Graphical abstract
Highlights
► Phylogenetic relationships are reconstructed within Myrceugenia. ► Genus Myrceugenia is monophyletic only when M. fernandeziana is excluded. ► Chilean and Brazilian species are two separate lineages. ► Brazilian species are included in a derived monophyletic group.
Keywords: Blepharocalyx, cpDNA, ETS, ITS, Luma, Myrteae, Myrceugenia, South America
Abstract
Myrceugenia is a genus endemic to South America with a disjunct distribution: 12 species occurring mainly in central Chile and approximately 25 in southeastern Brazil. Relationships are reconstructed within Myrceugenia from four plastid markers (partial trnK-matK, rpl32-trnL, trnQ-5′rps16 and rpl16) and two ribosomal nuclear regions (ETS and ITS) using maximum parsimony and Bayesian analyses. Relationships inferred previously from morphological data are not completely consistent with those from molecular data. All molecular analyses support the hypothesis that Myrceugenia is monophyletic, except for M. fernadeziana that falls outside the genus. Chilean species and Brazilian species form two separate lineages. Chilean species form three early diverging clades, whereas Brazilian species are a strongly supported monophyletic group in a terminal position. Least average evolutionary divergence, low resolution, short branches, and high species diversity found in the Brazilian clade suggest rapid radiation. Geographical distributions and phylogenetic reconstructions suggest that extant Myrceugenia species arose in northern Chile followed by colonization southward and finally to the Juan Fernández Islands and southeastern Brazil.
1. Introduction
Myrceugenia O. Berg is a South American genus consisting of about 40 species, which exhibit a disjunct distribution: 26 species occur in southeastern Brazil and adjacent regions of Paraguay, Uruguay and northeastern Argentina, 12 are found in Chile and Andean region of southwestern Argentina and two are endemic to the Juan Fernández archipelago (Landrum, 1981a, 1981b). Myrceugenia includes trees and shrubs with 4-merous flowers, usually persistent bracteoles, 2–4 locular ovaries, and few to several ovules per locule (Landrum, 1981a).
Relationships with other members of tribe Myrteae are not clear. Based on embryo structure Myrceugenia has been included in the subtribe Myrciinae, but both inflorescence and floral characters are similar to other Myrteae subtribes, Myrtinae and Eugeniinae. In fact, many Myrceugenia species were originally included under Eugenia L. and Myrtus L. (Landrum, 1981a). McVaugh (1968) divided tribe Myrteae into six informal groups according to inflorescence, flower, and seed characters, but he was unable to place Myrceugenia, Luma A. Gray, Nothomyrcia Kausel (a monotypic genus for Myrceugenia fernandeziana Hook. et Arn.) and five other genera into any group. He considered these genera to have arisen from the same ancestor as his six larger groups, but to have been less successful and therefore less species rich. Landrum (1981a) “tentatively” accepted three subtribes with in the Myrteae based on embryo structure and offered reason why these “may be true”. He listed seven anatomical, morphological and floral characteristics all found in at least one genus in each subtribe and also found in the anomalous genus Luma. He speculated that the ancestral group that would link the three subtribes together would have these characteristics and “provisionally” accepted these characteristics as plesiomorphies and the genera that have them as being similar to the ancestor of each subtribe. Thus, he hypothesized that Myrceugenia is similar to the ancestor of the subtribe Myrciinae. Myrcianthes would hold a similar position in the Eugeniinae and Blepharocalyx in the Myrtinae. Luma was also hypothesized to belong to this group of ancestral genera. Subsequent molecular work by Lucas et al. (2007) indicates that the Myrteae should be separated into seven groups. One of these is the “Myrceugenia group” including Myrceugenia, Blepharocalyx and Luma. However this group has low support and only appears when nuclear and chloroplast markers are combined.
Species relationships within Myrceugenia have been investigated by Landrum (1981b) based on morphological characters. He used various methods and proposed various hypotheses and concluded that “there have very probably been three or more cases of transcontinental migration in Myrceugenia”. Landrum (1981b) provisionally used two species from the Juan Fernández Islands as operational ancestors for his parsimony analysis, but stated that the results should be considered undirected as the species were accepted only “provisionally in order to use the program” and “ignored in the final result.” For a final hypothesis based on numerical methods and general knowledge of the ecology of the species and suspected cases of hybridization, Landrum (1981b) chose a point where branches to the two species of Juan Fernández Islands meet, based on their inflorescence structures.
Analyses based on flavonoids (Ruiz et al., 1994) and genetic divergences (Ruiz et al., 2004) have confirmed that the Juan Fernández species evolved in different lineages. These analyses, however, did not support the relationship between M. fernandeziana and Brazilian species, but instead this species and Myrceugenia schultzei Johow were more related to other Chilean species.
In recent years great advances have been made in molecular systematics (Felsenstein, 2004). This study of the molecular phylogeny of Myrceugenia was undertaken with the expectation that these methods, when applied to Myrceugenia, would result in a phylogenetic hypothesis with greater support than could be derived from morphological data. The present study is directed toward answering the following questions: What are the phylogenetic relationships among Myrceugenia species? Are the species of Myrceugenia in Brazil a lineage completely distinct from those of Chile or did they have a common evolutionary history? What is the relationship between the endemic Juan Fernández species and those of the continent?
2. Materials and methods
2.1. Taxon sampling
A total of fifty taxa were analyzed, with two individuals being sampled in Blepharocalyx salicifolius (Kunth) O. Berg and Myrceugenia ovata (Hook. and Arn.) var. regnelliana (O. Berg) Landrum. All species from Chile (12 species, including two varieties) and many from Brazil (25 species including nine varieties) were investigated. Eight species were included as outgroups: Luma apiculata (DC.) Burret, Luma chequen (A. Gray), Blepharocalyx cruckshanksii (Hook. and Arn.) Nied., B. salicifolius, all of these belonging to the “Group Myrceugenia” (Lucas et al., 2007), Myrcianthes cisplatensis (Cambess.) O. Berg, Ugni molinae Turcz, Ugni selkirkii (Hook. and Arn.) O. Berg, and Myrtus communis L., the last one having been recognized as the sister group of tribe Myrteae (Lucas et al., 2007). All DNA sequences analyzed were obtained during this study. Table 1 gives voucher specimens, herbarium, country of origin, and GenBank accession numbers.
Table 1.
List of taxa, voucher, country of origin, and GenBank accession numbers for plastid and nuclear sequences in species of Myrceugenia and outgroups.
| Taxa | Voucher (Herbarium) | Origen | trnQ-5’rps16 | rpl32-trnL | rpl16 | trnK-matK | ETS | ITS |
|---|---|---|---|---|---|---|---|---|
| Blepharocalyx kruckshankii | J. Murillo 4219 (CONC) | Chile | JN661105 | JN661055 | JN660956 | JN661006 | JN660857 | JN660907 |
| Blepharocalyx salicifolius | L. Landrum 11232 (ASU) | Argentina | JN661134 | JN661084 | JN660985 | JN661035 | JN660886 | JN660936 |
| Blepharocalyx salicifolius | M. Negritto 927 (CONC) | Argentina | JN661133 | JN661083 | JN660984 | JN661034 | JN660885 | JN660935 |
| Luma apiculata | J. Murillo 4205 (CONC) | Chile | JN661108 | JN661058 | JN660959 | JN661009 | JN660860 | JN660910 |
| Luma chequen | L. Landrum 7873 (CONC) | Chile | JN661109 | JN661059 | JN660960 | JN661010 | JN660861 | JN660911 |
| M. alpigena | E. Lucas 167 (K) | Brazil | JN661090 | JN661040 | JN660941 | JN660991 | JN660842 | JN660892 |
| M. alpigena var. fuligenea | G. Hatschbach 59697 (ASU) | Brazil | JN661089 | JN661039 | JN660940 | JN660990 | JN660841 | JN660891 |
| M. alpigena var longifolia | R. Harley 26218 (ASU) | Brazil | JN661091 | JN661041 | JN660942 | JN660992 | JN660843 | JN660893 |
| M. brevipedicellata | L. Landrum 2830 (ASU) | Brazil | JN661092 | JN661042 | JN660943 | JN660993 | JN660844 | JN660894 |
| M. campestris | R. Kummrow 2940 (ASU) | Brazil | JN661093 | JN661043 | JN660944 | JN660994 | JN660845 | JN660895 |
| M. chrysocarpa | L. Landrum 8166 (CONC) | Chile | JN661094 | JN661044 | JN660945 | JN660995 | JN660846 | JN660896 |
| M. colchaguensis | L. Landrum 8033 (CONC) | Chile | JN661095 | JN661045 | JN660946 | JN660996 | JN660847 | JN660897 |
