Abstract
Background
The abused volatile solvent toluene shares many behavioral effects with classic central nervous system depressants such as ethanol. Similarities between toluene and ethanol have also been demonstrated using in vitro electrophysiology. Together, these studies suggest that toluene and ethanol may be acting, at least in part, via common mechanisms.
Methods
We used the genetic model, C. elegans, to examine the behavioral effects of toluene in a simple system, and used mutant strains known to have altered responses to other CNS depressants to examine the involvement of those genes in the motor effects induced by toluene.
Results
Toluene vapor brings about an altered pattern of locomotion in wild-type worms that is visibly distinct from that generated by ethanol. Mutants of the slo-1, rab-3 and unc-64 genes that are resistant to ethanol or the volatile anesthetic halothane show no resistance to toluene. A mutation in the unc-79 gene results in hypersensitivity to ethanol, halothane and toluene indicating a possible convergence of mechanisms of the three compounds. We screened for, and isolated, two mutations that generate resistance to the locomotor depressing effects of toluene and do not alter sensitivity to ethanol.
Conclusions
In C. elegans, ethanol and toluene have distinct behavioral effects and minimal overlap in terms of the genes responsible for these effects. These findings demonstrate that the C. elegans model system provides a unique and sensitive means of delineating both the commonalities as well as the differences in the neurochemical effects of classical CNS depressants and abused volatile inhalants.
Keywords: toluene, ethyl benzene, ethanol, halothane, behavior, genetics, C. elegans
1. Introduction
Abuse of volatile solvents by inhalation is a significant health problem, particularly among adolescents. Volatile solvents, such as products containing the aromatic agent toluene, produce a rapid-onset, short-lived, euphoric intoxication when inhaled. Evidence suggests that these solvents have multiple neuronal targets that may overlap with the targets of alcohol, an idea that is supported by the commonality in the behavioral effects of toluene and ethanol in rodents. The effects of toluene are similar to those of classic central nervous system (CNS) depressants like alcohol, including low-dose locomotor activation and high-dose sedation and incoordination (Tegeris and Balster, 1994; Bowen and Balster, 1998). Drug discrimination studies in mice suggest that the discriminative stimulus effects of ethanol and toluene are similar (Bowen, 2009). Furthermore, there is some overlap in the molecular targets of ethanol and toluene that have been suggested by previous studies. Most prominent among these are a diverse group of ligand-gated ion channels, including the NMDA, GABAA, glycine, nACh and 5-HT receptors (Cruz et al., 1998; Beckstead et al., 2000; Bale et al., 2002; Lopreato et al., 2003; Bale et al., 2005), as well as multiple non-ligand-gated ion channels (Cruz et al., 2003; Shafer et al., 2005; Del Re et al., 2006). While these studies strongly suggest that ethanol and toluene may produce very similar CNS effects, many of these targets have only been studied in vitro, and the in vivo molecular targets of toluene that are important for its behavioral effects are less well delineated.
C. elegans continues to be a useful model for the identification of genes that are involved in mediating the effect of CNS depressants. Some examples relevant to the present study include the following studies. Loss-of-function mutations in the slo-1 gene, which encodes the voltage- and calcium-sensitive large conductance (BK) potassium channel (Wang et al., 2001), result in substantial resistance to the effect of ethanol on locomotion and egg laying (Davies et al., 2003). Similarly, loss-of-function mutations in the rab-3 gene, which encodes a small GTPase protein that is required for normal synaptic transmission (Nonet et al., 1997), result in resistance to the locomotor-depressing effects of ethanol (Kapfhamer et al., 2008). The gain-of-function mutation (md130) in the syntaxin-encoding gene, unc-64, produces significant resistance to the locomotor-depressing effects of the volatile anesthetics halothane and isoflurane (van Swinderen et al., 1999). Mutations in the unc-79 gene, which encodes a protein that localizes and regulates the activity of the NCA cation channel (Humphrey et al., 2007; Yeh et al., 2008), have been shown to generate increased sensitivity to the immobilizing effects of halothane and toluene (Morgan et al., 1990; Morgan et al., 2000). However, different behavioral assays of the effects of mutations in unc-79 on ethanol responses have been reported to increase resistance or increase hypersensitivity to the effect of ethanol (Morgan and Sedensky, 1995; Speca et al., 2010).
In the present study we used the nematode, Caenorhabditis elegans, to take a genetic approach to compare the behavioral effects of ethanol and toluene in wild-type animals and in mutant strains that have altered responses to ethanol and other CNS depressant drugs. Here, we show that toluene causes increases in the speed of locomotion at low concentrations, and coordination defects resulting in substantially reduced speeds of locomotion at high concentrations. Importantly, mutants that display resistance to ethanol or the volatile anesthetic, halothane, do not have altered toluene sensitivity. We have isolated two mutants that show profound resistance to the effects of high dose toluene that do not show resistance to ethanol. In summary, our data suggests that, at least for C. elegans, the degree of overlap in the genes that mediate the behavioral effects of ethanol and toluene may be limited. These results suggest that toluene, and possibly other inhaled solvents, should be considered as independent compounds rather than grouping them with ethanol in terms of the spectrum of likely molecular activities the drugs will have.
2. Methods
2.1 Maintenance of C. elegans
C. elegans were cultured as described (Brenner, 1974). Strains used were: N2 (wild type), slo-1(eg142), rab-3(js49), unc-64(md130), unc-79(ec1). Some strains were provided by the C. elegans Genetics Center (funded by NIH-NCRR).
2.2 Test compounds
HPLC-grade toluene was purchased from Sigma-Aldrich Chemicals (Milwaukee, WI). Halothane was purchased from Webster Veterinary Supply (Charlotte, NC). 200-proof ethyl alcohol was purchased from Aaper Alcohol and Chemical Company (Shelbyville, KY).
