Abstract
Several new technologies are providing useful diagnostic tools and new information related to the pathogenesis of certain oral diseases. In this review, we describe several of these technologies including gene and microRNA arrays, proteomics, and antigen arrays as they relate to the study of Sjögren's syndrome and head and neck cancer. A common theme is the systematic analysis of large scale inventories of RNAs, proteins, and autoantibody biomarkers revealing information not previously recognized. We also discuss metagenomic approaches that characterize the many different microorganisms present in the oral cavity that may impact oral and human health. Lastly, we describe applications of a new type of antibody profiling technology termed Luciferase Immunoprecipitation Systems (LIPS), which has a wide dynamic range of detection of both linear and conformational epitopes needed for optimum diagnostics and biomarker discovery. We propose that the information offered by these technologies will enhance our ability to diagnose, treat, and further understand the pathogenesis of multiple oral diseases.
Keywords: antigen array, gene array, LIPS, metagenomics, oral cancer, salivary gland, Sjögren's syndrome
Introduction
The oral cavity is constantly exposed to a wide variety of viruses, microbes, and other environmental insults. These stresses contribute to environment–gene interactions which are likely involved in the triggering and/or the pathogenesis of many oral diseases including autoimmune, infectious diseases, and cancer. One common autoimmune disease, Sjögren's syndrome (SjS), is characterized by an autoimmune attack on the salivary and lacrimal glands leading to decreased saliva and tear production (Fox, 2005). Although women are nearly nine times more commonly affected with SjS than men, the exact susceptibility genes and potential environmental triggers are unknown. Head and neck cancer, another important oral disease, is the sixth most common cancer in the world, possibly due to its widespread risk factors including tobacco use, excessive alcohol consumption, and human papilloma virus (HPV) infection (Haddad and Shin, 2008). A number of recent studies employing new technologies are providing novel insights into the pathogenesis, diagnosis, and/or treatment of some of these oral diseases. An important feature of many of these approaches is their ability to obtain large scale inventories of gene expression, microRNAs, proteins, and antibodies to provide a previously unrealized global perspective. One of these new technologies, Luciferase Immunoprecipitation Systems (LIPS), harnesses light-emitting recombinant antigen fusion proteins in a solution phase immunoassay to quantitatively measure antibody titers in serum and saliva for biomarker discovery. Application of this system can lead to better and faster diagnosis of SjS, robust biomarkers for the identification of autoimmune attack on extraglandular sites in SjS, and the potential identification of exposure to infectious agents related to oral diseases. In this review, we briefly describe these different technologies and focus on their utility for understanding oral diseases and infections. We also refer the readers to additional in-depth reviews of several of these topics including salivary proteomics (Spielmann and Wong, 2011), metagenomics (Zarco et al, 2011), and point of care diagnostics (Hart et al, 2011).
Gene Expression Profiling of Sjögren's Syndrome and Oral Cancers
In gene expression profiling studies, target mRNAs are isolated, labeled, and hybridized with gene arrays to evaluate mRNA expression profiles. Since the design of most commonly used arrays contains from 200 to 30,000 immobilized gene probes, one can simultaneously measure the expression levels of large numbers of mRNA species from target cells or tissues. One application of gene array profiling relevant to oral disease has been in studying gene expression in SjS. Studies using this approach in many SjS patients have revealed that the salivary gland (Gottenberg et al, 2006; Hjelmervik et al, 2005), peripheral blood monocytes (Emamian et al, 2009; Wildenberg et al, 2008), and plasmacytoid dendritic cells (Wildenberg et al, 2008) show a unique pattern of gene expression characterized by the up-regulation of many interferon inducible genes termed the interferon signature (see Table 1). For example, TLR8, IFITM1, BAFF, and IP-10, all known interferon up-regulated genes, were found to be elevated in a large number of SjS patients (Gottenberg et al, 2006), which is consistent with the in vivo finding of enriched levels of interferon-α-producing cells in the salivary gland, consistent with an increased local production of this cytokine (Bave et al, 2005). It is important to note that not all SjS patients show an interferon-signature (Hjelmervik et al, 2005), suggesting that the disease is heterogeneous and may involve different mechanisms. An interferon-alpha signature is also found in several other autoimmune diseases including systemic lupus erythematosis (SLE) (Baechler et al, 2003; Bennett et al, 2003) in which anti-interferon-αantibody based therapy has been used as a treatment for blocking flares of the disease (Merrill et al, 2011; Yao et al, 2009). These overlapping findings suggest that similar therapeutic strategies targeting interferon-α or its signaling pathways might also be effective in treating SjS.
