Abstract
Angiotensin II (AngII) plays a key role in maintaining body fluid homeostasis. The physiological and behavioral effects of central AngII include increased blood pressure and fluid intake. In vitro experiments demonstrate that repeated exposure to AngII reduces the efficacy of subsequent AngII, and behavioral studies indicate that prior icv AngII administration reduces the dipsogenic response to AngII administered later. Specifically, rats given a treatment regimen of three icv injections of a large dose of AngII, each separated by 20 min, drink less water in response to a test injection of AngII than do vehicle-treated controls given the same test injection. The present studies were designed to test three potential explanations for the reduced dipsogenic potency of AngII after repeated administration. To this end, we tested for motor impairment caused by repeated injections of AngII, for a possible role of visceral distress or illness, and for differences in the pressor response to the final test injection of AngII. We found that repeated injections of AngII neither affected drinking stimulated by carbachol nor did they produce a conditioned flavor avoidance. Furthermore, we found no evidence that differences in the pressor response to the final test injection of AngII accounted for the difference in intake. In light of these findings, we are able to reject these three explanations for the observed behavioral desensitization, and, we suggest instead that the mechanism for this phenomenon may be at the level of the receptor.
Keywords: angiotensin, thirst, desensitization
1. Introduction
Angiotensin II (AngII) is a peptide that is critically involved in the coordinated responses to hypovolemia, including increased blood pressure and the stimulation of water and salt intake [1]. The cardiovascular and behavioral actions of AngII have been shown to be primarily mediated by the angiotensin type 1 (AT1) receptor [2–6]. Previous research suggests that AT1 receptor activation leads to receptor desensitization [7–12] and this may be an important component of normal AngII functioning [13]. Much of this research, however, has used in vitro preparations to investigate receptor function. Therefore, less is known about AngII-induced desensitization in the behaving animal and whether these in vitro findings extend to in vivo models.
Repeated icv injections of AngII result in a desensitization of the dipsogenic [14–17] and renal [17] responses to the peptide. Our lab has shown that rats given a treatment regimen of three icv injections of AngII over a short period of time, drink less water after a final test injection of AngII than do rats that received a control treatment regimen before the same final test injection [16]. We showed that this behavioral desensitization is mediated by the AT1 receptor, relatively short lasting, and specific to AngII-induced water intake because intake of 1.5% saline was unaffected in a two-bottle test [16]. These previous findings are consistent with the idea that this phenomenon is regulated at the level of the receptor. Nevertheless, several alternative explanations could account for the observed differences in drinking behavior.
In the present study, we investigated several possible explanations that could account for the reduced water intake that occurs after repeated AngII administration. First, the reduction in water intake could reflect a motor impairment. If this were the case, we would expect drinking induced by other dipsogens to be affected similarly. To test this, we examined the effect of repeated injections of AngII on water intake stimulated by the cholinergic agonist, carbachol. Second, repeated injections of AngII may cause visceral distress or malaise, which could inhibit subsequent intake. This would be evidenced by the acquisition of a learned avoidance of a novel flavor paired with the repeated drug administration. Thus, we evaluated the development of conditioned flavor preference or avoidance for a novel flavor that was paired with repeated injections of AngII. Third, because AngII-induced fluid intake can be inhibited by hypertension [18], a sensitized pressor response to repeated AngII could explain the reduced water intake. Therefore, we continuously measured blood pressure during and after repeated injections of AngII to determine if an exaggerated pressor response paralleled the reduced water intake observed in our behavioral studies. Taken together, the results suggest that AngII-induced behavioral desensitization is not the result of these less specific inhibitory effects on water intake, but, instead, more likely reflects cellular changes consistent with receptor desensitization.
2. Materials and Methods
2.1. Experimental Animals
Adult male Sprague Dawley rats were obtained from Harlan Laboratories (Indianapolis, IN; 175–199 gm). Rats were maintained in a temperature- and humidity-controlled room in hanging stainless steel, wire-mesh cages on a 12:12 hr light:dark cycle. All rats had ad libitum access to standard rat chow and tap water, unless otherwise stated. The handling and care of laboratory animals conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the State University of New York at Buffalo.
2.2. Lateral ventricle cannula implantation
No fewer than 5 days after arrival from the breeder, rats were anesthetized by intramuscular injection of a combination of ketamine (70 mg/kg) and xylazine (5 mg/kg). Chronic indwelling cannulae aimed at the lateral ventricle (coordinates: 0.9 mm posterior to bregma, 1.4 mm lateral to midline, 1.8 mm ventral to dura) were implanted and affixed to the skull with bone screws and dental cement. Septocaine with epinephrine (topical) and carprofen (5 mg/kg, sc) were used during the surgery for analgesia. No fewer than 5 days after surgery, proper cannula placement and AngII responsiveness were verified by injection of 10 ng AngII, and only those animals that drank at least 6 ml in the 30 min after the injection were included in the experiments.
