Abstract
Hermit crabs play an important role in the Northern Adriatic Sea due to their abundance, wide range of symbionts, and function in structuring the benthic community. Small-scale (0.25 m2) hypoxia and anoxia were experimentally generated on a sublittoral soft bottom in 24 m depth in the Gulf of Trieste. This approach successfully simulates the seasonal low dissolved oxygen (DO) events here and enabled studying the behaviour and mortality of the hermit crab Paguristes eremita. The crabs exhibited a sequence of predictable stress responses and ultimately mortality, which was correlated with five oxygen thresholds. Among the crustaceans, which are a sensitive group to oxygen depletion, P. eremita is relatively tolerant. Initially, at mild hypoxia (2.0 to 1.0 ml l− 1 DO), hermit crabs showed avoidance by moving onto better oxygenated, elevated substrata. This was accompanied by a series of responses including decreased locomotory activity, increased body movements and extension from the shell. During a moribund phase at severe hypoxia (0.5 to 0.01 ml l− 1 DO), crabs were mostly immobile in overturned shells and body movements decreased. Anoxia triggered emergence from the shell, with a brief locomotion spurt of shell-less crabs. The activity pattern of normally day-active crabs was altered during hypoxia and anoxia. Atypical interspecific interactions occurred: the crab Pisidia longimana increasingly aggregated on hermit crab shells, and a hermit crab used the emerged infaunal sea urchin Schizaster canaliferus as an elevated substrate. Response patterns varied somewhat according to shell size or symbiont type (the sponge Suberites domuncula). Mortality occurred after extended anoxia (~ 1.5 d) and increased hydrogen sulphide levels (H2S ~ 128 μmol). The relative tolerance of crabs and certain symbionts (e.g. the sea anemone Calliactis parasitica) – as potential survivors and recolonizers of affected areas – may influence and promote community recovery after oxygen crises.
Keywords: Adriatic Sea, Dead zone, Eutrophication, Hydrogen sulphide, Hypoxia
Highlights
► Anoxia is a key threat, and the Northern Adriatic Sea is a model “dead zone”. ► Hermit crabs and their symbionts are important ecosystem engineers. ► The reactions of hermit crabs to anoxia are examined in the community setting. ► The crabs reacted with a defined sequence of stress behaviours and mortality. ► The crabs are among the most hypoxia/anoxia-tolerant crustaceans. ► Their density and tolerance help to understand post-disturbance community recovery.
1. Introduction
Hermit crabs inhabit a wide range of environments, from polar to tropical seas and from the supratidal to deep ocean canyons. They play important roles as predators, scavengers, detritivores and even filter-feeders (Schembri, 1982), and their manifold symbioses can enrich the biodiversity of their habitats today and probably in the past (Reiss et al., 2003). Hermit crab-occupied shells are important islands of hard structures for the attachment of epifauna in soft-bottom benthic communities (Brooks and Mariscal, 1986). There, empty shells are likely to be buried in the substrate unless they are used by hermit crabs as protection (Creed, 2000; Stachowitsch, 1977). Hermit crabs are therefore ecosystem engineers: by using gastropod shells they affect the abundance and distribution of other invertebrates (Gutierrez et al., 2003; Jones et al., 1994; 1997; Williams and McDermott, 2004).
In the Northern Adriatic Sea, which is characterised by high-biomass macroepibenthic assemblages (Fedra et al., 1976; Zuschin and Stachowitsch, 2009), hermit crabs exhibit a high density (1.9 individuals m− 2). Paguristes eremita (Linnaeus, 1767) (formerly Paguristes oculatus) is the dominant hermit crab species and harbours more than 110 symbionts (in the broad sense of De Bary, 1879, cited in Stachowitsch, 1980). The large number, size and range of species (Williams and McDermott, 2004) make hermit crab associations a stable yet mobile microbiocoenosis (Stachowitsch, 1977). The encrusting and endolithic species influence the time that a shell, as the key resource for hermit crabs, can function as a housing. This led to a new classification of symbionts based on their function in two simultaneous, opposing processes: a constructive process that strengthens and extends the shell's lifespan, and a destructive process that weakens the shell (Stachowitsch, 1980). Finally, this association plays an important role in structuring the overall community: when deteriorated shells are abandoned, many symbionts survive and grow to establish the multi-species clumps that characterise this soft bottom.
Coastal shallow seas face the greatest anthropogenic threats due to the impacts of accelerated human activities (Jenkins, 2003). Eutrophication, coupled with water column stratification, is one of the gravest threats. This is manifested in low dissolved oxygen levels (Diaz, 2001). Hypoxia (DO concentrations < 2 ml l− 1) and anoxia have spread exponentially since the 1960s and today are a key stressor in shallow marine ecosystems (Diaz and Rosenberg, 2008). Benthic animals respond to hypoxia and anoxia with a range of stress behaviours, physiological adaptations (Diaz and Rosenberg, 1995; Gray et al., 2002; Wu, 2002) and mass mortalities (in the Adriatic; Stachowitsch, 1984). The resulting dead zones (Diaz and Rosenberg, 2008) have impacts on all scales from the structure and function of benthic communities to impaired ecosystem services (Sala and Knowlton, 2006). The Northern Adriatic Sea is one of nearly 400 recognised dead zones worldwide. As a shallow, semi-enclosed water body with abundant nutrient discharges, mainly from the Po River, coupled with meteorological and climatic conditions, it exhibits most attributes associated with the development of low oxygen events (Justić et al., 1993). This makes it a model for ecosystems periodically affected by oxygen deficiency (Crema et al., 1991).
Due to the difficulty of predicting oxygen depletion events and their often rapid onset and course, an experimental anoxia generating unit (EAGU) was developed to simulate anoxia on a small scale and to document the full range of behavioural responses, inter- and intraspecific interactions, and mortalities in situ (Stachowitsch et al., 2007). First results on selected species and species groups have already been published for the Adriatic (Riedel et al., 2008a; 2008b). In general, crustaceans are sensitive to anoxia (Vaquer-Sunyer and Duarte, 2008). Our first results, however, indicate that various crustacean species show different tolerances. Thus, the bioherm-inhabiting crab Pisidia longimana was among the most sensitive species, whereas Pilumnus spinifer and Ebalia tuberosa (the latter buries itself in the sediment during the day) were more tolerant. The mutualistic pea crab Nepinnotheres pinnotheres (living inside ascidians) survived prolonged anoxia as well as higher H2S (Haselmair et al., 2010). Hermit crabs were not evaluated in that study, but our initial observations indicated that they were among the more tolerant crustaceans. This would have implications for survival of short-term hypoxia and the subsequent recolonization of affected areas. Considering the important roles that hermit crabs play in this community, little is known about their responses to hypoxia (Côté et al., 1998; Riedel et al., 2008b; Shives and Dunbar, 2010; Stachowitsch, 1984). The present study is designed to fully document their behaviour during oxygen crises and to correlate behaviour and mortality with specific oxygen thresholds.
2. Materials and methods
2.1. Study site
The study site is located 2.3 km off Cap Madona (Piran, Slovenia) in the Gulf of Trieste (45° 32′ 55.68″ N, 13° 33′ 1.89″ E) close to the oceanographic buoy of the Marine Biology Station Piran at a depth of 24 m.
The sublittoral bottom consists of poorly sorted silty sand with high-biomass aggregations of macrobenthic organisms termed multi-species clumps (Fedra et al., 1976). These multi-species clumps or bioherms are formed by biogenic structures, mainly mollusc shells which serve as a basis for sessile species including serpulid tubeworms, ascidians, sponges, anemones and bivalves (Zuschin et al., 1999). Multi-species clumps provide a substrate for semi-sessile and vagile species such as the brittle star Ophiothrix quinquemaculata. Filter- and suspension-feeding species dominate. Based on the 3 dominant taxa (O. quinquemaculata, the sponges Reniera spp., and the ascidians Microcosmus spp.), this benthic community was named the Ophiothrix–Reniera–Microcosmus community (Fedra et al., 1976). The sediment surface between the patchy distribution of multi-species clumps is characterised by a low epifaunal density (Zuschin et al., 1999) and is dominated by predators and deposit feeders, including the hermit crab P. eremita.
2.2. Experimental design and sampling
The experimental anoxia generating unit (EAGU) is an underwater device designed to artificially create hypoxia and anoxia on a small scale (0.25 m2) on the seafloor. It enables the documentation of the behaviour of macrobenthic organisms to oxygen depletion and increasing H2S.
The EAGU consists of a cubic aluminium frame or an interchangeable plexiglass chamber, both measuring 50 × 50 × 50 cm (for details see Stachowitsch et al., 2007). A separate instrument lid is placed on top of one of these two different bases. This lid houses a time-lapse camera, two flashes and a data logger with sensor array (Unisense®) to record DO, H2S and temperature. Oxygen was measured 2 and 20 cm above the sediment to capture potential stratification, and the H2S sensor was located 2 cm above the sediment. Photos were taken automatically every 6 min, and sensor data were logged every minute.
The EAGU was deployed in both configurations. First, in the “open” configuration, the aluminium frame was positioned above an aggregation of benthic organisms for 24 h to document behaviour under normoxic conditions. Then, in the “closed” configuration, the frame was exchanged with the plexiglass chamber and deployed above the same assemblage. The chamber allows no water exchange with the water column. Anoxia was induced within 1–2 d due to natural respiration rates of the enclosed organisms. This “closed” configuration was maintained for another 1–2 d to detect the reactions of more tolerant species. The starting point and duration of all phases of the deployments are not uniform due to the inherent nature of field experiments (e.g. weather, diving schedules). Remaining organisms (living and dead) were then collected for preservation in a 4% formalin:seawater solution, and species and biomass were determined. The fieldwork was conducted in September 2005 and from July to October 2006.