Table 1.
Biomarker discovery using large scale inventorying technologies.
| Technology | Findings | Reference |
|---|---|---|
| Gene Array | Gene array profiling of SjS patients identifies an “interferon signature” that includes up-regulated genes such as TLR8, IFITM1, and BAFF. | Bave et al, 2005; Gottenberg et al, 2006; Hjelmervik et al, 2005 |
| Relative expression of an eight-gene panel identifies SjS patients with a favorable response to anti-CD20 antibody therapy. | Devauchelle-Pensec et al, 2010 | |
| MicroRNA Array | The ratio of two microRNA (has-miR-768-3p, and has-miR-574) from gland biopsies predicts increased salivary inflammation. | Alevizos et al, 2011 |
| Metagenomics | High levels of Porphyromonas catoniae and Neiseerira flavescens in the oral cavity are associated with a lack of dental caries. | Crielaard et al, 2011 |
| Proteomics | SjS patients show altered protein composition of saliva | Hu et al, 2007; Ryu et al, 2006 |
| Antigen Array | Salivary autoantibodies against 24 proteins distinguished SjS from SLE and controls. | Hu et al, 2011 |
Gene expression profiling has also been used to identify treatment biomarkers for monitoring SjS therapy. In a study exploring anti-CD20 antibody treatment of SjS, gene array profiling identified eight genes which distinguished SjS patients with a favorable response to therapy compared to non-responders (see Table 1) (Devauchelle-Pensec et al, 2010). Validation of an eight-gene panel by PCR revealed that two genes were markedly down regulated, and six different genes were markedly up-regulated following treatment in the favorable response subgroup. These encouraging results demonstrate the possibility of using gene expression profiles in monitoring the efficacy of anti-CD20 antibody treatment in SjS and offer the possibility for further mechanistic studies, as well as monitoring other potential treatment modalities.
Remarkably, mRNAs shed from cells within the oral cavity remain stable and can also be evaluated by gene array profiling. Gene array analysis of cell-free saliva from healthy individuals revealed approximately 3000 different mRNAs present (Li et al, 2004b), and profiling of saliva identified a panel of potential mRNA biomarkers that could distinguish SjS patients from controls (Hu et al, 2007). Interestingly, many of the mRNA transcripts were previously identified interferon up-regulated genes known to be elevated in the salivary glands of SjS patients. Analogously, gene array profiling of mRNA salivary biomarkers for oral cancer identified a panel of four genes (SAT, IL-8, IL1-β and OAZ) that had diagnostic sensitivity and specificity of approximately 90% (Li et al, 2004a). These mRNA targets in saliva represent non invasive biomarkers potentially useful for diagnosis, but they require further improvement in performance to have real practical value.
Identifying Salivary MicroRNA Biomarkers
MicroRNAs are becoming increasingly recognized for their important role in gene regulation and disease pathogenesis (Filipowicz et al, 2008). Functionally, a single microRNA can bind a large number of different mRNA species and thereby control gene expression via translational repression and gene silencing. To begin to elucidate the role of microRNAs in salivary gland function, several recent studies have used PCR, high-throughput sequencing, and microRNA arrays to catalogue the microRNAs present in both saliva and the salivary gland. In one such study, microRNAs were isolated from small secreted vesicles, known as exosomes, in saliva and were studied by PCR in order to understand their expression in oral diseases (Michael et al, 2010). In a different study, researchers used commercially available RNA isolation kits to directly isolate RNA from saliva and identified five major microRNA species and many previously uncharacterized microRNA species in saliva from healthy individuals (Patel et al, 2011).