2.3. Drug injections and intake measures
AngII (Bachem Bioscience Inc., King of Prussia, PA) and carbachol (Sigma-Aldrich Corp, St. Lois, MO) were diluted in tris-buffered saline (TBS). All experiments were performed early in the light portion of the light:dark cycle. Injections were made through a 33 ga injection cannula that extended beyond the guide cannula into the lateral ventricle. The injector was connected to water-filled PE 50 tubing attached to a 10 μl Hamilton syringe (Hamilton Company, Reno, NV). Each injection was 1 μl and the injection cannulae were left in place for approximately 30 sec after each injection. For clarity, experimental injections are collectively referred to as the “treatment regimen” and the final challenge injection as the “test” injection. In all cases, the treatment regimen comprised three injections of either AngII (300 ng) or vehicle (TBS), with each injection separated by 20 min. The test injection was a single injection of either AngII (100 ng), carbachol (150 nM), or vehicle (TBS) given 20 min after the final treatment regimen injection. In most experiments, food and water were removed immediately before the start of the treatment regimen and returned after the test injection. In the experiment measuring blood pressure, food and water were not returned until pressure recordings had ended.
Total water intake during the testing period was calculated as the difference in pre- and post-test water bottle weight. The temporal distribution of intake was assayed by counting licks in discrete 10 min intervals using a contact lickometer (designed and constructed by the Psychology Electronics Shop, University of Pennsylvania, Philadelphia, PA). The lickometer interfaced with a computer using an integrated USB digital I/O device (National Instruments, Inc., Austin, TX) and was processed in a MATLAB (MathWorks, Natick, MA) software environment before being ported to Excel (Microsoft Corp, Redmond, WA) for final analysis. Water spouts were behind an electrically isolated metal plate with a 3.2 mm-wide opening through which the rat needed to lick to reach the spout, minimizing the possibility of non-tongue contact with the spout.
2.4. Flavor Preference Testing
Rats were tested for flavor preference conditioning in two different ways. First, rats were given either an AngII or a vehicle treatment regimen prior to all rats receiving a test injection of AngII. After the test injection, rats were returned to their cages and given access to a bottle containing 4 ml of either 0.4% almond or 0.4% vanilla extract (McCormick; McCormick & Co., Inc., Hunt Valley, MD) diluted in tap water. Because we expected rats in the AngII treatment regimen group would drink less than controls, we limited all rats to 4 ml of the flavored water to help keep exposure to the two flavors consistent between groups and, therefore, minimize any potential confounding influence of differential familiarization. If a rat consumed the entire 4 ml before the end of the session, the flavored water was replaced with normal tap water for the remainder of the 30 min intake phase. Three days later, each rat received the other treatment regimen (TBS or AngII) prior to a test injection of AngII and was given access to the previously unexposed flavor. Each rat received both treatment regimens and was exposed to both flavors, but only one flavor was paired with the AngII treatment regimen for any given animal. The order of treatment regimen and which flavor was associated with the vehicle or the AngII treatment regimen were each counterbalanced. Three days after the second conditioning trial, rats were injected with 10 ng AngII (icv) and tested for flavor preference in a two-bottle test (0.4% almond or 0.4% vanilla extract in tap water; for timeline see Figure 2A). Location of the bottles containing the flavored water was counterbalanced to control for any left/right preference.
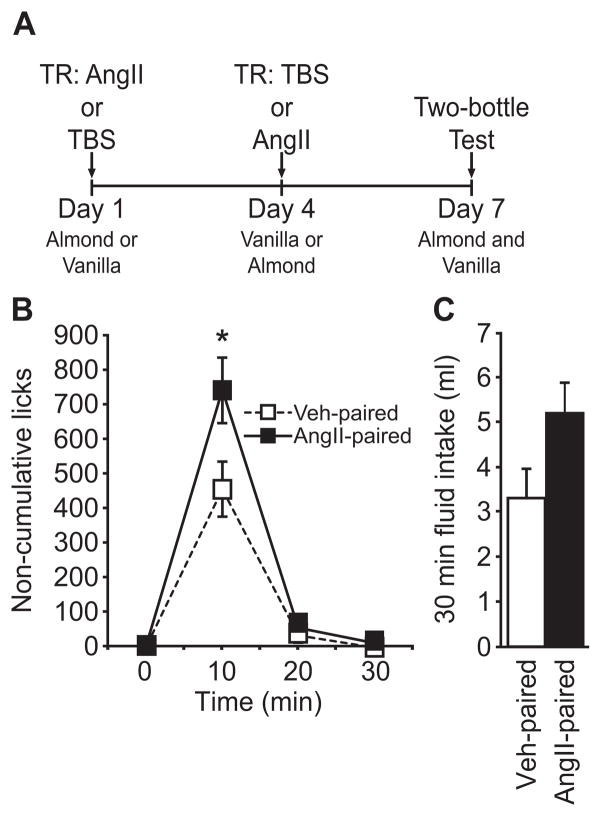
Figure 2.
A two-bottle preference test after pairing a novel flavor with repeated injections of AngII when intake was stimulated by an injection of AngII. On separate days, rats received an AngII or a vehicle treatment regimen before a test injection of AngII and exposure to a novel flavor. (A) The timeline used for the experiment. (B) Rats licked more at the spout containing the flavor that had been paired with the AngII treatment regimen (p<0.05, n=20 per group). (C) We did not detect a statistical difference in total fluid intake between the two flavors (p>0.05).
