Abstract
This study was aimed to synthesize polymeric excipients with improved mucoadhesive, cohesive and in situ-gelling properties to assure a prolonged retention time of dosage forms at a given target site, thereby achieving an increased uptake and improved oral bioavailability of certain challenging therapeutic agents such as peptides and proteins. Accordingly, poly(acrylic acid)-cysteine-2-mercaptonicotinic acid (PAA-cys-2MNA) conjugates were synthesized by the oxidative S–S coupling of PAA-cys (100-, 250- and 450 kDa) with 2-mercaptonicotinic acid (2MNA). Unmodified PAAs, PAAs-cys (thiomers) and PAA-cys-2MNA (100-, 250- and 450 kDa) conjugates were compressed into tablets to perform disintegration tests, mucoadhesion studies and rheological measurements. Moreover, cytotoxicty of the polymers was determined using Caco-2 cells. The resulting PAA-cys-2MNA (100-, 250- and 450 kDa) conjugates displayed 113.5 ± 12.7, 122.7 ± 12.2 and 117.3 ± 4.6 μmol/g of 2-mercaptonicotinic acid, respectively. Due to the immobilization of 2MNA, the PAA-cys-2MNA (pre-activated thiomers) conjugates exhibit comparatively higher swelling properties and disintegration time to the corresponding unmodified and thiolated polymers. On the rotating cylinder, tablets based on PAA-cys-2MNA (100-, 250- and 450 kDa) conjugates displayed 5.0-, 5.4- and 960-fold improved mucoadhesion time in comparison to the corresponding unmodified PAAs. Results achieved from tensile studies were found in good agreement with the results obtained by rotating cylinder method. The apparent viscosity of PAA-cys-2MNA (100-, 250- and 450 kDa) conjugates was improved 1.6-, 2.5- and 206.2-fold, respectively, in comparison to the corresponding unmodified PAAs. Moreover, pre-activated thiomers/mucin mixtures showed a time dependent increase in viscosity up to 24 h, leading to 7.0-, 18.9- and 2678-fold increased viscosity in comparison to unmodified PAAs (100-, 250- and 450 kDa), respectively. All polymers were found non-toxic over Caco-2 cells. Thus, on the basis of achieved results the pre-activated thiomers seem to represent a promising generation of mucoadhesive polymers which are safe to use for prolonged residence time of drug delivery systems to target various mucosa.
Keywords: Thiomers, Poly(acrylic acid)-cysteine, 2-Mercaptonictic acid, Pre-activated thiomers, Mucoadhesion, Oral drug delivery
1. Introduction
The concept of mucoadhesion and/or mucoadhesive polymers was emerged in pharmaceutical literature in the early 1980s as an interesting approach for controlled drug delivery. Mucoadhesive polymers are supposed to prolong drug action by increased contact time and residence time of the dosage form at the mucus membrane [1,2]. Extensive research efforts have been made in the past in order to evaluate and enhance adhesive properties of the existing natural polymers together with the development of novel mucoadhesive drug delivery systems. This led to the possibility to use mucoadhesive polymers to target several mucosal surfaces such as gastrointestinal, pulmonary, oropharyngeal, ocular, buccal, nasal, vaginal and rectal [3]. However, the research on mucoadhesives is still in its preliminary stage and further advances are needed for successful transformation of the academic research into commercial formulations.
Thiolated polymers (thiomers) belong to a unique generation of mucoadhesive polymers that was introduced in pharmaceutical literature in 1990s [4]. Unlike other mucoadhesive polymers, thiomers are capable of forming covalent bonds with cysteine-rich subdomains of mucus glycoproteins via thiol/disulfide exchange reactions [5]. These covalent bonds are supposed to be stronger than non- covalent interactions such as hydrogen bonds, van der Waal’s forces and ionic interactions of polymer with anionic substructures of the mucus layer [6]. Accordingly, thiomers in comparison to corresponding unmodified polymers provide strong adhesion which is sufficient to guarantee the localization of dosage form at a given target site for prolonged time. Thus, an enhanced oral bioavailability is expected resulting in many cases in a reduced dosing frequency and patient-compliance [7]. Thiomers exhibit furthermore enzyme inhibitory, permeation enhancing and efflux pump inhibitory properties [8]. Additionally, thiomers act as protective shield for incorporated drugs especially for peptides and proteins towards an enzymatic attack in the intestine [9]. Thus, all the mentioned advantages render thiomers as a highly suitable excipients for controlled drug delivery.
Apart from all these merits, thiomers are comparatively less stable in particular in solutions and gels as they are subject of thiol oxidation at pH ≥ 5 unless sealed under inert conditions. This too early oxidation of thiol groups before getting into contact with the mucus layer might deteriorate the interactions between thiomer and mucus layer thereby resulting in reduced efficacy of thiomers. Under these circumstances, the use of pre-activated thiol groups might be an interesting approach in order to enhance stability, mucoadhesion and cohesive properties of thiomers.
Accordingly, the overall objective of this study was to improve the stability of thiol groups towards oxidation as well as to enhance the mucoadhesive and cohesive properties of thiomers over a broader pH range. The concept of the preactivation of thiol groups is adapted from previous knowledge in covalent chromatography, where, for instance, peptides and proteins are very effectively quantitatively linked to thiol bearing resins, when they are pre-activated via pyridyl substructures [10]. Accordingly, 2-mercaptonicotinic acid (2MNA) was coupled to poly(acrylic) acid-cysteine (PAA-cys) conjugates via disulfide bonds formation. 2-Mercaptonicotinic acid was preferred over 2-pyridyl disulphide owing to the advantage of less toxic leaving group [11]. The resulting PAA-cys-2MNA conjugates or so-called pre-activated thiomers were characterized in terms of their thiol content, disulfide bonds, swelling behaviour, disintegration properties and increase in viscosity. Moreover, the influence of immobilized disulfide bonds was also evaluated on the mucoadhesive and cohesive properties of these preactivted thiomers.
2. Materials and methods
2.1. Materials
Poly(acrylic acid) (PAA) (100-, 250- and 450 kDa), 2-mercaptonicotinic acid (2MNA), hydrogen peroxide, l-cysteine hydrochloride, crude gastric mucin from porcine stomach (Type II), reduced glutathione (GSH) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDAC) were purchased from Sigma–Aldrich. Resazurin sodium salt powder was purchased from Acros Organic NV. All other reagents used were of analytical grade.
2.2. Synthesis of poly(acrylic acid)-cysteine conjugates
PAA-cysteine conjugates (PAA-cys; 100-, 250- and 450 kDa) were synthesized by the covalent attachment of cysteine to poly(acrylic acid) (PAA) according to a method described previously [12]. Briefly, one gram each of PAA (100-, 250- and 450 kDa) was hydrated separately in demineralized water and the pH value of the PAA solutions was adjusted to 6 by the addition of 5 m NaOH. Then, EDAC in the final concentration of 200 mm was added in order to activate the carboxylic acid moieties of each of the hydrated polymers. After 20 min of incubation under stirring at room temperature, 1 g of l-cysteine HCl was added to each of the hydrated PAA solution and the pH was adjusted to 6. Reaction mixtures were incubated for 3 h at room temperature under stirring. Neutralized polymers PAA (100-, 250- and 450 kDa) prepared in the same way as the PAA-cys conjugates but omitting EDAC during coupling reaction were served as references.