MicroRNA expression patterns may also have diagnostic potential as biomarkers for several oral diseases. In one study, a microRNA array based approach employing 540 human and 47 viral microRNAs identified several microRNAs that distinguished SjS patients from controls (Alevizos et al, 2011). Using quantitative PCR, two microRNAs were evaluated as simple biomarkers of inflammation. The researchers found that the has-miR-768-3p microRNA increased with focus score, while the has-miR-574 microRNA decreased. Comparison of the differential expression of these two microRNAs distinguished SjS patients with a high-degree of inflammation as assessed by focus score from those with a low-degree of inflammation (see Table 1). These studies, if confirmed in a larger number of patients, might simplify the assessment of salivary gland inflammation and significantly reduce the substantial subjectivity of scoring inflammatory infiltrates. In another study, expression of certain microRNAs correlated with and may play a role in the high levels of SSA and SSB autoantibodies found in SjS patients (Kapsogeorgou et al, 2011). In addition to SjS, oral cancer is another disease in which microRNAs have been explored as biomarkers. Two microRNAs, mir-125-a and mir-200-a, were found at lower levels in saliva of patients with oral squamous cell carcinoma than in healthy controls (Park et al, 2009). Blinded clinical studies are necessary to further validate the usefulness of these and other microRNAs in different oral diseases.
Metagenomics of the Oral Cavity
Metagenomics is a relatively new method of genetic characterization that involves nucleic acid isolation and massive, unbiased DNA sequencing to catalogue microorganisms and viruses in a given sample. One of the first metagenomic studies of the oral cavity focused on defining the DNA viral communities containing both phage and eukaryotic viruses in healthy individuals (Willner et al, 2011). Following viral DNA extraction from oropharyngeal swabs and subsequent large-scale DNA sequencing, mainly viral sequences derived from phages associated with particular bacteria were identified including Escherichia coli phage T3, Propionibacterium acnes phage PA6, and Streptococcus mitis phage SM1. In addition, DNA corresponding to the eukaryotic virus, Epstein-Barr virus (EBV) was also detected. These studies illustrate that phage associated with particular bacteria are present in high amounts in the oral cavity.
Normal flora in the oral cavity has also been studied in detail in healthy individuals to define the core bacterial species present. In one study thousands of bacterial species were identified by high-throughput DNA sequencing in healthy individuals (Keijser et al, 2011). An extension of this work has explored the different bacterial organisms present in children in relation to developmental stage and oral health (Crielaard et al, 2011). Researchers initially used high throughput DNA sequencing to identify the relevant bacteria then generated a microarray of 350 probes to determine the relative abundance of different bacterial targets in clinical samples. Subsequent interrogation with these bacterial arrays revealed that a caries-free oral cavity status was associated with a significantly higher signal of probes targeting certain bacteria including Porphyromonas catoniae and Neiseerira flavescens (see Table 1). While the exact mechanism underlying this association is unclear, these studies highlight the interplay between oral health and the resident bacteria present. Future studies are needed to explore the biological significance of these findings. It is possible, that there may be yet undiscovered infectious agents linked to other oral diseases. For example, infections by a number of other viruses including HIV, HTLV, and HCV can also cause sicca-like symptoms. Because of this, one area of additional exploration is to determine if currently unrecognized pathogens may be involved in certain subsets of SjS patients and other oral diseases.