Due to relatively low intakes in the preference test by some rats in the preceding experiment, a separate experiment was conducted to address concerns that a floor effect may have obscured any conditioned preference. This experiment was designed to promote higher baseline consumption and eliminate the need for AngII injection during the final preference test. Rats were habituated to 1 hr of water access per day for 7 days and maintained on this drinking schedule until the end of the experiment. After habituation, rats received either an AngII or a vehicle treatment regimen before all rats received a final test injection of AngII. Immediately after the test injection, rats were given 30-min access to a bottle containing either vanilla- or almond-flavored water. Two days later, rats received the opposite treatment regimen (TBS or AngII) before a test injection of AngII and were given access to the previously unexposed flavor (for timeline see Figure 3A). Order of treatment regimen and treatment regimen-paired flavor were counterbalanced. Tap water was provided each day after the experiment for 1 hr. Two days after the second conditioning trial, rats were given access to the two bottles of flavored water, at the time when they otherwise would have received their daily drinking water, and intake was measured. Location of the bottles containing the flavored water was counterbalanced to control for any left/right preference.
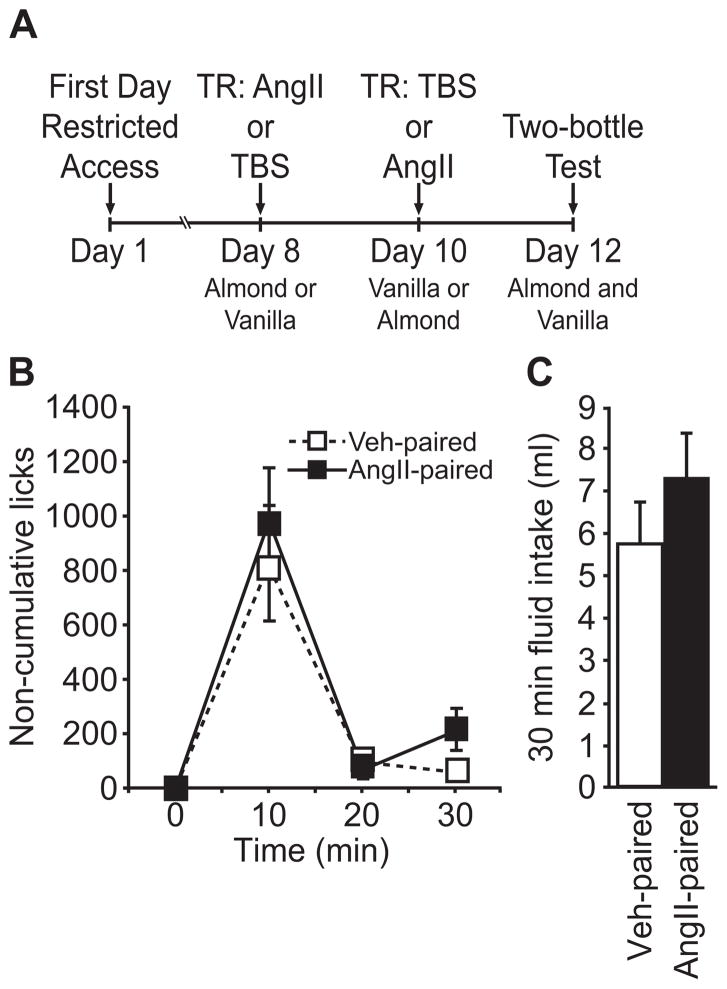
Figure 3.
A two-bottle preference test after pairing a novel flavor with repeated injections of AngII when intake was stimulated by scheduled access to water. On separate days, rats received an AngII or a vehicle treatment regimen before a test injection of AngII and exposure to a novel flavor. Subsequent flavor preference testing, in the absence of AngII, during the normally scheduled access to water did not reveal any difference in flavor preference. (A) The timeline used for the experiment. (B) There were no significant differences in non-cumulative licks (p>0.05, n=11 per group). (C) Total intake of the two flavors was not different during the preference test (p>0.05).
2.5. Blood pressure recordings
Blood pressure was measured by tail-cuff volume pressure recording (CODA Monitor; Kent Scientific Corporation, Torrington, CT). Briefly, rats were anesthetized by inhaled isoflurane immediately before blood pressure recording started. After 20 min of baseline recordings, rats received either an AngII or a vehicle treatment regimen 20 min before a test injection of either AngII or vehicle. Body temperature was maintained at approximately 38° C using an automated temperature monitoring system (Physitemp Instruments Inc., Clifton, NJ). Blood pressure recordings were taken at a frequency of once per min throughout the course of the injection procedure and for 30 min after the final test injection. For each subject, change in mean blood pressure was calculated as mean blood pressure minus baseline blood pressure. Baseline blood pressure was calculated as the mean pressure over 5 min of recordings taken immediately before the first treatment regimen injection. With the exception of baseline measures, all data are reported as change in mean blood pressure (mean ± SEM).