2.3. Purification
In order to eliminate unbound reacting species from the polymers, each of the above reaction mixtures was dialyzed five times using Spectra/Por® 3 membrane (MWCO: 1200) at (low acidic) pH ∼ 3 for 3 days in total at 10 °C in the dark against 5 mm HCl, then two times against the same medium but containing 1% NaCl. Then the samples were dialyzed exhaustively two times against 1 mm HCl. After dialysis the pH of PAA-cysteine (100-, 250- and 450 kDa) conjugates was readjusted to 6. Thereafter, the dialyzed products were freeze-dried for 3 days at −80 °C under reduce pressure and stored at 4 °C until use.
2.4. Synthesis of poly(acrylic acid)-cysteine-2-mercaptonicotinic acid (100-, 250- and 450 kDa)
In order to get 2MNA modified polymers, 2MNA dimer (2,2′-dithiodinicotinic acid) was first prepared by oxidation of 2MNA with hydrogen peroxide under neutral pH conditions. Briefly, 4 g of 2MNA were accurately weighed and dispersed in 50 mL of demineralized water by ultrasoncation. After 30 min, pH of the dispersed solution was adjusted to 7 – 8 using 5 m NaOH and a slight yellowish clear solution was obtained. An aliquot of 5.3 mL of hydrogen peroxide (30%, w/v) was added drop-wise and pH was maintained at 8 – 9 till a colorless solution (2MNA dimer; 2,2′-dithiodinicotinic acid) was obtained. The resulting colorless 2MNA dimer solution was further stirred at room temperature for 1 h and diluted to final volume of 100 mL. One gram each of PAA-cys (100-, 250- and 450 kDa) was hydrated separately in 100 mL of demineralized water. An aliquot of 1 mL of 2MNA solution was added drop-wise to each hydrated thiomer solution and pH was adjusted to 7–8 using 5 m NaOH. The reaction mixtures were incubated for over night at room temperature under continuous stirring. Each of the above three reaction mixtures were dialyzed using Spectra/Por® 3 membrane (MWCO: 1200) in 5 L of demineralized water for 3 days in total at 10 °C in the dark. Thereafter, the dialyzed products were freeze-dried for 3 days at −80 °C under reduce pressure and stored at 4 °C until use.
2.5. Determination of the thiol group content
The amount of thiol groups immobilized on the polymer conjugates was determined spectrophotometrically using Ellman’s reagent as described previously. l-Cysteine HCl was employed to establish calibration curve for all polymer conjugates [4].
2.6. Disulfide bond test
Disulfide bond test was performed firstly to quantify disulfide bonds [4] formed due to oxidation during the thiolation process and secondly to quantify disulfide bonds formed between thiomers and 2MNA.
2.7. Quantification of conjugated 2-mercaptonicotinic acid
The amount of conjugated 2-mercaptonicotinic acid (2MNA) was determined spectrophotometrically. Briefly, 0.5 mg of each of the PAA-cys-2MNA (100-, 250- and 450 kDa) was hydrated in 0.05 m phosphate buffer (pH 6.8) with 2% reduced glutathione. After 60 min of incubation at room temperature, absorbance of 300 μL solution was measured at 354 nm 2MNA was employed to establish calibration curve for all polymer conjugates.
2.8. Preparation of tablets
Lyophilized PAA-cys-2MNA (100-, 250- and 450 kDa) conjugates, corresponding PAAs-cys and the unmodified PAAs were compressed (Hanseaten Type EI, Hamburg, Germany) into 30 mg (5.0-mm diameter, 1.2-mm thickness) flat-faced tablets by applying a constant compaction pressure of 10 kN during the preparation all tablets.
2.9. Hardness of polymer tablets
Hardness of the tablets based on PAA-cys-2MNA (100-, 250- and 450 kDa) conjugates, corresponding PAAs-cys and the unmodified PAAs was determined using a Schleuniger 2-E/205 tablet-hardness tester (Dr. K. Schleuniger and Co., Switzerland).
2.10. Evaluation of the swelling behaviour
The water absorbing capacity of PAA-cys-2MNA (100-, 250- and 450 kDa), corresponding thiomers and the unmodified polymers was determined in simulated gastric fluid (0.1 m HCl, pH 1.2) as well as in simulated intestinal fluid (0.1 m phosphate buffer, pH 6.8) by a gravimetric method as described previously [13]. Briefly, polymer tablets were accurately weighed and fixed on the tip of a needle by applying a little force with thumb. The tablets were then placed in a beaker containing either 50 ml of 0.1 m HCl or 0.1 m phosphate buffer at 37 °C. At various time intervals hydrated tablets on the needle were taken out of the incubation medium and excess water was removed by gentle soaking with a tissue paper. The tablets were weighed again and the amount (%) of water uptake was determined gravimetrically as follows:
Where, Wt is the weight of the swollen polymer at a given time and W0 is the weight in the dry state.
2.11. Disintegration studies
Disintegration behaviour of the tablets comprising PAA-cys-2MNA (100-, 250- and 450 kDa), corresponding thiomers and the unmodified lyophilized polymers was evaluated in 100 mm phosphate buffer pH 6.8 at 37 ± 0.5 °C with a disintegration test apparatus according to the European Pharmacopoeia. The oscillating frequency was adjusted to 0.5 s−1 [14].
2.12. In vitro mucoadhesion studies
2.12.1. Rotating cylinder method
The mucoadhesion time of PAA-cys-2MNA (100-, 250- and 450 kDa), corresponding thiomers and the unmodified polymers was determined according to a method as described previously [12]. Briefly, freshly excised porcine intestine was provided from a local slaughter house. After necessary cleaning, the combined jejunum and ileum segment of intestine was cut into small pieces (approx. 12 cm2) without any discrimination. The intestinal mucosa was then fixed on a stainless steel cylinder (diameter: 4.4 cm; height: 5.1 cm; apparatus 4-cylinder) using a cynoacrylate adhesive glue (Henkel KGaA, Austria) and polymer tablets were attached to it. The cylinders were placed in the dissolution apparatus according to the European Pharmacopoeia containing 900 mL of 100 mm phosphate buffer pH 6.8 at 37 ± 0.5 °C. The fully immersed cylinders were agitated with 100 rpm. The detachment of the test tablets was monitored during an observation period of 24 h.