2.3 Solvent exposure
Two vapor chambers were used in this study. The first static vapor chamber was used for all experiments except that shown in Figure 1b to expose C. elegans to volatile compounds and has been described previously (Shelton, 2007). Briefly, this chamber consisted of a 27.2-liter cylindrical glass bell jar with a clear acrylic lid with an attached fan motor. A filter paper disk was suspended on a wire mesh below the fan. Solvent/anesthetic vapor was produced by injecting liquid through a sealable port onto the filter paper using a gas-tight glass syringe. The internal fan was then turned on which volatilized the liquid and produced vapor. As the chamber was sealed and of a fixed volume, the amount of liquid toluene necessary to produce a given vapor concentration could be precisely calculated using the ideal gas law equation for room temperature (Nelson, 1971). We did not make temperature or atmospheric pressure corrections to the calculations since normal laboratory variability in these factors were predicted to have a 1% or less effect on the parts per million of vapor produced by a given volume of toluene across the range examined. Although the ideal gas law governed the chamber concentration produced by a given volume of liquid toluene, the vapor concentration rise time and stability for the exposure durations used were verified prior to the start of the study by using a vacuum pump to recirculate the chamber air/toluene vapor mixture through a single wavelength monitoring infrared spectrometer (Miran 1A, Foxboro Analytical, North Haven, CT) connected to a computerized chart recorder (DataQ Instruments DI-194RS, Akron, OH). At all toluene exposure concentrations used in the present study reached peak vapor levels in less than 90 sec following activating the internal fan.
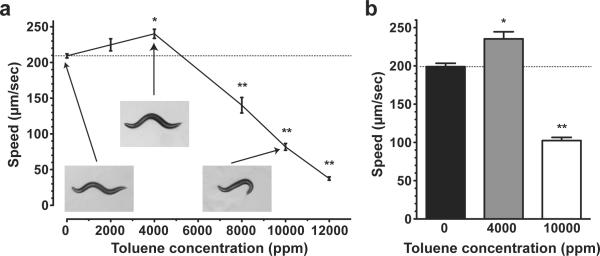
Figure 1.
Concentration-effect curve for the effect of toluene exposure in static vapor chambers on crawling speed of wild-type C. elegans. (a) Average speeds (± SEM) of wild-type animals determined over a 2-minute period immediately following a 10-minute exposure to air (0 ppm) or to particular concentrations of toluene (N = 8 trials of 10 animals per trial). Inset pictures show representative images of crawling posture of animals exposed to the indicated toluene concentrations. The 10,000 ppm toluene-exposed animals show a dramatic change in crawling posture. (b) Average speeds (± SEM) of wild-type animals determined over a 2-minute period during an exposure to air or two concentrations of toluene (N = 3 trials of 10 animals per trial). In both (a) and (b) dotted lines illustrate the speed of air-exposed animals for reference and symbols show significant differences compared to 0 ppm: *, p < 0.05; **, p<0.01.
The second chamber, which was used only in the generation of data shown in Figure 1b, was a clear acrylic cube with an internal volume of 1.61 liters, a removable lid and a port that could be sealed with a rubber stopper. The toluene was added with a gas-tight syringe to a filter paper disc that had been rolled and placed in the port tube. The small size of this chamber allows worms to be imaged during a vapor exposure, this is in contrast to the large chamber where worms were exposed to the vapor, removed from the chamber and then imaged. The advantage of imaging the worms outside of the chamber is that the worms are accessible to the observer. This was important for the screen for toluene-resistant mutant animals, where individual worms were isolated from a population of exposed animals (see section 2.7).
2.4 Locomotion speed assays (solvent/anesthetic)
Animals to be tested were age matched by picking well-fed L4 stage animals for each strain to new seeded (OP50) plates and left overnight (18–22 hours) at 20°C. The following day, young adult animals were moved to unseeded plates for 30 min to acclimate the animals to a lack of food and then moved to the assay plates. The acclimation and assay plates had been dried for 2 hours at 37°C before use. 3 or 4 copper rings (16 mm inner diameter) were embedded in the surface of the acclimation and assay plates to act as corrals for different genotypes or test groups as previously described (Davies et al., 2003). 10 animals for each strain were placed within a copper ring. The assay plate (without lid) was placed in the static vapor chamber. For all experiments except that shown in Figure 1b, the following methods were used: Control animals spent 10 minutes in the chamber with no solvent. Treated worms were exposed to the volatilized solvent or anesthetic for 10 minutes. Following exposure, the assay plate was quickly removed from the chamber, the plate lid replaced and the entire plate was imaged on an Olympus SZX-7 stereo microscope (0.8× magnification, 0.5× objective) using a Retiga 4000R camera (QImaging) and ImagePro Plus (6.2) (MediaCybernetics) software. A 2-minute recording (1 frame per second) was captured as soon as possible (less than 1 min) after the plate was removed from the chamber. ImagePro Plus software was then used to track the speed of each worm and an average speed for each group of 10 animals was calculated. The protocol for the worms and plates for the data presented in Figure 1b are the same as described above, however, the worms were imaged in the small chamber during an exposure to air, 4000 ppm or 10000 ppm toluene. A 2-minute movie (1 frame per second) was recorded between the 10–12 minute time points of exposure and the average speed of the animals during that time window was calculated using ImagePro Plus software.
2.5 Locomotion speed assays (ethanol)
Ethanol treatment of worms was performed as previously described (Davies et al., 2003). Briefly, age matched worms and plates were selected and prepared as described above. Two hours prior to the assay, cold 100% ethanol was added to the dried assay treatment plates to a final concentration of 200 mM or 400 mM ethanol. On the day of treatment, young adult worms were acclimated to a lack of food for 30 minutes on the dried plates then transferred to control plates lacking ethanol or plates containing 200 mM or 400 mM ethanol. Following 20 minutes of exposure, a 2-minute recording of the worms moving on the plate was collected and analyzed as described above.