Proteomics Analysis of Saliva for Studying Oral Diseases
Although two-dimensional electrophoresis was used historically to characterize the proteins present in a given sample, researchers now employ highly sensitive mass spectroscopy techniques to identify, measure, and catalogue proteins. In the case of human saliva, a detailed study using mass spectroscopy identified approximately 1100 proteins (Denny et al, 2008). Although a small subset of proteins was found exclusively in saliva, many of the proteins were also present in tears and plasma. Not unexpectedly, the exact composition of saliva can vary in different oral diseases when compared to baseline. In a study comparing SjS patients to healthy controls, a number of salivary proteins were increased while others were decreased (see Table 1) (Hu et al, 2007; Ryu et al, 2006). For example, amylase and carbonic anhydrase VI were decreased in the SjS patients, while lysozyme C, beta-2-microglobulin, and lactoferrin were increased in SjS patients compared to controls. In addition to SjS, head and neck cancer diagnosis is another disease in which there is interest in using such proteomic profiles. In patients with oral squamous cell carcinoma, several proteins including CD59, profilin, and catalase were detected at higher levels in saliva than they were in matched controls (Hu et al, 2008). Further refinement of these techniques may make them practical for use in diagnosing SjS and head and neck cancer.
Autoantibody Arrays of Serum and Saliva in Sjögren's Syndrome
Antibodies remain important biomarkers for many diseases due to their presence in biological fluids such as serum and saliva that can be obtained with minimal invasiveness, their stability, and their relative ease of detection for some targets. For the diagnosis of SjS, evaluation of serum autoantibody titers to SSA and SSB autoantigens by ELISA immunoassay serves as one of the critical clinical tests; however, only 75% of patients with SjS show autoantibodies to these antigens, and because these autoantibodies can also be found in other rheumatologic conditions, neither the sensitivity nor the specificity is 100% (Fox, 2005).
One new approach for autoantibody discovery involves using solid phase microarrays that can simultaneously evaluate antibody responses to thousands of immobilized human autoantigens and may provide clues to SjS pathogenesis. While solid phase antigen arrays are highly useful for autoantigen discovery, it should be noted that these solid phase arrays are not without issues including the sub-optimal detection of conformational epitopes, high backgrounds due to impure antigens, and narrow dynamic ranges of detection (Burbelo et al, 2010a). Using a commercially available array of approximately 8000 autoantigens, 24 different autoantibodies were identified in saliva that distinguished SjS from SLE patients and healthy controls (Hu et al, 2011). Several of the autoantigens identified represent biomarkers known to be elevated in the serum of patients with SLE, including autoantibodies against SSA, SSB, histone, and transglutaminase, but interestingly were found to be higher in the saliva of patients with SjS compared to those with SLE. Since serum autoantibodies against SSA and SSB are also found in SLE, these results may reflect the local increased production of these autoantibodies specifically from the salivary glands of SjS patients. Further studies are needed to validate many of these findings and determine whether some of the newly discovered antigens can be incorporated into simpler and more quantitative immunoassays for improved diagnosis of SjS.
LIPS Antibody Profiling of Sjögren's Syndrome
Due to the limited clinical performance of many solid phase immunoassays such as ELISAs and protein arrays, the antibodies present in serum have not been fully exploited for diagnostic purposes. To overcome these problems, we have developed the liquid phase luciferase immunoprecipitation systems (LIPS) technology that harnesses light emitting proteins to generate high definition antibody profiles optimal for both diagnostics and biomarker discovery. LIPS detects antibody response profiles against both linear and conformational epitopes of different proteins thereby providing highly sensitive diagnostics for domestic and global pathogens, insights into infection-related diseases, discovery of new biomarkers for human diseases, and identification of pathogenic autoantibodies (Burbelo et al, 2011).
LIPS studies in SjS patients found that autoantibodies against the recombinant La protein, which comprises the SSB autoantigen, were the most useful autoantigen for diagnosis (Figure 1). The LIPS anti-La autoantibody test demonstrated 75% sensitivity for the diagnosis of SjS and had markedly better clinical performance than the 46% sensitivity observed by ELISA (Burbelo et al, 2009). Additionally, the ability of the LIPS assay to produce and individually test the Ro52 and Ro60 proteins, which comprise the SSA autoantigen, revealed not only a high diagnostic performance of approximately 65% sensitivity for each autoantigen, but also showed the unique titers present for each protein. Remarkably, the high levels of Ro52 and Ro60 autoantibodies present in SjS patients and even healthy controls require extensive dilution of sera to obtain clinically useful results (Burbelo et al, 2009; Burbelo et al, 2010b). Furthermore, high levels of Ro52 and Ro60 autoantibodies are also present in saliva and had similar diagnostic performance for SjS as using serum (Ching et al, 2011). These studies highlight the usefulness of LIPS for diagnosing SjS.