2.12.2. Tensile studies
Tensile studies were performed using excised porcine intestinal mucosa according to a method as described previously [15]. Briefly, polymer tablet was glued to a stainless steel flat disc (10 mm in diameteter), which was hung from a laboratory stand with a nylon thread (15 cm). The porcine mucosa was fixed to a glass platform using a cyanoacrylate adhesive and was placed in a beaker filled with 100 mm phosphate buffer (pH 6.8). The beaker was placed on a balance, and then carefully raised by a mobile platform until the mucus came in contact with the tablet. The contact was determined when the nylon thread holding the tablet became bent. After 20 min incubation at 25 °C, the mucosa was pulled down from the tablet at a rate of 0.1 mm/s by manual turning of regulating knob provided with the mobile platform. The regulating knob was precisely marked and validated via length measurements to ensure the exact displacement of 0.1 mm at each turn. Data points were collected every second by computer software (SartoCollect V 1.0; Satorius AG, Germany) linked to the balance with integrated interface. Data was transferred to EXCEL 2007 (Microsoft, USA) and the force versus displacement curves were analyzed to calculate the maximum force of detachment (MDF) and the total work of adhesion as the area under the curve (AUC) in accordance with the trapezoidal rule.
2.13. Cytotoxicity screening-resazurin assay
Resazurin assay was performed on Caco-2 cells to determine the in-vitro cytotoxicity of the polymers. Briefly, Caco-2 cells (d = 1 × 105 cells/well) were cultured in a 24-well plate in a final volume of 0.5 mL MEM (pH 7.4) and incubated at 37 °C in 5% CO2 environment. The medium was replaced with fresh medium every second day. After 2 weeks, the cultured cells were washed twice with 1x PBS (pre-warmed at 37 °C). Test solutions (0.5%; m/v), negative control (MEM with out phenol red) and positive control (4% (v/v) Titron X-100) were added in triplicate to the cell culture in 0.5 mL quantity. The cells with medium were incubated again at 37 °C in 5% CO2 environment for 3-, 24- or 72 h, respectively. After incubation solutions were removed and cells were washed twice with 1x PBS. An aliquot of 250 μL of a 2.2 μm resazurin solution was added to each well and cells were incubated at 37 °C in a 5% CO2 for 3 h. The fluorescence of the supernatant was measured at a wavelength of 540 nm with background subtraction at 590 nm with Tecan infinite M200 spectrophotometer, Grödig, Austria [16].
2.14. Measurement of the apparent viscosity of polymers
PAA-cys-2MNA (100-, 250- and 450 kDa), corresponding thiomers and the unmodified polymers were hydrated in 0.1 m phosphate buffer pH 6.8 at 37 °C to give final concentration of 2% (m/v). After an incubation of 30 min, aliquots of 1000 μL were transferred to a cone-plate viscometer (RotoVisco RT20, Haake, Karlsruhe, Germany) for rheological measurements. The apparent viscosity (η) was determined at a shear rate of 50 s−1.
2.15. Measurement of the apparent viscosity of polymer/mucin mixtures
Porcine gastric mucin (4 g) was hydrated in 25 ml of demineralized water under continuous stirring at 4 °C overnight. The mucin solution was adjusted to pH 6.8 with 1 m NaOH and diluted to a final volume of 50 mL with 0.1 m phosphate buffer pH 6.8. The mucin stock solution (8% m/v) was stored at 4 °C no longer than 24 h. PAA-cys-2MNA (100-, 250- and 450 kDa), corresponding thiomers and the unmodified polymers were hydrated in demineralized water to give a concentration of 4% (m/v). The polymer solutions were added to an equal volume of mucin stock solution, mixed with a spatula and the pH of the mixture was adjusted to 6.8 with 2 m NaOH. After incubation for 0.5 h, 6 h and 24 h at 37 °C, 1 mL of polymer/mucin incubates were transferred to a cone-plate viscometer (RotoVisco RT20, Haake GmbH, Karlsruhe, Germany) for rheological measurements. The apparent viscosity of the polymer/mucin mixtures (η) was determined as described above [14].
2.16. Statistical data analysis
Statistical data analysis was performed using the student t-test with p < 0.05 as the minimal level of significance. All values were expressed as the means ± S.D.
3. Results and discussion
3.1. Synthesis and characterization of thiolated polymers
Synthesis of poly(acrylic acid-cysteine (PAA-cys) has already been described by our research group previously [12]. Briefly, l-cysteine was covalently attached to PAA (100-, 250- and 450 kDa) by the formation of amide bonds between carboxylic acid groups of respective PAAs and primary amino groups of l-cysteine. The carboxylic acid moieties of PAAs were activated using EDAC prior to the coupling reaction. Determination of the thiol groups attached to the polymers by the Ellman’s test demonstrated that on average 18.67 ± 1.81, 15.73 ± 4.40 and 16.82 ± 2.19 mmol of thiol groups were immobilized per mole of acrylic acid monomer as in the case of PAA-cys (100-, 250-, 450 kDa) conjugates, respectively. Inter and/or intra molecular disulfide bonds were 1.47 ± 0.39 mmol per mole of acrylic acid monomer as in the case of PAA-cys (100-, 250-, 450 kDa) conjugates, respectively. The low percentage of disulfide bonds indicated low extent of oxidation during the coupling reaction thus demonstrating efficiency of the thiolation step. The efficacy of the purification method for the resulting PAA-cys conjugates was verified by controls which were prepared in exactly the same way but omitting EDAC during the coupling reaction, exhibiting 4.00 ± 2.00 μmol of thiol groups per gram of the control polymers. The obtained polymers appeared as white, odourless powder of fibrous structure. For all experiments, the fibrous structured lyophilizates were used and no pulverization of the products was carried out. The modified polymers were easily soluble in aqueous solution. The lyophilized conjugates were stored at 4 °C and found stable towards air oxidation during the course of the study.