2.3. Data analysis
Overall, 6705 images were evaluated, yielding a documentation time of 670.5 h (45.5 h open, 625 h closed configuration). The behaviour of P. eremita was analysed image by image and recorded in categories (Table 1). In the open configuration (normoxia), all visible individuals within the camera's field (inside and outside of the aluminium frame) in two deployments (9, 11) were evaluated, yielding 25 and 26 hermit crabs, i.e. a total of 51 individuals. In the closed configuration (rapidly sinking values/hypoxia/anoxia), 25 hermit crabs were evaluated (eight deployments): 9 inhabited the smaller A. pespelecani (Linnaeus, 1758), 11 the larger Murex brandaris or Hexaplex trunculus, 3 sponge-covered shells (Suberites domuncula, Olivi 1792) and 2 unidentified shells. If 4 or fewer hermit crabs were present in an experiment, all were evaluated. If more individuals were present, those 4 individuals visible throughout the experiment were selected. Additional criteria for selection included range of sizes, occupied shell species and symbionts.
Table 1.
Behaviours and reactions of hermit crabs.
| Category/sub-category | Criteria |
|---|---|
| Visibility | |
| Visible | Hermit crab, its shell or parts thereof visible. This includes shell largely covered by other organisms (e.g. crab under brittle star aggregation, i.e. outline recognisable, no exit tracks). |
| Non-visible | Neither crab nor its shell or parts thereof visible (e.g. hidden behind a multi-species clump) |
| Location | |
| On sediment | Crab/shell located on sediment or on bivalve shells on sediment |
| Elevated | Crab/shell on multi-species clump (no contact with sediment) |
| Locomotion | |
| No locomotion | No displacement |
| Minor | Displacement < 1 shell length or < 1 body length (if crab outside shell) |
| Major | Displacement ≥ 1 shell length or ≥ 1 body length (if crab outside shell) |
| Turn | Turning movement without displacement |
| Body movement | Crab movement without displacement (retraction into or stretching out of shell; appendage movements: chelipeds/legs; slight shell movements if crab not visible) |
| Body posture | |
| Normal | Biologically normal body postures. One or more of the following visible: eyes, cheliped(s), leg(s), antennae. If crab not visible, shell aperture slightly elevated (i.e. not flat on sediment surface) |
| Extended | Soft (posterior) part of carapace (and occasionally also anterior part of abdomen) visible |
| Out | Crab fully emerged/shell abandoned |
| Shell position | |
| Upright | Aperture facing down |
| Overturned | Aperture facing up |
| Interaction | Visible interaction of crab, its shell, or a symbiont with another organism. Includes: |
| |
| |
| |
| Excludes organisms used by crab solely as a substrate, e.g. crab climbs up on or over a sponge or ascidian | |
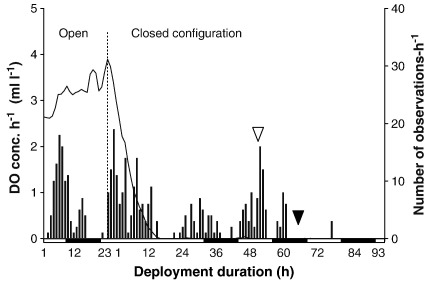
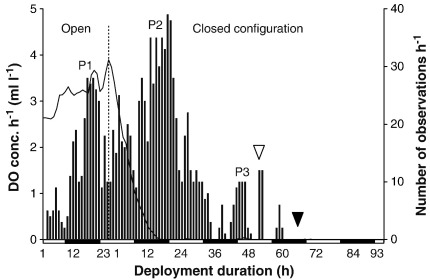
The hermit crabs themselves are typically only partially visible (i.e. antennae, eyestalks). Image evaluation was therefore based on shell locomotion and/or position changes (except in shell-less crabs). Displacement (i.e. major/minor locomotion) in small and large crabs was equated to shell displacement. For individuals in large S. domuncula, it was equated to estimated crab length. Behaviours were documented until mortality or until poor visibility due to decomposition of other benthic organisms. Mortality was recorded as occurring 2 h after the last observed locomotion or body movement. “Hours after hypoxia and anoxia” refer to the total time span measured between 2 ml l− 1 DO and the evaluated behaviour, whereby the anoxia component is provided separately (time between 0 ml l− 1 DO and the behaviour). Day and night were defined according to sunrise and sunset of the corresponding day of the deployment. Deployment 11 was chosen to graphically depict a representative deployment: it was one of the longest experiments in the closed configuration and was also one of the two deployments used to evaluate behaviour in normoxia (open configuration; Figs. 3 and 4).
Fig. 3.
Average number of locomotions per hour during deployment 11. n = 3 crabs in open, n = 4 in closed configuration. White and black bars: day and night. White and black arrowheads: emergence from shell and death, respectively (50% of individuals).
Fig. 4.
Average number of interactions per hour during deployment 11. n = 3 crabs in open, n = 4 in closed configuration. White and black bars: day and night. White and black arrowheads: emergence from shell and death (50% of individuals). P1–3: interaction peaks.
2.4. Statistical analysis
The behavioural categories were assigned to dissolved oxygen categories: normoxia (≥ 2.0 ml l− 1), mild hypoxia (2.0–1.0 ml l− 1), moderate hypoxia (1.0–0.5 ml l− 1), severe hypoxia (< 0.5 ml l− 1), and anoxia (0 ml l− 1).
The non-parametric Kruskal–Wallis test was used to determine differences in behaviour due to declining oxygen concentrations. The Mann–Whitney U-test was used to compare behaviours between different oxygen categories. To detect differences in responses between day- and night phases, cross tables were computed and the Pearson Chi-square test was performed. To identify which factor (length of the closed configuration, duration of hypoxia and anoxia, development of H2S) affected mortalities and survivorship, linear regressions were used. H2S concentrations were transformed using a log (x + 1) transformation. The software package SPSS 17.0 was used.
3. Results
3.1. Sensor data
During normoxia, DO values varied from 2.6 to 5.6 ml l− 1 (2 cm above the sediment). The values were typically higher (2.8 to 8.9 ml l− 1) 20 cm above the sediment. In all deployments, the subsequent closed configuration caused an immediate and constant decrease in DO concentrations. Anoxia was generated within 1 to 3 d. This depended in part on the initial concentration and on the amount of biomass enclosed in the respective deployment. It was induced fastest in deployment 11 (17.4 h) and the slowest in deployment 13 (69.8 h). In deployment 2 an intermediate oxygen peak developed. H2S was created in all deployments: values were lower in deployments with short anoxia (up to 21 μmol l− 1) and highest when anoxia lasted 2 d or more (maximum 304 μmol l− 1). The temperature remained constant in each deployment (range across deployments: 18.5 to 21.4 °C) and bottom water salinity was 38‰ (for details and characteristic sensor data see Haselmair et al., 2010).
3.2. Responses to decreasing dissolved oxygen concentrations
Decreasing DO values triggered a sequence of predictable stress responses and atypical interactions in hermit crabs. For significance levels of changes between oxygen categories, see also Suppl. material Table 1: all crabs combined, Table 2: small crabs, Table 3: large crabs, Table 4: crabs in the sponge S. domuncula.
3.3. Location changes
During normoxia, hermit crabs were located mainly on the sediment or partly hidden under multi-species clumps (only 24% of observations on clumps; Fig. 1A). The onset of hypoxia triggered a highly significant (P < 0.001) avoidance response, i.e. migration onto clumps. At mild and moderate hypoxia, crabs moved up clumps in nearly half the observations (47 and 48%, respectively). This value fell to 39% (P < 0.001) during severe hypoxia and anoxia (Suppl. material Table 1).
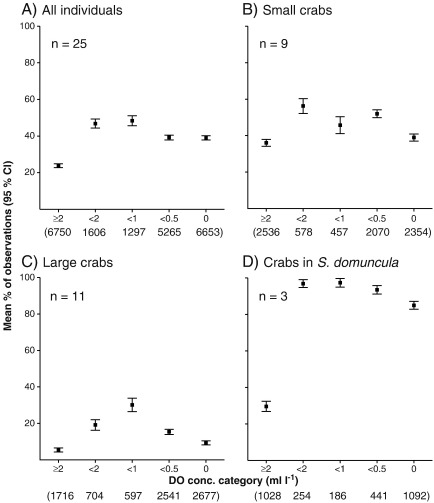
Fig. 1.
Presence of hermit crabs on multi-species clumps (mean percentage) related to five oxygen thresholds: (A) all individuals, (B–D) specific responses. n: number of evaluated crabs in closed configuration. Numbers in parentheses under oxygen thresholds indicate total photographs evaluated in each oxygen category.
The responses differed considerably according to housing type (large and small shells, S. domuncula). The three categories differed in the amount of time spent on the sediment and multi-species clumps in each oxygen category. Small crabs (in small shells) were mostly on the sediment during normoxia. During mild hypoxia, the number of observations of crabs located on bioherms increased from 36% in normoxia to 56% (P < 0.001) (Fig. 1B). During moderate hypoxia, slightly less than half the observations (46%) showed crabs on clumps, rising to 52% (P < 0.05) during severe hypoxia and decreasing to 39% (P < 0.001) during anoxia (Suppl. material Table 2). Large crabs, in contrast, were mostly on the sediment in all oxygen categories (normoxia: 94% of observations; Fig. 1C). Mild hypoxia triggered movement onto clumps (from 6 to 19%), with a peak during moderate hypoxia (30%), followed by a continuous decrease (15 and 9% during severe hypoxia and anoxia, respectively). The changes across all oxygen categories were highly significant (Suppl. material Table 3). During normoxia, most crabs in S. domuncula were on the sediment. Mild hypoxia caused movement onto clumps from 30 to 97% of observations (P < 0.001; Fig. 1D); this value remained constant during moderate hypoxia and fell during severe hypoxia (93%, P < 0.05) and anoxia (85%: P < 0.001, Suppl. material Table 4).