While the exogenous concentrations of ethanol required to cause behavioral effects are substantially higher than those required to cause locomotor impairment in rodents, we have previously reported that the internal tissue ethanol concentration is substantially less than the exogenous concentration (Davies et al., 2003, 2004; Kapfhamer et al., 2008). A refined method of ethanol concentration determination has shown the internal ethanol concentration resulting from a 10-minute exposure to 200 mM or 400 mM exogenous ethanol concentration to be 14.0 ± 0.9 mM (64.5 mg/dL) and 45.5 ± 6.7 mM (209.7 mg/dL) respectively (J. Alaimo and J. Bettinger, personal communication).
2.6 Crawling pattern analysis
Worms were treated with solvents or ethanol in the same manner as described for the locomotion speed analyses except that only two worms were placed in each ring. Higher magnification images of crawling were captured at 8 frames per second during the first 4 minutes following exposure at a magnification of 6.3× using a 0.5× objective. For Figure 2 and Supplementary Figure 11, every 8th frame was collected for each treatment.
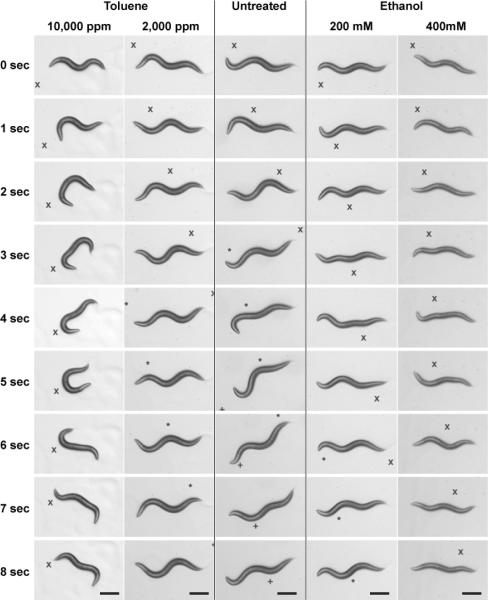
Figure 2.
Toluene and ethanol effects on coordination and body bends made during locomotion. Representative images of individual wild-type young-adult worms during an 8-second period of forward locomotion. Each column shows images highlighting the position of one animal approximately 1 second apart. Column 3 is an untreated animal crawling on agar media. Column 1 and 2 show animals that have been exposed to 10,000 ppm and 2,000 ppm toluene, respectively, and imaged immediately after removal from the static vapor chamber. Columns 4 and 5 are animals still in the presence of ethanol (200 mM and 400 mM, respectively) and have been exposed for 10 minutes prior to imaging. The worms have been centered in each cropped image, reference points have been marked with symbols (×, *, +) to allow relative movement of the worms to be judged. Scale bar equals 250 μm.
2.7 Screen for toluene sensitivity mutants
N2 animals were randomly mutagenized by exposure to ethyl methane sulfonate (EMS) (47 mM) in liquid for 4 hours then allowed to self-fertilize for two generations. Young adult animals of the F2 generation were exposed to 10,000 ppm toluene for 10 minutes and individual animals that displayed obviously faster locomotion and/or a lack of high amplitude body bends during the first 10 minutes post-exposure were picked and allowed to reproduce by self-fertilization. Progeny of these animals were retested for the altered toluene sensitivity phenotype and those candidates that bred true for the phenotype were maintained and were quantitatively assessed for toluene and ethanol sensitivity as described above.
2.8 Statistics
Statistics were carried out using Graphpad Prism software (5.0). Where strains differed in their basal rates of locomotion, as determined by t-tests (2 strains compared) or 1-way ANOVA (3 strains compared), relative speeds were calculated as the speed on the drug divided by the untreated speed and then expressed as a percentage. Genotype and concentration effects were compared using 1-way (single strain concentration effects) and 2-way ANOVA (multiple strains comparing both genotype and concentration). Bonferroni post-hoc tests were used to determine significant differences between data points.
3. Results
3.1 Toluene effects on locomotion
To determine the acute effect of toluene vapor on coordinated locomotion in C. elegans, we examined the effect of a short (10 min) toluene vapor exposure on the speed of crawling wild-type (N2) animals. We measured the average speed of crawling for N2 young-adult worms over a 2-minute period immediately following a 10-minute exposure to toluene (0–12,000 ppm) (Figure 1a). Toluene exposure significantly altered the average speed of locomotion in a biphasic concentration-dependent manner (1-way ANOVA, F(5,42) = 155.7, p < 0.0001). At 4,000 ppm toluene, we observed a significant increase in the speed compared with animals exposed to air (p < 0.01). Exposures of 8,000, 10,000 and 12,000 ppm resulted in significant decreases in the average speed of locomotion compared with untreated animals (p < 0.001). Worms that were initially slowed by toluene displayed a return to speeds equivalent to basal speeds over a period of 10–35 minutes after exposure, higher concentration-treated animals taking longer to achieve a speed equivalent to basal speeds (data not shown); this indicates that the effects of toluene concentrations we used were reversible. We excluded the possibility that removal from the toluene was contributing to the altered speeds or to the difference between low and high toluene concentrations by examining worms during the course of the exposure in a chamber that was small enough to be mounted on our microscope imaging system. Average speeds during the 10–12 minute time points of exposure to toluene showed similar effects as when the worms were imaged immediately following exposure (Figure 1b); 4,000 ppm toluene causes a significant increase in speed compared with air-exposed animals (p < 0.05) and 10,000 ppm toluene decreased speed significantly compared with air-exposed animals (p < 0.01) (1-way ANOVA, F(2,6) = 114.6, p < 0.0001).