| M. correifolia | S. Teillier 5360 (CONC) | Chile | JN661099 | JN661049 | JN660950 | JN661000 | JN660851 | JN660901 |
| M. cucullata | R. Wasum 105 (ASU) | Brazil | JN661096 | JN661046 | JN660947 | JN660997 | JN660848 | JN660898 |
| M. euosma | L. Soares 715 (ASU) | Brazil | JN661097 | JN661047 | JN660948 | JN660998 | JN660849 | JN660899 |
| M. exsucca | J. Murillo 4217 (CONC) | Chile | JN661098 | JN661048 | JN660949 | JN660999 | JN660850 | JN660900 |
| M. fernandeziana | T. Stuessy 15283 (CONC) | Juan Fernández | JN661101 | JN661051 | JN660952 | JN661002 | JN660853 | JN660903 |
| M. franciscensis | P. Miyagi 357 (ASU) | Brazil | JN661100 | JN661050 | JN660951 | JN661001 | JN660852 | JN660902 |
| M. gerttii | E. Barbosa 948 (ASU) | Brazil | JN661102 | JN661052 | JN660953 | JN661003 | JN660854 | JN660904 |
| M. glausecens | L. Landrum 11231 (ASU) | Brazil | JN661103 | JN661053 | JN660954 | JN661004 | JN660855 | JN660905 |
| M. kleinii | I. Cordeiro 734 (ASU) | Brazil | JN661104 | JN661054 | JN660955 | JN661005 | JN660856 | JN660906 |
| M. lanceolata | M. Mihoc 6220 (CONC) | Brazil | JN661106 | JN661056 | JN660957 | JN661007 | JN660858 | JN660908 |
| M. leptospermoides | J. Murillo 4214 (CONC) | Chile | JN661107 | JN661057 | JN660958 | JN661008 | JN660859 | JN660909 |
| M. miersiana | E. Lucas 164 (K) | Brazil | JN661110 | JN661060 | JN660961 | JN661011 | JN660862 | JN660912 |
| M. myrcioides var. acrophylla | O. Ribas 229 (ASU) | Brazil | JN661111 | JN661061 | JN660962 | JN661012 | JN660863 | JN660913 |
| M. myrcioides | E. Lucas 503 (K) | Brazil | JN661113 | JN661063 | JN660964 | JN661014 | JN660865 | JN660915 |
| M. myrtoides | M. Rossato 47 (MO) | Brazil | JN661117 | JN661067 | JN660968 | JN661018 | JN660869 | JN660919 |
| M. obtuse | P. Brownless 1227 (CONC) | Chile | JN661114 | JN661064 | JN660965 | JN661015 | JN660866 | JN660916 |
| M. ovalifolia | E. Lucas 259 (K) | Chile | JN661115 | JN661065 | JN660966 | JN661016 | JN660867 | JN660917 |
| M. ovata var. acutata | F. Chagas 1979 (ASU) | Brazil | JN661116 | JN661066 | JN660967 | JN661017 | JN660868 | JN660918 |
| M. ovata var. nannophylla | M. Mihoc 5162 (CONC) | Brazil | JN661118 | JN661068 | JN660969 | JN661019 | JN660870 | JN660920 |
| M. ovata var. ovata | F. Gardner 19 (CONC) | Chile | JN661120 | JN661070 | JN660971 | JN661021 | JN660872 | JN660922 |
| M. ovata var. regnelliana | J. Silva 18 (ASU) | Brazil | JN661119 | JN661069 | JN660970 | JN661020 | JN660871 | JN660921 |
| M. ovata var. regnelliana | V. Souza 10621 (ASU) | Chile | JN661137 | JN661087 | JN660988 | JN661037 | JN660889 | – |
| M. oxysepala | O. Ribas 2234 (ASU) | Brazil | JN661121 | JN661071 | JN660972 | JN661022 | JN660873 | JN660923 |
| M. parvifolia | L. Landrum 5916 (CONC) | Chile | JN661122 | JN661072 | JN660973 | JN661023 | JN660874 | JN660924 |
| M. pilotantha | E. Lucas 230 (K) | Brazil | JN661123 | JN661073 | JN660974 | JN661024 | JN660875 | JN660925 |
| M. pilotantha var. pilotantha | C. Lohmann 35 (ASU) | Brazil | JN661124 | JN661074 | JN660975 | JN661025 | JN660876 | JN660926 |
| M. pinifolia | F. Gardner 164 (CONC) | Chile | JN661125 | JN661075 | JN660976 | JN661026 | JN660877 | JN660927 |
| M. planipes | C. Aedo 7378 (CONC) | Chile | JN661126 | JN661076 | JN660977 | JN661027 | JN660878 | JN660928 |
| M. reitzii | E. Barbosa 945 (ASU) | Brazil | JN661135 | JN661085 | JN660986 | JN661036 | JN660887 | JN660937 |
| M. rufa | S. Teillier 150795 (CONC) | Chile | JN661127 | JN661077 | JN660978 | JN661028 | JN660879 | JN660929 |
| M. rufescens | E. Lucas 469 (K) | Brazil | JN661128 | JN661078 | JN660979 | JN661029 | JN660880 | JN660930 |
| M. schultzei | E. Ruiz 8266 (CONC) | Juan Fernández | JN661136 | JN661086 | JN660987 | – | JN660888 | JN660938 |
| M. seriatoramosa | J. Silva 2358 (MO) | Brazil | JN661130 | JN661080 | JN660981 | JN661031 | JN660882 | JN660932 |
| M. smithii | R. García 533 (ASU) | Brazil | JN661129 | JN661079 | JN660980 | JN661030 | JN660881 | JN660931 |
| Myrcianthes cisplathensis | L. Landrum 11233 (ASU) | Uruguay | JN661112 | JN661062 | JN660963 | JN661013 | JN660864 | JN660914 |
| Myrtus communis | Botanical Garden Austria | Austria | JN661088 | JN661038 | JN660939 | JN660989 | JN660840 | JN660890 |
| Ugni molinae | J. Murillo 4213 (CONC) | Chile | JN661131 | JN661081 | JN660982 | JN661032 | JN660883 | JN660933 |
| U. selkirkii | F. Gardner 31 (CONC) | Chile | JN661132 | JN661082 | JN660983 | JN661033 | JN660884 | JN660934 |
2.2. DNA extraction
Total DNA genomic was extracted mainly from herbarium specimens, in some cases from silica-gel dried or fresh leaves, using a modified CTAB (Cetyl trimethyl ammonium bromide) method (Doyle and Doyle, 1987). Ground material was previously treated several times with sorbitol, followed by incubation at 65–70 °C for 30 min with CTAB buffer and Sarkosyl. The precipitated material was left overnight at −20 °C and centrifuged at 14,000 rpm for 30 min, then followed by two washes with 70% ethanol. Total DNA was resuspended in 30–50 μL of 1% TE buffer. Some samples were extracted using DNeasy extraction kit (Qiagen) according to the manufacturer’s instructions.
2.3. Marker selection and primer design
Nine chloroplast markers were evaluated (matK gen and flanking trnK intron, ndhA intron, rpl16 intron, intergenic spacer rpl32-trnL and flanking rpl32 gen, and the intergenic spacer psbA-trnH, psbD-trnT, TrnL-trnF, trnQ-5′rps16, following Shaw et al., 2007), but all of these showed low variability. However, we selected the four most variable regions (partial trnK-matK, rpl32-trnL, trnQ-5′rps16 and rpl16) for reconstructing phylogenetic relationships among species of Myrceugenia. Internal transcribed spacer (ITS: ITS1-5.8S-ITS2) of nuclear ribosomal genes 18S and 26S and the external transcribed spacer 18S-26S rDNA (ETS) were also included. Primers used for each region are listed in Table 2. Two internal primers for region trnQ-5′rps16 were designed.
Table 2.
List of primers.
| Molecular Marker | Pimer name | DNA sequence | Reference |
|---|---|---|---|
| trnQ-5’rps16 intergenic spacer | |||
| trnQ(UUG) | 5′-GCGTGGCCAAGYGGTAAGGC-3′ | Shaw et al. (2007) | |
| MYtrnQR | 5′-AGTTGATGTAAAGGAAGATTTAGACTC-3′ | This study | |
| MYrps16F | 5′-GCGTAAAAWGAGGAAATGCTTAATG-3′ | This study | |
| rpS16x1 | 5′-GTTGCTTTYTACCACATCGTTT-3′ | Shaw et al. (2007) | |
| rpl32-trnL intergenic spacer | |||
| trnL(UAG) | 5′-CTGCTTCCTAAGAGCAGCGT-3′ | Shaw et al. (2007) | |
| rpL32-F | 5′-CAGTTCCAAAAAAACGTACTTC-3′ | Shaw et al. (2007) | |
| rpl16 intron | |||
| rpl16-F71 | 5′-GCTATGCTTAGTGTGTGACTCGTTG-3′ | Jordan et al. (1996) | |
| rpl16-R1516 | 5′-CCCTTCATTCTTCCTCTATGTTG-3′ | Jordan et al. (1996) | |
| Partial matK gene + flanking trnK intron | |||
| matK 700F | 5′-CAATCTTCTCACTTACGATCAACATC-3′ | Gruenstaeudl et al. (2009) | |
| matK1710R | 5′-GCTTGCATTTTTCATTGCACACG-3′ | Samuel et al. (2005) | |
| trnK-R3 | 5′-CGG GGC TCG AAC CCG GA-3 | Wicke and Quandt (2009) | |
| ITS | |||
| AB101 | 5′-ACGAATTCATGGTCCGGTGAAGTGTTCG-3′ | Sun et al. (1994) | |
| AB102 | 5′-GAATTCCCCGGTTCGCTCGCCGTTAC-3′ | Sun et al. (1994) | |
| ITS-4 | 5′-TCCTCCGCTTATTGATATGC-3′ | White et al. (1990) | |
| ETS + flanking 18S gene | |||
| MyrtF | 5′-CTCCGTGCTGGTGCATCGAACTGC-3′ | Lucas et al. (2007) | |
| ETS-18S | 5′-GAGCCATTCGCAGTTTCACAG-3′ | Wright et al. (2001) | |
2.4. Amplification, sequencing and alignment
ITS sequences were amplified mainly in a volume of 25 μL containing 2.5 units of Taq polymerase (Paq5000 DNA polymerase, Stratagene Inc.), 2.5 μL 10X PCR buffer (Stratagene Inc.), 0.2 mL of each dNTP (25 mM), 0.5 μL of each primer (4 μM). All other sequences were amplified using 18 μL 1.1X ReddyMix PCR Master Mix (Thermo Fisher Scientific Inc, ABGene, UK), 0.4 μL of each primer (20 μM), 0.6 μL 0.4% of bovine serum albumin (BSA, MBI-Fermentas, St. Leon-Rot, Germany), and 1–2 μL template DNA. For reducing secondary structure problems 0.5 μL of dimethyl sulfoxide (DMSO) were added to all nuclear markers amplifications. PCR conditions for ITS were according to the Stratagene polymerase manufacturer’s recommendations. An initial DNA denaturation at 95 °C for 2 min was followed by 30 cycles 95 °C for 20 s, 48–52 °C for 20 s, and 72 °C for 20 s, then a final extension at 72 °C for 5 min. PCR conditions for ETS were according to Lucas et al. (2007). An initial denaturation at 94 °C for 5 min was followed by 30 cycles 94 °C for 1 min, 50 °C for 1 min, and 72 °C for 1 min, then a final extension at 72 °C for 5 min. PCR conditions for chloroplast regions were according to Shaw et al. (2007) with some modifications. A first template denaturation at 80 °C for 5 min was followed by 35 cycles 94 °C for 30 s, 50–53 °C for 30 s, and a ramp of 0.3 °C/s to 65 °C, then 65 °C for 3 min, finally an extension at 65 °C for 8 min.