Figure 1.
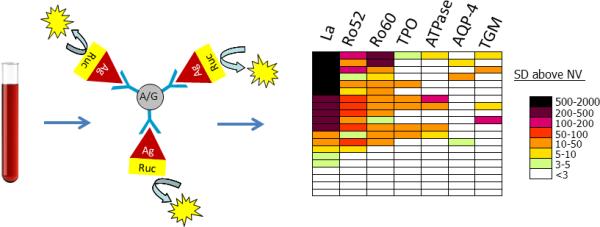
LIPS provides a facile and robust technology for measuring autoantibodies in SjS. In these studies, antibodies in serum are incubated in solution with Renilla luciferase (Ruc)-tagged antigens (Ag). IgG immunoglobulins are then captured with protein A/G beads and following washing, antigen-specific antibody titers are measured by luciferase-catalyzed light production. Due to the wide dynamic range of antibody detection and the ability to profile many different target antigens, LIPS data can often be best visualized with a heatmap of color-coded antibody titers. In this heatmap, the antigen-antibody measurement greater than the control mean plus 3 standard deviations was color-coded to signify a titer above these cut-off values. Colors on the heatmap signify the relative number of standard deviations further above the control mean plus 3 standard deviations. Each row of the heatmap represents one patient's antibody profile against 7 different autoantigens including La, Ro52, Ro60, thyroid peroxidase (TPO), gastric ATPase (ATPase), aquaporin-4 (AQP-4), and transglutaminase (TGM). Although many of the SjS patients showed high titer and heterogeneous autoantibody responses, four of the SjS patients at the bottom of the heatmap did not show statistical response to any of the autoantigens tested.
Besides sicca symptoms, other extraglandular symptoms are common in subsets of SjS patients including neurological and gastrointestinal symptoms, and thyroid disease (Fox, 2005). Since many of these extraglandular symptoms seen in SjS patients are heterogeneous and may have an autoimmune basis, the SjS autoantibody profiles generated by LIPS may be a perfect tool for personalized medicine providing a new perspective for identifying these additional clinical problems. From LIPS testing, certain SjS patients were found to have elevated levels of antibodies to a variety of autoantigenic targets such as thyroid peroxidase, gastric ATPase, and aquaporin-4 (AQP-4) that are likely representative of autoimmune attacks on the thyroid, gastric parietal cells, and blood brain barrier, respectively (Figure 1) (Burbelo et al, 2009). Autoantibodies to AQP-4 were found to be a specific biomarker of nervous system involvement in SjS (Burbelo et al, 2009) and therefore it is likely that detection of other autoantibodies by LIPS may have further clinical correlates. Additional unpublished findings suggest that there may be similar clinical implications for other neuronal autoantibodies, autoantibodies associated with celiac disease such as transglutaminase (TGM), cancer protein targets related to SjS-associated lymphoma, and even anti-cytokine autoantibodies. Since the sensitivity of LIPS and other antibody tests are not 100% sensitive (e.g. many SjS patients are negative for SSA and SSB autoantibodies), the assembly of a large autoantigen screening panel may identify the SjS patients that are negative from existing autoantibody testing. A more complete understanding of the autoantibody targets in SjS may also allow the identification of patients earlier in the disease and the possibility for better treatments. Lastly, based on encouraging results of a very small LIPS study, a similar strategy employing a large panel of antigenic targets may also be useful for early detection of patients with head and neck and other cancers (Burbelo et al, 2005).