To examine the impact of toluene on locomotion in more detail, we examined the pattern of locomotion of the toluene-exposed worms compared with untreated worms and worms exposed to ethanol for the same length of time. While the average speed of high-concentration toluene-exposed animals was significantly reduced, as it was during high-dose ethanol treatment, the toluene-exposed worms showed a distinctly different pattern of locomotion and posture than the ethanol-treated animals (Figure 2). Untreated worms (Figure 2, column 3), either following air exposure in the static vapor chamber or on an assay plate with no ethanol added, displayed the characteristic sinusoidal pattern of locomotion. Low-concentration toluene exposure (2,000 ppm) (Figure 2, column 2) did not alter the sine wave pattern of locomotion. Low-dose ethanol treatment (200 mM) (Figure 2, column 4) caused a detectable, but small, decrease in the amplitude of the sine wave pattern of locomotion. The 10,000 ppm toluene-treated worms (Figure 2, column 1) displayed an uncoordinated movement pattern that reflects prolonged bending of the anterior third of the animal. These toluene-treated animals bent their heads to one side and remained temporarily in that position while the posterior ends of the animals continued to propagate body bends to either side. The end result of this defect was that the toluene-treated animals changed their direction repeatedly and did not make significant forward progress. As previously described (Davies et al., 2003), 400 mM ethanol-treated animals (Figure 2, column 5) had significantly reduced body bend amplitude but, in general, continued to show a shallow or flattened sine wave posture. These comparisons make it clear that ethanol and toluene have profoundly different overall behavioral effects on wild-type worms.
3.2 Testing for targets of toluene
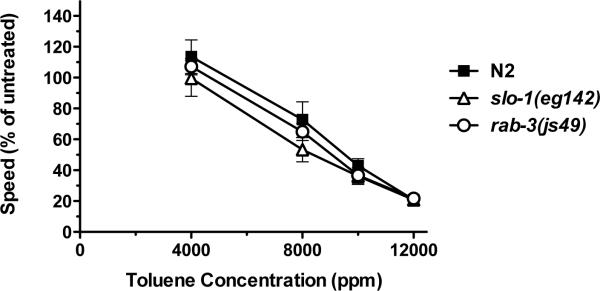
We assessed the degree of overlap in the behavioral effects of toluene and ethanol by testing the requirement for two genes, slo-1 and rab-3, that we have previously shown to be necessary for wild-type sensitivity to the effects of ethanol on locomotion in C. elegans (Davies et al., 2003; Kapfhamer et al. 2008). The slo-1(eg142) loss-of-function mutant animals we tested are slower than the wild-type and rab-3(js49) loss-of-function mutant animals in the absence of treatment due to a mild locomotion defect associated with the loss of function in the slo-1 gene (N2, untreated speed = 204.2 ± 2.3 μm/sec; slo-1(eg142) untreated speed = 140.0 ± 9.9 μm/sec; rab-3(js49) untreated speed = 203.7 ± 6.7 μm/sec; 1-way ANOVA F(2,9) = 27.64, p < 0.001). To account for these genotype-specific basal speed differences, we calculated the effect of toluene relative to the untreated speed for each strain. At all concentrations of toluene examined, there were no significant differences in relative speed between the ethanol-resistant slo-1(eg142) and rab-3(js49) mutant animals and wild-type animals (Figure 3). The lack of an altered toluene response in these mutants suggests that unlike ethanol, toluene does not act through SLO-1 or RAB-3 to bring about its effects on locomotion.
Figure 3.
Concentration-effect curve comparing the effect of toluene on the crawling speed of wild-type (N2) and two ethanol-resistant mutant strains, slo-1(eg142) and rab-3(js49). Shown are averages (± SEM) for the speed of each strain at a particular concentration of toluene relative to the untreated speed for that strain. Speeds were determined over a 2-minute period immediately following a 10-minute exposure to particular concentrations of toluene (N = 4 trials of 10 animals per trial).
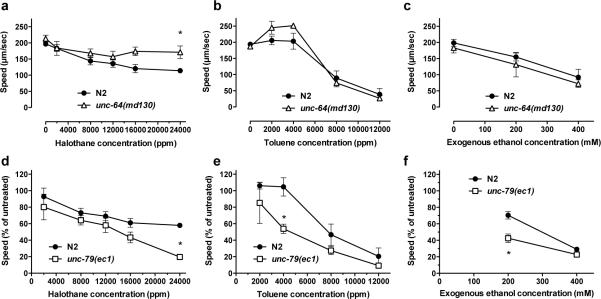
To further characterize the genetics of toluene sensitivity, we examined the effects of toluene and ethanol in additional mutant strains reported to have altered sensitivity to volatile vapor anesthetics. We examined the effect of toluene, halothane and ethanol on the volatile anesthetic-resistant unc-64(md130) mutant. N2 and unc-64(md130) worms showed similar untreated speeds, so relative speed measurements were not used for these comparisons (t2 = 1.14, p > 0.05). We confirmed that unc-64(md130) mutant animals were significantly resistant to the locomotor depressing effects of halothane in our behavioral assay compared with wild-type animals (Figure 4a) (2-way ANOVA, FGenotype(1, 24) = 13.78, p < 0.01). In contrast, the response to toluene of the unc-64(md130) mutant animals was not significantly different from wild-type animals at 4 concentrations of toluene (2,000–12,000 ppm) (Figure 4b) (2-way ANOVA, FGenotype(1,20) = 1.24, p > 0.05). These results demonstrate that toluene and halothane differ in their requirement for UNC-64 to generate a behavioral effect. When we examined the effect of two exogenous concentrations of ethanol on unc-64(md130) mutant animals compared with wild-type animals we observed no significant differences in the response of the two strains to 20 minutes of ethanol exposure (Figure 4c) (2-way ANOVA, FGenotype(1, 15) = 3.8, p > 0.05). These results provide support for a lack of overlap between the genes involved in the behavioral response of animals to toluene and halothane and also for lack of overlap between the genes involved in the behavioral responses to ethanol and halothane.