The locations at the end of the evaluation also differed; most small (9 of 10 individuals) and large crabs (9 of 11) returned to the sediment. Two of 3 individuals in S. domuncula, however, remained on multi-species clumps until the end.
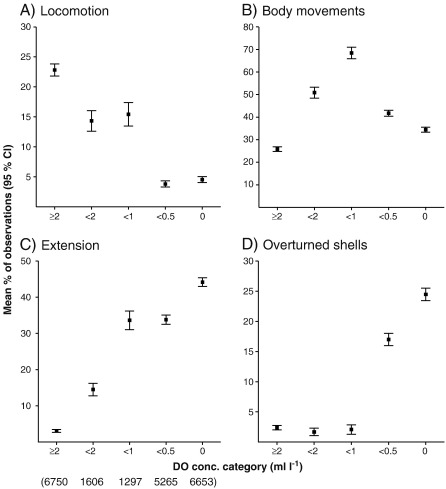
3.4. Decreased locomotion
Most horizontal locomotion was observed during normoxia (23%; Fig. 2A). Mild hypoxia caused a decrease to 14% (P < 0.001, Suppl. material Table 1) and severe hypoxia a further drop to 4% (P < 0.001) (anoxia 5%). Crabs in S. domuncula moved much less than those in shells; the decrease from normoxia (17% of all observations) to severe hypoxia (1%) was constant and steep (P < 0.001, Suppl. material Table 4) and the value remained minimal during anoxia (1%).
Fig. 2.
Reactions of all hermit crab individuals (mean percentage) related to five oxygen thresholds: (A–D) selected behaviours. Normoxia n = 51; hypoxia/anoxia n = 25. Numbers in parentheses under oxygen thresholds indicate total photographs evaluated in each oxygen category (valid for A–D). Note different scales.
3.5. Altered activity pattern
Locomotion occurred in episodes, reflecting activity peaks. Initially, during normoxia, more locomotion occurred during the day than at night (32 and 14% of observations, respectively). During the day, slightly more major than minor locomotion occurred (19 and 11%, respectively); during the night the relationship was more balanced (7 versus 6%). Oxygen depletion dampened this rhythm, i.e. a smaller daytime activity peak during early anoxia (Fig. 3). All crabs that emerged from their shells (one exception), however, moved around shell-less on the sediment or multi-species clumps. This is reflected in the somewhat higher last activity peak (Fig. 3). This last phase was initiated after a mean of 55 h (SD = 9.4) of combined hypoxia and anoxia (mean anoxia duration = 26.9 h; SD = 14.2). Locomotion here ranged from a single location change (minutes) to 13.9 h of movement (mean = 3.7 h; SD = 4.1). Within this period, the mean number of location changes was 15 (range: 1 to 40).
Overall the highly significantly different locomotory activity between day and night (normoxia) largely disappeared during mild hypoxia, re-occurred during moderate and severe hypoxia, and disappeared again at anoxia (Suppl. material Table 5).
3.6. Changes in body movements
Initially, during normoxia, locomotion and body movements accounted for a similar percentage of observations (23 and 26%, respectively; Fig. 2A and B). At mild hypoxia, locomotion decreased (see above) but body movements started to increase highly significantly (51% of observations), peaking at moderate hypoxia (68%; Fig. 2B), then continuously dropping at severe hypoxia and anoxia (42 and 34%, respectively; all changes P < 0.001, Suppl. material Table 1). Note that, at anoxia locomotion occurred in only 5% of observations (Fig. 2A). In 20 (out of 25) individuals, body movements lasted longer than locomotion (mean = 25.2 h; SD = 24.1; range: 18 min to 81.4 h). The remaining 5 crabs moved around shell-less, and locomotion ceased at about the same time as body movements. In sponge-inhabiting individuals, mild hypoxia triggered a steeper increase of body movements (70% of observations) and a higher peak value during moderate hypoxia (91%) (Suppl. material Table 4).
3.7. Body posture: extension from shell
During normoxia, normal body posture was maintained (93% of observations). Extension from the shell and other postures were rare (7%; Fig. 2C). Eight individuals were extended 19 times briefly (maximum 9 images) while examining the sediment or empty, damaged shells. Interactions (both inter- and intraspecific) caused 3 individuals to stretch out of their shell towards the opponent (maximum 2 images). With decreasing DO concentrations, extension (all crabs) increased to 15% at mild and 34% at moderate hypoxia, reaching 44% of observations at anoxia (all changes except from moderate to severe hypoxia P < 0.001, Suppl. material Table 1). This pattern was more distinct in crabs inhabiting S. domuncula: values were higher during moderate hypoxia (57%) and anoxia (55%), but the major increase again occurred at moderate hypoxia.
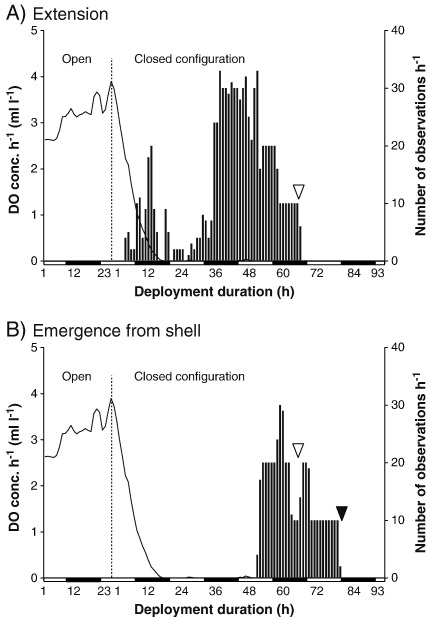
Extension (Suppl. material Fig. 1A) typically occurred in two peaks. The second peak is higher and longer than the first. In between, body posture returned to normal. The decrease after the second peak reflects fully emerged individuals (Suppl. material Fig. 1B) rather than a return to normal posture.
Supplementary Fig. 1.
Fig. 1. (A) Extension and (B) emergence from shell (average number of the selected behaviour per hour) during deployment 11. n = 3 crabs in open, n = 4 in closed configuration. White and black bars: day and night. White and black arrowheads: death (50 % and 100 % of individuals).
3.8. Atypical shell position: overturned
Initially, most housings were normally positioned (aperture facing down: 97, 98 and 98% of observations during normoxia, mild and moderate hypoxia, respectively). As DO values dropped further, more shells were overturned, with highly significant increases at severe hypoxia (from 2 to 17% of observations) and at anoxia (24%; Fig. 2D) (Suppl. material Table 1, Fig. 2B). The start and the number of overturned shells differed according to housing: large crabs often started overturning at severe hypoxia (26% of observations), small crabs (17%) and those in S. domuncula (38%) at anoxia.
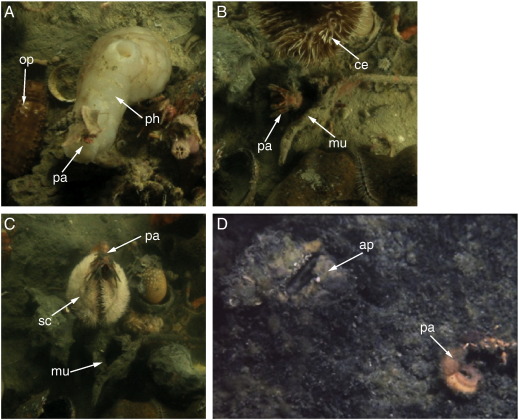
Supplementary Fig. 2.
Fig. 2. (A) Paguristes eremita (pa) in Aporrhais pespelecani shell on top of ascidian Phallusia mammilata (ph) at 13.6 h after anoxia (H2S 18.3 µmol l–1). The holothurian Ocnus planci (op) on left. (B) P. eremita (pa) extending from an overturned Murex brandaris shell (mu) at 16.1 h after anoxia (H2S 36.7 µmol l–1). Note extended sea anemone (Cereus pedunculatus, ce) at top. (C) Atypical interactions: shell-less hermit crab P. eremita (pa) on top of emerged infaunal sea urchin Schizaster canaliferus (sc) at 35 h after anoxia (H2S 243.2 µmol l–1). Note abandoned, overturned M. brandaris (mu) shell on the sediment. Bent-over C. pedunculatus partially extended from tube on right. (D) Dead P. eremita (pa) (curved abdomen visible on bottom) next to its abandoned shell (overturned A. pes-pelecani (ap) at top left). Note typical serpulid-encrusted aperture.
3.9. Emergence from shell
P. eremita remained inside its shell from normoxia to severe hypoxia. Anoxia, however, triggered emergence (13 of 25 individuals; 52%). The crabs emerged between 38.9 and 71.3 h of combined hypoxia and anoxia (mean = 52.8 h; SD = 8.5), whereby anoxia lasted from 10.3 to 48.6 h (mean = 24.4 h; SD = 11.8). At emergence, 77% of the crabs were positioned on the sediment and 62% of shells were still upright. Once emerged, the crabs moved around. One large individual (in M. brandaris) returned to its shell. Two images (12 min) before it emerged for the first time (after 34 h of anoxia), nearly the whole abdomen was visible. The crab remained emerged for 24 min, moved around on top of its upright shell, turned around, and partially inserted its abdomen back into the aperture. It remained inside for 12 min before finally emerging again and climbing on an exposed infaunal sea urchin Schizaster canaliferus.