3.2. Synthesis and characterization of PAA-cys-2MNA (100-, 250- and 450 kDa)
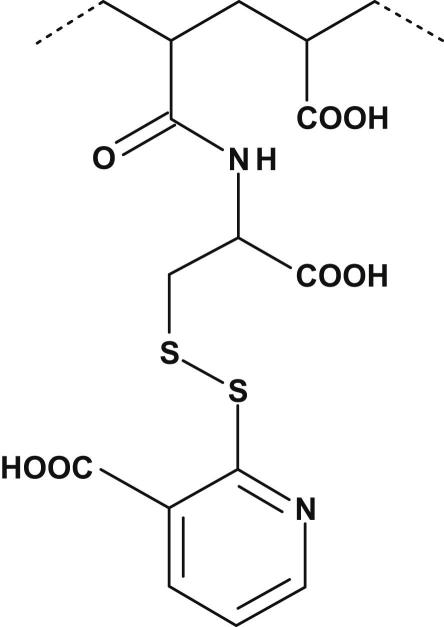
Synthesis of PAA-cys-2MNA (100-, 250- and 450 kDa) conjugates was achieved by coupling of PAA-cys (100-, 250-, and 450 kDa) to 2-mercaptonicotinic acid dimer (2,2′-dithiodinicotinic acid) via formation of disulfide bonds between thiol functions of PAA-cys with thiol groups of 2MNA dimer. The presumptive chemical substructure of PAA-cys-2MNA (100-, 250- and 450 kDa) conjugates is illustrated in Fig. 1. The lyophilized PAA-cys-2MNA conjugates were appeared as off-white powder of fibrous structure, odourless and easily soluble in aqueous solution. The resulting PAA-cys-2MNA (100-, 250- and 450 kDa) conjugates were found to exhibit 113.5 ± 15.7, 122.7 ± 12.2 and 117.3 ± 14.6 μmol/g of 2-mercaptonicotinic acid, respectively. Moreover, the inter- and/or intramolecular disulfide bonds were found 160 ± 40 μmol/g. This increment in the percentage of disulfide bonds indicated efficiency of oxidation in the presence of 2MNA dimer. Several methodologies and different experimental systems are currently in practice for the oxidative conversion of thiols to disulfides. Some are rather time consuming and associated with certain problems such as toxicity of reagents, limited availability and instability of oxidizing agents, require specific reaction conditions, formation of undesirable side products and difficulties in product isolation [17–19]. These problems were avoided by the synthesis of 2MNA dimer. The proposed mechanisms lying behind the oxidative coupling of thiol groups seems to be based on: 1) the conversion of 2MNA into activated oxidant form (2MNA dimer; 2,2′-dithiodinicotinic acid) after oxidation in the presence of hydrogen peroxide, 2) covalently coupling of 2,2′-dithiodinicotinic acid to free thiol (R1–SH) belongs to poly(acrylic acid)-cysteine (PAA-Cys) conjugates and removal of free 2MNA molecule, 3) covalently coupling of remaining free thiols (R2–SH) belongs to PAA-Cys to the free 2MNA molecule.
Fig. 1.
Presumptive chemical substructure of poly(acrylic acid)-cysteine-2-mercaptonicotinic acid (PAA-cys-2MNA) conjugates. 2-Mercaptonicotinic acid (2MNA) was covalently coupled to poly(acrylic acid)-cysteine (PAA-cys) conjugates by the oxidative S–S coupling of PAA-cys (100-, 250- and 450 kDa) with 2-mercaptonicotinic acid (2MNA).
3.3. Hardness of polymer tablets
A certain extend of hardness (strength) is a prerequisite for tablets to endure mechanical stress during its fabrication, packaging and transportation. Moreover, tablet hardness is believed to be an important parameter that influences disintegration, swelling and release properties [20]. Therefore, hardness of the tablets based on PAA-cys-2MNA (100-, 250- and 450 kDa) conjugates, corresponding thiomers and unmodified polymers was determined. Results are reported in Table 1 indicating that the hardness of the tablets was found to be increases by increasing the average molecular weight of the polymer. Moreover, tablets based on unmodified, thiolated and pre-activated thiomers showed different hardness although the same force (10 kN) was applied to compress these tablets. For instance, covalent attachment of cysteine to PAAs (100-, 250- and 450 kDa) considerably enhanced the crosslinking properties and the hardness of the tablets was increased 2.1-, 2.4- and 1.2- fold, respectively. A more pronounced effect was even observed in the presence of pre-activated thiomers i.e. PAA-cys-2MNA (100-, 250- and 450 kDa) based tablets where the hardness was improved 3.0-, 3.3- and 1.4-fold, respectively. These observations might be explained by the particulate nature of 2MNA together with higher degree of inter- and/or intramolecular disulfide bonds within PAA-cys-2MNA (100-, 250- and 450 kDa) conjugates being responsible for increased stability and hardness of PAA-cys-2MNA (100-, 250- and 450 kDa) conjugates.
Table 1.
Comparison of the total work of adhesion (TWA), maximum detachment force (MDF) and improvement ratios (indicated polymer/corresponding unmodified polymer) of 30 mg tablets consisting of PAA-cysteine (100-, 250- and 450 kDa) and PAA-cys-2MNA (100-, 250- and 450 kDa) in comparison to corresponding unmodified polymers. Indicated values are mean ± S.D. of at least three experiments.
| Tested polymers | TWA (μJ) | Improvement ratio | MDF (mN) | Improvement ratio |
|---|---|---|---|---|
| unmodified PAA (100 kDa) | 25.7 ± 3.0 | – | 18.8 ± 3.0 | – |
| PAA-cysteine (100 kDa) | 75.4 ± 9.5 | 2.9 | 102.1 ± 6.4 | 5.4 |
| PAA-cysteine-2MNA (100 kDa) | 152.2 ± 44.3 | 5.9 | 129.1 ± 2.0 | 6.8 |
| unmodified PAA (250 kDa) | 47.3 ± 7.9 | – | 26.6 ± 8.1 | – |
| PAA-cysteine (250 kDa) | 98.5 ± 3.9 | 2.1 | 111.6 ± 6.3 | 4.2 |
| PAA-cysteine-2MNA (250 kDa) | 189.5 ± 7.5 | 4.0 | 144.8 ± 39.5 | 5.4 |
| unmodified PAA (450 kDa) | 106.9 ± 13.2 | – | 92.7 ± 19.3 | – |
| PAA-cysteine (450 kDa) | 202.2 ± 24.3 | 1.9 | 131.1 ± 2.0 | 1.4 |
| PAA-cysteine-2MNA (450 kDa) | 325.7 ± 36.6 | 3.0 | 233.8 ± 8.7 | 2.5 |
