Abstract
Caps and stipes of 141 fruiting bodies of Parasol Mushroom (Macrolepiota procera) and surface layer of soils collected from 11 spatially distant and background (pristine) areas in Northern Poland were analyzed for Ag, Al, Ba, Ca, Cd, Co, Cr, Cu, Fe, Hg, K, Mg, Mn, Na, Ni, P, Pb, Rb, Sr, and Zn by inductively coupled plasma optical emission spectroscopy and cold vapor atomic absorption spectroscopy. In terms of bioconcentration and bioexclusion concept, K, Ag, Cu, Rb, and P were highly bioconcentrated in caps, and their bioconcentration factor values varied for the 11 sites between 120 and 500—67–420, 70–220, 10–170, and 45–100, respectively. Cd, Zn, Mg, and Na showed bioconcentration factors (BCFs) between 3.3 and 36, 3.7–15, 0.92–6.3, and 1.4–44 while Al, Ba, Ca, Co, Cr, Mn, Ni, Pb, and Sr were excluded (BCF < 1). The Parasol Mushroom is a species harvested in the wild, and its caps are of unique taste and can contain a spectrum of essential and hazardous mineral compounds accumulated at elevated concentrations, even if collected at the background (pristine) areas. These elevated mineral concentrations of the caps are due to the efficient bioconcentration potential of the species (K, Ag, Cu, Rb, P, Cd, Zn, Mg, and Na) and abundance in the soil substrates (Al, Ca, Fe, Mn). The estimated intake rates of Cd, Hg, and Pb contained in Parasol Mushroom’s caps show a cause for concern associated with these metals resulting from the consumption of between 300- and 500-g caps daily, on a frequent basis in the mushrooming season.
Electronic supplementary material
The online version of this article (doi:10.1007/s11356-011-0574-5) contains supplementary material, which is available to authorized users.
Keywords: Food, Fungi, Heavy metals, Mineral composition, Nutrition, Mushrooms, Wild food
Introduction
Fungi represent a highly biodiverse group of biota with an estimated number of above 300,000 species worldwide that are able to dissolve take-up and introduce metals from organic and soil mineral substrates into the biosphere (Gadd 2007; Gumińska and Wojewoda 1988). Among fungi, about 10,000 species are fleshy mushrooms (macrofungi, macromycetes, higher fungi). Around 2,000 mushrooms growing in the wild from more than 30 genera are considered edible (Chang 1990). Edible wild mushrooms can contain, in the flesh, a spectrum of mineral macro- and micronutrients, nonessential trace elements, and problematic heavy metals. Mycorrhizal and saprobe mushrooms take part in a biogeochemical turnover of all mineral constituents contained in soil compartments or other substrates in which mycelium develops. Despite the fact that they hold a key role, the biological mechanisms and environmental factors that drive and influence the metal bioconcentrations and content of fruiting bodies are poorly known. Mercury can be an example of a metal that is much more enriched in fruiting bodies of mushrooms than in plants or animals (Chudzyński et al. 2009, 2011; Falandysz 2002; Falandysz and Brzostowski 2007; Falandysz et al. 2002a, b, c, d, 2003a, b, c, 2004, 2007a; Jarzyńska and Falandysz 2011a, b; Melgar et al. 2009; Stijve and Besson 1976; Stijve and Roschnik 1974). Some mushrooms were found recently as efficient accumulators and even hyperaccumulators of noble metals such as gold and silver and examples are the Amanitas mushrooms (Amanita submembranacea and Amanita strobiliformis) or the Agaricus mushrooms (Borovička et al. 2005, 2007, 2009, 2010; Falandysz et al. 1994a, b; Falandysz and Danisiewicz 1995). Certain mushrooms are specifically rich in arsenic, iron, selenium, or vanadium (Stijve et al. 1990, 1998; Falandysz 2008; Falandysz et al. 2001, 2007b, 2008a, b). Edible wild-grown mushrooms, as mentioned, can accumulate both essential and toxic metals and metalloids in the flesh, but knowledge on the toxicological risk and nutritional benefits of such substances in edible mushrooms and mushroom meals is still low (Alonso et al. 2003; Baptista et al. 2009; Carvalho et al. 2005; Cenci et al. 2010; Cocchi et al. 2006; Falandysz et al. 2011; Frankowska et al. 2010; García et al. 2009; Malinowska et al. 2004; Melgar et al. 1998; Vetter and Siller 1997; Zhang et al. 2010).
Documentation on trace element composition of wild food is important, both to know the amount and intake rates as well as their time trends due to anthropogenic emission and environmental pollution. This study is part of an ongoing wild mushrooms survey aimed to investigate the mineral content and composition and bioconcentration of mineral constituents of Parasol Mushroom (Macrolepiota procera) growing in Northern Poland. Assessed also was the contamination status of this mushroom as well as its mineral intake rates, nutritional significance, and risk to consumers.
