund II and by grant P51-RR00164 from the National Institutes of Health.
References
- 1.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. The Lancet infectious diseases. 2004;4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 2.Bogaert D, Hermans PW, Adrian PV, Rumke HC, de Groot R. Pneumococcal vaccines: an update on current strategies. Vaccine. 2004;22:2209–2220. doi: 10.1016/j.vaccine.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 3.Bogaert D, van Belkum A, Sluijter M, Luijendijk A, de Groot R, Rumke HC, Verbrugh HA, Hermans PW. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet. 2004;363:1871–1872. doi: 10.1016/S0140-6736(04)16357-5. [DOI] [PubMed] [Google Scholar]
- 4.Bogaert D, Weinberger D, Thompson C, Lipsitch M, Malley R. Impaired innate and adaptive immunity to Streptococcus pneumoniae and its effect on colonization in an infant mouse model. Infection and immunity. 2009;77:1613–1622. doi: 10.1128/IAI.00871-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowers LC, Purcell JE, Plauche GB, Denoel PA, Lobet Y, Philipp MT. Assessment of the nasopharyngeal bacterial flora of rhesus macaques: moraxella, Neisseria, Haemophilus, and other genera. Journal of clinical microbiology. 2002;40:4340–4342. doi: 10.1128/JCM.40.11.4340-4342.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1997;46:1–24. [PubMed] [Google Scholar]
- 7.Chen S, Paterson GK, Tong HH, Mitchell TJ, DeMaria TF. Sortase A contributes to pneumococcal nasopharyngeal colonization in the chinchilla model. FEMS microbiology letters. 2005;253:151–154. doi: 10.1016/j.femsle.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 8.Chi F, Leider M, Leendertz F, Bergmann C, Boesch C, Schenk S, Pauli G, Ellerbrok H, Hakenbeck R. New Streptococcus pneumoniae clones in deceased wild chimpanzees. Journal of bacteriology. 2007;189:6085–6088. doi: 10.1128/JB.00468-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hament JM, Kimpen JL, Fleer A, Wolfs TF. Respiratory viral infection predisposing for bacterial disease: a concise review. FEMS immunology and medical microbiology. 1999;26:189–195. doi: 10.1111/j.1574-695X.1999.tb01389.x. [DOI] [PubMed] [Google Scholar]
- 10.Harkema JR. Comparative aspects of nasal airway anatomy: relevance to inhalation toxicology. Toxicologic pathology. 1991;19:321–336. doi: 10.1177/0192623391019004-102. [DOI] [PubMed] [Google Scholar]
- 11.Jones EE, Alford PL, Reingold AL, Russell H, Keeling ME, Broome CV. Predisposition to invasive pneumococcal illness following parainfluenza type 3 virus infection in chimpanzees. Journal of the American Veterinary Medical Association. 1984;185:1351–1353. [PubMed] [Google Scholar]
- 12.Lipsitch M, Dykes JK, Johnson SE, Ades EW, King J, Briles DE, Carlone GM. Competition among Streptococcus pneumoniae for intranasal colonization in a mouse model. Vaccine. 2000;18:2895–2901. doi: 10.1016/s0264-410x(00)00046-3. [DOI] [PubMed] [Google Scholar]
- 13.Malley R, Stack AM, Ferretti ML, Thompson CM, Saladino RA. Anticapsular polysaccharide antibodies and nasopharyngeal colonization with Streptococcus pneumoniae in infant rats. The Journal of infectious diseases. 1998;178:878–882. doi: 10.1086/597600. [DOI] [PubMed] [Google Scholar]
- 14.Malley R, Weiser J. Animal Models of Pneumococcal Colonization; in Pneumococcal Vaccines: the Impact of Conjugate Vaccine. Washington DC: ASM Press; 2008. [Google Scholar]
- 15.McCool TL, Weiser JN. Limited role of antibody in clearance of Streptococcus pneumoniae in a murine model of colonization. Infection and immunity. 2004;72:5807–5813. doi: 10.1128/IAI.72.10.5807-5813.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padovan D, Cantrell C. Causes of death of infant rhesus and squirrel monkeys. Journal of the American Veterinary Medical Association. 1983;183:1182–1184. [PubMed] [Google Scholar]
- 17.Philipp MT, Purcell JE, Martin DS, Buck WR, Plauche GB, Ribka EP, DeNoel P, Hermand P, Leiva LE, Bagby GJ, Nelson S. Experimental infection of rhesus macaques with Streptococcus pneumoniae: a possible model for vaccine assessment. Journal of medical primatology. 2006;35:113–122. doi: 10.1111/j.1600-0684.2006.00164.x. [DOI] [PubMed] [Google Scholar]
- 18.Regev-Yochay G, Dagan R, Raz M, Carmeli Y, Shainberg B, Derazne E, Rahav G, Rubinstein E. Association between carriage of Streptococcus pneumoniae and Staphylococcus aureus in Children. JAMA : the journal of the American Medical Association. 2004;292:716–720. doi: 10.1001/jama.292.6.716. [DOI] [PubMed] [Google Scholar]
- 19.Sorensen RU, Leiva LE, Javier FC, 3rd, Sacerdote DM, Bradford N, Butler B, Giangrosso PA, Moore C. Influence of age on the response to Streptococcus pneumoniae vaccine in patients with recurrent infections and normal immunoglobulin concentrations. The Journal of allergy and clinical immunology. 1998;102:215–221. doi: 10.1016/s0091-6749(98)70089-2. [DOI] [PubMed] [Google Scholar]
- 20.van Rossum AM, Lysenko ES, Weiser JN. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infection and immunity. 2005;73:7718–7726. doi: 10.1128/IAI.73.11.7718-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weycker D, Strutton D, Edelsberg J, Sato R, Jackson LA. Clinical and economic burden of pneumococcal disease in older US adults. Vaccine. 2010;28:4955–4960. doi: 10.1016/j.vaccine.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 22.Wu HY, Virolainen A, Mathews B, King J, Russell MW, Briles DE. Establishment of a Streptococcus pneumoniae nasopharyngeal colonization model in adult mice. Microbial pathogenesis. 1997;23:127–137. doi: 10.1006/mpat.1997.0142. [DOI] [PubMed] [Google Scholar]
- 23.Zou S, Luo Q, Chen Z, Cheng A, Wang M, Zhu D, Jia R, Liu F, Chen X, Zhou Y, Bi F, Yang Z. Isolation, identification of Streptococcus pneumoniae from infected rhesus monkeys and control efficacy. Journal of medical primatology. 2010;39:417–423. doi: 10.1111/j.1600-0684.2010.00427.x. [DOI] [PubMed] [Google Scholar]