Figure 4.
Concentration-effect curves comparing the effect of halothane, ethanol and toluene on the crawling speed of wild-type and unc-64(md130) and unc-79(ec1) mutant strains. (a–c) Shown are unadjusted average speeds (±SEM) for the N2 strain and the halothane-resistant unc-64(md130) mutant strain. (d–f) Shown are relative speeds (% of untreated speed ± SEM) for the N2 strain and the ethanol-, toluene- and halothane-hypersensitive unc-79(ec1) mutant strain. Speeds are determined over a 2-minute period immediately following a 10-minute exposure to halothane or toluene or between minute 10 and minute 12 of ethanol exposure (N = 3–5 trials of 10 animals per trial). Symbol shows significant differences compared to 0 ppm: *, p < 0.05.
Mutations in unc-79 have been shown to cause hypersensitivity to the immobilizing effects of halothane and toluene following a long exposure (Morgan et al., 1990; Morgan et al., 2000). Further, using different quantitative measures (immobilization or body bend frequency) and different concentrations of ethanol, mutations in unc-79 have been shown to confer either hypersensitivity or resistance to the effects of ethanol (Morgan and Sedensky, 1995; Speca et al., 2010). unc-79(ec1) mutant animals are significantly slower than wild-type animals (t4 = 10.3, p < 0.001) so we used relative speeds (% of untreated speed) to compare the affects of halothane, ethanol and toluene on unc-79(ec1) mutants and wild-type animals. Using our locomotion crawling assay, we observed hypersensitivity of unc-79(ec1) mutant animals to a high concentration of halothane (24000 ppm) (Figure 4d) (2-way ANOVA, FGenotype(1,20) = 13.1, p < 0.01) and hypersensitivity to low dose effects of ethanol (Figure 4f) (2-way ANOVA, FGenotype(1,14) = 21.6, p < 0.001). Our assessment of higher dose effects of ethanol may be complicated by a floor effect in our ability to measure low speeds. We examined the response of the unc-79(ec1) mutant animals to toluene and found that they showed a significant decrease in speed at 4000 ppm toluene compared with wild-type animals (Figure 4e) (2-way ANOVA, FGenotype(1,16) = 9.6, p < 0.01). This decrease is made even more noticeable because wild-type animals at the same dose show an increase in speed. There was a trend for the unc-79(ec1) animals to show greater effects at higher toluene concentrations but these differences did not achieve statistical significance and may be impacted by the same behavioral floor effect that we observed with the ethanol exposure. In this case, the hypersensitivity to halothane, ethanol and toluene of unc-79(ec1) mutant animals suggests a potential overlap in genes involved in the behavioral responses to the three compounds.
3.3 Isolation of toluene-resistant mutants
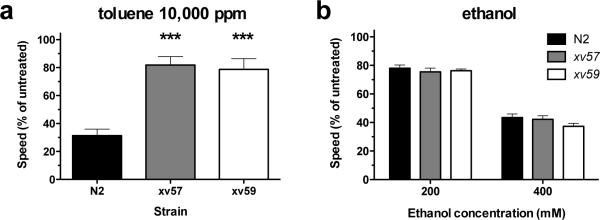
In order to identify novel genes that are required to mediate toluene effects on locomotion we have conducted an initial screen for new mutants that are resistant to the effects of toluene. Mutagenized N2 animals were allowed to breed for two generations in order to allow any induced mutations to become homozygous in a quarter of the second-generation (F2) progeny. We screened these F2 progeny for animals that displayed wild-type locomotion patterns following toluene exposure. In a relatively small pilot screen of only 450 haploid genomes we isolated 2 independently-generated mutants, xv57 and xv59, that were less affected by toluene than wild-type animals. These toluene-resistant mutants, when examined in the absence of treatment, displayed locomotion patterns that were superficially similar to wild-type animals (Supplementary Figure 12); the mutants generate sinusoidal patterns of locomotion but both mutants had an average speed that was significantly slower than wild-type animals measured under the same conditions (N2: 193.8±4.0 μm/sec; xv57: 156.8±5.4 μm/sec; xv59: 147.7±6.4μm/sec; 1-way ANOVA, F(2,6) = 39.7, p < 0001). For this reason we used relative speed calculations for direct comparisons of xv57, xv59 and wild-type animals. The reduced effect of high concentration toluene on xv57 and xv59 mutant animals is evident in Figure 5a; both of these mutants displayed smaller reductions in speed following 10,000 ppm toluene exposure compared with identically treated wild-type animals (1-way ANOVA, F(2, 14) = 20.8, p < 0.001). The toluene-resistant mutants continued to make sinusoidal body bends and propagate forward motion following toluene exposure (Supplementary Figure 13). We asked whether the resistance to toluene would generalize to ethanol by testing the response of the xv57 and xv59 mutants to ethanol. We tested these mutants for their response to ethanol at two doses and found that neither mutation conferred any change in ethanol sensitivity compared with wild-type animals (2-way ANOVA, FGenotype (2,33) = 1.47, p > 0.05) (Figure 5b). That we can identify mutants that show resistance to the effects of toluene but do not affect ethanol-sensitivity provides substantial support to the idea that ethanol and toluene can act through different genes to bring about their effects on locomotion.
Figure 5.