All amplicons were run on a 1% agarose gel to confirm amplification of PCR products. ITS amplified products were purified with QIAquick PCR purification kits (Qiagen) according to manufacturer’s recommendations. All other PCR products were purified using 0.5 μL Exonuclease I and 1 μL Thermosensitive alkaline phosphatase, FastAP (Fermentas) by incubation at 37 °C for 45 min, and later at 80 °C for 15 min. Most amplified ITS products were sent for sequencing either to Macrogen (Korea) or to the Molecular Lab at the University of Santiago de Chile. All others sequences were sequenced in the Molecular Laboratory of the Department of Systematic and Evolutionary Botany in the Biodiversity Center of the University of Vienna. For cycle sequencing a 10 μL reaction volume including 1 μL of the primer (3.2 μM), 0.5 μL of BigDye Terminator v3.1 Ready Reaction mix (Applied Biosystems, Austria), 1.75 μL of sequencing buffer, 4 μL of amplified purified product, and 2.75 μL of ddH2O was used. Cycle sequencing parameters consisted of an initial 96 °C for 1 min, followed by 35 cycles 96 °C for 10 s, 50 °C for 5 s, and 60 °C for 4 min, finally 60 °C for 5 min. Electrophoresis was performed on a 3730 DNA analyzer (Applied Biosystems, ABI). All sequences were analyzed and edited with Lasergene Seqman Pro 7.1.1 (DNASTAR) to obtain consensus sequences from both forward and reverse DNA strands. It was not possible to sequence either Myrceugenia bracteosa (DC.) D. Legrand and Kausel, Myrceugenia hoehnei (Burret) D. Legrand and Kausel, Myrceugenia pilotantha (Kiaersk.) Landrum var. nothorufa (D. Legrand) Landrum, Myrceugenia scutellata D. Legrand, Myrceugenia venosa D. Legrand and Blepharocalyx eggersii for all regions nor M. schultzei Johow for the trnK-matK region.
Sequences were aligned with ClustalX (Thompson et al., 1997) and then corrected manually with Winclada (Nixon, 1999–2002) according to the suggestions of Kelchner (2000). Gaps were coded manually according to the simple indel coding method proposed by Simmons and Ochoterena (2000). Total numbers of both indel sites and indel events, and indel diversity were obtained with DnaSP v.5.10.01 (Librado and Rozas, 2009). Alignments of ITS and ETS sequences were adjusted according to the secondary structure.
Folding predictions of secondary structures of the ITS1, 5.8S, ITS2, and ETS were made at the mfold web server (http://mfold.rna.albany.edu/?q=mfold/RNA-Folding-Form) (Mathews et al., 1999; Zucker, 2003). All parameters were used as default, with and without input constraints. The secondary structure was chosen among those with the highest negative free energy value. Each structure was compared with those ITS1 and ITS2 structures proposed by Biffin et al. (2007) for Myrtaceae. All sequences were aligned and corrected using secondary structure with 4Sale (Seibel et al., 2006, 2008). Indels were coded following Tippery and Les (2008), who established pairwise interactions for each stem, where pairing bases were coded as 1 and non-pairing bases as 0. In the ITS1 region the conserved angiosperm motifs GGCR-(4–7 n)-GYGYCAAGGAA (Liu and Schardl, 1994) were found. We also looked for the motifs GAATTGCAGAATCC, TTTGAAyGCA, CGATGAAGAACGTAGC (Harpke and Peterson, 2008; Jobes and Thien, 1997), and the conserved EcoRV GATATC (Liston et al., 1996) in the 5.8S region. All ITS2 secondary structures showed four helices, helix III being the longest, a U–U mismatch in helix II, and an UGGU motif near the apex of helix III (Schultz et al., 2005). The presence of all these motifs suggests that all ITS sequences obtained in this study are functional. ITS2 region was delimited in the Database http://its2.bioapps.biozentrum.uni-wuerzburg.de/cgi-bin/index.pl?annotator (Keller et al., 2009).
2.5. Phylogenetic analyses
2.5.1. Maximum parsimony analyses (MP)
Heuristic searches were performed using the parsimony algorithm included in NONA (Goloboff, 1999), which uses Winclada as interface (Nixon, 1999–2002). Parsimony ratchet analyses were performed with the following strategy: 10 replicates, 1000 iterations replicate, holding 10 trees per iteration, sampling 10% of the characters, and 10 random constraint levels. The characters were assessed as unordered and equally weighted (Fitch, 1971). Unsupported branches were collapsed and polytomies were allowed. Multiple searches were carried out until no shorter equally parsimonious trees were obtained. Branch supports were assessed using parsimony bootstrapping with 1000 replicates, 10 random search replications each, one starting tree per replicate, 100 maxtrees, and Tree Bisection and Reconnection (TBR) on. Several independent analyses were performed. Each region was evaluated separately, and then several combined data sets were also evaluated, including all chloroplast markers (cpDNA), all nuclear regions (nDNA), cpDNA + ETS, cpDNA + ITS, all combined data (cpnDNA), and cpnDNA + gaps. Congruence among each combined data set was determined by using the Partition homogeneity test (ILD test) (Farris et al., 1994) implemented in PAUP∗v4.10 (Swofford, 2002). For each ILD test 1000 replicates were carried out, each with 10 random addition sequence replicates, holding 10 trees per replicate, TBR branch swapping, and MulTrees on.
2.5.2. Bayesian analyses (BA)
Bayesian inference of phylogenetic reconstruction (Huelsenbeck and Ronquist, 2001) was performed using MrBayes 3.1.2 (Ronquist and Huelsenbeck, 2003), with Markov Chain Monte Carlo (MCMC) providing the heuristic search for a maximum likelihood model of nucleotide substitution. To select the best model of base substitution of DNA, each individual marker data set was evaluated according to the Akaike information criterion using Modeltest 3.7 (Posada and Crandall, 1998) under MrMTgui Interface 1.0 (Nuin, 2007). The best models were TVM + G (trnQ-5′rps16, rpl32-trnL), K81uf + I + G (rpl16), GTR + G (trnK-matK), TIM + G (ETS, ITS1), JC + I (5.8S), TVM + I + G (ITS2). Each region was evaluated separately and in combination, in this case a partitioning model was used, allowing for the best substitution model for each marker. Two independent runs integrated each analysis, each with four chains. Analyses were run between two and ten million generations until the average standard deviation of split frequencies became less than 0.01. For each chain one tree was saved every 10 generations. All trees generated in each run were evaluated in Tracer v1.4 (Rambaut and Drummond, 2007) to determinate effective sample size, convergence of both runs, mixing of the MCMC chains, and Burn-in values for eliminating sample trees found before the stationary phase. From the remaining trees a majority rule consensus tree was reconstructed and posterior probabilities (PPs) calculated.
2.5.3. Network analysis
To evaluate conflicting phylogenetic signals, concatenated data sets of all molecular markers were evaluated with Neighbor Net analysis, using uncorrected p-distances as implemented in SplitsTree4 version 4.11.3 (Huson and Bryant, 2006). Parsimony uninformative sites were excluded. Bootstrap support for splits was calculated using 1000 replicates.
2.6. Statistical analysis
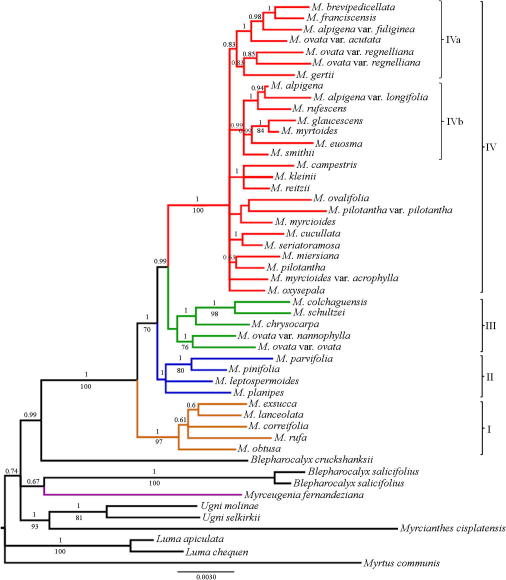
Saturation of substitutions was evaluated with the Xia index using DAMBE v 5.2.13 (Xia, 2001; Xia and Xie, 2001). The diversity of nucleotides for each marker and the rate of divergence for each clade were estimated by Mega 4 (Tamura et al., 2007) following phylogenetic results recovered from all combined marker analyses (Fig. 3) and among groups of species ordained by geographic distribution in clades I–III from Chile and clade IV from Brazil.
Fig. 3.
Bayesian tree resulting from all sequence data (cpnDNA). Numbers above branches are Bayesian posterior probabilities; numbers below branches are bootstrap percentages. Branches are colored according to Fig. 2. The scale indicates substitutions per site.
3. Results
3.1. Chloroplast markers
All chloroplast markers showed low variability. The trnQ-5′rps16 spacer displayed more variables (7.44%) and more parsimony informative characters (4.36%); all other regions are less variable, with only 2.95–3.72% parsimony informative characters (Table 3). this is also shown with the nucleotide diversity value, the trnQ-5′rps16 region revealing 1.37–1.5 more diversity than other chloroplast markers (Table 4). All chloroplast sequences were not significantly saturated (Table 5). Plastid markers have high content of T/A bases (Table 4). Six poly T/A regions are present in the rpl32-trnL region with a length from 7 to 12 bp, whereas the rpl16 intron has two poly T/A regions from 6 to 7 to 12 bp. The trnK-matK region has the least number of indels (22 sites, nine events, indel diversity 0.855) (Table 6). Regions with both the greatest indel diversity and indel length are trnQ-5′rps16 (9.418, 261) and rpl32-trnL (8.797, 156). Due to low DNA quality several sequences were partially amplified, e.g., most trnK-matK sequences only amplified between 758 and 1031 bp. B. salicifolius did not amplify 521 bp for the rpl32-trnL region and M. ovata var. regnelliana only amplified 673 bp for trnQ-5′rps16 region.
Table 3.