Emergence was clearly a precursor to mortality: 8 of 13 crabs (62%) that abandoned their shell died. Three of the remaining emerged crabs survived due to relatively short anoxia in the deployments. Two other individuals disappeared from view and could not be further evaluated.
3.10. Inter- and intraspecific interactions
Short inter- and intraspecific interactions were difficult to observe because photographs were taken in 6-min intervals. Nonetheless, during normoxia, such interactions took place in 50% of observations, largely due to individual, long-lasting contacts. During mild hypoxia both inter- and intraspecific interactions highly significantly increased (57%) and remained stable during moderate and severe hypoxia (57 and 56%, respectively) until anoxia caused a major drop to 25% (P < 0.001, Suppl. material Table 1).
Interaction patterns differed according to housing category. In small crabs a marked increase occurred at mild hypoxia (from 53 to 67% of observations) followed by a subsequent decrease until anoxia (19%; all changes P < 0.001, Suppl. material Table 2). In large crabs the normoxic value was retained (43, 45 and 47% during normoxia, mild and moderate hypoxia, respectively) until severe hypoxia triggered an increase to 63% and anoxia a drop to 29% (the latter two changes P < 0.001, Suppl. material Table 3). In sponge-inhabiting crabs, interactions accounted for 61 and 63% of observations during normoxia and mild hypoxia, respectively. A highly significant increase occurred at moderate hypoxia (92%), dropping at anoxia to 29% (P < 0.001, Suppl. material Table 4).
During normoxia and mild hypoxia, more interactions occurred at night. This changed at moderate hypoxia (more interactions during the day). During severe hypoxia more interactions were again recorded at night, but this pattern reversed again during anoxia. The day/night differences were highly significant during normoxia, moderate hypoxia and anoxia, and significant during mild and severe hypoxia (Suppl. material Table 5). These day/night differences are illustrated here using deployment 11: two major peaks (peak 1: normoxia/open configuration, peak 2: late hypoxia to early anoxia/closed configuration) describe interactions that occurred at night (Fig. 4). The final, small peak (peak 3) represents a lengthier contact in which the crab crawled onto the sea urchin S. canaliferus.
3.11. Interspecific interactions
Interspecific interactions with numerous organisms were documented (Table 2). The interactions with the infaunal S. canaliferus, for example, occurred during anoxia, one involving a hermit crab directly, one its symbiotic sea anemone Calliactis parasitica (Couch, 1842). In the former, the crab initially used the sea urchin as an elevated substratum for 5.3 h (Suppl. material Fig. 2C). It then emerged from its shell and climbed on top of the sea urchin, where it remained for 1.8 h. This was followed by a moribund, immobile state on the sediment with sporadic body movements (mortality 15.9 h after onset of the interaction).
Table 2.
Interspecific interactions between hermit crabs and benthic invertebrates.
| Class/Taxa |
|---|
| Anthozoa |
| Cereus pedunculatus (Pennant, 1777) |
| Epizoanthus arenaceus (Delle Chiaje, 1823) |
| Gastropoda |
| Hexaplex trunculus (Linnaeus, 1758) |
| Murex brandaris (Linnaeus, 1758) |
| Diodora sp. |
| Bivalvia |
| Corbula gibba (Olivi, 1792) |
| Polychaeta |
| Protula tubularia (Montagu, 1803) |
| Infaunal polychaete individuals |
| Crustacea |
| Pisidia longimana (Risso, 1816) |
| Pilumnus spinifer (Milne-Edwards, 1834) |
| Nepinnotheres pinnotheres (Linnaeus, 1758) |
| Macropodia sp. |
| Alpheus glaber (Olivi, 1792) |
| Holothuroidea |
| Ocnus planci (Brandt, 1835) |
| Echinoidea |
| Psammechinus microtuberculatus (Blainville, 1825) |
| Schizaster canaliferus (Lamarck, 1816) |
| Ophiuroidea |
| Ophiothrix quinquemaculata (Delle Chiaje, 1828) |
| Ophiura spp. |
| Ascidiacea |
| Microcosmus sulcatus (Coquebert, 1797) |
In response to decreasing DO concentrations, the crabs P. longimana often positioned themselves on hermit crab-occupied shells (maximum duration 23.2 h). No such interaction ever occurred during normoxia. During mild and moderate hypoxia, 11 and 15 interactions with individuals or aggregations of up to 9 individuals, respectively, were documented. During severe hypoxia and anoxia, the number of interactions increased further to 43.
Ophiura spp., a species normally partially buried in the sediment, also used shells to serve as elevated sites. During severe hypoxia and anoxia, these brittle stars were commonly observed on occupied shells.
Most interspecific interactions (241) occurred with the brittle star O. quinquemaculata. Initially, during normoxia, most individuals showed escape reactions when approached by a hermit crab: they interrupted their filter-feeding posture and changed location. Slight contact between arm tips and crabs was recorded several times. At mild hypoxia, escape reactions ceased entirely although the number and duration of interactions increased. During severe hypoxia, moribund brittle stars even clung to hermit crab shells: some were positioned on top of the shells and completely covered them (maximum duration: 21.7 h). Interactions ceased during anoxia because the brittle stars had died.
Interactions with H. trunculus occurred during normoxia, ceased during mild hypoxia, re-occurred at severe hypoxia, and were the highest during anoxia.
3.12. Mortality
Overall, 36% of the hermit crabs (n = 9) died, all of them during anoxia. These 9 individuals were observed in 3 different deployments in which anoxia occurred faster and lasted longer (including one with an intermediate oxygen peak). Hermit crabs died between 52.2 and 73.5 h after combined hypoxia and anoxia (mean = 60.9 h; SD = 7.8), whereby anoxia ranged from 18.4 to 62.1 h (mean = 37.7 h; SD = 16.6). At anoxia, H2S began to develop. Mortality occurred at H2S values ranging from 116.5 to 248 μmol l− 1 (mean = 128.1 μmol l− 1; SD = 9.8). Mortality was highly significantly affected by H2S concentration and significantly affected by the duration of anoxia (P < 0.001 and < 0.05, respectively; Suppl. material Table 6). At death, 8 of the 9 crabs (89%) were positioned on the sediment and were outside their shell; 5 shells were overturned (Suppl. material Fig. 2D). The first shell-less individuals died 2.3 h and the last 18.4 h after emergence (mean = 8.4 h; SD = 5.1).
3.13. Survival
Overall, 13 individuals (52%) survived 30.2 to 78.0 h of hypoxia and anoxia (mean = 58.8 h; SD = 14.15) (5 different deployments). Survivors were typically present only in deployments with shorter anoxia ranging from 8.5 to 25.1 h (mean = 20.8 h; SD = 4.9) and lower final H2S concentrations (0 to 126.1 μmol l− 1; mean = 42.2 μmol l− 1; SD = 48.2). Most of the survivors were on the sediment in upright shells (9 of 13: 69%), 4 were on multi-species clumps (1 inside its shell). Ten crabs survived the relatively low H2S concentrations at the end of the respective deployments (maximum 21.0 μmol l− 1), although 3 of the 13 individuals experienced and survived much higher values (121.1 μmol l− 1).
4. Discussion
4.1. Responses to decreasing dissolved oxygen concentrations
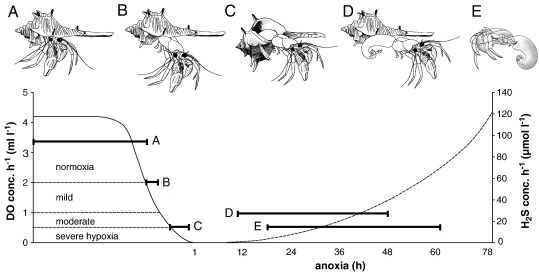
This study provides a detailed account of the full range of hermit crab behaviours during declining DO- and rising H2S concentrations and correlates the specific responses with oxygen thresholds. The reactions encompass a succession of stress behaviours and atypical interactions, and many crabs died (Fig. 5).
Fig. 5.
Sequence of behavioural responses to oxygen thresholds, anoxia duration and H2S development: (A) normal body posture, (B) crab extended from upright shell, (C) crab extended from overturned shell, (D) crab outside shell, and (E) mortality. Hours in pictograms are mean values from onset of anoxia.
In situ observations and earlier time-lapse films confirm that P. eremita prefers the sediment surface between multi-species clumps (Stachowitsch, 1979). As oxygen levels fall, however, the crabs tend to migrate onto the higher multi-species clumps/bioherms, although most ultimately return to the sediment. Such avoidance is common in crustaceans (Diaz and Rosenberg, 1995; Pihl et al., 1991; Renaud, 1986) and both vertical and horizontal migrations are strategies to prolong survival. In the Neuse River Estuary (North Carolina, USA), trawl collections showed that the blue crab Callinectes sapidus migrated horizontally from deeper to shallower, better oxygenated sites (Bell and Eggleston, 2005). Large numbers of the West Coast rock lobster Jasus lalandii migrate shoreward during hypoxic conditions at high tides in the greater Elands Bay region (West coast, South Africa), leading to mass strandings (Cockcroft, 2001). Hypoxic bottom water affects the vertical distribution of fish and crustaceans (mantis shrimp Squilla empusa and blue crab C. sapidus): migrations in the water column have been documented to avoid unfavourable conditions (Hazen et al., 2009; Pihl et al., 1991).