Toluene and ethanol responses of wild-type and two toluene-resistant mutant strains. (a) Average speed of locomotion (% of untreated speed ± SEM) determined over a 2-minute period following a 10-minute exposure to 10,000 ppm toluene is shown for N2, xv57 and xv59 young-adult animals (n = 5 trials of 10 animals per trial). (b) Average speed of locomotion (% of untreated speed ± SEM) between minute 10 and minute 12 of 200 mM or 400 mM ethanol exposures (N = 6–7 trials of 10 animals per trial). Symbols show significant differences compared to 0 ppm toluene: ***, p < 0.001.
4. Discussion
Here we describe a study aimed at examining the degree of overlap of gene involvement in the motor impairment effects of treatment with toluene, ethanol and volatile anesthetics (summarized in Table 1). We used the nematode C. elegans to address this question and describe a robust dose-dependent effect of toluene on locomotor activity and coordination. The concentrations of toluene required to bring about these behavioral effects in C. elegans are in a comparable range as those that bring about behavioral effects in mice (Bowen and Balster, 1998; Shelton, 2007).
Table 1.
Summary of toluene, ethanol and halothane effects on locomotion in different mutant C. elegans strains compared with the effects on wild-type animals.
| Genotype | Affected gene and function | Toluene response | Ethanol response | Halothane response | |||
|---|---|---|---|---|---|---|---|
| low dose | high dose | low dose | high dose | low dose | high dose | ||
| +/+ (wild type) | increased speed | high amplitude uncoordinated bends, decreased speed | decreased speed | flattened bends, decreased speed | decreased speed | decreased speed | |
| slo-1(eg142) | BK potassium channel, cell excitability | WT | WT | resistant | resistant | WT | WT |
| rab-3(js49) | small GTPase, synaptic transmission | WT | WT | WT | resistant | WT | WT |
| unc-64(md130) | syntaxin, synaptic transmission | WT | WT | WT | WT | WT | resistant |
| unc-79(ec1) | regulator of cation leak (NCA) channels | decreased speed | WT* | hyper-sensitive | WT* | WT | hyper-sensitive |
| xv57 | not known | ND | resistant | WT | WT | ND | ND |
| xv59 | not known | ND | resistant | WT | WT | ND | ND |
unc-79(ec1) mutant animals may show a floor effect for high doses of toluene and ethanol.
WT, not different from the response of wild-type animals
ND, not determined
At higher doses of toluene we observed dramatic decreases in speed. It is clear from visualization of the movement pattern of high-dose toluene-treated animals that their decrease in speed does not appear to be due to a `sedative' effect, instead the animals continue to move but appear to be quite uncoordinated in their movement patterns. The toluene-treated worms show a characteristic defect, in that their heads and anterior third of their bodies remain in the same positions, bent to one side for 1–2 seconds while the posterior two thirds continues to propagate body bends. Eventually the worms' heads swing back to the opposite side but the same defect is often observed with each head swing. This movement defect restricts the animals' ability to make forward progress, hence the decrease in measured speed. The lack of coordination associated with toluene treatment is distinct from that observed when wild-type worms are treated with ethanol, which show a dose-dependent flattening of body bend amplitude (Figure 2 and Davies et al., 2003). We have previously demonstrated that at least part of the ethanol effect on C. elegans locomotion is due to increased activation of the SLO-1 potassium channel by ethanol (Davies et al., 2003), an effect that is likely to cause a pan-neuronal decrease in activity. The movement defect we observed with toluene appears to be distinct from that which we observe with ethanol treatment; it has a more limited anatomical focus on the anterior of the animal. This may be the most obvious difference between ethanol and toluene behavioral effects in C. elegans and suggests that the two compounds are not having identical effects in C. elegans. The specific movement effect of toluene is likely to be a helpful tool in understanding the molecular effects of the compound. A variety of mutant strains exist that display characteristic uncoordinated movement patterns associated with mutations in known genes. If one of these mutants has a phenotype similar to that shown by toluene-exposed animals then it may represent a target of toluene.
We observed a low-concentration toluene effect on C. elegans that causes increased speed of these animals (Figure 1), which may be akin to the low dose locomotor activation effects seen in rodents with many of the CNS depressants, including toluene (Bowen and Balster, 1998). Wild-type (N2) worms do not show an increase in speed associated with low-dose ethanol treatment (Davies et al., 2003), which represents another difference we have observed in the comparison of toluene and ethanol. While it is possible that ethanol cannot cause low-dose activation in C. elegans, we hypothesize that the N2 strain, which is the wild-type strain used most commonly in laboratories, and is used here, may have a genetic background that minimizes low dose ethanol activation effects. A similar situation has been observed with mice strains commonly used in ethanol studies, for example, C57BL/6J mice show no locomotor activation with low dose ethanol administration, whereas another strain of mice, DBA/2J, shows robust locomotor activation induced by ethanol (Tritto and Dudek, 1994). We are particularly interested in the increase in speed shown by N2 animals exposed to low-dose toluene. In rodents, mesolimbic dopamine release has been linked to the locomotor activation effects of drugs of abuse, including toluene (Riegel et al., 2003). The fact that dopamine is an important regulator of locomotion in C. elegans (Sawin et al., 2000; Hills et al., 2004; Gaglia and Kenyon, 2009) suggests an immediate candidate target system for toluene's effects on speed at low doses.