Conclusion
The continued application of many of the described technologies including gene and microRNA arrays, proteomics, and antigen arrays are likely to impact our understanding of SjS, head and neck cancer, and other oral diseases. The ability to data mine these inventories of biomarkers in large-scale studies is likely to reveal information previously unrecognized, including new targets for therapy. In addition, the ability to identify biomarkers that correlate with response to treatment, as evidenced in SjS patients treated with anti-CD20 antibody therapy, will provide the opportunity for personalized care and is a field of therapeutics that will continue to expand in the years to come.
Other technologies not discussed in the review that generate high content genomic data, including genome-wide association studies (GWAS) and DNA sequencing of the full genome, are also likely to provide further information related to these oral diseases. In one example, high-throughput DNA sequencing of tumors from head and neck cancer recently identified previously unrecognized, high frequency mutations in the Notch signaling pathway (Agrawal et al, 2011). The identification of an individualized mutation spectrum offers new possibilities of developing early diagnostics and new therapeutic strategies for these subsets of patients with head and neck cancer. Similarly, GWAS and high throughput sequencing may identify important polymorphisms in genetic susceptibility genes associated with SjS and other oral diseases. Ultimately, integrating and analyzing multiple types of information including gene expression, autoantibodies, and genetic polymorphisms together may yield additional insights not yet recognized.
For some of the discussed technologies, translation of these findings to clinical practice will require further validation, refinement, and technical improvements; however, improved laboratory-based antibody detection assays will likely be implemented in the near future. For example, a new rapid microfluidics LIPS format could be used in point of care testing for the diagnosis of SjS and is also applicable for antibody-based testing to study infection by pathogenic agents (Zubair et al, 2011). Serologic detection of a number of pathogens including biomarkers of HPV-driven head and neck tumorigenesis, if incorporated into a simple LIPS screening tool, would be quite useful as part of a routine oral exam. Additionally, the robust evaluation by LIPS offers the opportunity to monitor autoantibodies in prospective samples even before the onset of SjS. Since autoantibodies in other autoimmune diseases such as SLE and type I diabetes appear before clinical symptoms, it is possible that these antibody-based screening tools might be used for early prediction of individuals at risk of SjS, but more work needs to be done. Such detailed studies of SjS and other oral diseases might provide a greater understanding of potential environmental triggers and offer new insights into their prevention and treatment.
Acknowledgments
We thank Hal Kominsky and Jason Wagner for their critical reading of this manuscript. This work was supported by the Division of Intramural Research, National Institute of Dental and Craniofacial Research.
Footnotes
Author contributions
P.D.B. drafted the manuscript, A.B., E.E.L, M.J.I. and P.D.B. edited the manuscript and all authors approved the final manuscript.
REFERENCES
- Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alevizos I, Alexander S, Turner RJ, Illei GG. MicroRNA expression profiles as biomarkers of minor salivary gland inflammation and dysfunction in Sjogren's syndrome. Arthritis Rheum. 2011;63:535–544. doi: 10.1002/art.30131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bave U, Nordmark G, Lovgren T, Ronnelid J, Cajander S, Eloranta ML, et al. Activation of the type I interferon system in primary Sjogren's syndrome: a possible etiopathogenic mechanism. Arthritis Rheum. 2005;52:1185–1195. doi: 10.1002/art.20998. [DOI] [PubMed] [Google Scholar]
- Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo PD, Goldman R, Mattson TL. A simplified immunoprecipitation method for quantitatively measuring antibody responses in clinical sera samples by using mammalian-produced Renilla luciferase-antigen fusion proteins. BMC Biotechnol. 2005;5:22. doi: 10.1186/1472-6750-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo PD, Leahy HP, Issa AT, Groot S, Baraniuk JN, Nikolov NP, et al. Sensitive and robust luminescent profiling of anti-La and other autoantibodies in Sjogren's syndrome. Autoimmunity. 2009;42:515–524. doi: 10.1080/08916930902911738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo PD, Ching KH, Bush ER, Han BL, Iadarola MJ. Antibody-profiling technologies for studying humoral responses to infectious agents. Expert Rev Vaccines. 2010a;9:567–578. doi: 10.1586/erv.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbelo PD, Ching KH, Han BL, Bush ER, Reeves WH, Iadarola MJ. Extraordinary antigenicity of the human Ro52 autoantigen. Am J Transl Res. 2010b;2:145–155. [PMC free article] [PubMed] [Google Scholar]
- Burbelo PD, Ching KH, Bren KE, Iadarola MJ. Searching for biomarkers: humoral response profiling with luciferase immunoprecipitation systems. Expert Rev Proteomics. 2011;8:309–316. doi: 10.1586/epr.11.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching KH, Burbelo PD, Gonzalez-Begne M, Roberts ME, Coca A, Sanz I, et al. Salivary anti-Ro60 and anti-Ro52 antibody profiles to diagnose Sjogren's Syndrome. J Dent Res. 2011;90:445–449. doi: 10.1177/0022034510390811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crielaard W, Zaura E, Schuller AA, Huse SM, Montijn RC, Keijser BJ. Exploring the oral microbiota of children at various developmental stages of their dentition in the relation to their oral health. BMC Med Genomics. 2011;4:22. doi: 10.1186/1755-8794-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny P, Hagen FK, Hardt M, Liao L, Yan W, Arellanno M, et al. The proteomes of human parotid and submandibular/sublingual gland salivas collected as the ductal secretions. J Proteome Res. 2008;7:1994–2006. doi: 10.1021/pr700764j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devauchelle-Pensec V, Cagnard N, Pers JO, Youinou P, Saraux A, Chiocchia G. Gene expression profile in the salivary glands of primary Sjogren's syndrome patients before and after treatment with rituximab. Arthritis Rheum. 2010;62:2262–2271. doi: 10.1002/art.27509. [DOI] [PubMed] [Google Scholar]
- Emamian ES, Leon JM, Lessard CJ, Grandits M, Baechler EC, Gaffney PM, et al. Peripheral blood gene expression profiling in Sjogren's syndrome. Genes Immun. 2009;10:285–296. doi: 10.1038/gene.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- Fox RI. Sjogren's syndrome. Lancet. 2005;366:321–331. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- Gottenberg JE, Cagnard N, Lucchesi C, Letourneur F, Mistou S, Lazure T, et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjogren's syndrome. Proc Natl Acad Sci U S A. 2006;103:2770–2775. doi: 10.1073/pnas.0510837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad RI, Shin DM. Recent advances in head and neck cancer. N Engl J Med. 2008;359:1143–1154. doi: 10.1056/NEJMra0707975. [DOI] [PubMed] [Google Scholar]
- Hart R, Mauk M, Liu C, Qiu X, Thompson J, Chen D, et al. Point-of-care oral-based diagnostics. Oral Dis. 2011 doi: 10.1111/j.1601-0825.2011.01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelmervik TO, Petersen K, Jonassen I, Jonsson R, Bolstad AI. Gene expression profiling of minor salivary glands clearly distinguishes primary Sjogren's syndrome patients from healthy control subjects. Arthritis Rheum. 2005;52:1534–1544. doi: 10.1002/art.21006. [DOI] [PubMed] [Google Scholar]