Hermit crabs responded in a manner similar to a wide range of bioherm-associated crustaceans from the Northern Adriatic Sea, which first emerged from their hiding places and then escaped to more elevated substrates (Haselmair et al., 2010). In the Northern Adriatic Sea, sublittoral sediment bottoms are flat and extensive, so that hypoxia can affect several hundred to thousands of km2 (Stachowitsch, 1984). This makes it unlikely that benthic invertebrates can escape horizontally. Multi-species clumps represent the only elevated structures, and oxygen concentrations were generally higher 20 cm above than directly at the bottom. The normally declining oxygen gradients within the benthic boundary layer and increasing concentrations into the water column (Diaz and Rosenberg, 1995; Jørgensen, 1980) are therefore retained and strengthened during oxygen crises. Migration onto multi-species clumps may therefore provide a refuge for hermit crabs from short-term hypoxia.
Mild hypoxia induced such initial avoidance behaviour in hermit crabs. The same threshold was reported in most bioherm-associated crustaceans, apart from the more tolerant pea crab N. pinnotheres (a symbiont in bivalves and sea squirts) and the nut crab E. tuberosa (Haselmair et al., 2010). The blue crab Callinectes similis actively detects and avoids DO below 2.3 ppm (Das and Stickle, 1994). The penaeid shrimp Metapenaeus ensis moves away from oxygen-depleted water (0.5 mg l− 1 DO), seeking normoxic conditions (3.0 mg l− 1 DO; Wu et al., 2002). The white shrimp Penaeus setiferus and the brown shrimp Penaeus aztecus avoid hypoxic conditions below 1.5 and 2.0 ppm DO, respectively (Renaud, 1986). Physiological mechanisms to actively detect dropping oxygen concentrations (and thus impending hypoxia) (Breitburg, 1992) and to orientate towards more favourable conditions (Bell et al., 2003) are essential to successfully avoid hypoxia. For example, the deep-water hermit crab Parapagurus pilosimanus adjusts its orientation and movement according to oxygen gradients and currents (Rowe and Menzies, 1968).
The density of P. eremita was high during a recent transect survey on this Northern Adriatic sediment bottom (2.4 individuals m− 2), slightly higher than in an earlier sampling (1.9 m− 2; Stachowitsch, 1977). P. eremita is a highly mobile crustacean with an average speed of 1.8 m h− 1 (Stachowitsch, 1979). Based on earlier time-lapse films, these crabs were estimated to move 21.6 m per day but remained within a defined radius (based on the high tagging relocation rate). Locomotion is non-directed, apparently determined by multi-species clumps on the sediment and by intraspecific encounters. Hermit crabs responded to 2.0 ml l− 1 DO by moving less, and at 0.5 ml l− 1 DO locomotion ceased. This pattern was only briefly interrupted by a locomotion spurt when shells were abandoned. Juvenile Norway lobster Nephrops norvegicus also alter their behaviour pattern: the first response to 30% oxygen saturation is prolonged inactivity (decreased walking and digging), with escape bursts of swimming (Eriksson and Baden, 1997). This may correspond with the initial less-active behaviour of hermit crabs.
Most crustaceans try to escape by first increasing their locomotion, later becoming immobile at species-specific thresholds. Such reduced locomotion may save energy needed for respiration (Johansson, 1997; Mistri, 2004). Other crustaceans in the Northern Adriatic Sea increase their locomotion threefold with declining oxygen, followed by a decrease between 1.0 and 0.5 ml l− 1 DO (Haselmair et al., 2010). In the brackish-water shrimps Palaemonetes varians (Hagerman and Uglow, 1984) and Crangon crangon (Hagerman and Szaniawska, 1986), ~ 50% oxygen saturation caused restless swimming followed by immobility at < 10 mm Hg DO and 30% saturation, respectively. Hermit crabs atop bioherms and decreased locomotion were concurrent events at this threshold. The relatively small clumps (decimetre range), restrict locomotion if the crabs want to stay on them. We therefore interpret decreased locomotion and immobility as a combination of saving energy and the relatively small elevated sites.
The overall activity pattern changed with decreasing oxygen concentrations. P. eremita is diurnal, being mostly active during the day and moving less at night: lengthy stops during the night suggested a resting phase (Stachowitsch, 1979). Thus, during a 12-h daytime phase, the number of individuals moving within the camera's field of vision in that study was fivefold compared to nighttime. The present study confirms this activity pattern at normoxia. At the beginning and end of oxygen decline – during mild hypoxia and anoxia – this day/night rhythm disappeared, but for different reasons. In the former case we interpret this to reflect activity both day and night to avoid the unfavourable conditions. In the latter case, moribund animals during anoxia also showed no day/night difference. Altered activity patterns have been reported in other crustaceans in this community, for example P. longimana. That crab has a cryptic lifestyle and emerges only during nighttime (Haselmair et al., 2010). From moderate hypoxia on, however, P. longimana was exposed and visible both day and night.
As opposed to locomotion, body movements steadily increased as DO values fell from 2.0 to 0.5 ml l− 1. Thereafter, during severe hypoxia and anoxia, such movements decreased. General body movements, combined with movements of the pleopods (Lancester, 1988) or abdomen (Gerlach et al., 1976), create water currents to deliver gill respiratory current in the hermit crab Pagurus bernhardus; pleopod movements also remove faeces from inside the shell. Hypoxia triggers increased pleopod beating in the Norway lobster N. norvegicus to ventilate the burrow (Gerhardt and Baden, 1998). Brief injection of hypoxic water (2 ppm DO), however, did not change the pleopod beating rate in the hermit crab Dardanus arrosor (Innocenti et al., 2004). Certain crustaceans, in addition to pleopod movements, increase the frequency of scaphognathite beating when oxygen concentrations decline (McMahon, 2001). Accordingly, body movements in P. eremita are interpreted as a response to hypoxic stress but may also create additional water movement inside the shell.
Decreasing oxygen initiated a sequence of different postures and an altered shell position: extension from the shell during mild hypoxia, overturned shells during severe hypoxia and emergence beginning approximately 24 h after anoxia. Extension increased (percentage of observations) up until anoxia, when nearly 50% of all crabs were extended. The number of overturned shells increased throughout anoxia. Before emergence, crabs showed only body movements, but no locomotion. Emergence (after a mean of 26.9 h after anoxia) was associated with a locomotion spurt or “escape movement” in almost all shell-less individuals. Such spurts lasted an average of 3.7 h. Hermit crabs expend energy in carrying a shell (Herreid and Full, 1986). During slow locomotion, for example, the land hermit crabs Coenobita compressus in shells require twice as much oxygen as shell-less individuals. We therefore interpret emergence along with increased locomotion as a last attempt to escape in an energy-saving mode.
Hermit crabs emerge from their shells when exposed to various environmental stresses: hypoxia (Riedel et al., 2008b; Stachowitsch, 1984), extreme thermal stress (Bertness, 1982), high temperature and desiccation (Taylor, 1981), when pursued (Greenaway, 2003) and during dredging (Young, 1979). The intertidal hermit crab Pagurus samuelis is commonly subjected to sedimentation, which can lead to oxygen deficiency (Shives and Dunbar, 2010). When buried with the shell aperture facing upward, P. samuelis emerged from the shell in a laboratory study. In our study, nearly two-thirds of the emerged P. eremita did so from normally positioned shells, although shell position may be less important when shells are on the sediment surface. Hypoxic conditions can also impact other shell-related behaviours, for example shell selection: crabs spend less time investigating new shells and inhabit significantly lighter and smaller shells (Côté et al., 1998). This reduces shell volume, increases the predation risk and impacts reproduction. Nonetheless, these drawbacks are apparently outweighed, at least temporarily, by the reduced energy costs.
4.2. Housing-specific responses
Shell size and weight affected avoidance behaviour onto multi-species clumps and the number of interactions. Crabs in S. domuncula, for example, spent the most time on such clumps and also had the most interactions, followed by small and then large individuals. Large shells were overturned already during severe hypoxia, while small shells and S. domuncula-covered shells were first overturned during anoxia. This may reflect the difficult-to-overturn form of the A. pespelecani shells occupied by smaller crabs or different tolerances of juvenile versus adult crustaceans to hypoxia (although juveniles are typically more sensitive; Eriksson and Baden, 1997). Weight may also play a role (Bridges and Brand, 1980): larger individuals (with heavier shells) climbed less onto elevated substrates. They may require less oxygen and also save energy by remaining on the sediment. The pronounced upward movement of S. domuncula-inhabiting crabs may be an attempt to keep the sponges alive. This escape onto bioherm refuges may explain the increased interactions (see below). The opposite occurred in C. parasitica-covered (mostly larger) shells: normally, the sea anemone not only provides protection (Williams and McDermott, 2004) but may also reduce interspecific interactions.
The responses of S. domuncula occupants differed the most, including intensified body movements, higher frequencies of extension from the shell as well as an earlier and more constant decrease of locomotion. The oxygen content in the sponge tissue is only 50 to 60% that of the surrounding water (Gatti et al., 2002) and the “aperture” is typically tight fitting; this may reduce water exchange and prompt the crab to extend out more often during moderate hypoxia and anoxia and increase body movements during mild, moderate and severe hypoxia.
4.3. Atypical interactions
Interactions with other species increased during hypoxia but then decreased during anoxia. Hypoxia dampened reactions upon an encounter (no avoidance, shorter flight distances of other organisms), initiated interactions with normally hidden or buried organisms, increased interactions with other organisms aggregating on elevated substrates, and caused organisms to climb onto crab-occupied shells.