Our analyses of the toluene-sensitivity of mutants that alter sensitivity to other CNS depressants support the hypothesis that genes that mediate toluene effects have little overlap with genes that mediate the effects of ethanol and halothane (Table 1). Previous studies have identified a small number of shared responses of mutants for particular genes to these different drugs, although such behavioral similarities may not represent shared mechanisms of action. For instance, loss-of-function mutations in slo-1 generate significant resistance to ethanol and moderate levels of resistance to halothane (Davies et al., 2003; Hawasli et al., 2004). The latter effect is hypothesized to be a result of a non-specific increase in neurotransmitter release that occurs in the slo-1 mutant animals (Wang et al., 2001; Hawasli et al., 2004), whereas, ethanol was shown to potentiate the activity of the SLO-1 potassium channel (Davies et al., 2003) suggesting a more direct effect of ethanol on the SLO-1 protein. When we looked for a correlation between the responses of mutant animals to ethanol and toluene or halothane and toluene we found limited evidence to support such overlap. Mutations in slo-1 or rab-3, which produce substantial resistance to ethanol (Davies et al., 2003; Kapfhamer et al., 2008), do not affect toluene sensitivity. The gain-of-function syntaxin mutation, unc-64(md130), which produces significant resistance to halothane and isoflurane (van Swinderen et al., 1999) does not alter toluene or ethanol sensitivity. These outcomes all support the hypothesis that toluene, ethanol and halothane act through different genes. This does not discount the possibility that these compounds also share a genetic focus that is independent of SLO-1, RAB-3 or UNC-64, in fact, the observation that a mutation in the unc-79 gene, which encodes a regulator of NCA channel activity (Humphrey et al., 2007; Yeh et al., 2008), causes hypersensitivity to all three drugs in our assay suggests that all of the drugs have some convergent effect that can be enhanced by loss of UNC-79, and presumably the loss of NCA channel activity. Our data for the effects of a mutation in unc-79 were consistent with previous studies for unc-79 mutant responses to halothane (Morgan et al., 1990), to ethanol at a dose equivalent to what was used here (Speca et al., 2010) and to toluene at a longer exposure (Morgan et al., 2000) but our data were inconsistent with an effect of higher ethanol doses on immobilization (Morgan and Sedensky, 1995). While one possibility is that loss of function of unc-79 is causing a non-specific effect that alters responses to all of these CNS depressant drugs, it should be noted that some specificity must exist because unc-79 mutations do not alter sensitivity to the volatile anesthetic isoflurane under the same conditions where changes in halothane sensitivity are observed (Morgan et al., 1990).
Further evidence for a limited overlap between the genes that mediate the effects of ethanol and toluene is the isolation of mutants that display significant resistance to toluene but do not have altered sensitivity to ethanol. The great advantage that C. elegans provides in these studies is that genes that have behavioral relevance to the in vivo effects of the drug can be identified and characterized in a simple, yet powerful, system. The next step in this process is the identification of the gene(s) that have been mutated in these strains so as to better understand the mechanism of action of toluene. The gene product(s) affected by the xv57 and xv59 mutations may represent direct targets of toluene or regulators of a toluene target. Moving forward, the true value of C. elegans as a model for understanding the pharmacological effects of toluene will lie in taking what will be learned in worms and testing that knowledge in systems closer to humans, such as mice or rats.
Given that ethanol and toluene have been proposed to share mechanisms of action, it is important that studies such as those described here are carried out to directly test such ideas. The data we present here strongly supports a distinction between the genes involved in the responses to ethanol and toluene, which favors further testing of toluene as a drug that is distinct in its actions from ethanol.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A supplementary figure for this article can be found by accessing the online version at http://dx.doi.org and by entering doi:…
Supplementary Figure 1 can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary Figure 1 can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
Supplementary Figure 1 can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
References
- Bale AS, Tu Y, Carpenter-Hyland EP, Chandler LJ, Woodward JJ. Alterations in glutamatergic and gabaergic ion channel activity in hippocampal neurons following exposure to the abused inhalant toluene. Neuroscience. 2005;130:197–206. doi: 10.1016/j.neuroscience.2004.08.040. [DOI] [PubMed] [Google Scholar]
- Bale AS, Smothers CT, Woodward JJ. Inhibition of neuronal nicotinic acetylcholine receptors by the abused solvent, toluene. Br. J. Pharmacol. 2002;137:375–383. doi: 10.1038/sj.bjp.0704874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Weiner JL, Eger EI, Gong DH, Mihic SJ. Glycine and ©-aminobutyric acidA receptor function is enhanced by inhaled drugs of abuse. Mol. Pharmacol. 2000;57:1199–1205. [PubMed] [Google Scholar]
- Bowen SE, Balster RL. A direct comparison of inhalant effects on locomotor activity and schedule-controlled behavior in mice. Exp. Clin. Psychopharmacol. 1998;6:235–247. doi: 10.1037//1064-1297.6.3.235. [DOI] [PubMed] [Google Scholar]