M. procera (Scop. Fr.) Singer (1946), formerly called Lepiota procera and known under the common name Parasol Mushroom, has an edible and delicious cap. This mushroom is a soil-inhabiting saprophytic species growing alone or scattered in woods or at the edges of woods, or in pastures and is common in Poland. Parasol Mushrooms can be purchased commercially in Poland, but because of the relatively fragile structure of its cap, it is rarely offered at the rural markets, while easily available from roadside sellers in the countryside. In this article, the concentrations of 19 metals and phosphorous in soils and edible caps and inedible stipes (which have hard and fibrous texture) of Parasol Mushrooms from 11 distant sites in Northern Poland, are reported. Soil phosphorous and trace element composition were investigated to determine possible differences between the various locations as well as bioconcentration factors (BCFs) in the Parasol Mushroom.
Materials and methods
A set of 141 well-grown and roughly similar in size (cap diameter) and shape (parasol-shaped) specimens of the Parasol Mushroom (M. procera) Bull. Fr. and corresponding surface layer (0–10 cm) of humifying and mineral soil beneath fruiting bodies were collected from 11 geographically distant sites in Poland (Fig. 1). The mushrooms were air-dried for several days after clean up with a plastic knife from any visible plant vegetation and soil substrate debris. Further, each specimen was separated into two parts—the cap with pileipellis (skin) and stipe—and dried at 65°C to constant weight. The dried mushrooms were pulverized in an agate mortar and were kept in brand new sealed polyethylene bags in dry condition.
Fig. 1.
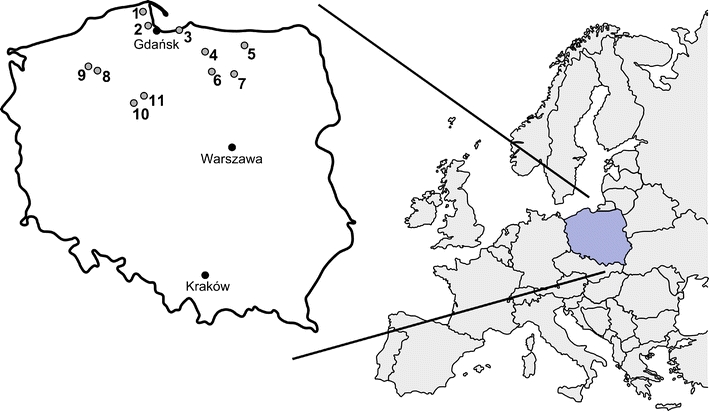
Location of the sampling sites of Parasol Mushrooms. Darżlubska Forest (1), Trójmiejski Park Krajobrazowy (2), Mierzeja Wiślana (3), Morąg and Łukta (4), Kętrzyn (5), Olsztynek (6), Szczytno (7), Czarne (8), Szczecinek (9), Bydgoszcz (10), and Rafa (11)
The pulverized subsamples (∼400 mg) of caps and stipes were weighted into pressure-resistant and analytical quality prodigestive vessels made of polytetrafluoroethylene, predigested for 24 h with concentrated nitric acid (65%; Suprapure®, Merck; 7 mL) at room temperature and further digested under pressure in an automatic microwave digestion system type (MARS 5 of CEM Corp., Matthews, NC, USA). The digest was diluted to 25 mL using deionized water and was subjected to instrumental analysis (Brzostowski et al. 2009).
Soil samples were air-dried in room temperature in clean condition for several weeks and were sieved through a pore size of 2 mm and further dried in an electric oven 40°C to constant weight. Next, the soil subsamples (∼5 g) in quartz vessels were cold-treated with nitric acid (HNO3 20%; 20 mL) and were left to stand for 24 h. An extract obtained was filtered through Whatmann no. 42 filter paper into a plastic measuring vessel (50 mL), and after adding an internal standard solution (yttrium; 20 mg L−1), a 50-mL mixture was made using deionized water and was subjected to instrumental analysis. Two blank digests were run with every set of the 15 soil samples.
Ag, Al, Ba, Ca, Cd, Co, Cu, Cr, Fe, K, Mg, Mn, Na, Ni, P, Pb, Rb, Sr, and Zn were determined by inductively coupled plasma optical emission spectroscopy (Optima 2000 DV, PerkinElmer, USA), and the yttrium was used as internal standard (Brzostowski et al. 2009).
These methods of metallic element measurements were validated and controlled for several occasions, and analyses were made of officially certified reference materials: International Atomic Energy Agency (IAEA) 359 cabbage leaves; and these from participating international calibration trials: GEMS/Food Euro proficiency testing exercise, the IAEA 338 proficiency test of trace elements in lichen, the Aquacon Project 9 soil analysis (European Commission Environment Institute), and oriental tobacco leaves (CTA–OTL–1), tea leaves (INTC–TL–1), and Polish herbal blend (INCT–MPH–2) by the Institute of Nuclear Chemistry and Technology in Warsaw, Poland (Brzostowski et al. 2009, 2011a, b; Chudzyński and Falandysz 2008). Discrepancies between certified values and concentrations quantified were below 10%. Duplicates and blanks followed with every set of ten mushrooms or soil samples examined. For blank samples, no major interferences were found for the element quantified. Limits of detection for Ag, Al, Ba, Ca, Cd, Co, Cu, Cr, Fe, K, Mg, Mn, Na, Ni, P, Pb, Rb, Sr, and Zn were between 0.01 and 0.10 μg g−1 dry weight (dw). Coefficients of variation for these measurements on routine runs were well below 10%.