3.4. Swelling behaviour
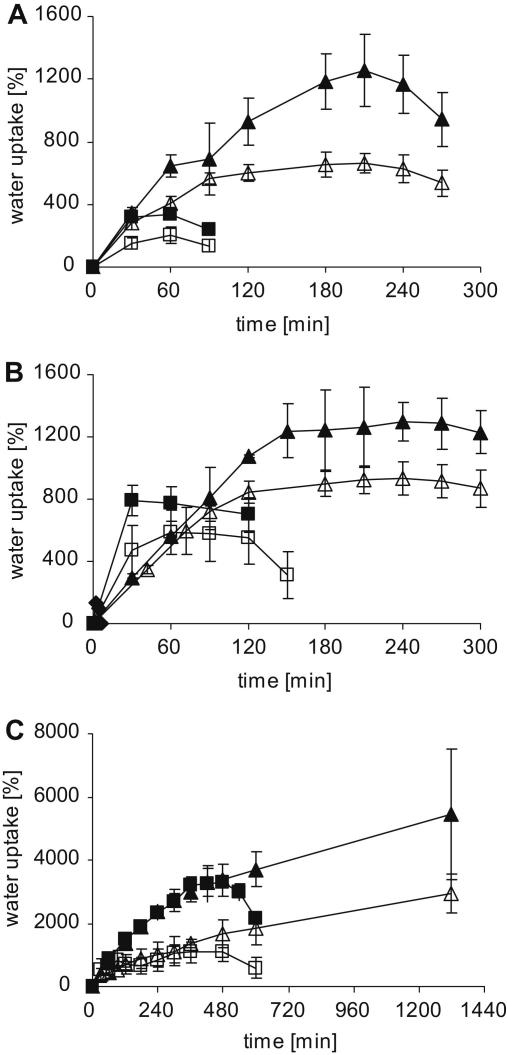
The swelling behaviour of mucoadhesive polymers has a great impact on their stability, release of embedded drugs, adhesive properties and cohesiveness [21,22]. After attachment with the mucus layer, mucoadhesive polymers swell up by initiating water absorption from the underlying mucosal tissue via capillary action and diffusion process which leads to a considerably strong adhesion. In contrast, excessive water uptake results in over swelling, constrained cohesive properties and resulted in the failure of mucoadhesive bond between the polymer and the mucus membrane [23]. Therefore, moderate swelling is necessary to avoid overhydration and loss of adhesive properties before the delivery system reaches the target. In order to evaluate swelling behaviour, water uptake studies were carried out in 0.1 m HCl (pH 1.2) and 0.1 m phosphate buffer (pH 6.8) at 37 °C, respectively. Water uptake studies were performed with 30 mg (5.0 - mm diameter) flat-faced tablets based on unmodified PAAs (100-, 250- and 450 kDa), PAA-cys (100-, 250- and 450 kDa) and pre-activated thiomers i.e. PAA-cys-2MNA (100-, 250- and 450 kDa) conjugates.Results are shown in Fig. 2. Unmodified PAA (100-, 250- and 450 kDa) displayed poor water uptake and tablets were completely dissolved and/or eroded within 6-, 10- and 30 min, respectively (data not shown). The swelling behaviour of tablets based on thiolated and pre-activated polymers seem to be strongly dependent on the molecular mass and pH. For instance, tablets based on high molecular mass (450 kDa) polymers showed enhanced water absorbing capacity in comparison to the tablets based on low molecular mass (100- and 250 kDa) polymers. Similarly, all tablets showed more weight gain in the presence of 0.1 m phosphate buffer (pH 6.8). The presence of comparatively higher quantity of ionic substructures within less acidic polymers is supposed to be responsible for improved water uptake at elevated pH. Furthermore, it was observed that the covalent attachment of cysteine to PAAs (100-, 250- and 450 kDa) significantly enhanced the swelling properties as well as stability of these polymers, leading to 4.7-/7.9-, 17.3-/23.3-/and 37.1-/109.7-fold weight gain in the presence of 0.1 m HCl (pH 1.2) and 0.1 m phosphate buffer (pH 6.8), respectively. A more pronounced effect was even observed in the presence of pre-activated thiomers i.e. PAA-cys-2MNA (100-, 250- and 450 kDa) conjugates where the initial weight was improved 22.1-/41.8-, 31.1/43.2- and 98.3-/182.2-fold, respectively. These observations might be explained by the fact that lipophilic nature of 2MNA together with higher degree of inter- and/or intramolecular disulfide bonds within PAA-cys-2MNA (100-, 250- and 450 kDa) conjugates is responsible for increased stability, cohesiveness and swelling capacity of PAA-cys-2MNA (100-, 250- and 450 kDa) conjugates.
Fig. 2.
Swelling behaviour of A: PAA-cys (100 kDa) (■, □), PAA-cys-2MNA (100 kDa) (▴, ▵); B: PAA-cys (250 kDa) (■, □), PAA-cys-2MNA (250 kDa) ((▴, ▵); C: PAA-cys (450 kDa) (■, □), PAA-cys-2MNA (450 kDa) (▴, ▵). Water uptake studies were carried out in simulated gastric fluid (0.1 m HCl, pH 1.2) (white symbols) as well as in simulated intestinal fluid (0.1 m phosphate buffer, pH 6.8) (black symbols) at 37 °C. All indicated values represent an average of at least three experiments (±S.D.).
3.5. Disintegration studies
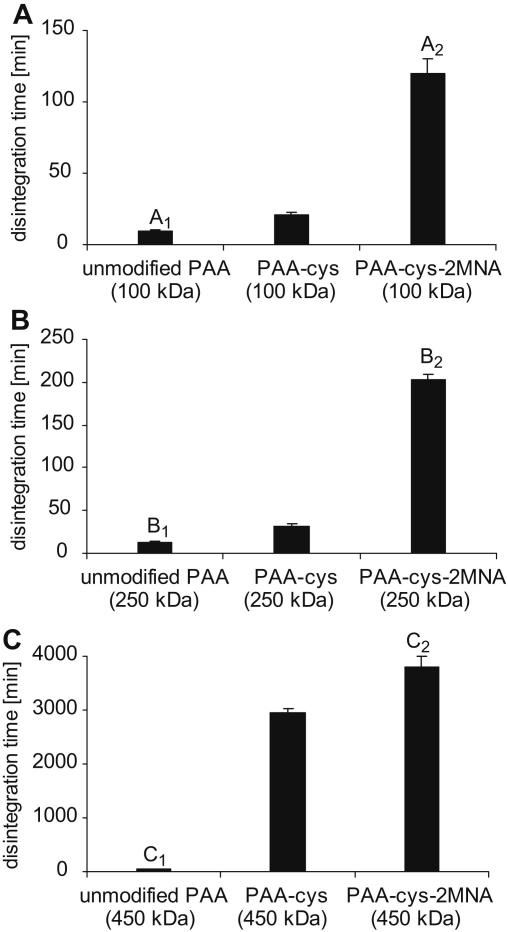
Disintegration studies revealed that disintegration time increased with increasing molecular mass of the polymers as the difference in disintegration time between unmodified, thiolated and pre-activated polymers become more pronounced with increasing molecular mass. Results are shown in Fig. 3. As expected, tablets comprising unmodified PAA (100-, 250- and 450 kDa) were completely disintegrated with in 8-, 12- and 55 min, respectively. It can be seen that thiolation process greatly improves the crosslinking properties of polymers. As reported earlier, tablets comprising PAA-cysteine (100-, 250- and 450 kDa) in comparison to the tablets comprising corresponding unmodified polymers displayed 2.6-, 3.1- and 53.8-fold improved disintegration time [14,15]. Moreover, the covalent attachment of 2-mercaptonicotinic acid (2MNA) to PAAs-cys significantly enhanced the swelling properties and stability of these thiomers and 15-, 19.7- and 69.1-fold prolonged disintegration times were achieved in the presence of PAA-cys-2MNA (100-, 250- and 450 kDa) conjugates, respectively. The results obtained from disintegration studies are in accordance with the theory that formation of covalent bonds (disulfide bonds) within polymer structure resulted in enhanced stability and cohesion [11]. Thus, it is believed that the lipophilic nature of 2MNA in combination with inter- and/or intramolecular disulfide bonds within PAA-cys-2MNA (100-, 250- and 450 kDa) conjugates is responsible for increased stability and prolong disintegration time.
Fig. 3.