Summary of MP analyses of plastid and nuclear markers.
| trnQ-5′rps16 | rpl32-trnL | rpl16 | trnK-matK | cpnDNA | cpDNA + gaps | ETS | ITS | nDNA | cpDNA + ETS | cpDNA + ITS | cpDNA | cpDNA + ETS + ITS + gaps | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of taxa | 50 | 50 | 50 | 49 | 50 | 50 | 50 | 49 | 50 | 50 | 50 | 50 | 50 |
| Character | 1465 | 885 | 1005 | 1083 | 4438 | 4628 | 502 | 642 | 1145 | 4940 | 5080 | 5582 | 6226 |
| Constant characters (%) | 1292 (88.19) | 795 (89.83) | 904 (89.95) | 978 (90.34) | 3969 (89.43) | 379 (75.49) | 516 (80.37) | 895 | 4348 | 4485 | 4865 | ||
| Variable characters (%) | 109 (7.44) | 57 (6.44) | 65 (6.46) | 73 (6.74) | 304 (6.84) | 68 (13.54) | 61 (9.5) | 129 | 372 | 365 | 433 | ||
| NP-informative characters (%) | 64 (4.36) | 33 (3.72) | 36 (3.58) | 32 (2.95) | 165 (3.71) | 263 (5.68) | 55 (10.95) | 65 (10.12) | 121 | 220 | 230 | 285 | 419 |
| No. of most parsimonious trees | 12 | 135 | 98 | 188 | 12 | 290 | 81 | 140 | 794 | 440 | 185 | 8 | 1192 |
| Length | 95 | 51 | 62 | 46 | 272 | 574 | 202 | 160 | 325 | 419 | 499 | 641 | 1050 |
| Consistency index CI | 0.77 | 0.66 | 0.62 | 0.76 | 0.66 | 0.48 | 0.66 | 0.51 | 0.48 | 0.59 | 0.54 | 0.52 | 0.44 |
| Retention index (RI) | 0.92 | 0.81 | 0.87 | 0.9 | 0.86 | 0.73 | 0.61 | 0.68 | 0.63 | 0.79 | 0.76 | 0.73 | 0.66 |
Table 4.
Nucleotide compositions of plastid and nuclear regions.
| Marker | Alignment sites (range) bp | A | T | G | C | GC% | Nucleotide diversity |
|---|---|---|---|---|---|---|---|
| trnQ-5′rps16 | 1483 (1206–1384) | 0.3795 | 0.3516 | 0.1353 | 0.1335 | 0.2631–0.3025 | 0.01393 |
| rpl32-trnL | 885 (795–840) | 0.32466 | 0.39901 | 0.13816 | 0.13817 | 0.2825–0.3203 | 0.01015 |
| rpl16 | 1005 (817–967) | 0.27693 | 0.41009 | 0.14939 | 0.16359 | 0.3048–0.3276 | 0.00995 |
| trnK-matK | 1083 (1056–1064) | 0.34544 | 0.32224 | 0.15217 | 0.18015 | 0.3308–0.3919 | 0.00928 |
| ETS | 502 (455–470) | 0.18842 | 0.31794 | 0.25902 | 0.23461 | 0.4792–0.5185 | 0.02671 |
| ITS | 642 (602–616) | 0.21896 | 0.21436 | 0.27892 | 0.28777 | 0.5548–0.5873 | 0.02613 |
| ITS1 | 256 (239–245) | ||||||
| 5.8S | 160 (153–160) | ||||||
| ITS2 | 226 (208–217) |
Table 5.
Substitution saturation.
| Iss | Iss.c | p | |
|---|---|---|---|
| trnQ-5′rps16 | 0.011 | 0.666 | <0.0001 |
| rpl32-trnL | 0.071 | 0.678 | <0.0001 |
| rpl16 | 0.047 | 0.718 | <0.0001 |
| trnK-matK | 0.031 | 0.717 | <0.0001 |
| ETS | 0.091 | 0.681 | <0.0001 |
| ITS | 0.041 | 0.709 | <0.0001 |
Iss = index of substitution saturation.
Iss.c = critical value.
Table 6.
Indel characteristics of plastid and nuclear regions.
| trnQ-5′rps16 | rpl32-trnL | rpl16 | trnK-matK | ETS | ITS | |
|---|---|---|---|---|---|---|
| Range of indel length | 1–261 | 1–156 | 1–29 | 1–7 | 1–10 | 1–6 |
| Total number of indel sites | 374 | 391 | 208 | 22 | 73 | 78 |
| Total number of indel events | 71 | 52 | 32 | 9 | 60 | 62 |
| Indel diversity k(i) | 9.418 | 8.797 | 4.73 | 0.855 | 5.433 | 6.648 |
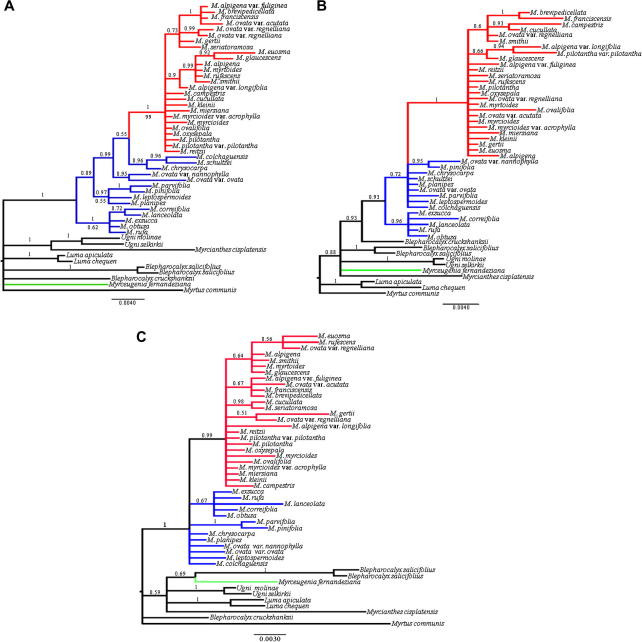
MP analyses from individual chloroplast regions gave strict consensus trees with low resolution (data not shown). The rpl32-trnL region displayed the lowest resolution (data not shown), whereas trnQ-5′rps16 region had the most resolved tree (Fig. 1A). However, all topologies are nearly congruent and show Myrceugenia to be monophyletic (Myrceugenia clade), except for M. fernandeziana appears in different placement within the outgroups. Brazilian species appear as a derived monophyletic group. Furthermore, there is no evidence of a monophyletic origin of the “Myrceugenia group” (sensu Lucas et al., 2007). All of these relationships are completely congruent with results rising from Bayesian analysis (BA) (Fig. 1), but with more resolution.
Fig. 1.
Majority consensus tree of species of Myrceugenia and outgroups from Bayesian analyses of (A) trnQ-5’rps16, (B) rpl16, and (C) trnK-matK regions. Numbers above branches are Bayesian posterior probabilities; numbers below branches are bootstrap percentages. Branches are colored according to their main geographical distribution: red, Brazilian species; blue, Chilean species; green, M. fernandeziana. The scale indicates substitutions per site.
There was no significant incongruence among the four-chloroplast regions (P = 0.57). Therefore, we combined all markers into a single data set with a total length of 4438 sites. Both MP and BA analyses of the combined data set agree with separate analyses of individual molecular markers (Fig. 2A). M. fernandeziana appears in a clade with B. salicifolius (BS = 97%, PP = 0.69), whereas most Myrceugenia species are included within a monophyletic clade (BS = 100%, PP = 1.0), with 13 synapomorphic substitutions, of which nine are non-homoplasious. Chilean species are recovered in three clades: the earliest diverging clade (clade I), comprising Myrceugenia obtusa, Myrceugenia correifolia, Myrceugenia rufa, Myrceugenia exsucca and Myrceugenia lanceolata, is highly supported (BS = 99%, PP = 1.0) by six non-homoplasious substitutions. This clade was also recovered from the trnQ-5′rps16, rpl32-trnL, rpl16 and trnK-matK analyses (Fig. 1). The next clade (clade II), consisting of Myrceugenia leptospermoides, Myrceugenia planipes, Myrceugenia parvifolia and Myrceugenia pinifolia received high support (BS = 97, PP = 0.97) and is supported by one non-homoplasious substitution. This clade was only recovered from the trnQ-5′rps16 spacer (Fig. 1A). The third clade (clade III), comprising the remaining Chilean species, was only recovered from BA for all chloroplast markers, but only poorly supported (PP = 0.64%) by ten substitutions, six of these without homoplasy. In the last clade (clade IV) all the Brazilian species appear; this clade is highly supported (BS = 99, PP = 1.0), but with low resolution. Average evolutionary divergences over sequence pairs within clades revealed that Chilean clades (I, II, III) contain more divergence than the Brazilian clade (IV), except for rpl16 that is lower (Table 7).
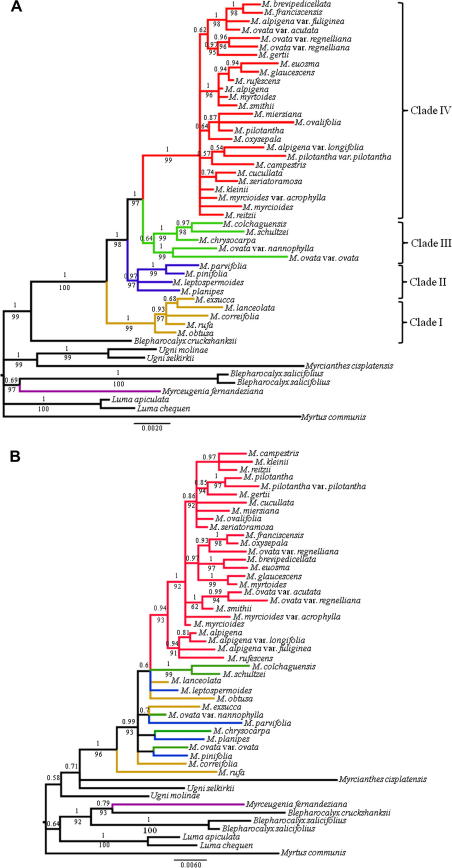
Fig. 2.
Bayesian trees of species of Myrceugenia and outgroups resulting from combined analyses of (A) cpDNA and (B) nDNA sequences. Numbers above branches are Bayesian posterior probabilities; numbers below branches are bootstrap percentages. Brach red, Brazilian species; green, blue and yellow, Chilean species, and violet, M. fernandeziana. The scale indicates substitutions per site.