Details on the methodology of mercury determination in fungal materials and soils by cold vapor atomic absorption spectroscopy were given in other reports (Falandysz 1990; Falandysz and Chwir 1997). For mercury, the method limit of detection was 0.005 μg g−1 dw. Data on total mercury in these mushrooms and soils were reported elsewhere (Falandysz et al. 2007a; Falandysz and Gucia 2008). In Table 1, the median values of mercury concentration in the cap, which were used for risk assessment, are cited.
Table 1.
Mineral constituent content of caps and stipes of Parasol Mushrooms and soil (mean, SD, median value, in parentheses and range) *(Falandysz et al. 2007a)
| Element | Matrix | Site | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Szczecinek, 2003 (n = 15) | Czarne, 2003 (n = 16) | TPK, 1995 n = 8 | Mierzeja Wiślana, 2001 (n = 10) | Morąg and Łukta, 2002 (n = 9) | Olsztynek, 2003 (n = 16) | Szczytno, 2003 (n = 15) | Rafa, 2002 (n = 15) | Darżlubska Forest, 2000 (n = 6) | Kętrzyn, 2000 (n = 16) | Bydgoszcz, 2000 (n = 15) | ||
| mg g−1 dw | ||||||||||||
| K | Cap | 40 ± 4 | 36 ± 5 | 45 ± 4 | 49 ± 14 | 35 ± 8 | 31 ± 4 | 39 ± 4 | 26 ± 4 | 36 ± 6 | 41 ± 8 | 26 ± 4 |
| (41) | (36) | (45) | (47) | (37) | (30) | (41) | (26) | (34) | (38) | (25) | ||
| 33–50 | 27–46 | 42–54 | 38–86 | 21–46 | 26–37 | 28–43 | 17–34 | 30–45 | 32–48 | 21–33 | ||
| Stipe | 28 ± 7 | 30 ± 10 | 35 ± 5 | 41 ± 9 | 24 ± 3 | 15 ± 5 | 27 ± 6 | 24 ± 11 | 22 ± 8 | 29 ± 6 | 21 ± 8 | |
| (29) | (28) | (35) | (43) | (24) | (16) | (27) | 25 | (21) | (28) | (20) | ||
| 16–38 | 13–53 | 30–43 | 26–50 | 18–29 | 12–23 | 18–40 | 5.9–38 | 16–37 | 20–44 | 6.4–35 | ||
| Soil | 0.11 ± 0.03 | 0.090 ± 0.023 | 0.14 ± 0.02 | 0.34 ± 0.21 | 0/19 ± 0.03 | 0.17 ± 0.08 | 0.11 ± 0.03 | 0.18 ± 0.11 | 0.15 ± 0.19 | NA | NA | |
| (0.13) | (0.080) | (0.15) | (0.25) | (0.20) | (0.14) | (0.11) | (0.18) | (0.074) | ||||
| 0.069–0.15 | 0.073–0.13 | 0.12–0.16 | 0.17–0.71 | 0.14–0.23 | 0.097–0.29 | 0.065–0.14 | 0.059–0.33 | 0.060–0.54 | ||||
| P | Cap | 15 ± 2 | 11 ± 2 | 12 ± 1 | 12 ± 3 | NAa | 11 ± 2 | 14 ± 1 | NA | NA | NA | NA |
| (16) | (11) | (12) | (11) | (10) | (14) | |||||||
| 11–18 | 7.9–14 | 11–14 | 9.8–18 | 8.4–16 | 12–15 | |||||||
| Stipe | 8.0 ± 1.4 | 6.9 ± 1.4 | 6.8 ± 0.8 | 6.6 ± 1.0 | NA | 4.7 ± 0.8 | 6.2 ± 1.1 | NA | NA | NA | NA | |
| (7.5) | (6.9) | (6.9) | (6.2) | (4.7) | (6.0) | |||||||
| 5.5–10 | 4.3–9.2 | 5.9–8.1 | 5.1–8.4 | 3.5–6.2 | 4.7–7.9 | |||||||
| Soil | 0.19 ± 0.08 | 0.16 ± 0.02 | 0.14 ± 0.04 | 0.28 ± 0.07 | 0.26 ± 0.05 | 0.28 ± 0.06 | 0.18 ± 0.05 | 0.23 ± 0.09 | NA | NA | NA | |