- Bowen SE. Time course of the ethanol-like discriminative stimulus effects of abused inhalants in mice. Pharmacol. Biochem. Behav. 2009;91:345–350. doi: 10.1016/j.pbb.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz SL, Mirshahi T, Thomas B, Balster RL, Woodward JJ. Effects of the abused solvent toluene on recombinant N-methyl-D-aspartate and non-N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J. Pharmacol. Exp. Ther. 1998;286:334–340. [PubMed] [Google Scholar]
- Cruz SL, Orta-Salazar G, Gauthereau MY, Millan-Perez P, Lourdes, Salinas-Stefanón EM. Inhibition of cardiac sodium currents by toluene exposure. Br. J. Pharmacol. 2003;140:653–660. doi: 10.1038/sj.bjp.0705481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AG, Pierce-Shimomura JT, Kim H, VanHoven MK, Thiele TR, Bonci A, Bargmann CI, McIntire SL. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell. 2003;115:655–666. doi: 10.1016/s0092-8674(03)00979-6. [DOI] [PubMed] [Google Scholar]
- Del Re AM, Dopico AM, Woodward JJ. Effects of the abused inhalant toluene on ethanol-sensitive potassium channels expressed in oocytes. Brain Res. 2006;1087:75–82. doi: 10.1016/j.brainres.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Gaglia MM, Kenyon C. Stimulation of movement in a quiescent, hibernation-like form of caenorhabditis elegans by dopamine signaling. J. Neurosci. 2009;29:7302–7314. doi: 10.1523/JNEUROSCI.3429-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawasli AH, Saifee O, Liu C, Nonet ML, Crowder CM. Resistance to volatile anesthetics by mutations enhancing excitatory neurotransmitter release in Caenorhabditis elegans. Genetics. 2004;168:831–843. doi: 10.1534/genetics.104.030502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills T, Brockie PJ, Maricq AV. Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J. Neurosci. 2004;24:1217–1225. doi: 10.1523/JNEUROSCI.1569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey JA, Hamming KS, Thacker CM, Scott RL, Sedensky MM, Snutch TP, Morgan PG, Nash HA. A putative cation channel and its novel regulator: cross-species conservation of effects on general anesthesia. Curr. Biol. 2007;17:624–629. doi: 10.1016/j.cub.2007.02.037. [DOI] [PubMed] [Google Scholar]
- Kapfhamer D, Bettinger JC, Davies AG, Eastman CL, Smail EA, Heberlein U, Mcintire SL. Loss of RAB-3/A in Caenorhabditis elegans and the mouse affects behavioral response to ethanol. Genes Brain Behav. 2008;7:669–676. doi: 10.1111/j.1601-183X.2008.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopreato GF, Phelan R, Borghese CM, Beckstead MJ, Mihic SJ. Inhaled drugs of abuse enhance serotonin-3 receptor function. Drug Alcohol Depend. 2003;70:11–15. doi: 10.1016/s0376-8716(02)00330-7. [DOI] [PubMed] [Google Scholar]
- Morgan PG, Radke GW, Sedensky MM. Effects of nonimmobilizers and halothane on Caenorhabditis elegans. Anesth. Analg. 2000;91:1007–1012. doi: 10.1097/00000539-200010000-00044. [DOI] [PubMed] [Google Scholar]
- Morgan PG, Sedensky M, Meneely PM. Multiple sites of action of volatile anesthetics in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 1990;87:2965–2969. doi: 10.1073/pnas.87.8.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PG, Sedensky MM. Mutations affecting sensitivity to ethanol in the nematode, Caenorhabditis elegans. Alcohol. Clin. Exp. Res. 1995;19:1423–1429. doi: 10.1111/j.1530-0277.1995.tb01002.x. [DOI] [PubMed] [Google Scholar]
- Nonet ML, Staunton JE, Kilgard MP, Fergestad T, Hartwieg E, Horvitz HR, Jorgensen EM, Meyer BJ. Caenorhabditis elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesicles. J. Neurosci. 1997;17:8061–8073. doi: 10.1523/JNEUROSCI.17-21-08061.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegel AC, Ali SF, French ED. Toluene-induced locomotor activity is blocked by 6-hydroxydopamine lesions of the nucleus accumbens and the mGluR2/3 agonist LY379268. Neuropsychopharmacology. 2003;28:1440–1447. doi: 10.1038/sj.npp.1300193. [DOI] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26:619–631. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Shafer TJ, Bushnell PJ, Benignus VA, Woodward JJ. Perturbation of voltage-sensitive Ca2+ channel function by volatile organic solvents. J. Pharmacol. Exp. Ther. 2005;315:1109–1118. doi: 10.1124/jpet.105.090027. [DOI] [PubMed] [Google Scholar]
- Shelton KL. Inhaled toluene vapor as a discriminative stimulus. Behav. Pharmacol. 2007;18:219–229. doi: 10.1097/FBP.0b013e328157f460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speca DJ, Chihara D, Ashique AM, Bowers MS, Pierce-Shimomura JT, Lee J, Rabbee N, Speed TP, Gularte RJ, Chitwood J, Medrano JF, Liao M, Sonner JM, Eger EI, Peterson AS, Mcintire SL. Conserved role of unc-79 in ethanol responses in lightweight mutant mice. PLoS Genet. 2010;6:e1001057. doi: 10.1371/journal.pgen.1001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegeris JS, Balster RL. A comparison of the acute behavioral effects of alkylbenzenes using a functional observational battery in mice. Fundam. Appl. Toxicol. 1994;22:240–250. doi: 10.1006/faat.1994.1028. [DOI] [PubMed] [Google Scholar]
- Tritto T, Dudek BC. Differential activating effects of ethanol in C57BL/6Abg and DBA/2Abg mice. Alcohol. 1994;11:133–139. doi: 10.1016/0741-8329(94)90054-x. [DOI] [PubMed] [Google Scholar]
- van Swinderen B, Saifee O, Shebester L, Roberson R, Nonet ML, Crowder CM. A neomorphic syntaxin mutation blocks volatile-anesthetic action in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 1999;96:2479–2484. doi: 10.1073/pnas.96.5.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZW, Saifee O, Nonet ML, Salkoff L. SLO-1 potassium channels control quantal content of neurotransmitter release at the C. elegans neuromuscular junction. Neuron. 2001;32:867–881. doi: 10.1016/s0896-6273(01)00522-0. [DOI] [PubMed] [Google Scholar]
- Yeh E, Ng S, Zhang M, Bouhours M, Wang Y, Wang M, Hung W, Aoyagi K, Melnik-Martinez K, Li M, Liu F, Schafer WR, Zhen M. A putative cation channel, NCA-1, and a novel protein, UNC-80, transmit neuronal activity in C. elegans. PLoS Biol. 2008;6:e55. doi: 10.1371/journal.pbio.0060055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.