Comparison of the disintegration behaviour of unmodified, thiolated and pre-activated poly acrylate tablets (30 mg) with an average molecular mass of A: 100 kDa; B: 250 kDa and C: 450 kDa. Studies were performed with a disintegration apparatus according to the European Pharmacopoeia in 100 mm phosphate buffer pH 6.8 at 37 °C. The indicated disintegration time represents an average of at least three experiments (±S.D.). A1 differs from A2p = 0.0024; B1 differs from B2p = 0.0002; C1 differs from C2p = 0.001.
3.6. Cytotoxicity screening-resazurin assay
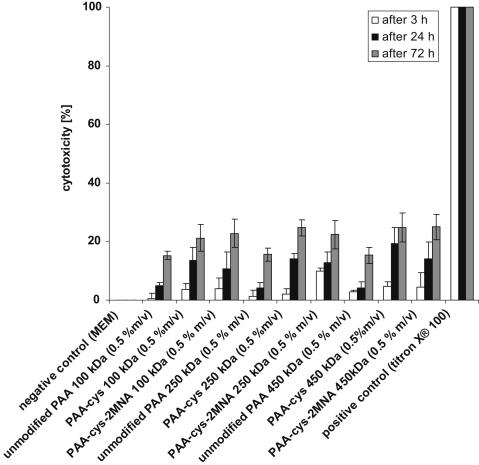
Caco-2 cells were incubated with PAA-cys-2MNA (100-, 250- and 450 kDa), PAA-cys (100-, 250- and 450 kDa) and corresponding unmodified PAAs either for 3-, 24- or 72 h in order to evaluate their in-vitro cytotoxic effect. Results are shown in Fig. 4. Cell viability of more than 90%, 85% and 75% in the presence of PAA-cys-2MNA (100-, 250- and 450 kDa) after 3-, 24- and 72 h respectively, indicating that these polymers are not at all harmful for the cells. All polymers were tested in a final concentration of (0.5%; m/v) as concentrations higher than 0.5% (m/v) led to a too high viscosity of the sample. Moreover, the chosen concentration allows comparing cytotoxicity of the pre-activated polymers to that of well-established thiomers.
Fig. 4.
Cytotoxicity potential of poly(acrylic acid)-cysteine-2-mercaptonicotinic acid (PAA-cys-2MNA) conjugates in comparison to respective thiomers and corresponding unmodified polymers. Cytotoxicity was tested on CaCo-2 cells using resazurin assay and expressed in percent. Indicated values are the means of at least three experiments (±S.D.).
3.7. In vitro mucoadhesion studies
Mucoadhesion studies were performed in order to evaluate on one hand the influence of covalent attachment of 2MNA to PAA-cys (100-, 250- and 450 kDa) conjugates and on the other hand the influence of molecular mass using two different test methods.
3.7.1. Rotating cylinder method
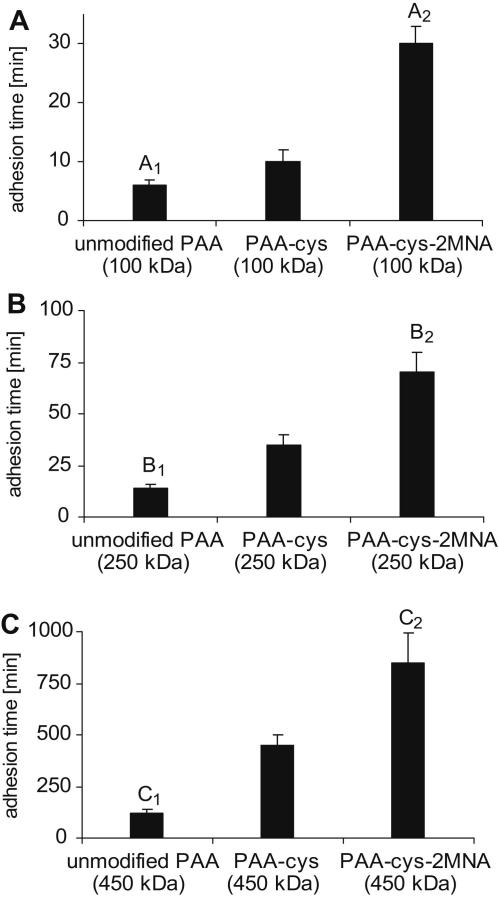
Adhesion studies were performed utilizing jejunum and ileum sections of freshly excised porcine intestinal mucosa thereby obtaining comparable results in both cases. The presence of lymphoid aggregates (peyer’s patches) is not supposed to have any influence on mucoadhesion, as the phenomenon of mucoadhesion is believed to be based on the associative interections between mucoahesive polymers and mucus gel layer. Results obtained by rotating cylinder method are illustrated in Fig. 5. Adhesion studies revealed that immobilization of thiol groups on PAA (100-, 250- and 450- kDa) resulted in 1.7-, 2.5- and 452-fold improved mucoadhesive properties, respectively. Furthermore, due to the incorporation of hydrophobic ligand 2MNA to PAAs-cys polymeric backbone, the mucoadhesive properties of these thiolated polymers were significantly improved. On the rotating cylinder, tablets based on PAA-cys-2MNA (100-, 250- and 450 kDa) conjugates displayed 5.0-, 5.4- and 960-fold improved mucoadhesion time in comparison to the corresponding unmodified PAAs.
Fig. 5.
Comparison of the mucoadhesive properties of unmodified, thiolated and pre-activated poly acrylates with an average molecular mass of A: 100 kDa; B: 250 kDa and C: 450 kDa as determined by the rotating cylinder method. Thirty milligrams polymer tablets were attached to excised porcine intestinal mucosa, which was spanned on a vertical cylinder rotated with 100 rpm in 100 mm phosphate buffer pH 6.8 at 37 °C. The indicated time of adhesion represents an average of at least three experiments (±S.D.). A1 differs from A2p = 0.0023; B1 differs from B2p = 0.0067; C1 differs from C2p = 0.0145.
3.7.2. Tensile studies
Tensile studies were performed on porcine intestinal mucosa in order to confirm adhesion results obtained by rotating cylinder method. Results being illustrated in Table 1 were found in good agreement with the results obtained by rotating cylinder method. In case of tensile studies the total work of adhesion (TWA) was found in good correlation with the maximum detachment force (MDF). Several factors are supposed to be responsible for enhanced mucoadhesive property of PAA-cys-2MNA (100-, 250- and 450 kDa) conjugates. For instance, more active thiol groups are available in the form of disulfide bonds which can interact with mucus and lead to high cohesiveness to the polymer carrier, presence of disulfide bonds prevent early oxidation of thiol groups before coming into contact with the mucus gel layer, incorporation of hydrophobic ligand decreases the dispersive properties of polymeric backbone thus provide more stability thereby resulting in a prolonged residence time of the dosage form to the mucosal membrane.