| (0.19) | (0.16) | (0.13) | (0.26) | (0.28) | (0.28) | (0.17) | (0.25) | |||||
| 0.12–0.28 | 0.14–0.20 | 0.10–0.20 | 0.21–0.41 | 0.18–0.31 | 0.18–0.34 | 0.10–0.26 | 0.11–0.33 | |||||
| Mg | Cap | 2.0 ± 0.2 | 1.5 ± 0.2 | 1.6 ± 0.1 | 1.7 ± 0.3 | 1.4 ± 0.3 | 1.4 ± 0.2 | 1.6 ± 0.1 | 1.0 ± 0.1 | 1.4 ± 0.1 | 1.2 ± 0.1 | 0.86 ± 0.09 |
| (2.0) | (1.5) | (1.6) | (1.5) | (1.5) | (1.4) | (1.6) | (1.0) | (1.4) | (1.1) | (0.84) | ||
| 1.4–2.2 | 1.1–1.9 | 1.6–1.8 | 1.4–2.3 | 0.89–1.7 | 1.2–1.7 | 1.3–1.7 | 0.86–1.3 | 1.3–1.7 | 1.0–1.4 | 0.75–1.1 | ||
| Stipe | 1.2 ± 0.2 | 0.89 ± 0.23 | 1.3 ± 0.2 | 1.1 ± 0.2 | 0.94 ± 0.15 | 0.64 ± 0.14 | 0.77 ± 0.09 | 0.70 ± 0.12 | 0.69 ± 0.14 | 0.82 ± 0.10 | 0.50 ± 0.07 | |
| (1.2) | (0.89) | (1.3) | (1.1) | (1.0) | (0.67) | (0.75) | (0.72) | (0.71) | (0.80) | (0.53) | ||
| 0.79–1.7 | 0.41–1.2 | 1.0–1.6 | 0.67–1.3 | 0.62–1.1 | 0.25–0.83 | 0.59–0.95 | 0.52–0.85 | 0.47–0.88 | 0.54–1.0 | 0.34–0.59 | ||
| Soil | 0.28 ± 0.13 | 0.14 ± 0.02 | 0.26 ± 0.07 | 0.31 ± 0.09 | 0.26 ± 0.05 | 0.65 ± 0.51 | 0.24 ± 0.06 | 0.16 ± 0.05 | 0.18 ± 0.06 | NA | NA | |
| (0.27) | (0.13) | (0.23) | (0.30) | (0.27) | (0.36) | (0.24) | (0.16) | (0.15) | ||||
| 0.16–0.41 | 0.12–0.17 | 0.22–0.39 | 0.18–0.48 | 0.19–0.31 | 0.27–1.4 | 0.14–0.29 | 0.093–0.23 | 0.13–0.31 | ||||
| μg g−1 dw | ||||||||||||
| Ag | Cap | 3.9 ± 1.4 | 1.8 ± 1.4 | 0.63 ± 0.22 | 1.3 ± 0.7 | NA | 1.5 ± 0.5 | 3.8 ± 2.5 | NA | NA | 5.5 ± 3.4 | 0.77 ± 0.22 |
| (3.5) | (1.4) | (0.65) | (1.1) | (1.3) | (4.4) | (4.8) | (0.76) | |||||
| 1.8–5.9 | 0.58–5.9 | 0.36–0.95 | 0.50–3.0 | 0.88–3.0 | 0.76–8.0 | 0.74–11 | 0.34–1.2 | |||||
| Stipe | 2.3 ± 1.1 | 3.9 ± 3.2 | 1.1 ± 0.4 | 4.9 ± 2.5 | NA | 1.6 ± 0.6 | 2.3 ± 0.7 | NA | NA | 2.4 ± 1.2 | 1.2 ± 0.3 | |
| (2.4) | (2.9) | (1.0) | (3.9) | (1.6) | (2.2) | (2.2) | (1.2) | |||||
| 0.73–4.0 | 1.2–11 | 0.71–1.8 | 2.4–10 | 0.31–3.0 | 1.4–3.7 | 0.71–4.7 | 0.79–1.8 | |||||
| Soil | 0.0080 ± 0.0014 | 0.0069 ± 0.0015 | 0.0098 ± 0.0027 | 0.040 ± 0.031 | 0.010 ± 0.006 | 0.020 ± 0.010 | 0.0094 ± 0.0010 | 0.018 ± 0.007 | NA | NA | NA | |
| (0.0081) | (0.0062) | (0.010) | (0.038) | (0.0076) | 0.021 | (0.0088) | (0.017) | |||||
| 0.0059–0.0094 | 0.0055–0.0092 | 0.0066–0.014 | 0.005–0.092 | 0.0050–0.017 | 0.0061–0.031 | 0.0084–0.011 | 0.0095–0.031 | |||||
| Al | Cap | 23 ± 17 | 180 ± 120 | 280 ± 230 | 260 ± 260 | 76 ± 26 | 54 ± 50 | 45 ± 23 | 110 ± 160 | 37 ± 16 | 140 ± 65 | 82 ± 46 |
| (15) | (130) | (180) | (180) | (73) | (25) | (35) | (51) | (31) | (130) | (67) | ||
| 6.4–67 | 47–440 | 120–790 | 36–900 | 37–110 | 9.4–160 | 19–88 | 21–650 | 22–61 | 45–310 | 24–170 | ||