Interestingly, interaction peaks did not correspond with crab locomotion peaks. During normoxia, most interactions occurred at night (crabs day-active). At mild hypoxia, interactions increased and remained high throughout severe hypoxia. Crab locomotion, in contrast, decreased during mild hypoxia and again during severe hypoxia. At anoxia, interactions were halved and most crabs were moribund. Most interactions were apparently initiated by nocturnal organisms, by hypoxia-induced increased activity of other organisms during the day, and by the artefact of long-lasting contact with and between moribund organisms.
Finally, the range of potential interactions was broadened by emerging infauna species, e.g. a hermit crab climbing onto S. canaliferus (Pados, 2010) and by the occupied shells themselves becoming an atypical substratum for other species. The brittle stars Ophiura lacterosa and O. quinquemaculata normally escape when hermit crabs approach, pointing to predator–prey relationships (Stachowitsch, 1979; Wurzian, 1982). During severe hypoxia and anoxia, however, these brittle stars positioned themselves on the shells. The crab P. longimana also aggregates on any available elevated structures, such as ascidians (Haselmair et al., 2010). Such hypoxia-induced movements to and aggregations in oxygenated refuge habitats that increase the number of biological interactions have been reported elsewhere (Lenihan et al., 2001). The ultimate effect of such spatial overlaps is determined by the relative tolerance of predator and prey (Breitburg et al., 1994; Kolar and Rahel, 1993). In the Northern Adriatic, for example, the sea anemone C. parasitica consumed less tolerant, moribund O. quinquemaculata in our deployments (Riedel et al., 2008a). Crustaceans, however, are more sensitive than anemones, and P. eremita was never observed to consume moribund prey at hypoxia. Conversely, hermit crabs were not consumed by other organisms in our enclosed experiments. In a more extensive, natural hypoxia/anoxia event, however, a much wider range of potential larger predators would be present and predation in certain phases cannot be excluded.
Finally, during anoxia, interactions decreased and involved more tolerant gastropods (H. trunculus) and emerged sea urchins (S. canaliferus).
4.4. Mortality and tolerance
Among the hypoxia-sensitive crustaceans (Gray et al., 2002; Theede et al., 1969), P. eremita is relatively tolerant of oxygen deficiencies and H2S. Hermit crabs first died during prolonged anoxia (mean = 37.7 h). During a mass mortality event in 1983 in the Gulf of Trieste, few crabs were alive on the 5th day after the onset of hypoxia/anoxia, and on day 7 no living individuals were observed (Stachowitsch, 1984). Previous studies with hermit crabs have mainly involved laboratory experiments and dealt with short-term exposure to low DO, focusing on survival. Pagurus spp. survived 0.6 mg l− 1 DO for one hour and was then returned to normal oxygen conditions (Marshall and Leverone, 1994). Other hermit crabs survived burial (and the related hypoxia) for 12 h (Shives, 2010). The intertidal hermit crab Clibanarius vittatus typically survived 5.5 h in oxygen-free seawater (Wernick and Penteado, 1983).
A general critical DO concentration for the onset of benthic mortality is 1 ml l− 1, with wide-ranging mortality at about 0.5 ml l− 1 (Diaz and Rosenberg, 1995). These thresholds, however, might be underestimated for crustaceans: recent approaches suggest a median lethal concentration (LC50) of 2.45 mg l− 1 DO (SD = 0.14), with a median lethal time (LT50) of 55.5 h (SD = 12.4) (Vaquer-Sunyer and Duarte, 2008). Generally, crustaceans and fishes are more sensitive to hypoxia, whereas molluscs, cnidarians and priapulids are relatively tolerant (Vaquer-Sunyer and Duarte, 2008). Beyond this broad pattern, tolerance differs among species within a particular taxonomic group. Bioherm-associated crabs such as P. longimana and Galathea spp. are sensitive, while E. tuberosa and N. pinnotheres are more tolerant, the latter two dying last (after 34.2 and 78 h of anoxia, respectively; Haselmair et al., 2010).
Mortality was significantly dependent on the duration of anoxia but highly significantly on H2S levels. Tolerance to oxygen depletion alone is difficult to study because anoxia is generally accompanied by H2S (Vismann, 1991). This additional impact reduces survival time by an average of 30% (Vaquer-Sunyer and Duarte, 2010). Hermit crabs died at a mean of 128.1 μmol l− 1 H2S. In the examined benthic community, however, mortalities could often be attributed to oxygen depletion alone: many organisms, including the crab P. longimana, died before H2S developed. Others such as N. pinnotheres tolerated higher H2S concentrations (Haselmair et al., 2010) or are physiologically adapted to hypoxic environments with high sulphide concentrations (sediment-burrowing Thalassinidea) (Johns et al., 1997).
Tolerance depends on behavioural and physiological adaptations (Wu, 2002). In our study, the former included migration to better-oxygenated bioherms and reduced energy consumption (immobility and emergence from shells). Physiologically, hermit crabs may have evolved – in analogy to sediment-burrowing crustaceans – to be somewhat more tolerant to low DO concentrations than other, free-living crustaceans because they live in tight-fitting, impermeable calcareous shells. C. vittatus, for example, is an “oxygen conformer” that lowers its oxygen uptake when values fall (Wernick and Penteado, 1983). P. samuelis meets the demand for aerobic respiration with anaerobic fermentation, which is indicated by increasing lactate levels in the hemolymph, after burial-induced hypoxia (Shives, 2010).
4.5. Symbionts
During normal oxygen conditions, the tentacle crown of the well-known hermit crab symbiont C. parasitica is open and directed downwards, towards the sediment, sweeping the bottom as the crabs move (Fig. 6 in Stachowitsch, 1980). The anemone responds to hypoxia by directing the open tentacle crown upwards (Riedel et al., 2008a; 2008b). As oxygen decreases, individuals start rotating their crown. During anoxia, the crown is contracted and again faces down. Ultimately, some individuals detach from the shell (Jørgensen, 1980; Riedel et al., 2008a; Sagasti et al., 2001). In the Northern Adriatic, C. parasitica individuals survived between 28 and 35 h of anoxia. Other studies demonstrated that anemones are especially resistant, surviving prolonged exposure to anoxia (Jørgensen, 1980; Wahl, 1984). This tolerance is attributed to a combination of switching from aerobic to anaerobic pathways, metabolic depression and elongation to increase the surface area for gas exchange (Sassaman and Mangum, 1972; Shick, 1991).
Other reactions of symbionts include drooping (colonial ascidians and the sponge S. domuncula; Stachowitsch, 1984). Epizoanthus arenaceus, another common symbiont (Ates, 2003; Stachowitsch, 1980), was among the survivors one week after an earlier mass mortality event. In the laboratory, another known hermit crab symbiont, the barnacle Balanus improvisus (Williams and McDermott, 2004), reacts to hypoxia by vertically extending its feeding appendages without the normal movement of feeding (Sagasti et al., 2001). These authors also demonstrated that bryozoans, a common shell-encrusting group, survive hypoxia by forming a resting state.
The relative tolerance of hermit crabs to anoxia, coupled with their high mobility and abundant symbionts, indicates an important role in community recovery after oxygen depletion. The crabs and certain more tolerant symbionts such as sea anemones directly determine post-hypoxia community composition by potentially surviving short-term hypoxia. The gastropod shells are also important structures for larval attachment, especially since most non-motile structures become covered by sediment (Stachowitsch, 1984). Indirectly, hermit crabs, as scavengers and predators, are attracted by discards and moribund benthos, for example after disturbance by trawl fishery (Groenewold and Fonds, 2000; Rumohr and Kujawski, 2000). A similar process can be expected after anoxia-induced collapses. Crabs with complete, undamaged symbiont communities (Stachowitsch, 1979) may move in from the unaffected surroundings. When the crabs occupy freshly available shells, they deposit their overgrown shells, accelerating recovery. Such processes may be crucial in the world's increasing number of anoxia-related “dead zones”.
The following are the supplementary materials related to this article.
Tab. 1. Results of Mann-Whitney U-test for behavioural differences in the number of observations in the five oxygen categories: all Paguristes eremita individuals. Comparison refers to oxygen categories: 1: normoxia; 2: mild hypoxia; 3: moderate hypoxia; 4: severe hypoxia; 5: anoxia. Bold: highly significant (P < 0.001); Underlined numbers: significant differences (P < 0.05).
Tab. 2. Results of Mann-Whitney U-test for behavioural differences in the number of observations in the five oxygen categories: Paguristes eremita in small shells. For explanation see suppl. material Tab. 1.
Tab. 3. Results of Mann-Whitney U-test for behavioural differences in the number of observations in the five oxygen categories: Paguristes eremita in large shells. For explanation see suppl. material Tab. 1.
Tab. 4. Results of Mann-Whitney U-test for behavioural differences in the number of observations in the five oxygen categories: Paguristes eremita in Suberites domuncula. For explanation see suppl. material Tab. 1.
Tab. 5. Results of Pearson х2-test for differences between day- and night phases of the selected behaviour in the five oxygen categories. For explanation see suppl. material Tab. 1.
Tab. 6. Results of linear regressions to identify factors affecting mortality and survival of P. eremita. Bold: highly significant (P < 0.001); Underlined numbers: significant differences (P < 0.05).