2.6. Data analysis
Data were analyzed using Statistica software (version 9.0; Statsoft, Tulsa, OK). Two-way repeated measures ANOVA was used to test for treatment-related differences in non-cumulative licks and change in mean blood pressure. One-way ANOVA was used to analyze baseline blood pressure, change in blood pressure at the last measure before the test injection, and peak change in pressure after the test injection. Statistically significant main or interaction effects (p<0.05) were further analyzed using Student Neuman-Keuls post hoc tests. Student’s t tests were used to analyze 30 min fluid intake.
3. Results
3.1. Repeated injections of AngII did not affect carbachol-induced water intake
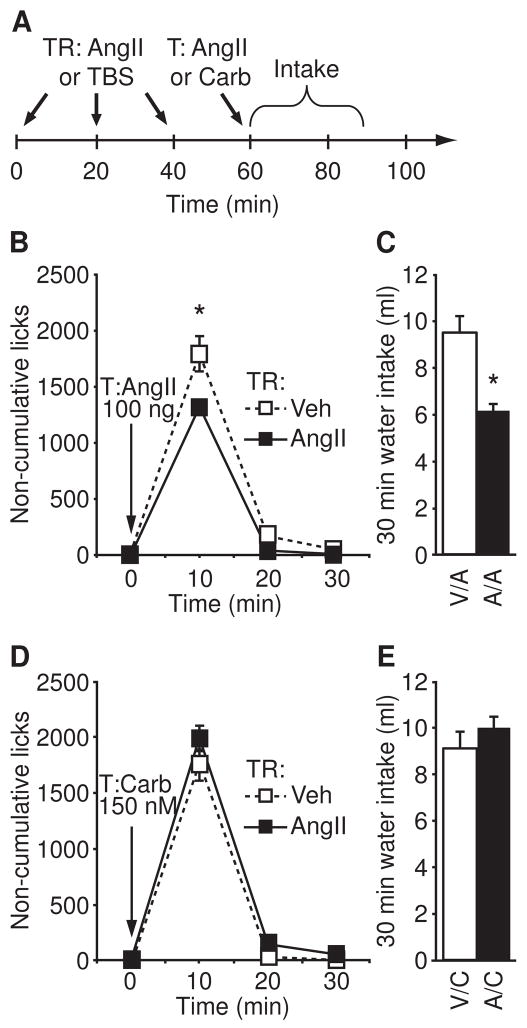
Rats received either an AngII or a vehicle treatment regimen before a test injection of AngII or carbachol (for timeline see Figure 1A). When rats were given a test injection of AngII, we did not detect a significant main effect of Condition (F1,8=5.06, p>0.05), but we found a significant Time × Condition interaction (F2,16=4.67, p<0.05, n=4–6 per group; Figure 1B). Analysis of individual time bins revealed that this effect was most prominent at 10 min after the test injection (p<0.05), at which time rats given an AngII treatment regimen licked the water spout less than did rats given a vehicle treatment regimen. Measures of total 30 min water intake also revealed less water intake by rats in the AngII treatment regimen group than by controls (t=3.338, p<0.05; Figure 1C). We found no difference, however, in non-cumulative licks (main effect of Condition, F1,12=4.24, p>0.05; Time × Condition interaction, F2,24=1.06, p>0.05; Figure 1D) or total water intake (t=1.14, p>0.05; Figure 1E) by rats that received either an AngII or a vehicle treatment regimen prior to a test injection of carbachol.
Figure 1.
Repeated injections of AngII caused a reduction in AngII-stimulated water intake, but had no effect on carbachol-induced intake. (A) The timeline used for the experiment. Rats were given an AngII or a vehicle treatment regimen before a test injection of AngII or carbachol. (B) The number of licks by rats given repeated injections of AngII was less than it was by rats in the control group after both groups received a test injection of AngII (p<0.05; n=4–6 per group). (C) Rats in the AngII treatment regimen group also drank less over the entire 30 min test (p<0.05). (D) We found no between-group differences in non-cumulative licks (p>0.05, n=7 per group) after a test injection of carbachol. (E) No between-group differences in 30 min intake were found after a test injection of carbachol (p=0.28).
3.2. Repeated injections of AngII did not produce a conditioned flavor avoidance
Rats were administered either an AngII or a vehicle treatment regimen prior to a test injection of AngII, and were given access to a novel flavor diluted in their drinking water. Three days after the completion of this training phase, rats were tested for preference/avoidance of the treatment regimen-paired flavors in a two-bottle preference test after fluid intake was stimulated by a single injection of AngII (10 ng).
Rats showed a tendency to prefer, rather than avoid, the flavor previously consumed after the AngII treatment regimen. Analysis of non-cumulative licks revealed a significant main effect of Flavor (F1,38=4.909, p<0.05, n=20 per group; Figure 2B) and a significant Time × Flavor interaction (F2,76=4.429, p<0.05). Post hoc analysis of individual time bins determined that this effect was most prominent 10 min after the injection, at which time rats licked more at the spout containing the flavor previously paired with the AngII treatment regimen (p<0.05). Total intake in the 30 min test did not, however, differ significantly (t=2.005, p>0.05; Figure 2C).