3.8. Measurement of the apparent viscosity of polymers
The viscosity of 2% (m/v) PAA-cys-2MNA (100-, 250- and 450 kDa), corresponding thiomers and the respective unmodified PAAs in 100 mm phosphate buffer (pH 6.8) was measured in order to confirm the results obtained from disintegration studies. Results are shown in Table 2 (a). Unmodified control PAAs exhibit least apparent viscosity due to the lack of ability to form intramolecular bonds. In contrast, PAA-cys (100-, 250 and 450 kDa) conjugates due to their ability to form intramolecular disulfide bonds at physiological pH, led to enhanced apparent viscosity up to 1.6-, 2.5- and 206.2- fold, respectively. This increase was found even more significant in the presence of pre-activated PAA-cys-2MNA (100-, 250- and 450 kDa) conjugates where the improvement in apparent viscosity was 5.0-, 7.2- and 477-fold, respectively. Hence, the achieved results confirmed the hypotheses that increase in viscosity proportionally correlates with the molecular mass and amount of sulfhydryl moieties on the polymer [15].
Table 2.
Comparison of the apparent viscocity (η) of PAA-cysteine (100-, 250- and 450 kDa) and PAA-cys-2MNA (100-, 250- and 450 kDa) in comparison to corresponding unmodified polymers. Indicated values are mean ± S.D. of at least three experiments.
| Tested polymers | Apparent viscosity [η (mPas)] |
|||
|---|---|---|---|---|
| Without mucin (a) |
With mucin (b) |
|||
| At time point “0” | After 0.5 h | After 6 h | After 24 h | |
| unmodified PAA (100 kDa) | 10 ± 4 | 10 ± 4 | 10 ± 2 | 10 ± 2 |
| PAA-cysteine (100 kDa) | 16 ± 4 | 20 ± 5 | 20 ± 2 | 30 ± 5 |
| PAA-cysteine-2MNA (100 kDa) | 50 ± 10 | 50 ± 10 | 55 ± 10 | 70 ± 10 |
| unmodified PAA (250 kDa) | 12 ± 4 | 24 ± 2 | 23 ± 3 | 23 ± 4 |
| PAA-cysteine (250 kDa) | 27 ± 5 | 50 ± 30 | 70 ± 10 | 140 ± 30 |
| PAA-cysteine-2MNA (250 kDa) | 80 ± 10 | 287 ± 49 | 318 ± 69 | 434 ± 69 |
| unmodified PAA (450 kDa) | 26 ± 2 | 63 ± 10 | 61 ± 2 | 62 ± 2 |
| PAA-cysteine (450 kDa) | 6020 ± 281 | 7982 ± 405 | 17027 ± 719 | 19458 ± 1852 |
| PAA-cysteine-2MNA (450 kDa) | 12860 ± 400 | 35870 ± 1942 | 58872 ± 3227 | 166033 ± 24017 |
3.9. Measurement of the apparent viscosity of polymer/mucin mixtures
The apparent viscosity of polymer/mucin blend is supposed to be a resultant of certain associations such as chain entanglements, conformational changes and non-covalent interactions. The same properties are believed to be responsible for the mucoadhesion phenomenon, as well. Thus, viscosity measurements can be used as an index to evaluate mucoadhesive bond strength [24,25]. Accordingly, the apparent viscosities of PAA-cys-2MNA (100-, 250- and 450 kDa), corresponding thiomers and the corresponding unmodified PAAs were determined in the presence of mucin at predetermined time intervals. Results are shown in Table 2 (b). Viscosity measurements were done in the presence commercially available mucin instead of natural mucin in order to achieve better reproducibility and comparability [14]. As anticipated, polymers with higher molecular mass exhibit higher viscosity. Unmodified PAAs were found to have no interaction with mucin as their viscosity was remain constant over a time period of 24 h. PAA-cys (100-, 250- and 450 kDa) showed time dependent increase in viscosity due to associative interactions with mucus glycoproteins and gradual oxidation of thiol moieties. Mucin mixtures with pre-activated thiomers i.e. PAA-cys-2MNA (100-, 250- and 450 kDa) displayed a more pronounced time dependent increase in viscosity in comparison to respective PAA-cys and corresponding unmodified PAA. The increase is most likely due to the efficient physico-chemical interactions between the mucin and the PAA-cys-2MNA (100-, 250- or 450 kDa) resulting in 7.0-, 18.9- and 2678-fold enhanced viscosity in comparison to unmodified PAAs, respectively.
It is believed that the pre-activated thiomers can interact with mucin glycoproteins in many different ways resulting in very high viscosity of pre-activated polymer/mucin blends. Preactivated thiomers contain hydroxyl groups in their structure which can easily undergo a nucleophilic attack on the carbonyl group of aspartic acid and glutamic acid substructures of mucus, resulting in the formation of stable ester bonds. Preactivated thiomers also contain electronegative nitrogen atoms which are able to interact with nitrogen atoms of proline substructures in mucin. Additionally, nitrogen atom (acceptor) of precativated thiomers can interact with the oxygen atom (donor) of the carbonyl group containing asparagine and glutamine. Another possible interaction is the bond formation between the oxygen atom of the carbonyl group (acceptor) of the pre-activated thiomer and the nitrogen atom of the proline substructures (donor) of mucin. Furthermore, carboxylic groups of pre-activated thiomers can interact with carboxyl groups of aspartic acid and glutamic acid substructures in mucin via formation of hydrogen bond. Last but not least, pre-activated thiomers contain free thiol groups in addition with disulfide bonds which can interact with cysteine-rich subdomains found in mucin leading to the formation of new disulfide bonds [5,14]. Mucus is composed of numerous cationic charged substructures like arginine and lysine [26], the formation of disulfide bonds with thiol groups neighboured by an anionic charged carboxyl group like it is the case for the pre-activated PAA-cys-2MNA conjugates is highly promoted. Overall, the process of formation of new bonds between pre-activated polymers and mucus, is supposed to be responsible for the strengthening of mucoadhesive bond and time-dependent changes in viscosity of polymer/mucin mixtures.
4. Conclusion
This study summarizes the synthesis and characterization of pre-activated poly(acrylic acid)-cysteine-2MNA (100-, 250-, and 450 kDa). The covalent attachment of 2MNA to poly(acrylic acid)-cysteine back bone significantly improved the mucoadhesion, cohesion, stability and self crosslinking properties of these polymers. Moreover, all the tested polymers were found non-toxic over Caco-2 cells. Among all polymers tested, poly(acrylic acid)-cysteine-2MNA (450 kDa) displayed the strongest mucoadhesive properties followed by poly(acrylic acid)-cysteine-2MNA (250 kDa) and poly(acrylic acid)-cysteine-2MNA (100 kDa). In contrast to so far used thiomers, the pre-activated version contains disulfide bonds which are unable to further oxidize at high pH. All these properties represent pre-activated polymers as promising generation of mucoadhesive polymers which are safe to use for prolong residence time of drug delivery systems to target various mucosal surfaces.
Acknowledgments
This work was supported by the FWF (Fonds zur Förderung der wissenschaftlichen Forschung) project no. ZFP 235150 Higher Education Commission Pakistan (HEC) and Austrian Agency for International Cooperation in Education and Research (ÖAD).