Acknowledgements
This research was conducted in the framework of projects P17655-B03 and P21542 B17 (Austrian Science Fund — FWF). We thank I. Gallmetzer, A. Haselmair, L. Schiemer and P. Steiner for technical and diving support and L. Celestina for keeping our boat in running order. Special thanks go to the director and staff of the Marine Biology Station (MBS) in Piran, Slovenia, for their hospitality and support during our field work. We thank Steve Dunbar and another, anonymous reviewer for very helpful comments that helped streamline the manuscript. [SS]
Contributor Information
Katrin Pretterebner, Email: kati.kati@gmx.at.
Bettina Riedel, Email: bettina.riedel@univie.ac.at.
Martin Zuschin, Email: martin.zuschin@univie.ac.at.
Michael Stachowitsch, Email: stachom5@univie.ac.at.
References
- Ates R.M.L. A preliminary review of zoanthid-hermit crab symbioses (Cnidaria; Zoantharia/Crustacea; Paguridea) Zool. Verhandel. Leiden. 2003;345:41–48. [Google Scholar]
- Bell G.W., Eggleston D.B. Species-specific avoidance responses by blue crabs and fish to chronic and episodic hypoxia. Mar. Biol. 2005;146:761–770. [Google Scholar]
- Bell G.W., Eggleston D.B., Wolcott T.G. Behavioural responses of free-ranging blue crabs to episodic hypoxia. I. Movement. Mar. Ecol. Prog. Ser. 2003;259:215–225. [Google Scholar]
- Bertness M.D. Shell utilization, predation pressure, and thermal stress in Panamanian hermit crabs: an interoceanic comparison. J. Exp. Mar. Biol. Ecol. 1982;64:159–187. [Google Scholar]
- Breitburg D.L. Episodic hypoxia in Chesapeake Bay: interacting effects of recruitment, behavior, and physical disturbance. Ecol. Monogr. 1992;62:525–546. [Google Scholar]
- Breitburg D.L., Steinberg N., Dubeau S., Cooksey C., Houde E.D. Effects of low dissolved oxygen on predation on estuarine fish larvae. Mar. Ecol. Prog. Ser. 1994;104:235–246. [Google Scholar]
- Bridges C.R., Brand A.R. Oxygen consumption and oxygen-independence in marine Crustaceans. Mar. Ecol. Prog. Ser. 1980;2:133–141. [Google Scholar]
- Brooks W.R., Mariscal R.N. Interspecific competitions for space by hydroids and a sea anemone living on gastropod shells inhabited by hermit crabs. Mar. Ecol. 1986;28:241–244. [Google Scholar]
- Cockcroft A.C. Jasus lalandii ‘walkouts’ or mass strandings in South Africa during the 1990s: an overview. Mar. Freshwater Res. 2001;52:1085–1094. [Google Scholar]
- Côté I.M., Reverdy B., Cooke P.K. Less choosy or different preference? Impact of hypoxia on hermit crab shell assessment and selection. Anim. Behav. 1998;56:867–873. doi: 10.1006/anbe.1998.0828. [DOI] [PubMed] [Google Scholar]
- Creed J.C. Epibiosis on cerith shells in a seagrass bed: correlation of shell occupant with epizoite distribution and abundance. Mar. Biol. 2000;137:775–782. [Google Scholar]
- Crema R., Castelli A., Prevedelli D. Long term eutrophication effects on macrofaunal communities in northern Adriatic Sea. Mar. Pollut. Bull. 1991;22:503–508. [Google Scholar]
- Das T., Stickle W.B. Detection and avoidance of hypoxic water by juvenile Callinectes sapidus and C. similis. Mar. Biol. 1994;120:593–600. [Google Scholar]
- De Bary A. Karl J. Trubner; Strassburg: 1879. Die Erscheinung der Symbiose. [Google Scholar]
- Diaz R.J. Overview of hypoxia around the world. J. Environ. Qual. 2001;30:275–281. doi: 10.2134/jeq2001.302275x. [DOI] [PubMed] [Google Scholar]
- Diaz R.J., Rosenberg R. Marine benthic hypoxia: a review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr. Mar. Biol. 1995:245–303. [Google Scholar]
- Diaz R.J., Rosenberg R. Spreading dead zones and consequences for marine ecosystems. Science. 2008;321:926–929. doi: 10.1126/science.1156401. [DOI] [PubMed] [Google Scholar]
- Eriksson S.P., Baden S.P. Behaviour and tolerance to hypoxia in juvenile Norway lobster (Nephrops norvegicus) of different ages. Mar. Biol. 1997;128:49–54. [Google Scholar]
- Fedra K., Ölscher E.M., Scherübel C., Stachowitsch M., Wurzian R.S. On the ecology of a North Adriatic benthic community: distribution, standing crop and composition of the macrobenthos. Mar. Biol. 1976;38:129–145. [Google Scholar]
- Gatti S., Brey T., Müller W.E.G., Heilmayer O., Holst G. Oxygen microoptodes: a new tool for oxygen measurements in aquatic animal ecology. Mar. Biol. 2002;140:1075–1085. [Google Scholar]
- Gerhardt L., Baden S.P. Gender- and oxygen-related irrigation behaviour of the decapod Nephrops norvegicus. Mar. Biol. 1998;131:553–558. [Google Scholar]
- Gerlach S.A., Ekstrøm D.K., Eckardt P.B. Filter feeding in the hermit crab, Pagurus bernhardus. Oecologia. 1976;24:257–264. doi: 10.1007/BF00345477. [DOI] [PubMed] [Google Scholar]
- Gray J.S., Wu R.S.S., Ying Y.O. Effects of hypoxia and organic enrichment on the coastal marine environment. Mar. Ecol. Prog. Ser. 2002;238:249–279. [Google Scholar]
- Greenaway P. Terrestrial adaptations in the Anomura (Crustacea: Decapoda) Mem. Mus. Victoria. 2003;60:13–26. [Google Scholar]
- Groenewold S., Fonds M. Effects on benthic scavengers of discards and damaged benthos produced by the beam-trawl fishery in the southern North Sea. ICES J. Mar. Sci. 2000;57:1395–1406. [Google Scholar]
- Gutierrez J.L., Jones C.G., Strayer D.L., Iribarne O.O. Mollusks as ecosystem engineers: the role of shell production in aquatic habitats. Oikos. 2003;101:79–90. [Google Scholar]
- Hagerman L., Uglow R.F. The influence of hypoxia on the blood regulation of the brackish water shrimp Palaemonetes varians Leach. J. Exp. Mar. Biol. Ecol. 1984;76:157–165. [Google Scholar]
- Hagerman L., Szaniawska A. Behaviour, tolerance and anaerobic metabolism under hypoxia in the brackish-water shrimp Crangon crangon. Mar. Ecol. Prog. Ser. 1986;34:125–132. [Google Scholar]
- Haselmair A., Stachowitsch M., Zuschin M., Riedel B. Behaviour and mortality of benthic crustaceans in response to experimentally induced hypoxia and anoxia in situ. Mar. Ecol. Prog. Ser. 2010;414:195–208. [Google Scholar]
- Hazen E.L., Craig J.K., Good C.P., Crowder L.B. Vertical distribution of fish biomass in hypoxic waters on the Gulf of Mexico shelf. Mar. Ecol. Prog. Ser. 2009;375:195–207. [Google Scholar]
- Herreid C.F., Full R.J. Energetics of hermit crabs during locomotion: the cost of carrying a shell. J. Exp. Mar. Biol. Ecol. 1986;120:297–308. [Google Scholar]
- Innocenti G., Bicocchi A., Vannini M. The use of pleopods for shell water circulation and respiration by hermit crabs. Mar. Freshwat. Behav. Physiol. 2004;37:161–171. [Google Scholar]
- Jenkins M. Prospects for biodiversity. Science. 2003;302:1175–1177. doi: 10.1126/science.1088666. [DOI] [PubMed] [Google Scholar]
- Johansson B. Behavioural response to gradually declining oxygen concentration by Baltic Sea macrobenthic crustaceans. Mar. Biol. 1997;129:71–78. [Google Scholar]
- Johns A.R., Taylor A.C., Atkinson R.J.A., Grieshaber M.K. Sulphide metabolism in thalassinidean Crustacea. J. Mar. Biol. Assoc. UK. 1997;77:127–144. [Google Scholar]
- Jones C.G., Lawton J.H., Shachak M. Organisms as ecosystem engineers. Oikos. 1994;69:373–386. [Google Scholar]
- Jones C.G., Lawron J.H., Shachak M. Positive and negative effects of organisms as physical ecosystem engineers. Ecology. 1997;78:1946–1957. [Google Scholar]
- Jørgensen B.B. Seasonal oxygen depletion in the bottom waters of a Danish fjord and its effect on the benthic community. Oikos. 1980;34:68–76. [Google Scholar]
- Justić D., Rabalais N.N., Turner R.E., Wiseman W.J. Seasonal coupling between riverborne nutrients, net productivity and hypoxia. Mar. Pollut. Bull. 1993;26:184–189. [Google Scholar]
- Kolar C.S., Rahel F.J. Interaction of a biotic factor (predator presence) and an abiotic factor (low oxygen) as an influence on benthic invertebrate communities. Oecologia. 1993;95:210–219. doi: 10.1007/BF00323492. [DOI] [PubMed] [Google Scholar]
- Lancester I. Pagurus bernhardus (L.) — an introduction to the natural history of hermit crabs. Field Stud. 1988;7:189–238. [Google Scholar]
- Lenihan H.S., Peterson C.H., Byers J.E., Grabowski J.H., Thayer G.W., Colby D.R. Cascading of habitat degradation: oyster reefs invaded by refugee fishes escaping stress. Ecol. Appl. 2001;11:764–782. [Google Scholar]
- Marshall M.J., Leverone J.R. Final report. Mote Marine Laboratory, Mote Technical Report; No. 381. 1994. The distribution and effects of hypoxia on marine organisms in Sarasota Bay. [Google Scholar]
- McMahon B.R. Respiratory and circulatory compensation to hypoxia in crustaceans. Respir. Physiol. 2001;128:349–364. doi: 10.1016/s0034-5687(01)00311-5. [DOI] [PubMed] [Google Scholar]
- Mistri M. Effects of hypoxia on predator–prey interactions between juvenile Carcinus aestuarii and Musculista senhousia. Mar. Ecol. Prog. Ser. 2004;275:211–217. [Google Scholar]
- Pados, T., 2010. A time-lapse camera experiment on benthic reactions to anoxia in the Northern Adriatic Sea. MSc thesis, University of Vienna.