Table 7.
Estimates of average evolutionary divergence over sequence pairs within Chilean and Brazilian clades (average/standard deviation).
| Group | trnQ-5′rps16 | rpl32-trnL | rpl16 | trnK-matK | ETS | ITS |
|---|---|---|---|---|---|---|
| Outgroups | 0.02010/0.00215 | 0.01236/0.00210 | 0.01449/0.00244 | 0.01654/0.00235 | 0.05130/0.00465 | 0.04613/0.00630 |
| Chile | ||||||
| Clade I–III | 0.00717/0.00133 | 0.00901/0.00220 | 0.00346/0.00111 | 0.00397/0.00120 | 0.01783/0.00208 | 0.02069/0.00350 |
| Clade I | 0.00191/0.00079 | 0.00426/0.00157 | 0.00250/0.00108 | 0.00219/0.00094 | 0.01802/0.00281 | 0.02907/0.00604 |
| Clade II | 0.00359/0.00118 | 0.00510/0.00180 | 0.00219/0.00115 | 0.00503/0.00200 | 0.01809/0.00308 | 0.01382/0.00376 |
| Clade III | 0.00714/0.00150 | 0.00496/0.00161 | 0.00220/0.00104 | 0.00205/0.00119 | 0.01582/0.00297 | 0.01622/0.00409 |
| Brazil | ||||||
| Clade IV | 0.00313/0.000066 | 0.00278/0.00069 | 0.00474/0.00097 | 0.00275/0.00081 | 0.01075/0.00142 | 0.01243/0.00207 |
3.2. Nuclear markers
Nuclear markers displayed more variability (10.12–10.95%, Table 3), diversity (1.9–2.9-fold, Table 4), GC content (1.2–2.2-fold, Table 4), average evolutionary divergence over sequence pairs (Table 7), and lower indel variability (Table 6) than the plastid markers. ETS and ITS sequences did not show significant saturation of nucleotide substitution (Table 5). Both the strict consensus trees from MP and majority rule consensus tree from BA obtained from individual markers showed poor resolution (data not shown). Consensus trees from combined nuclear markers are partially congruent with those from plastid markers (Fig. 2B). Myrceugenia appears monophyletic, with most species of the genus, except for M. fernandeziana, residing in a highly supported (BS = 96% PP = 1.0) group held together by seven synapomorphic substitutions, three of these non-homoplasious. M. rufa is sister to the rest of the species of Myrceugenia; this relationship only emerges in BA analyses from ITS and from combined nuclear marker analyses. The Chilean species are poorly resolved, whereas Brazilian species appear in a highly supported (BS = 93% PP = 0.94) monophyletic group held together by four homoplasious synapomorphies. A clade consisting of Myrceugenia alpigena and Myrceugenia rufescens (BS = 91% PP = 0.94) is sister to the rest of the Brazilian species. This relationship is not found in all other analyses. The most derived clade has low resolution, but three highly supported (BS > 0.86) subclades were recovered.
3.3. Congruence
The partition homogeneity test for nDNA, cpDNA + ETS, cpDNA + ITS, and cpnDNA show character incongruence (P = 0.01). All analyses show congruence among major clades, but within clades poor resolution emerges, which could explain lack of congruence reported by the ILD test. All topologies displayed short branches, mostly poorly supported, especially among Brazilian species. Such conditions have been interpreted by Wendel and Doyle (1998) as a soft incongruence, which might disappear with additional data. Gatesy et al. (1999) demonstrated that concatenating of truly incongruent data sets could still increase resolution and branch support. Furthermore, there is no evidence of the presence of pseudogenes in nuclear regions that could explain the incongruence. Therefore we combined all genomic markers.
3.4. Combined analysis
MP and BA analyses from cpDNA + ETS, cpDNA + ITS and cpnDNA showed similar consensus trees, except that the strict consensus trees of MP recovered from cpDNA + ETS and cpDNA + ITS show least resolution within clade IV; all of these, however, are nearly congruent with those from combined chloroplast markers. The BA consensus tree from cpnDNA (Fig. 3) shows that the Myrceugenia clade is strongly supported, as well as clades I to IV. Clade IV appears with two subclades (Clade IVa, IVb); these emerge from all combined partitions and are quite similar to those from cpDNA (Fig. 2A) and trnQ-5’rps16 (Fig. 1A) analyses. These subclades from BA have relatively high support, but in MP support is low.
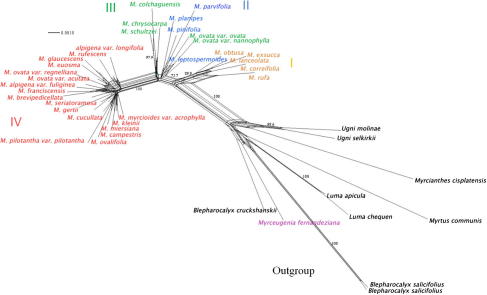
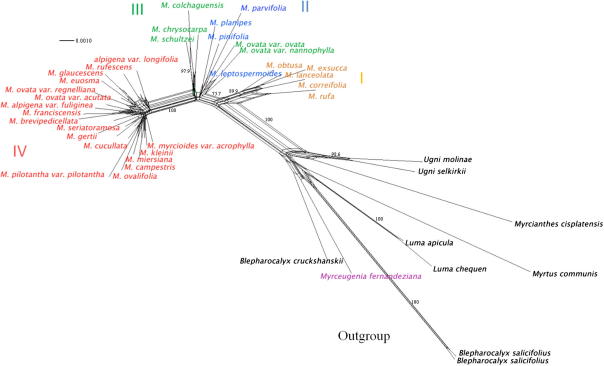
Neighbor Net analysis. Splits graph analysis reveals that species of Myrceugenia are distributed in four splits (Fig. 4), but M. fernandeziana groups with outgroups taxa as also shown in the phylogenetic analyses. All of these splits are strongly compatible with topologies recovered from MP and BA analysis, except that M. ovata var. ovata and M. ovata var. nannophylla cluster within split II. Support between split II and III is low, but other splits are strongly supported. Conflicting phylogenetic signals occur within split I and also within split IV.
Fig. 4.
Split graph resulting from Neighbor Net analysis using concatenated data sets of all molecular markers. Numbers are Bootstrap values. Splits are colored according to Fig. 2.
3.5. Indel information
Similar topologies are recovered from analyses with or without gaps. Inclusion of coded indels reduced bootstrap support, CI, RI, and resolution. Analyses including all markers and coded indels reveal the Myrceugenia clade to have three non-homoplasious indel synapomorphies: an insertion of TAAA for the trnK-matK region, and two gaps, AAGTGATGA and TTMAAAKT, for the rpl32-trnL region. In Addition, two indel synapomorphies exist in the ETS secondary structure: a bulge arising from a mismatch at the middle of Helix II, and other mismatch whereby the Myrceugenia clade has the Helix VI shorter than the outgroups or M. fernandeziana. Clade I is supported by a large deletion of 168 bp, occurring between positions 342 and 510 in the spacer trnQ-5’rps16. Clades II, III and IV have a synapomorphic deletion of 28 bp (positions 244–272) of the rpl16 intron. Clade IVa has the insertion ATTAC in the trnQ-5’rps16 region. A deletion of 11 bp (position 290–300) in rpl32-trnL is a synapomorphy for Ugni.
4. Discussion
4.1. Polyphyly of Myrceugenia
All analyses strongly indicate that Myrceugenia is monophyletic only when the Juan Fernández species, M. fernandeziana, which shows no relationship with other species of the genus, is excluded. The clade containing the remaining species of Myrceugenia is supported by 12 substitutions (three nuclear and nine plastid), an insertion of trnK-matK, two gaps from rpl32-trnL, and two structural differences within ETS secondary structure. None of these traits are present in M. fernandeziana nor in other genera of tribe Myrteae examined. This strongly suggests that M. fernandeziana does not belong to Myrceugenia.
M. fernandeziana was included in the genus Nothomyrcia by Kausel (1948), which was considered by McVaugh (1968) to have unclear position within tribe Myrteae, and related in some fashion to the ancestor of the tribe. Landrum (1981b) considered it as a early diverging species within Myrceugenia, closely related to two Brazilian species, Myrceugenia campestris and M. rufescens, supported by features of the indumentum, inflorescence, and flowers. According to results in the present study, these morphological similarities appear to be convergences, possibly favored by similar climatic conditions in which all these species occur, i.e., a foggy subtropical forests (Landrum, 1981a). Morphological differences to the remaining Myrceugenia species are scarious bracteoles, which are caducous at anthesis, and a leaf midvein that is not impressed, whereas in the rest of the species the bracteoles are rarely scarious and usually persistent, and the midvein of the leaves is impressed (Landrum, 1981a). Preliminary results regarding taxonomic position of this species, including 30 genera of tribe Myrteae (Murillo, 2011), suggest that M. fernandeziana is an independent lineage related to Blepharocalyx. These results support both what Kausel (1948) considered as an independent genus, and McVaugh (1968) considered as an independent, relatively unsuccessful lineage.
4.2. Phylogenetic relationships within Myrceugenia
Myrceugenia splits into four clades (Fig 3). The Chilean species are distributed into three clades, whereas all Brazilian species are included in a derived monophyletic group. All of these relationships are supported by Neighbor Net analysis (Fig. 4), which displays a split graph strongly congruent with the combined analysis. These results affirm concatenation of both chloroplast and nuclear markers, which were shown to be incongruent by the ILD test, but which improved topology resolution as suggested by Gatesy et al. (1999). Incongruence has also been found when there are pseudogenes in the nuclear regions, but no evidence of these non-functional regions has been found in any of the sequences analyzed. Neighbor Net analysis shows incongruence in splits I and IV, which could be due to recombination, hybridization, gene conversion, gene transfer, or sampling error (Bryant and Moulton, 2004). According to the phi test, however, there was no statistically significant evidence for recombination (p = 0.239). This might suggest therefore that incongruencies are due to low levels of sequence variability, which can obviously cause lack of true phylogenetic signal (Wendel and Doyle, 1998).