References
- 1.Smart J.D., Kellaway I.W., Worthington H.E.C. An in vitro investigation of mucosa adhesive materials for use in controlled drug delivery. J Pharm Pharmacol. 1984;36:295–299. doi: 10.1111/j.2042-7158.1984.tb04377.x. [DOI] [PubMed] [Google Scholar]
- 2.Harris D., Fell J.T., Sharma H., Taylo D.C., Linch J. Studies on potential bioadhesive systems for oral drug delivery. STP Pharmacol. 1989;5:852–856. [Google Scholar]
- 3.Khanna R., Agrawal S.P., Ahuja A. Mucoadhesive buccal drug delivery: a potential alternative to conventional therapy. Indian J Pharm Sci. 1998;60:1–11. [Google Scholar]
- 4.Bernkop-Schnürch A., Schwarz V., Steininger S. Polymers with thiol groups: a new generation of mucoadhesive polymers? Pharm Res. 1999;16:876–881. doi: 10.1023/a:1018830204170. [DOI] [PubMed] [Google Scholar]
- 5.Gum J.R., Jr., Hicks J.W., Toribara N.W., Rothe E.M., Lagace R.E., Kim Y.S. The human MUC2 intestinal mucin has cysteine–rich subdomains located both upstream and downstream of its central repetitive region. J Biol Chem. 1992;267:21375–21383. [PubMed] [Google Scholar]
- 6.Mikos A.G., Peppas N.A. Kinetics of mucus-polymer interactions. In: Gurny R., Junginger H.E., editors. Bioadhesion–possibilities and future trends. Wissenschaftliche Verlagsges; Stuttgart, Germany: 1990. pp. 65–85. [Google Scholar]
- 7.Bernkop-Schnürch A., Krauland A.H., Leitner V.M., Palmberger T. Thiomers: potential excipients for non-invasive peptide delivery systems. Eur J Pharm Biopharm. 2004;58:253–263. doi: 10.1016/j.ejpb.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 8.Borchard G., Lueßen H.L., de Boer A.G., Verhoef J.C., Lehr C.M., Junginger H.E. Effects of chitosan-glutamate and carbomer on epithelial tight junctions in vitro. J Control Release. 1996;39:131–138. [Google Scholar]
- 9.Bernkop-Schnürch A. Polymer-inhibitor conjugates: a promising strategy to overcome the enzymatic barrier to perorally administered (poly)peptide drugs? STP Pharma Sci. 1999;9:78–87. [Google Scholar]
- 10.Brandt J., Svenson A., Carlsson J., Drevin H. Covalent coupling of unsaturated compounds to thiol agarose using ã–radiation: a new method for preparation of adsorbents for affinity chromatography. J Solid–Phase Biochem. 1977;2:105–109. [Google Scholar]
- 11.Grassetti DR. Postoperative treatment of carcinoma patients. US Patent No. 4378364, 1983.
- 12.Bernkop-Schnürch A., Steininger S. Synthesis and characterization of mucoadhesive thiolated polymers. Int J Pharm. 2000;194:239–247. doi: 10.1016/s0378-5173(99)00387-7. [DOI] [PubMed] [Google Scholar]
- 13.Kast C.E., Bernkop-Schnürch A. Thiolated polymers: development and in vitro evaluation of chitosan-thioglycolic acid acid conjugates. Biomaterials. 2001;22:2345–2352. doi: 10.1016/s0142-9612(00)00421-x. [DOI] [PubMed] [Google Scholar]
- 14.Leitner V.M., Marschütz M.K., Bernkop-Schnürch A. Mucoadhesive and cohesive properties of poly(acrylic acid)-cysteine conjugates with regard to their molecular mass. Eur J Pharm Sci. 2003;18:89–96. doi: 10.1016/s0928-0987(02)00245-2. [DOI] [PubMed] [Google Scholar]
- 15.Palmberger T.F., Albert K., Loretz B., Bernkop-Schnürch A. Thiolated polymers: evaluaion of the influence of the amount of covalently attached L–cysteine to poly(acrylic acid) Eur J Pharm Biopharm. 2007;66:405–412. doi: 10.1016/j.ejpb.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Vetter A., Perera G., Leithner K., Klima G., Bernkop-Schnürch A. Development and in vivo bioavailability study of an oral fondaparinux delivery system. Eur J Pharm Sci. 2010;41:489–497. doi: 10.1016/j.ejps.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi M., Okunaga K., Nishida S., Kawamura K., Eda K. Oxidative transformation of thiols to disulfides promoted by activated carbon–air system. Tetrahedron Lett. 2010;51:6734–6736. [Google Scholar]
- 18.Demir A.S., Igdir A.C., Mahasneh A.S. Novel conversion of thiols into disulfides, via S–nitrosothiol intermediates using trichloronitromethane. Tetrahedron. 1999;55:12399–12404. [Google Scholar]
- 19.Ghorbani-Choghamarani A., Nikoorazm M., Goudarziafshar H., Tahmasbi B. An efficient and new method on the oxidative coupling of thiols under mild and heterogeneous conditions. Bull Korean Chem Soc. 2009;30:1388–1390. [Google Scholar]
- 20.Parrott E.L. Compression. In: Lieberman H.A., Lachman L., Schwartz J.B., editors. Pharmaceutical dosage forms: tablets. 2nd ed. Marcel Dekker; New York: 1990. pp. 201–243. [Google Scholar]
- 21.Chickering D.E., Mathiowitz E. Definitions, mechanisms, and theories of bioadhesion. In: Mathiowitz E., Chickering D.E., Lehr C.M., editors. Bioadhesive drug delivery systems. Marcel Dekker; New York: 1999. pp. 1–10. [Google Scholar]
- 22.Duchêne D., Ponchel G. Principle and investigation of the bioadhesion mechanism of solid dosage forms. Biomaterials. 1992;13:709–714. doi: 10.1016/0142-9612(92)90132-8. [DOI] [PubMed] [Google Scholar]
- 23.Roldo M., Hornof M., Caliceti P., Bernkop-Schnürch A. Mucoadhesive thiolated chitosans as platforms for oral controlled drug delivery: synthesis and in vitro evaluation. Eur J Pharm Biopharm. 2004;57:115–121. doi: 10.1016/s0939-6411(03)00157-7. [DOI] [PubMed] [Google Scholar]
- 24.Hassan E.E., Gallo J.M. A simple rheological method for the in vitro assessment of mucin–polymer bioadhesive bond strength. Pharm Res. 1990;7:491–495. doi: 10.1023/a:1015812615635. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari F., Rossi S., Martini A., Muggetti L., De Ponti R., Caramella C. Technological induction of mucoadhesive properties on waxy starches by grinding. Eur J Pharm Sci. 1997;5:277–285. [Google Scholar]
- 26.Zalewska A., Zwierz K., Zółkowski K., Gindzieński A. Structure and biosynthesis of human salivary mucins. Acta Biochim Pol. 2000;47:1067–1079. [PubMed] [Google Scholar]