| Stipe | 23 ± 11 | 150 ± 84 | 590 ± 670 | 500 ± 410 | 100 ± 91 | 55 ± 45 | 34 ± 12 | 500 ± 420 | 36 ± 10 | 170 ± 110 | 55 ± 23 | |
| (21) | (130) | (240) | (320) | (66) | (43) | (31) | (64–1,800) | (36) | (130) | (54) | ||
| 11–47 | 24–310 | 100–1,900 | 31–1,100 | 25–290 | 15–180 | 23–68 | 400 | 23–49 | 44–460 | 24–100 | ||
| Soil | 1,600 ± 160 | 1,600 ± 120 | 1,100 ± 170 | 570 ± 150 | 1,400 ± 290 | 2,500 ± 350 | 2,200 ± 180 | 1,300 ± 320 | 2,100 ± 720 | NA | NA | |
| (1,600) | (1,600) | (1,000) | (570) | (1,500) | (2,700) | (2,300) | (1,300) | (1,900) | ||||
| 1,300–1,800 | 1,500–1,800 | 990–1,400 | 360–720 | 990–1,700 | 2,100–2,800 | 1,900–2,300 | 870–1,700 | 1,700–3,600 | ||||
| Ba | Cap | 1.3 ± 2.0 | 1.1 ± 0.6 | 1.7 ± 1.1 | 3.7 ± 3.4 | 1.7 ± 1.0 | 0.80 ± 0.34 | 0.72 ± 0.18 | 0.68 ± 0.81 | 0.37 ± 0.19 | 1.3 ± 0.8 | 0.56 ± 0.24 |
| (0.45) | (0.91) | (1.3) | (2.7) | (1.5) | (0.75) | (0.69) | (0.30) | (0.35) | (1.2) | (0.52) | ||
| 0.10–7.0 | 0.39–2.1 | 0.83–4.3 | 0.75–11 | 0.52–3.8 | 0.39–1.7 | 0.50–1.1 | 0.11–3.1 | 0.19–0.73 | 0.62–3.7 | 0.18–1.2 | ||
| Stipe | 1.0 ± 0.6 | 0.99 ± 0.56 | 3.3 ± 2.6 | 7.3 ± 4.5 | 1.4 ± 0.6 | 0.87 ± 0.35 | 0.84 ± 0.22 | 3.0 ± 1.5 | 0.60 ± 0.34 | 2.2 ± 3.1 | 0.47 ± 0.19 | |
| (1.1) | (0.84) | (2.4) | (7.0) | (1.6) | (0.91) | (0.77) | (2.6) | (0.59) | (1.4) | (0.41) | ||
| 0.41–2.2 | 0.22–2.3 | 0.90–7.6) | 1.6–14 | 0.72–2.1 | 0.37–1.4 | 0.61–1.3 | 0.65–7.0 | 0.21–1.1 | 0.64–13 | 0.23–0.87 | ||
| Soil | 13 ± 2 | 8.6 ± 1.3 | 10 ± 1 | 24 ± 13 | 21 ± 2 | 15 ± 3 | 13 ± 2 | 14 ± 4 | 23 ± 11 | NA | NA | |
| (13) | (8.9) | (10) | (16) | (21) | (16) | (12) | (16) | (23) | ||||
| 10–15 | 7.2–10 | 8.3–12 | 11–42 | 19–24 | 11–20 | 11–16 | 13–15 | 12–39 | ||||
| Ca | Cap | 190 ± 190 | 90 ± 39 | 210 ± 110 | 320 ± 280 | 200 ± 85 | NA | 99 ± 33 | 68 ± 61 | 110 ± 68 | 130 ± 67 | 78 ± 15 |
| (100) | (87) | (180) | (230) | (170) | (97) | (54) | (73) | (120) | (76) | |||
| 52–610 | 40–160 | 84–390 | 80–970 | 91–320 | 42–180 | 18–170 | 61–220 | 54–270 | 41–99 | |||
| Stipe | 170 ± 82 | 77 ± 41 | 340 ± 200 | 820 ± 430 | 210 ± 84 | NA | 180 ± 76 | 400 ± 310 | 170 ± 110 | 160 ± 230 | 78 ± 27 | |
| (160) | (70) | (350) | (860) | (220) | (160) | (350) | (120) | (100) | (77) | |||
| 68–360 | 19–180 | 120–590 | 280–1,600 | 100–370 | 96–380 | 87–1,400 | 81–370 | 41–960 | 35–120 | |||
| Soil | 1,900 ± 1,300 | 240 ± 63 | 600 ± 120 | 2,300 ± 1,300 | 680 ± 100 | 2,900 ± 3,900 | 340 ± 180 | 840 ± 590 | 3,100 ± 1,800 | NA | NA | |
| (1,800) | (200) | (650) | (2,000) | (620) | (360) | (300) | (810) | (2,900) | ||||
| 780–3,300 | 190–320 | 410–710 | 780–5,100 | 550–870 | 170–8,800 | 200–660 | 210–1,600 | 1,500–6,300 | ||||