In a second set of experiments, consumption in the final two-bottle preference test was stimulated by water restriction (1 hr/day access) instead of by AngII injection. Here, rats showed no evidence of learned avoidance of or preference for the flavor previously paired with the AngII treatment regimen. We found no effect of Flavor on non-cumulative licks (main effect of Flavor, F1,20=0.895, p>0.05; Time × Flavor interaction, F2,40=0.321, p>0.05; n=11 per group; Figure 3B) or on total intake between groups (t=1.072, p>0.05; Figure 3C).
3.3. Repeated injections of AngII did not enhance the pressor response to an AngII test injection
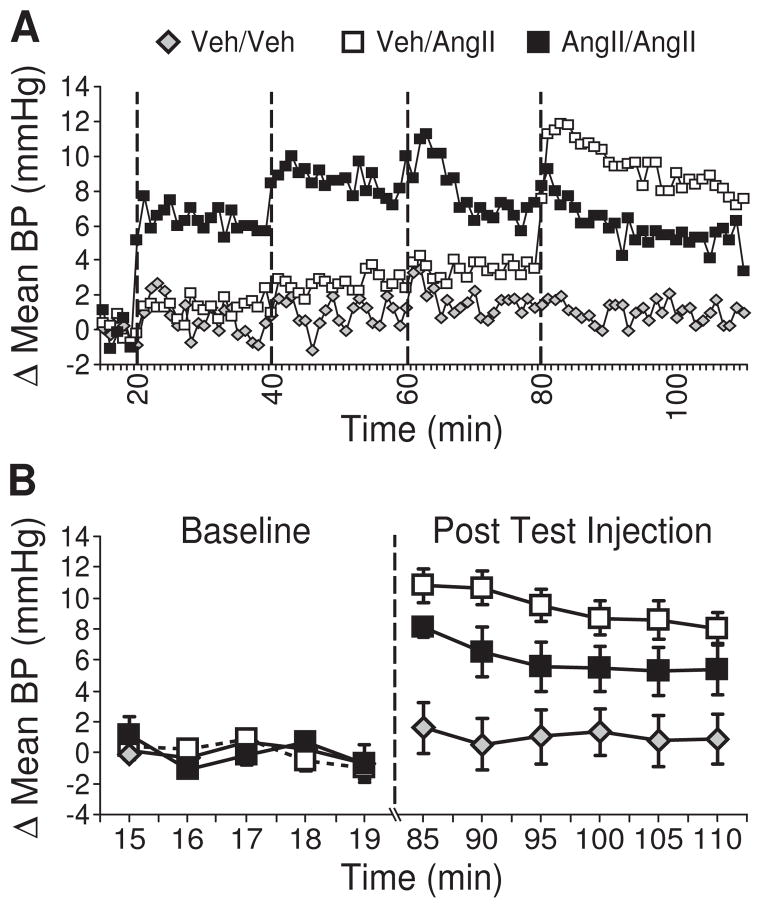
Elevated blood pressure inhibits AngII-induced water intake [19]. Therefore, an enhanced pressor response to repeated injections of AngII could explain the observed differences in water intake. To test this hypothesis, we recorded blood pressure in rats given either an AngII or a vehicle treatment regimen prior to a test injection of AngII. A third group of rats received a vehicle treatment regimen prior to a vehicle test injection. We found no statistical difference in baseline blood pressure between groups (main effect of Condition, F2,19= 1.782, p>0.05, n=7–8 per group; Table 1). Pressure recordings over the course of the treatment regimen revealed a significant main effect of Condition (F2,18=9.791, p<0.05; Figure 4A) and post hoc tests confirmed that blood pressure was elevated in rats that received an AngII treatment regimen. We did not detect a significant Time × Condition interaction (F118,1062=1.125, p>0.05) in the time before the test injection. As expected, we found no significant differences in blood pressure over the course of the treatment regimen injections between the two groups of rats that received vehicle treatment regimens. Analysis of the final blood pressure measure taken before the test injection indicated that blood pressure remained elevated at this time in the rats that received an AngII treatment regimen (main effect of Condition, F2,19= 5.945, p<0.05; Table 1). Analysis of blood pressure after the test injection revealed a significant main effect of Condition (F2,19=9.965, p<0.05, n=7–8 per group; Figure 4B) and a significant Time × Condition interaction (F10,95=2.59, p<0.05). Post hoc tests on the interaction found differences in the response to the test injection (vehicle vs. AngII) in rats given a vehicle treatment regimen. Specifically, the AngII test injection caused an increase in blood pressure at all of the times tested (p<0.05). Rats given an AngII treatment regimen, however, had a pressor response to a test injection of AngII that did not differ significantly from either of the other two groups (p>0.05); however, when we limited our analysis to the peak change in pressure after the test injection, we found that both groups of rats that received an AngII test injection differed from controls, but not from each other (F2,19= 12.803, p<0.05; Table 1).