Landrum (1981b), based on morphological data, proposed that Myrceugenia could be split into five groups, each one including species from both Chile and Brazil. A clade recovered from ITS analysis consisting of the three varieties of M. alpigena, M. rufescens from Brazil, and the Chilean M. schultzei and Myrceugenia colchaguensis, and another clade emerging from ETS analyses that includes Myrceugenia cucullata and M. rufescens from Brazil and M. parvifolia and M. correifolia from Chile, could justify those relationships. These clades, however, are poorly supported (0.69 PP, 0.7 PP, respectively) and they are not recovered from either chloroplast markers or any partitioned data set analyses. Relationships recovered by Landrum (1981b) show only seven Brazilian species appearing close to Chilean species, whereas the others remain in a separate clade. According to our analyses, we might infer that lack of separation of these two lineages in the Landrum analyses might be due to paucity of informative characters. It is not easy to establish comparisons between molecular and morphological data for each clade; because there seems to be no correlation between molecular data and morphological data; it will be necessary, therefore, to look for new morphological and anatomical data to help clarify relationships found in this study.
Clade I, composed of M. lanceolata, M. exsucca, M. rufa, M. correifolia, and M. obtusa, was recovered from all analyses, except from that with nuclear markers. Little internal resolution exists, but BA analyses from ITS and from combined ITS + ETS analyses show M. rufa to be the earliest diverging species. However, the incongruent position of this species or any other species, when they are compared with the topology found with chloroplast markers, may be due to lack of resolution among species (Fig. 2B). Resolution increased, however, especially among Chilean species, when all data were combined (Fig. 3), which is a general result suggested by Wendel and Doyle (1998). The three first-listed species were treated as being closely related from the morphological analyses (Landrum, 1981a). All results are consistent, in suggesting that these species are sister to the remaining members of the genus.
Clade II is recovered from all combined analyses (Fig. 3) and also from the trnQ-5’rps16 analysis (Fig. 1A) with strong support, but in MP analyses alone, it has poor support. Neighbor Net analysis confirms a relationship among these species, but in this split Chilean varieties of M. ovata are also included (Fig. 4). Such a relationship does not appear in any other phylogenetic analysis, which falls poorly supported within clade III from combined phylogenetic analyses. These varieties are recovered as an independent clade from the spacer trnQ-5’rps16 analysis. The other individual chloroplast markers reveal no resolution, whereas nuclear markers show them to be biphyletic. Despite the fact that a monophyletic origin of the other Brazilian varieties was not seen, Landrum (1981a) still kept them under the same species because he could not find morphological characters to separate them. All molecular analyses, however, strongly support their separation.
Relationships among M. chrysocarpa, M. colchaguensis and M. schultzei recovered from cpDNA, cpDNA + ETS, cpDNA + ITS and trnQ-5’rps16 analyses all are strongly supported. This is confirmed with Neighbor Net analysis, but this and MP analyses show low support for relationships between M. chrysocarpa and the other two species. Results from BA of trnQ-5’rps16 spacer are highly congruent with those from combined analysis, showing its high phylogenetic signal. The tree from this analysis (Fig. 1A) shows that these three species are sister to those in Brazil. This relationship was partially found in the phylogenetic analysis by Landrum (1981a), whereby M. chrysocarpa appears as the earliest diverging species of a clade formed by M. colchaguensis and 22 Brazilian taxa. This would suggest a common ancestry for these Chilean and Brazilian species. M. schultzei was considered by Landrum (1981b) to be an ancient splitting taxon within Myrceugenia and closely allied to M. lanceolata and M. exsucca. In our study, however, none of these relationships are observed; M. schultzei in fact, appears to be one of the most derived species within the Chilean group.
The Brazilian clade is strongly supported (BS = 100% PP = 1.0), also consistent with Neighbor Net analysis (BS = 100%), but with poor resolution. This lack of resolution has been interpreted as resulting from saturation of variable sites, such that phylogenetic signal is not recovered (von Dohlen and Moran, 2000). There is no significant evidence, however, for saturation in either chloroplast or nuclear sequences (Table 5). We interpret these ambiguous relationships as being due to low number of informative characters (5.1% combined data) and lack of synapomorphic characters as shown by CI = 0.52 and RI = 0.73 in the parsimony analysis. The average evolutionary divergence over sequence pairs demonstrates that Chilean species show between1.44- and 3.24-fold higher divergence than Brazilian species (Table 7), except for rpl16 that is 1.36-fold lower, suggesting independent evolutionary histories for these disjunct groups. The low resolution, short branches, and high species diversity may be correlated with rapid radiation (Schwarzbach and Kadereit, 1995; Baldwin and Sanderson, 1998; Fishbein et al., 2001; Hughes and Eastwood, 2006; Miwa et al., 2009) as emphasized by both lack of phylogenetic signal among species of split IV and low resolution within the diverse clade IV. Rapid radiation could help explain the lack of definitive molecular and morphological characters (von Dohlen and Moran, 2000), at least in the Brazilian species.
4.3. Relationship within the “Myrceugenia group”
The “Myrceugenia group” was proposed by Lucas et al. (2007) to include Blepharocalyx, Luma, and Myrceugenia. All analyses in this study, however, show no evidence of monophyly for this group. Depending on data analyzed, genera of this complex appear in different positions associated with other genera. Species belonging to Luma form the only consistently monophyletic clade. Species of Blepharocalyx appear biphyletic as was displayed by Lucas et al. (2007); evidence for their monophyly was only recovered from ITS Bayesian analysis with high support (PP = 0.91).
According to Landrum (1981b) these three genera and Myrcianthes belong to separate lineages having arisen from ancestors of each of the three subtribes recognized by Berg (1855–1856), whereas Luma would have arisen from the tribe ancestor. All of these genera share anatomical and floral features (e.g., some vessels with scalariform perforation; simple, uniflorous, dichasial or bracteate shoot inflorescences; tetramerous flowers; free calyx-lobes; 2–4 locular ovaries, numerous ovules; free cotyledons; and membranous testa), which would be regarded as plesiomorphies. Lucas et al. (2007) proposed that these characteristics are, in fact, synapomorphies of the “Myrceugenia group”, but in their study this group had low support and only appears when data are combined. Analyses presented here do not establish whether all of these genera represent a very old linage arising from ancestral groups of tribe Myrteae, but what is possible to propose is that all these genera do not belong to a monophyletic group.
4.4. Biogeographical implications
Myrceugenia is a genus that has originated in southern South America (Landrum, 1981b; Lucas et al., 2007), with two centers of species diversity in Central Chile and southeastern Brazil. This disjunct distribution is also seen in other taxa such as Alstroemeria, Araucaria, Azara, Escallonia, and Weinmannia (Landrum, 1981b). Distribution of the South American flora has been explained by climatic and geological changes occurring during the Neogene (Landrum, 1981b; Villagran and Hinojosa, 1997).
The earliest fossil records of Myrtaceae in the southern South America are pollen from the Campanian (83.5–70.6 Ma) (Poole and Cantrill, 2006; Prámparo et al., 2007). Remains of leaves and wood from the Late Cretaceous have been found that have been assigned to Eugenia, Luma (Poole et al., 2001, 2003) and Myrcia (Barreda and Palazzesi, 2007). The most ancient fossil of Myrceugenia is M. chubutense, described by Ragonese (1980) from wood remains in southwestern Argentina (Province Chubut) from early Paleocene (61.7–65.5 Ma) deposits. Other fossils assigned to this genus have been reported from leaves from central Argentina (early Eocene, 47–52 Ma; González et al., 2003; Wilf et al., 2005) and central Chile (early Eocene; Gayo et al., 2005), and early Miocene (21 Ma; Villagrán and Hinojosa, 2005). Fossil records, therefore suggest that Myrceugenia is a very old lineage, as was inferred by McVaugh (1968) and Landrum (1981b), having been widely distributed in southern South America (Landrum, 1981b), from early Paleocene.
Murillo (2011) selected to M. chubutense and Myrceugenelloxylon antarcticus, to perform a calibration analysis for the tribe Myrteae. The latter is a fossil from the Upper Cretaceous (Maastrichtian, 68.5–65 Ma, Poole et al., 2003) to the Middle Eocene of Antarctica (49–43 Ma, Poole and Cantrill, 2006) that shows wood anatomy similar to moderm L. apiculata. These results suggest that Myrteae is older than what has been proposed by Sytsma et al. (2004) and Biffin et al. (2010) based on fruits remains of Paleomyrtinaea princetonensis, a fossil described from the Palaeocene (56 Ma) of North America (Pigg et al., 1993), which would agree with the extensive and old fossil record for the family in southern South America.
Biogeographic analyses (Murillo, 2011) of taxa in clade I suggest that species dispersed to northern Chile between the Lower and Middle Miocene. Ancestors that gave rise to the remaining clades dispersed to southern Chile in the Lower Miocene as did also some species of clade II and III to Argentina. Molecular studies in Ourisia (Plantaginaceae) have also shown dispersal in southern Chile (Meudt and Simpson, 2006), which could suggest a local dispersal pattern for development of the vegetation (Arroyo pers. comm.). During the Pliocene, the clade III shows a dispersal to the Juan Fernandez Archipelago now represented by M. schultzei from Masafuera. The analysis suggests Brazilian Myrceugenia diverged in the Lower Miocene from ancestors in southern Chile, but its diversification in southeastern Brazil began during Middle Miocene, in a process of colonization from South to North.
Separation of southern from northern South America by the Paranaense Sea (15–13 Ma) (Hernández et al., 2005), plus the final early Pliocene uplift of the Andes and the “Pampean Mountain Range” in central Argentina (Pascual et al., 1996), all resulted in completion of the arid diagonal. This began to develop at the end of the Miocene (Hinojosa and Villagrán, 1997; Gregory-Wodzicki, 2000), which could have affected distributions of both arthropods (Donato, 2006; Roig-Juñent et al., 2006) and plants (Ritz et al., 2007; Marquínez et al., 2009). It is likely, therefore, that the same events occurring during the Miocene caused Myrceugenia to remain confined to Central Chile and southeastern Brazil, regions of similar climatic conditions (Landrum, 1981b).