| Cd | Cap | 1.1 ± 1.6 | 3.2 ± 3.0 | 1.1 ± 0.8 | 1.8 ± 1.0 | 2.5 ± 2.9 | 3.2 ± 1.8 | 2.8 ± 1.4 | 2.4 ± 1.2 | 4.9 ± 3.0 | 1.9 ± 3.3 | 1.6 ± 1.3 |
| (0.54) | (2.1) | (0.9) | (1.8) | (1.0) | (2.8) | (2.9) | (1.8) | (3.7) | (0.49) | (1.2) | ||
| 0.36–6.0 | 0.77–11 | 0.40–3.1 | 0.75–3.8 | 0.72–8.7 | 1.4–8.1 | 0.88–5.1 | 0.74–4.3 | 2.3–10 | 0.29–9.0 | 0.69–5.5 | ||
| Stipe | 0.31 ± 0.19 | 1.2 ± 1.4 | 0.14 ± 0.21 | 0.32 ± 0.19 | 0.89 ± 0.77 | 0.88 ± 0.77 | 0.99 ± 0.47 | 0.84 ± 0.34 | 1.7 ± 1.1 | 0.43 ± 0.78 | 0.74 ± 0.31 | |
| (0.26) | (0.67) | (0.10) | (0.31) | (0.43) | (0.60) | (1.1) | (0.68) | (1.2) | (0.19) | (0.72) | ||
| 0.094–0.75 | 0.22–5.9 | 0.01–0.60 | 0.075–0.70) | 0.18–2.2 | 0.22–3.1 | 0.39–1.8 | 0.32–1.4 | 0.67–3.5 | 0.68–3.1 | 0.25–1.4 | ||
| Soil | 0.17 ± 0.06 | 0.088 ± 0.009 | 0.070 ± 0.018 | 0.13 ± 0.04 | 0.10 ± 0.01 | 0.10 ± 0.06 | 0.070 ± 0.016 | 0.097 ± 0.032 | 0.19 ± 0.08 | NA | NA | |
| (0.16) | (0.083) | (0.068) | (0.12) | (0.10) | (0.083) | (0.073) | (0.10) | (0.16) | ||||
| 0.11–0.24 | 0.082–0.10 | 0.044–0.094 | 0.087–0.19 | 0.088–0.12 | 0.057–0.21 | 0.003–0.051 | 0.062–0.15 | 0.11–0.28 | ||||
| Co | Cap | 0.22 ± 0.07 | 0.11 ± 0.11 | 0.20 ± 0.06 | 0.19 ± 0.07 | NA | 0.10 ± 0.10 | 0.16 ± 0.15 | NA | NA | 0.29 ± 0.17 | 0.17 ± 0.03 |
| (0.20) | (0.06) | (0.19) | (0.20) | (0.11) | (0.11) | (0.23) | (0.16) | |||||
| 0.10–0.40 | 0.01–0.39 | 0.15–0.32 | 0.10–0.30 | 0.01–0.33 | 0.01–0.44 | 0.01–0.56 | 0.11–0.23 | |||||
| Stipe | 0.36 ± 0.12 | 0.14 ± 0.18 | 0.28 ± 0.13 | 0.30 ± 0.16 | NA | 0.18 ± 0.26 | 0.14 ± 0.13 | NA | NA | 0.27 ± 0.16 | 0.12 ± 0.03 | |
| (0.37) | (0.06) | (0.25) | (0.28) | (0.11) | (0.11) | (0.19) | (0.12) | |||||
| 0.20–0.65 | 0.01–0.50 | 0.15–0.54 | 0.10–6.3 | 0.01–1.0 | 0.01–0.45 | 0.094–0.52 | 0.064–0.17 | |||||
| Soil | 0.48 ± 0.03 | 0.39 ± 0.05 | 0.73 ± 0.09 | 0.84 ± 0.93 | 0.96 ± 0.19 | 1.1 ± 0.5 | 0.63 ± 0.17 | 0.60 ± 0.11 | 0.52 ± 0.16 | NA | NA | |
| (0.48) | (0.39) | (0.72) | (0.41) | )1.0) | (0.94) | (0.66) | (0.60) | (0.48) | ||||
| 0.45–0.52 | 0.33–0.46 | 0.64–0.87 | 0.22–2.6 | 0.65–1.1 | 0.67–1.8 | 0.39–0.84 | 0.45–0.73 | 0.39–0.81 | ||||
| Cr | Cap | 0.52 ± 0.08 | 0.44 ± 0.12 | 0.80 ± 0.54 | 0.62 ± 0.28 | NA | 0.10 ± 0.16 | <0.01 | NA | NA | 0.26 ± 0.10 | 0.13 ± 0.05 |
| (0.50) | (0.44) | (0.70) | (0.52) | (0.05) | (0.24) | (0.14) | ||||||
| 0.44–0.70 | 0.22–0.72 | 0.49–1.6 | 0.35–1.2 | 0.01–0.44 | 0.15–0.59 | 0.077–0.21 | ||||||
| Stipe | 0.26 ± 0.11 | 0.32 ± 0.10 | 1.2 ± 1.0 | 0.76 ± 0.43 | NA | 0.20 ± 0.06 | <0.01 | NA | NA | 0.42 ± 0.27 | 0.11 ± 0.03 | |
| (0.23) | (0.33) | (0.80) | (0.65) | (0.19) | (0.33) | (0.10) | ||||||