- Hu S, Wang J, Meijer J, Ieong S, Xie Y, Yu T, et al. Salivary proteomic and genomic biomarkers for primary Sjogren's syndrome. Arthritis Rheum. 2007;56:3588–3600. doi: 10.1002/art.22954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Arellano M, Boontheung P, Wang J, Zhou H, Jiang J, et al. Salivary proteomics for oral cancer biomarker discovery. Clin Cancer Res. 2008;14:6246–6252. doi: 10.1158/1078-0432.CCR-07-5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Vissink A, Arellano M, Roozendaal C, Zhou H, Kallenberg CG, et al. Identification of autoantibody biomarkers for primary Sjogren's syndrome using protein microarrays. Proteomics. 2011;11:1499–1507. doi: 10.1002/pmic.201000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsogeorgou EK, Gourzi VC, Manoussakis MN, Moutsopoulos HM, Tzioufas AG. Cellular microRNAs (miRNAs) and Sjogren's syndrome: Candidate regulators of autoimmune response and autoantigen expression. J Autoimmun. 2011 doi: 10.1016/j.jaut.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Li Y, St John MA, Zhou X, Kim Y, Sinha U, Jordan RC, et al. Salivary transcriptome diagnostics for oral cancer detection. Clin Cancer Res. 2004a;10:8442–8450. doi: 10.1158/1078-0432.CCR-04-1167. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhou X, St John MA, Wong DT. RNA profiling of cell-free saliva using microarray technology. J Dent Res. 2004b;83:199–203. doi: 10.1177/154405910408300303. [DOI] [PubMed] [Google Scholar]
- Merrill JT, Wallace DJ, Petri M, Kirou KA, Yao Y, White WI, et al. Safety profile and clinical activity of sifalimumab, a fully human anti-interferon {alpha} monoclonal antibody, in systemic lupus erythematosus: a phase I, multicentre, double-blind randomised study. Ann Rheum Dis. 2011 doi: 10.1136/ard.2010.144485. [DOI] [PubMed] [Google Scholar]
- Michael A, Bajracharya SD, Yuen PS, Zhou H, Star RA, Illei GG, et al. Exosomes from human saliva as a source of microRNA biomarkers. Oral Dis. 2010;16:34–38. doi: 10.1111/j.1601-0825.2009.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park NJ, Zhou H, Elashoff D, Henson BS, Kastratovic DA, Abemayor E, et al. Salivary microRNA: discovery, characterization, and clinical utility for oral cancer detection. Clin Cancer Res. 2009;15:5473–5477. doi: 10.1158/1078-0432.CCR-09-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RS, Jakymiw A, Yao B, Pauley BA, Carcamo WC, Katz J, et al. High resolution of microRNA signatures in human whole saliva. Arch Oral Biol. 2011 doi: 10.1016/j.archoralbio.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu OH, Atkinson JC, Hoehn GT, Illei GG, Hart TC. Identification of parotid salivary biomarkers in Sjogren's syndrome by surface-enhanced laser desorption/ionization time-of-flight mass spectrometry and two-dimensional difference gel electrophoresis. Rheumatology (Oxford) 2006;45:1077–1086. doi: 10.1093/rheumatology/kei212. [DOI] [PubMed] [Google Scholar]
- Spielmann N, Wong DT. Saliva: diagnostics and therapeutic perspectives. Oral Dis. 2011;17:345–354. doi: 10.1111/j.1601-0825.2010.01773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildenberg ME, van Helden-Meeuwsen CG, van de Merwe JP, Drexhage HA, Versnel MA. Systemic increase in type I interferon activity in Sjogren's syndrome: a putative role for plasmacytoid dendritic cells. Eur J Immunol. 2008;38:2024–2033. doi: 10.1002/eji.200738008. [DOI] [PubMed] [Google Scholar]
- Willner D, Furlan M, Schmieder R, Grasis JA, Pride DT, Relman DA, et al. Metagenomic detection of phage-encoded platelet-binding factors in the human oral cavity. Proc Natl Acad Sci U S A. 2011;108:4547–4553. doi: 10.1073/pnas.1000089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Richman L, Higgs BW, Morehouse CA, de los Reyes M, Brohawn P, et al. Neutralization of interferon-alpha/beta-inducible genes and downstream effect in a phase I trial of an anti-interferon-alpha monoclonal antibody in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1785–1796. doi: 10.1002/art.24557. [DOI] [PubMed] [Google Scholar]
- Zarco M, Vess T, Ginsburg G. The oral microbiome in health and disease and the potential impact on personalized dental medicine. Oral Dis. 2011 doi: 10.1111/j.1601-0825.2011.01851.x. [DOI] [PubMed] [Google Scholar]
- Zubair A, Burbelo PD, Vincent LG, Iadarola MJ, Smith PD, Morgan NY. Microfluidic LIPS for serum antibody detection: demonstration of a rapid test for HSV-2 infection. Biomed Microdevices. 2011 doi: 10.1007/s10544-011-9575-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