Molecular calibration studies for the flora of South America are scarce. Estimated age for divergence of the Chilean species of Myrceugenia (Murillo, 2011) and Drimys (Marquínez et al., 2009) from those present in Brazil, is between 16 and 13 Ma. This age estimation coincides with the formation of the Paranaense Sea (Hernández et al., 2005). Disjunct distribution patterns in many South American genera (Villagran and Hinojosa, 1997; Landrum, 1981b), which also match ages of divergence of Myrceugenia and Drimys would support the hypothesis that the Paranaense Sea could have acted as cause of vicariance. This would have had great importance for the distribution and diversity of the flora and fauna of South America (Webb, 1995). In Australia, there is a very rich endemic flora distributed into two zones separated by a wide arid zone where many taxa occurred disjunctly. This is similar to the pattern in southern South America. Divergences among those lineages in Australia have also been interpreted to be due to an ancient vicariant event (Crisp and Cook, 2007).
Acknowledgments
The authors thank CONYCIT (24090098) of Chile and University of Concepción (DIUC 209.111.054-1.0) for financial support. Herbaria ASU, CONC, K, MO and RB sent us material for DNA extraction. Eva Lucas from Royal Botanic Gardens, Kew, and Edith Karpino from Jodrell Laboratory, Kew sent us DNA aliquots of some species. Herbarium VEZ sent specimens of Blepharocalyx eggerssi for morphological study. Jim Solomon, Missouri Botanical Garden, Rafaela Forzza, Rio de Janeiro Botanical Garden, Frank Schumacher, Botanical Garden, University of Vienna, all assisted us in providing material. Labwork at Concepción was facilitated by Patricia Gómez, Mariela González and Angela Carrasco, and in the Molecular Systematics Laboratory Department of Systematic and Evolutionary Botany, University of Vienna, by Rosabelle Samuel, Elfriede Grasserbauer, Gudrun Kohl, Verena Klejna, Patricio López, and Walter Till. We thank two anonymous reviewers for their comments and suggestions on the manuscript. JM was supported by a fellowship from the University of Concepcion-MECESUP (UCO0708) and from CONICYT for a stay at Vienna University. Partially supported by FWF Grant No. P21723-B16 to TFS.
References
- Baldwin B.G., Sanderson M.J. Age and rate of diversification of the Hawaiian silversword alliance (Compositae) Proc. Natl. Acad. Sci. U.S.A. 1998;95:9402–9406. doi: 10.1073/pnas.95.16.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreda V., Palazzesi L. Patagonian vegetation turnovers during the Paleogene-early Neogene: Origin of arid-adapted floras. Bot. Rev. 2007;73:31–50. [Google Scholar]
- Berg O. Revisio Myrtacearum Americae. Linnaea. 1855-1886;27:1–472. [Google Scholar]
- Biffin E., Harrington M., Crisp M., Craven L., Gadek P. Structural partitioning, paired-sites models and evolution of the ITS transcript in Syzygium and Myrtaceae. Mol. Phylogenet. Evol. 2007;43:124–139. doi: 10.1016/j.ympev.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Biffin E., Lucas E.J., Craven L.A., Ribeiro I., Harrington M.G., Crisp M.D. Evolution of exceptional species richness among lineages of fleshy-fruited Myrtaceae. Ann. Bot. 2010;106:79–93. doi: 10.1093/aob/mcq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D., Moulton V. Neighbor-Net: an agglomerative method for the construction of phylogenetic networks. Mol. Biol. Evol. 2004;21:255–265. doi: 10.1093/molbev/msh018. [DOI] [PubMed] [Google Scholar]
- Crisp M.D., Cook L.G. A congruent molecular signature of vicariance across multiple plant lineages. Mol. Phylogenet. Evol. 2007;43:1106–1117. doi: 10.1016/j.ympev.2007.02.030. [DOI] [PubMed] [Google Scholar]
- Donato D. Historical biogeography of the family Tristiridae (Orthoptera: Acridomorpha) applying dispersal – vicariance analysis. J. Arid Environ. 2006;66:421–434. [Google Scholar]
- Doyle J.J., Doyle J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987;19:11–15. [Google Scholar]
- Farris J.D., Kallersjo M., Kluge A.G., Bult C. Testing significance of incongruence. Cladistics. 1994;10:315–319. [Google Scholar]
- Felsenstein J. Sinauer Associates, Inc., Publishers; Sunderland, Massachusetts: 2004. Inferring Phylogenies. [Google Scholar]
- Fishbein M., Hibsch-Jetter C., Soltis D.E., Hufford L. Phylogeny of Saxifragales (Angiosperms, Eudicots), analysis of a rapid, ancient radiation. Syst. Biol. 2001;50:817–847. doi: 10.1080/106351501753462821. [DOI] [PubMed] [Google Scholar]
- Fitch W.M. Toward defining the course of evolution, minimum change for a specific tree topology. Syst. Zool. 1971;20:406–416. [Google Scholar]
- Gatesy J., O’Grady P., Baker R.H. Corroboration among data sets in simultaneous analysis, hidden support for phylogenetic relationships among higher level Artiodactyl taxa. Cladistics. 1999;15:271–313. doi: 10.1111/j.1096-0031.1999.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Gayo E., Hinojosa L.F., Villagrán C. On the persistence of tropical paleofloras in central Chile during the early Eocene. Rev. Paleobot. Palynol. 2005;137:41–50. [Google Scholar]
- Goloboff, P., 1999. NONA (NO NAME), v.2. Tucumán, published by the author.
- González, C., Gandolfo, M.A., Cúneo, R., Wilf, P., 2003. Revisio´n de las Myrtaceae de Laguna del Hunco y Ri´o Pichileufu´ (Eoceno Inferior), Patagonia, Argentina. XII Simposio Argentino de Paleobotánica y Palinología. Ameghiniana. 40 (Suppl.), 7–8.
- Gregory-Wodzicki K.M. Uplift history of the Central and Northern Andes, a review. Geol. Soc. Am. Bull. 2000;112:1091–1105. [Google Scholar]
- Gruenstaeudl M., Urtubey E., Jansen R.K., Samuel R., Barfuss M.H.J., Stuessy T.F. Phylogeny of Barnadesioideae (Asteraceae) inferred from DNA sequence data and morphology. Mol. Phylogenet. Evol. 2009;51:572–587. doi: 10.1016/j.ympev.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Harpke D., Peterson A. 5.8S motif for the identification of pseudogenic ITS regions. Botany. 2008;86:300–305. doi: 10.1007/s10265-008-0156-x. [DOI] [PubMed] [Google Scholar]
- Hernández R.M., Jordan T.E., Dalenz A., Echavarría L., Idleman B.D., Reynolds J.H. Age, distribution, tectonics, and eustatic controls of the Paranense and Caribbean marine transgressions in southern Bolivia and Argentina. J. S. Am. Earth Sci. 2005;19:495–512. [Google Scholar]
- Hinojosa L.F., Villagrán C. Historia de los bosques del sur de sudamérica I, antecedentes paleobotánicos, geológicos y climáticos del terciario del cono sur de América. Rev. Chil. Hist. Nat. 1997;70:225–239. [Google Scholar]
- Huelsenbeck J.P., Ronquist F. MRBAYES, Bayesian inference of phylogeny. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Hughes C., Eastwood R. Island radiation on a continental scale, exceptional rates of plant diversification after uplift of the Andes. Proc. Natl. Acad. Sci. U.S.A. 2006;103:10334–10339. doi: 10.1073/pnas.0601928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson D.H., Bryant D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- Jobes D.V., Thien L.B. A conserved motif in the 5.8S ribosomal RNA (rRNA) gene is a useful diagnostic marker for plant internal transcribed spacer (ITS) sequences. Plant Mol. Biol. Rep. 1997;15:326–334. [Google Scholar]
- Jordan W.C., Courtney M.W., Neigel J.E. Low levels of intraspecific genetic variation at a rapidly evolving chloroplast DNA locus in North American duckweeds (Lemnaceae) Am. J. Bot. 1996;83:430–439. [Google Scholar]
- Kausel E. Notas mirtológicas. Lilloa. 1948;13:125–149. [Google Scholar]
- Kelchner S.A. The evolution of noncoding chloroplast DNA and its application in plant systematics. Ann. Mo. Bot. Gard. 2000;87:482–498. [Google Scholar]
- Keller A., Schleicher T., Schultz J., Müller T., Dandekar T., Wolf M. 5.8S–28S rRNA interaction and HMM-based ITS2 annotation. Gene. 2009;430:50–57. doi: 10.1016/j.gene.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Landrum, L.R., 1981a. A monograph of the Genus Myrceugenia (Myrtaceae). New York Botanical Garden, Bronx, New York (Flora Neotropica Monographs 29).
- Landrum L.R. The phylogeny and geography of Myrceugenia (Myrtaceae) Brittonia. 1981;33:105–129. [Google Scholar]
- Librado P., Rozas J. DnaSP v5, A software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- Liston A., Robinson W.A., Oliphant J.M., Alvarez-Buylla E.R. Length variation in the nuclear ribosomal internal transcribed spacer region of non-flowering seed plants. Syst. Bot. 1996;21:109–120. [Google Scholar]
- Liu J.S., Schardl C.L. A conserved sequence in internal transcribed spacer 1 of plant nuclear rRNA genes. Plant Mol. Biol. 1994;21:109–120. doi: 10.1007/BF00013763. [DOI] [PubMed] [Google Scholar]
- Lucas E.J., Harris S., Mazine F., Belsham S., Lughadha E., Telford A., Gasson P., Chase M. Suprageneric phylogenetics of Myrteae, the generically richest tribe in Myrtaceae (Myrtales) Taxon. 2007;56:1105–1128. [Google Scholar]
- Marquínez X., Lohmann L.G., Faria-Salatino M.L., Salatino A., Gonza´lez F. Generic relationships and dating of lineages in Winteraceae based on nuclear (ITS) and plastid (rpS16 and psbA-trnH) sequence data. Mol. Phylogenet. Evol. 2009;53:435–449. doi: 10.1016/j.ympev.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Mathews D., Sabina J., Zuker M., Turner D.H. Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J. Mol. Biol. 1999;288:911–940. doi: 10.1006/jmbi.1999.2700. [DOI] [PubMed] [Google Scholar]
- McVaugh R. The genera of American Myrtaceae, an interim report. Taxon. 1968;17:354–418. [Google Scholar]
- Meudt H.M., Simpson B.B. The biogeography of the austral, subalpine genus Ourisia (Plantaginaceae) based on molecular phylogenetic evidence, South American origin and dispersal to New Zealand and Tasmania. Biol. J. Linn. Soc. 2006;8:479–513. [Google Scholar]
- Miwa H., Odrzykoski I.J., Matsui A., Hasegawa M., Akiyama H., Jia Y., Sabirov R., Takahashi H., Boufford D.E., Murakami N. Adaptive evolution of rbcL in Conocephalum (Hepaticae, bryophytes) Gene. 2009;441:169–175. doi: 10.1016/j.gene.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Murillo, J., 2011. Molecular Phylogeny and biogeographic analysis of Myrceugenia (Myrtaceae). Doctoral Dissertation, University of Concepción, Concepción.