| 0.18–0.63 | 0.11–0.54 | 0.45–3.1 | 0.20–1.4 | 0.11–0.38 | 0.16–1.1 | 0.071–0.16 | ||||||
| Soil | 1.4 ± 0.3 | 1.4 ± 0.1 | 1.5 ± 0.3 | 0.92 ± 0.18 | 1.7 ± 0.4 | 2.6 ± 0.3 | 2.0 ± 0.2 | 1.1 ± 0.3 | 1.9 ± 0.7 | NA | NA | |
| (1.4) | (1.4) | (1.4) | (0.95) | (1.8) | (2.6) | (2.1) | (1.1) | (1.7) | ||||
| 1.1–1.8 | 1.2–1.5 | 1.2–1.9 | 0.60–1.1 | 1.1–2.1 | 2.2–3.0 | 1.7–2.2 | 0.81–1.4 | 1.5–3.3 | ||||
| Cu | Cap | 130 ± 50 | 200 ± 60 | 100 ± 48 | 170 ± 76 | 150 ± 42 | 160 ± 43 | 140 ± 35 | 180 ± 71 | 140 ± 54 | 110 ± 27 | 130 ± 30 |
| (100) | (200) | (94) | (170) | (150) | (140) | (140) | (150) | (120) | (110) | (140) | ||
| 93–250 | 120–340 | 63–210 | 94–360 | 97–220 | 54–260 | 67–190 | 95–320 | 100–240 | 43–140 | 81–180 | ||
| Stipe | 77 ± 23 | 150 ± 62 | 100 ± 20 | 210 ± 60 | 96 ± 25 | 78 ± 14 | 91 ± 11 | 160 ± 46 | 100 ± 32 | 61 ± 15 | 100 ± 20 | |
| (67) | (150) | (100) | (190) | (97) | (68) | (90) | (150) | (110) | (58) | (100) | ||
| 53–120 | 61–310 | 71–130 | 130–310 | 62–140 | 54–140 | 71–110 | 110–270 | 61–140) | 30–90 | 76–140 | ||
| Soil | 5.7 ± 1.1 | 1.1 ± 0.6 | 1.3 ± 0.3 | 5.6 ± 2.6 | 2.2 ± 3.1 | 2.2 ± 1.4 | 0.95 ± 0.14 | 1.1 ± 0.4 | 1.6 ± 0.9 | NA | NA | |
| (5.8) | (0.80) | (1,2) | (5.6) | (0.80) | (1.6) | (0.93) | (1.1) | (1.3) | ||||
| 4.2–6.9 | 0.69–2.0 | 1.0–1.6 | 1.5–9.2 | 0.66–7.8 | 1.0–4.4 | 0.81–1.1 | 0.64–1.7 | 0.88–3.2 | ||||
| Fe | Cap | 41 ± 18 | 140 ± 56 | 260 ± 170 | 210 ± 170 | 97 ± 18 | 86 ± 23 | 80 ± 10 | 82 ± 50 | 77 ± 11 | 82 ± 40 | 84 ± 22 |
| (36) | (140) | (200) | (140) | (99) | (83) | (81) | (62) | (78) | (71) | (76) | ||
| 28–93 | 79–320 | 130–660 | 63–610 | 72–120 | 52–130 | 61–97 | 50–290 | 60–93 | 41–190 | 57–120 | ||
| Stipe | 32 ± 10 | 110 ± 50 | 480 ± 570 | 330 ± 250 | 86 ± 60 | 71 ± 36 | 49 ± 10 | 260 ± 190 | 48 ± 13 | 100 ± 75 | 52 ± 14 | |
| (29) | (110) | (180) | (240) | (59) | (59) | (48) | (200) | (45) | (77) | (52) | ||
| 19–58 | 110–200 | 65–1,600 | 28–750 | 37–200 | 28–170 | 35–80 | 48–870 | 38–72 | 35–280 | 29–80 | ||
| Soil | 2,400 ± 640 | 1,400 ± 160 | 1,400 ± 300 | 1,100 ± 550 | 2,000 ± 160 | 2,900 ± 580 | 2,400 ± 330 | 1,300 ± 260 | 1,800 ± 270 | NA | NA | |
| (2,300) | (1,400) | (1,300) | (1,100) | (2,000) | (2,800) | (2,300) | (1,300) | (2,100) | ||||
| 1,800–3,100 | 1,300–1,700 | 1,200–1,900 | 540–2,100 | 1,700–2,100 | 2,300–3,700 | 2,000–2,900 | 940–1,500 | 1,800–2,100 | ||||
| Hg* | Cap | (3.7) | (5.7) | (2.9) | (5.2) | (3.5) | (6.5) | (7.0) | (2.7) | NA | (4.6) | (1.3) |
| Mn | Cap | 25 ± 10 | 22 ± 9 | 22 ± 12 | 32 ± 31 | 29 ± 16 | 22 ± 14 | 21 ± 6 | 15 ± 8 | 11 ± 2 | 33 ± 31 | 15 ± 3 |