Table 1.
Baseline mean blood pressure, change in mean pressure immediately before the test injection, and peak change in mean pressure after the test injection. Statistically significant differences (p<0.05) from rats receiving only vehicle (vehicle treatment regimen and vehicle test injection) are noted by an asterisk within each measure.
| Condition | Mean Baseline | Δ mean pressure before test injection | Peak Δ mean pressure after test injection |
|---|---|---|---|
| Vehicle/Vehicle | 76.89 ± 4.54 | 1.26 ± 1.46 | 4.69 ± 1.60 |
| Vehicle/AngII | 68.13 ± 2.39 | 3.5 ± 1.10 | 12.75 ±1.12 * |
| AngII/AngII | 73.59 ± 3.05 | 7.27 ± 1.02 * | 10.56 ± 0.52 * |
Figure 4.
The pressor response to vehicle or AngII after repeated injections of vehicle or AngII. Rats were given either an AngII or a vehicle treatment regimen prior to a test injection of either AngII or vehicle. (A) The AngII treatment regimen caused an elevated pressor response that was greater than that by rats given a vehicle treatment regimen (p<0.05, n=7–8 per group). Vertical dashed lines are used to show the timing of injections. (B) In response to a test injection of AngII, rats given a vehicle treatment regimen had a reliable pressor response (p<0.05 vs. vehicle test injection); however, the response to the same test injection of AngII by rats in the AngII treatment regimen group was not statistically significant compared with either negative controls (vehicle treatment regimen and vehicle test injection) or positive controls (vehicle treatment regimen and AngII test injection; p>0.05). The key shown in panel A applies to panel B.
4. Discussion
The present experiments tested three potential reasons that rats given repeated injections of AngII drink less water than do rats given a single injection of AngII. We found no support for any of these explanations, suggesting that this behavioral desensitization results from a more specific tachyphylaxis. Moreover, these data are consistent with the hypothesis that the changes in behavior reflect an underlying change in AngII receptor function.
Consistent with a previous report [14], we found that repeated injections of AngII caused a reduction in AngII-induced water intake, but water intake stimulated by an injection of carbachol was unaffected. Because carbachol-induced drinking is independent from intake stimulated by AngII, this finding suggests that the behavioral desensitization is specific to the angiotensin system. If the reduction in AngII-stimulated water intake after an AngII treatment regimen were the result of some broader behavioral or motor deficit, water intake would have been equally affected by repeated injections of AngII in rats given a test injection of AngII or of carbachol. The data did not, however, support this possibility because repeated injections of AngII only affected drinking stimulated by a test injection of AngII. Taken together with our previous finding that a repeated AngII treatment regimen did not affect saline intake [16], it seems unlikely that a more general motor impairment or broader behavioral deficit accounted for the effect of repeated injections of AngII.
Although carbachol-induced drinking would likely be affected by any treatment that caused a more general negative effect, such as malaise or visceral distress, we wanted to consider additional testing paradigms to address this possibility. To this end, we used well-established flavor conditioning procedures [20] to evaluate any positive or negative associations conditioned by the repeated injections of AngII. These experiments failed to find evidence for a negative association between the novel flavor and repeated injections of AngII. To the contrary, in one of the two testing conditions used, rats appeared to prefer the flavor that had been paired with repeated injections of AngII. When rats were tested under conditions of scheduled water access, however, we found no differences in the number of licks or intake of the two flavors. A potential explanation for the different results may relate to state-dependent learning. Tasks learned during exposure to particular drugs, for example, are performed better while exposed to those same drugs than in the absence of the drug [21]. It is possible that the development of a preference would be specific to times while AngII levels were elevated as they were during the conditioning trials. Accordingly, when intake during the preference test was stimulated by fluid restriction, instead of by AngII injection, the difference in AngII exposure created a different context from that during conditioning, thus a preference was no longer apparent. This type of state-dependent learning of flavor preferences has been documented previously using differences in deprivation state. For example, when rats are food-deprived, they display a preference for a non-nutritive flavor that had previously been paired with protein repletion. Moreover, this preference is attenuated by a protein preload, but not a carbohydrate preload [22]. Perhaps more relevant to the present study, Holman demonstrated that water-deprived rats preferred a flavored water that had been paired with water intake under a similar deprivation state, but this preference was not expressed when the rats were not water deprived [23]. Nevertheless, the rats in the present studies never showed evidence of an aversion to the flavor paired with repeated injections of AngII and we, therefore, reject the hypothesis that a more general aversive effect is responsible for the reduced intake observed after a treatment regimen of AngII.