- Nixon, K.C., 1999-2002. WinClada ver. 1.0000 Published by the author, Ithaca, New York.
- Nuin, P., 2007. MrMTgui. v 1.0. MrModelTest/ModelTest Graphical interface for Windows/Linux. <http://genedrift.org/mtgui.php>.
- Pascual R., Ortiz-Jaureguizar E., Prado J.L. Landmammals, paradigm for Cenozoic South American geobiotic evolution. Münch. Geowissen. Abhand. Reihe, A. 1996;30:265–319. [Google Scholar]
- Pigg K.B., Stockey R.A., Maxwell S.L. Paleomyrtinaea, a new genus of permineralized myrtaceous fruits and seeds from the Eocene of British Columbia and Paleocene of North Dakota. Can. J. Bot. 1993;71:1–9. [Google Scholar]
- Poole I., Hunt R.J., Cantrill D.J. A fossil wood flora from King George Island: ecological implications for an Antarctic Eocene vegetation. Ann. Bot. 2001;88:33–54. [Google Scholar]
- Poole I., Mennega A.M.W., Cantrill D.J. Valdivian ecosystems in the Late Cretaceous and Early Tertiary of Antarctica: further evidence from myrtaceous and eucryphiaceous fossil wood. Rev. Palaeobot. Palyn. 2003;124:9–27. [Google Scholar]
- Poole I., Cantrill D.J. Cretaceous and Cenozoic vegetation of Antarctica integrating the fossil wood record. Geol. Soc. London Spec. Publi. 2006;258:63–81. [Google Scholar]
- Posada D., Crandall K. Modeltest, testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Prámparo M., Quattrocchio M., Gandolfo M.A., Zamaloa M., Romero E. Historia evolutiva de las angiospermas (Cretácico-Paleogeno) en Argentina a través de los registros paleoflorísticos. Ameghiniana. 2007;11:157–172. [Google Scholar]
- Ragonese A.M. Leños fósiles de dicotiledóneas del Paleoceno de Patagonia, Argentina. I. Myrceugeniachubutiense n. sp. (Myrtaceae) Ameghiniana. 1980;17:297–311. [Google Scholar]
- Rambaut, A., Drummond, A.J., 2007. Tracer v1.4. <http://beast.bio.ed.ac.uk/Tracer>.
- Ritz C.M., Martins L., Mecklenburg R., Goremykin V., Hellwig F.H. The molecular phylogeny of Rebutia (Cactaceae) and its allies demonstrates the influence of paleogeography on the evolution of South American mountain cacti. Am. J. Bot. 2007;94:1321–1332. doi: 10.3732/ajb.94.8.1321. [DOI] [PubMed] [Google Scholar]
- Ronquist F., Huelsenbeck J.P. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Roig-Juñent S., Domínguez M.C., Flores G.E., Mattoni C. Biogeographic history of South American arid lands, a view from its arthropods using TASS analysis. J. Arid Environ. 2006;66:404–420. [Google Scholar]
- Ruiz E., Becerra J., Silva M., Crawford D.J., Stuessy T.F. Flavonoid chemistry of the endemic species of Myrceugenia (Myrtaceae) of the Juan Fernández Islands and relatives in continental South America. Brittonia. 1994;46:187–193. [Google Scholar]
- Ruiz E., Crawford D.J., Stuessy T.F., Gonzalez F., Samuel R., Becerra J., Silva O.M. Phylogenetic relationships and genetic divergence among endemic species of Berberis, Gunnera, Myrceugenia and Sophora of the Juan Fernandez Islands (Chile) and their continental progenitors based on isozymes and nrITS sequences. Taxon. 2004;53:321–332. [Google Scholar]
- Samuel R., Kathriarachchi H., Hoffmann P., Barfuss M.H.J., Wurdack K.J., Davis C.D., Chase M. Molecular phylogenetics of Phyllanthaceae, evidence from plastid matK and nuclear PHYC sequences. Am. J. Bot. 2005;92:132–141. doi: 10.3732/ajb.92.1.132. [DOI] [PubMed] [Google Scholar]
- Schultz J., Maisel S., Gerlach D., Müller T., Wolf M. A common core of secondary structure of the internal transcribed spacer 2 (ITS2) throughout the Eukaryota. RNA. 2005;11:361–364. doi: 10.1261/rna.7204505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbach A.E., Kadereit J.W. Rapid radiation of North American desert genera of the Papaveraceae Evidence from restriction site zapping of PCR-amplified chloroplast DNA fragments. Plant Syst. Evol. 1995;(Suppl. 9):159–170. [Google Scholar]
- Seibel P.N., Müller T., Dandekar T., Schultz J., Wolf M. 4SALE – a tool for synchronous RNA sequence and secondary structure alignment and editing. BMC Bioinform. 2006;7:498. doi: 10.1186/1471-2105-7-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibel P.N., Müller T., Dandekar T., Wolf M. Synchronous visual analysis and editing of RNA sequence and secondary structure alignments using 4SALE. BMC Res. Notes. 2008;1:91. doi: 10.1186/1756-0500-1-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J., Lickey E.B., Schilling E.E., Small R.L. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms, the tortoise and the hare III. Am. J. Bot. 2007;94:275–288. doi: 10.3732/ajb.94.3.275. [DOI] [PubMed] [Google Scholar]
- Simmons M.P., Ochoterena H. Gaps as characters in sequence based phylogenetic analyses. Syst. Biol. 2000;49:369–381. [PubMed] [Google Scholar]
- Sun Y., Skinner D., Liang G., Hulbert S. Phylogenetic analysis of Sorghum and related taxa using internal transcribed spacers of nuclear ribosomal DNA. Theor. Appl Genet. 1994;89:26–32. doi: 10.1007/BF00226978. [DOI] [PubMed] [Google Scholar]
- Swofford, D.I., 2002. PAUP∗. Phylogenetic Analysis Using Parsimony (∗and Other Methods) Version 4.0b10. Sinauer Associates, Inc. Publishers, Sunderland, Massachusetts.
- Sytsma K.J., Litt A., Zjhra M.L., Pires J.C., Nepokroeff M., Conti E., Walker J., Wilson P.G. Clades, clocks, and continents: historical and biogeographical analysis of Myrtaceae, Vochysiaceae, and relatives in the southern hemisphere. Int. J. Plant Sci. 2004;165:S85–S105. [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. MEGA4, Molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Thompson J.D., Gibson T., Lewniak F., Jeanmougin F., Higgins D. The ClustalX windows interface, flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl. Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippery N.P., Les D.H. Phylogenetic analysis of the internal transcribed spacer (ITS) region in Menyanthaceae using predicted secondary structure. Mol. Phylogenet. Evol. 2008;49:526–537. doi: 10.1016/j.ympev.2008.07.019. [DOI] [PubMed] [Google Scholar]
- Villagran C., Hinojosa L.F. Historia de los bosques del sur de Sudamérica, II: Análisis fitogeográfico. Rev. Chil. Hist. Nat. 1997;70:241–267. [Google Scholar]
- Villagrán, C., Hinojosa, L.F., 2005. Esquema biogeográfico de Chile, in: Llorente, J., Morrone, J.J. (Eds.), Regionalización Biogeográfica en Iberoámeríca y Tópicos Afines. Ediciones de la Universidad Nacional Autónoma de México, Jiménez Editores, México, pp. 551–577.
- Von Dohlen C.D., Moran N. Molecular data support a rapid radiation of aphids in the Cretaceous and multiple origins of host alternation. Biol. J. Linn. Soc. 2000;71:689–717. [Google Scholar]
- Wendel J., Doyle J. Phylogenetic incongruence, window into genome history and evolution. In: Soltis D., Soltis P., Doyle J., editors. Molecular Systematics of Plants II DNA Sequencing. Kluwer Academic Press; New York: 1998. pp. 265–296. [Google Scholar]
- Webb S.D. Biological implications of the Middle Miocene Amazon seaway. Science. 1995;269:361–362. doi: 10.1126/science.269.5222.361. [DOI] [PubMed] [Google Scholar]
- White T., Bruns T., Lee S., Taylor J. Amplification and direct sequencing of fungal ribosomal DNA genes for phylogenetics. In: Innis M., Gelfand D., Snrnsky J., White T., editors. PCR Protocols. Academic Press; New York: 1990. pp. 315–322. [Google Scholar]
- Wicke S., Quandt D. Universal primers for the amplification of the plastid trnK/matK region in land plants. Ann. Jard. Bot. Madrid. 2009;66:285–288. [Google Scholar]
- Wilf P., Johnson K.R., Cúneo N.R., Smith M.E., Singer B.S., Gandolfo M.A. Eocene plant diversity at Laguna del Hunco and Río Pichileufú, Patagonia, Argentina. Am. Nat. 2005;165:634–650. doi: 10.1086/430055. [DOI] [PubMed] [Google Scholar]
- Wright S.D., Yong C.G., Wichman S.R., Dawson J.W., Gardner R.C. Stepping stones to Hawaii: a transequatorial dispersal pathway for Metrosideros (Myrtaceae) inferred from nrDNA (ITS + ETS) J. Biogeogr. 2001;28:769–774. [Google Scholar]
- Xia X. Kluwer Academic Publishers; Boston: 2001. Data Analysis in Molecular Biology and Evolution. [Google Scholar]
- Xia X., Xie Z. DAMBE, Data analysis in molecular biology and evolution. J. Hered. 2001;92:371–373. doi: 10.1093/jhered/92.4.371. [DOI] [PubMed] [Google Scholar]
- Zucker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucl. Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]