- Pihl L., Baden S.P., Diaz R.J. Effects of periodic hypoxia on distribution of demersal fish and crustaceans. Mar. Biol. 1991;108:349–360. [Google Scholar]
- Reiss H., Knäuper S., Kröncke I. Invertebrate associations with gastropod shells inhabited by Pagurus bernhardus (Paguridae) — secondary hard substrate increasing biodiversity in North Sea soft-bottom communities. Sarsia. 2003;88:404–415. [Google Scholar]
- Renaud M.L. Detecting and avoiding oxygen deficient sea water by brown shrimp, Penaeus aztecus (Ives), and white shrimp Penaeus setiferus (Linnaeus) J. Exp. Mar. Biol. Ecol. 1986;98:283–292. [Google Scholar]
- Riedel B., Stachowitsch M., Zuschin M. Sea anemones and brittle starts: unexpected predatory interactions during induced in situ oxygen crisis. Mar. Biol. 2008;153:1075–1085. [Google Scholar]
- Riedel B., Zuschin M., Haselmair A., Stachowitsch M. Oxygen depletion under glass: behavioural responses of benthic macrofauna to induced anoxia in the Northern Adriatic. J. Exp. Mar. Biol. Ecol. 2008;367:17–27. [Google Scholar]
- Rowe G.T., Menzies R.J. Orientation in two bathyal, benthic decapods, Munida valida Smith and Parapagurus pilosimanus Smith. Limnol. Oceanogr. 1968;13:549–552. [Google Scholar]
- Rumohr H., Kujawski T. The impact of trawl fishery on the epifauna of the southern North Sea. ICES J. Mar. Sci. 2000;57:1389–1394. [Google Scholar]
- Sagasti A., Schaffner L.C., Duffy J.E. Effects of periodic hypoxia on mortality, feeding and predation in an estuarine epifaunal community. J. Exp. Mar. Biol. Ecol. 2001;258:257–283. doi: 10.1016/s0022-0981(01)00220-9. [DOI] [PubMed] [Google Scholar]
- Sala E., Knowlton N. Global marine biodiversity trends. Annu. Rev. Environ. Resour. 2006;31:93–122. [Google Scholar]
- Sassaman C., Mangum C.P. Adaptations to environmental oxygen levels in infaunal and epifaunal sea anemones. Biol. Bull. 1972;143:657–678. doi: 10.2307/1540189. [DOI] [PubMed] [Google Scholar]
- Schembri P.J. Feeding behaviour of fifteen species of hermit crabs (Crustacea: Decapoda: Anomura) from the Otago region, southeastern New Zealand. J. Nat. Hist. 1982;16:859–878. [Google Scholar]
- Shick J.M. Chapman and Hall; New York: 1991. A Functional Biology of Sea Anemones. [Google Scholar]
- Shives, J.A., 2010. Behaviour and Physiology of Hermit Crabs During Burial: Shell Abandonment and Lactate Accumulation. MSc thesis, Loma Linda University.
- Shives J.A., Dunbar S.G. Behavioral responses to burial in the hermit crab, Pagurus samuelis: implications for the fossil record. J. Exp. Mar. Biol. Ecol. 2010;388:33–38. [Google Scholar]
- Stachowitsch M. The hermit crab microbiocoenosis — the role of mobile secondary hard bottom elements in a North Adriatic benthic community. In: Keegan B.F., O'Ceidigh P., Boaden P.J.S., editors. Biology of Benthic Organisms. Pergamon Press; London: 1977. pp. 549–558. [Google Scholar]
- Stachowitsch M. Movement, activity pattern, and role of a hermit crab population in a sublittoral epifaunal community. J. Exp. Mar. Biol. Ecol. 1979;39:135–150. [Google Scholar]
- Stachowitsch M. The epibiotic and endolithic species associated with the gastropod shells inhabited by the hermit crabs Paguristes oculatus and Pagurus cuanensis. PSZN I. Mar. Ecol. 1980;1:73–101. [Google Scholar]
- Stachowitsch M. Mass mortality in the Gulf of Trieste: the course of community destruction. PSZN I. Mar. Ecol. 1984;5:243–264. [Google Scholar]
- Stachowitsch M., Riedel B., Zuschin M., Machan R. Oxygen depletion and benthic mortalities: the first in situ experimental approach to documenting an elusive phenomenon. Limnol. Oceanogr. Methods. 2007;5:344–352. [Google Scholar]
- Taylor P.R. Hermit crab fitness: the effect of shell condition and behavioral adaptations on environmental resistance. J. Exp. Mar. Biol. Ecol. 1981;52:205–218. [Google Scholar]
- Theede H., Ponat A., Hiroki K., Schlieper C. Studies on the resistance of marine bottom invertebrates to oxygen-deficiency and hydrogen sulphide. Mar. Biol. 1969;2:325–337. [Google Scholar]
- Vaquer-Sunyer R., Duarte C.M. Thresholds of hypoxia for marine biodiversity. Proc. Natl. Acad. Sci. U. S. A. 2008;105:15452–15457. doi: 10.1073/pnas.0803833105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaquer-Sunyer R., Duarte C.M. Sulfide exposure accelerates hypoxia-driven mortality. Limnol. Oceanogr. 2010;55:1075–1082. [Google Scholar]
- Vismann B. Sulfide tolerance: physiological mechanisms and ecological implications. Ophelia. 1991;34:1–27. [Google Scholar]
- Wahl M. The fluffy sea anemone Metridium senile in periodically oxygen depleted surroundings. Mar. Biol. 1984;81:81–86. [Google Scholar]
- Wernick A.M., Penteado C.H.S. Oxygen consumption by the hermit crab, Clibanarius vittatus (Bosc, 1802) in declining oxygen tensions. Comp. Biochem. Physiol. A. 1983;74:749–753. [Google Scholar]
- Williams J.D., McDermott J.J. Hermit crab biocoenoses: a worldwide review of the diversity and natural history of hermit crab associates. J. Exp. Mar. Biol. Ecol. 2004;305:1–128. [Google Scholar]
- Wu R.S.S. Hypoxia: from molecular responses to ecosystem responses. Mar. Pollut. Bull. 2002;45:35–45. doi: 10.1016/s0025-326x(02)00061-9. [DOI] [PubMed] [Google Scholar]
- Wu R.S.S., Lam P.K.S., Wan K.L. Tolerance to, and avoidance of, hypoxia by the penaeid shrimp (Metapenaeus ensis) Environ. Pollut. 2002;118:351–355. doi: 10.1016/s0269-7491(01)00298-6. [DOI] [PubMed] [Google Scholar]
- Wurzian, R.S., 1982. Die Funktion der Räuber der Makro-Epifauna in einer sublitoralen Benthos Gemeinschaft im Golf von Triest. PhD thesis, University of Vienna.
- Young A.M. Differential utilization of gastropod shells by three hermit crab species in North Inlet, South Carolina, U.S.A. Crustaceana. 1979;5:101–104. [Google Scholar]
- Zuschin M., Stachowitsch M., Pervesler P., Kollmann H. Structural features and taphonomic pathways of a high-biomass epifauna in the northern Gulf of Trieste, Adriatic Sea. Lethaia. 1999;32:299–317. [Google Scholar]
- Zuschin M., Stachowitsch M. Epifauna-dominated benthic shelf assemblages: lessons from the modern Adriatic Sea. Palaios. 2009;24:148–158. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tab. 1. Results of Mann-Whitney U-test for behavioural differences in the number of observations in the five oxygen categories: all Paguristes eremita individuals. Comparison refers to oxygen categories: 1: normoxia; 2: mild hypoxia; 3: moderate hypoxia; 4: severe hypoxia; 5: anoxia. Bold: highly significant (P < 0.001); Underlined numbers: significant differences (P < 0.05).
Tab. 2. Results of Mann-Whitney U-test for behavioural differences in the number of observations in the five oxygen categories: Paguristes eremita in small shells. For explanation see suppl. material Tab. 1.
Tab. 3. Results of Mann-Whitney U-test for behavioural differences in the number of observations in the five oxygen categories: Paguristes eremita in large shells. For explanation see suppl. material Tab. 1.
Tab. 4. Results of Mann-Whitney U-test for behavioural differences in the number of observations in the five oxygen categories: Paguristes eremita in Suberites domuncula. For explanation see suppl. material Tab. 1.
Tab. 5. Results of Pearson х2-test for differences between day- and night phases of the selected behaviour in the five oxygen categories. For explanation see suppl. material Tab. 1.
Tab. 6. Results of linear regressions to identify factors affecting mortality and survival of P. eremita. Bold: highly significant (P < 0.001); Underlined numbers: significant differences (P < 0.05).