Central injection of AngII increases blood pressure [24], and hypertension can inhibit water intake stimulated by central AngII [18, 19]. If repeated injections of AngII led to a sensitized response to the test injection, the exaggerated change in blood pressure could explain the differences in water intake. To test this hypothesis, we measured blood pressure in rats given repeated injections of AngII or vehicle before a test injection of AngII and compared the pressor response to controls given only vehicle. The results of this experiment did not support the hypothesis because we did not detect any difference in the AngII-induced pressor response in rats that received an AngII or a vehicle treatment regimen. Analysis of peak change in pressure after the test injection found that both groups receiving an AngII test injection had changes in blood pressure greater than those observed after a vehicle test injection. When the analysis included all measures after the test injection, however, we did not find differences between the group receiving repeated injections of AngII and rats receiving only vehicle. This suggests that the AngII treatment regimen attenuated the pressor response to the final AngII test injection, but caution is needed when drawing this conclusion because there were no statistically significant differences detected between the two groups given a test injection of AngII. It is important to note, however, that any effect of the repeated injections of AngII, even though not statistically significant, was in the opposite direction from that which would cause decreases in intake. Nevertheless, these data warrant further investigation to determine if repeated injections of AngII affect the pressor response, but it is clear that the decrease in water intake caused by repeated AngII administration cannot be explained by a sensitized pressor response to the final AngII test injection.
Additional studies have been able to produce an almost complete suppression of water intake using lower doses of AngII and fewer injections [15, 17], but a major difference exists between the methods used in these studies and those reported here and previously by our laboratory [16]: we do not allow the rats to drink over the course of the injection protocol. Although previous studies have attempted to control for the effects of water intake by providing a gastric preload or an open gastric fistula, these studies do not account for the intake-suppressive effects of water in the mouth and throat [25], nor do they control for the possibility that the act of drinking provides negative feedback. Regardless of differences in the magnitude of the response or the doses of AngII needed to suppress drinking in the various studies, the literature collectively provides strong evidence that rats given repeated injections of AngII drink less water than is consumed by rats given a single injection of AngII. The present data indicate that this difference in intake is not due to a more generalized behavioral suppression or motor deficit, the generation of a negative affective response, or differences in blood pressure.
5. Conclusions
The present study investigated the specificity of AngII-induced behavioral desensitization. We found that this phenomenon appears to be specific to the angiotensin system because rats drank less water in response to a test injection of AngII after prior exposure to an AngII treatment regimen, but water intake stimulated by the cholinergic agonist carbachol was unaffected. Furthermore, it is unlikely that AngII-induced behavioral desensitization is the result of some aversive consequence of the repeated AngII treatment regimen because rats did not avoid a novel flavor that was paired with an AngII treatment regimen, and at least under certain testing conditions it appears that rats prefer the flavor paired with repeated injections of AngII. Finally, we showed that this behavioral desensitization is not explained by differences in blood pressure at the time when the intake occurs because there was no difference in the pressor response to a test injection of AngII in rats that received an AngII or a vehicle treatment regimen. Taken together, the findings rule out these alternative explanations and are consistent with the hypothesis that the observed behavioral desensitization reflects an underlying change in AngII responsiveness.
Research Highlights.
Repeated icv AngII causes a reduction in the dipsogenic response to subsequent AngII.
This AngII-induced desensitization is not the result of motor impairment.
Repeated icv AngII did not produce a conditioned flavor avoidance.
AngII-induced desensitization is not explained by differences in blood pressure.
Acknowledgments
Aniko Marshall, Naomi McKay, Elizabeth Mietlicki, and Kimberly Plyler provided valuable technical assistance. Funding was provided by NIH award HL-91911 (DD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fitzsimons JT. Angiotensin, thirst, and sodium appetite. Physiol Rev. 1998;78:583–686. doi: 10.1152/physrev.1998.78.3.583. [DOI] [PubMed] [Google Scholar]
- 2.Sakai RR, Ma LY, He PF, Fluharty SJ. Intracerebroventricular administration of angiotensin type 1 (AT1) receptor antisense oligonucleotides attenuate thirst in the rat. Regul Pept. 1995;59:183–92. doi: 10.1016/0167-0115(95)00111-n. [DOI] [PubMed] [Google Scholar]
- 3.McKinley MJ, McAllen RM, Pennington GL, Smardencas A, Weisinger RS, Oldfield BJ. Physiological actions of angiotensin II mediated by AT1 and AT2 receptors in the brain. Clin Exp Pharmacol Physiol Suppl. 1996;3:S99–104. [PubMed] [Google Scholar]
- 4.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 5.Kirby RF, Thunhorst RL, Johnson AK. Effects of a non-peptide angiotensin receptor antagonist on drinking and blood pressure responses to centrally administered angiotensins in the rat. Brain Res. 1992;576:348–50. doi: 10.1016/0006-8993(92)90703-c. [DOI] [PubMed] [Google Scholar]
- 6.Sakai RR, He PF, Yang XD, Ma LY, Guo YF, Reilly JJ, et al. Intracerebroventricular administration of AT1 receptor antisense oligonucleotides inhibits the behavioral actions of angiotensin II. J Neurochem. 1994;62:2053–6. doi: 10.1046/j.1471-4159.1994.62052053.x. [DOI] [PubMed] [Google Scholar]
- 7.Thomas WG. Regulation of angiotensin II type 1 (AT1) receptor function. Regul Pept. 1999;79:9–23. doi: 10.1016/s0167-0115(98)00140-2. [DOI] [PubMed] [Google Scholar]
- 8.Guo DF, Sun YL, Hamet P, Inagami T. The angiotensin II type 1 receptor and receptor-associated proteins. Cell Res. 2001;11:165–80. doi: 10.1038/sj.cr.7290083. [DOI] [PubMed] [Google Scholar]
- 9.Thomas WG, Thekkumkara TJ, Baker KM. Cardiac effects of AII. AT1A receptor signaling, desensitization, and internalization. Adv Exp Med Biol. 1996;396:59–69. [PubMed] [Google Scholar]
- 10.Gebke E, Muller AR, Jurzak M, Gerstberger R. Angiotensin II-induced calcium signalling in neurons and astrocytes of rat circumventricular organs. Neuroscience. 1998;85:509–20. doi: 10.1016/s0306-4522(97)00601-5. [DOI] [PubMed] [Google Scholar]
- 11.Hunyady L, Catt KJ, Clark AJ, Gaborik Z. Mechanisms and functions of AT(1) angiotensin receptor internalization. Regul Pept. 2000;91:29–44. doi: 10.1016/s0167-0115(00)00137-3. [DOI] [PubMed] [Google Scholar]
- 12.Sasamura H, Dzau VJ, Pratt RE. Desensitization of angiotensin receptor function. Kidney Int. 1994;46:1499–501. doi: 10.1038/ki.1994.429. [DOI] [PubMed] [Google Scholar]
- 13.Billet S, Bardin S, Verp S, Baudrie V, Michaud A, Conchon S, et al. Gain-of-function mutant of angiotensin II receptor, type 1A, causes hypertension and cardiovascular fibrosis in mice. J Clin Invest. 2007;117:1914–25. doi: 10.1172/JCI28764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quirk WS, Wright JW, Harding JW. Tachyphylaxis of dipsogenic activity to intracerebroventricular administration of angiotensins. Brain Res. 1988;452:73–8. doi: 10.1016/0006-8993(88)90010-8. [DOI] [PubMed] [Google Scholar]
- 15.Torsoni MA, Carvalheira JB, Calegari VC, Bezerra RM, Saad MJ, Gontijo JA, et al. Angiotensin II (AngII) induces the expression of suppressor of cytokine signaling (SOCS)-3 in rat hypothalamus - a mechanism for desensitization of AngII signaling. J Endocrinol. 2004;181:117–28. doi: 10.1677/joe.0.1810117. [DOI] [PubMed] [Google Scholar]
- 16.Vento PJ, Daniels D. Repeated administration of angiotensin II reduces its dipsogenic effect without affecting saline intake. Exp Physiol. 2010;95:736–45. doi: 10.1113/expphysiol.2010.052191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zapparoli A, Figueiredo JF, Boer PA, Gontijo JA. Impaired dipsogenic and renal response to repetitive intracerebroventricular angiotensin II (AngII) injections in rats. J Renin Angiotensin Aldosterone Syst. 2011;12:161–8. doi: 10.1177/1470320310392617. [DOI] [PubMed] [Google Scholar]
- 18.Thunhorst RL, Johnson AK. Effects of arterial pressure on drinking and urinary responses to intracerebroventricular angiotensin II. Am J Physiol. 1993;264:R211–7. doi: 10.1152/ajpregu.1993.264.1.R211. [DOI] [PubMed] [Google Scholar]
- 19.Thunhorst RL, Lewis SJ, Johnson AK. Role of arteria baroreceptor input on thirst and urinary responses to intracerebroventricular angiotensin II. Am J Physiol. 1993;265:R591–5. doi: 10.1152/ajpregu.1993.265.3.R591. [DOI] [PubMed] [Google Scholar]
- 20.Myers KP, Sclafani A. Development of learned flavor preferences. Dev Psychobiol. 2006;48:380–8. doi: 10.1002/dev.20147. [DOI] [PubMed] [Google Scholar]
- 21.Overton DA. State-Dependent or “Dissociated” Learning Produced with Pentobarbital. J Comp Physiol Psychol. 1964;57:3–12. doi: 10.1037/h0048023. [DOI] [PubMed] [Google Scholar]
- 22.Baker BJ, Booth DA, Duggan JP, Gibson EL. Protein appetite demonstrated: Learned specificity of protein-cue preference to protein need in adult rats. Nutrition Research. 1987;7:481–7. [Google Scholar]
- 23.Holman EW. Irrelevant-incentive learning with flavors in rats. J Exp Psychol Anim Behav Process. 1980;6:126–36. [PubMed] [Google Scholar]
- 24.Wright JW, Morseth SL, Abhold RH, Harding JW. Pressor action and dipsogenicity induced by angiotensin II and III in rats. Am J Physiol. 1985;249:R514–21. doi: 10.1152/ajpregu.1985.249.5.R514. [DOI] [PubMed] [Google Scholar]
- 25.Miller NE, Sampliner RI, Woodrow P. Thirst-reducing effects of water by stomach fistula vs. water by mouth measured by both a consummatory and an instrumental response. J Comp Physiol Psychol. 1957;50:1–5. doi: 10.1037/h0046009. [DOI] [PubMed] [Google Scholar]