| (22) | (19) | (17) | (23) | (21) | (20) | (23) | (11) | (11) | (24) | (15) | ||
| 13–41 | 13–44 | 13–50 | 7.3–110 | 15–56 | 8.8–60 | 10–28 | 7/2–31 | 9.5–15 | 14–150 | 9.3–20 | ||
| Stipe | 32 ± 13 | 23 ± 9 | 33 ± 22 | 55 ± 39 | 53 ± 25 | 29 ± 21 | 39 ± 18 | 51 ± 33 | 11 ± 6 | 52 ± 59 | 12 ± 6 | |
| (30) | (20) | (37) | (45) | (46) | (28) | (36) | (43) | (9.3) | 36 | (11) | ||
| 17–56 | 14–42 | 5.8–59 | 17–150 | 24–93 | 4.4–80 | 8.0–64 | 14–120 | 7.2–22 | 16–260 | 5.2–25 | ||
| Soil | 140 ± 82 | 110 ± 18 | 95 ± 16 | 140 ± 63 | 240 ± 30 | 170 ± 89 | 130 ± 61 | 190 ± 66 | 160 ± 82 | NA | NA | |
| (130) | (110) | (94) | (150) | (250) | (160) | (130) | (180) | (170) | ||||
| 65–220 | 85–130 | 78–110 | 73–220 | 190–260 | 67–310 | 68–220 | 110–310 | 74–290 | ||||
| Na | Cap | 80 ± 120 | 170 ± 200 | 140 ± 52 | 150 ± 92 | 120 ± 94 | NA | 250 ± 140 | 190 ± 200 | 72 ± 27 | 80 ± 110 | 59 ± 35 |
| (20) | (77) | (140) | (120) | (60) | (290) | (45) | (71) | (37) | (49) | |||
| 15–440 | 36–580 | 84–160 | 75–380 | 38–300 | 54–510 | 10–560 | 43–110 | 16–220 | 25–160 | |||
| Stipe | 150 ± 140 | 400 ± 430 | 570 ± 400 | 740 ± 300 | 160 ± 180 | NA | 650 ± 390 | 710 ± 870 | 210 ± 150 | 82 ± 63 | 490 ± 440 | |
| (79) | (210) | (420) | (740) | (55) | (670) | (180) | (150) | (56) | (310) | |||
| 45–460 | 77–1,600 | 280–1,400 | 240–1,100 | 37–480 | 87–1,300 | 28–3,100 | 71–460 | 29–240 | 86–1,400 | |||
| Soil | 15 ± 2 | 6.9 ± 0.6 | 8.7 ± 2.5 | 11 ± 4 | 7.2 ± 1.2 | 9.7 ± 5.8 | 5.7 ± 1.4 | 3.4 ± 0.8 | 24 ± 22 | NA | NA | |
| (14) | (6.9) | (9.4) | (12) | (6.5) | (7.2) | (5.0) | (3.2) | (15) | ||||
| 13–18 | 6.1–7.8 | 8.2–12 | 4.7–17 | 6.3–8.9 | 3.7–17 | 4.5–7.8 | 2.6–4.6 | 13–70 | ||||
| Ni | Cap | 0.43 ± 0.50 | 0.057 ± 0.086 | 0.39 ± 0.40 | 0.31 ± 0.36 | NA | <0.01 | <0.01 | NA | NA | 1.1 ± 0.3 | 0.15 ± 0.22 |
| (0.25) | (0.030) | (0.39) | (0.23) | (1.1) | (0.01) | |||||||
| 0.05–2.0 | 0.01–0.33 | 0.01–1.2 | 0.01–1.1) | 0.66–1.6 | 0.01–0.69 | |||||||
| Stipe | 0.11 ± 0.18 | 0.38 ± 0.35 | 0.32 ± 0.27 | 0.41 ± 0.36 | NA | <0.01 | <0.01 | NA | NA | 1.3 ± 0.5 | 0.079 ± 0.15 | |
| (0.01) | (0.29) | (0.35) | (0.40) | (1.3) | (0.02) | |||||||
| 0.01–0.63 | 0.01–1.3 | 0.01–0.80 | 0.01–1.2 | 0.48–2.3 | 0.01–0.57 | |||||||
| Soil | 1.4 ± 0.3 | 0.74 ± 0.12 | 1.1 ± 0.2 | 1.8 ± 1.4 | 0.96 ± 0.20 | 2.1 ± 0.9 | 1.0 ± 0.2 | 0.93 ± 0.29 | 1.4 ± 0.6 | NA | NA | |
| (1.4) | (0.69) | (1.0) | (1.0) | (1.0) | (1.5) | (1.1) | (0.84) | (1.2) | ||||
| 1.1–1.8 | 0.62–0.89 | 0.75–1.4 | 0.72–4.4 | 0.65–1.1 | 1.3–3.4 | 0.65–1.2 | 0.65–1.3 | 0.97–2.5 | ||||
| Pb | Cap | 2.3 ± 0.9 | 3.5 ± 1.5 | 4.2 ± 0.8 | 5.1 ± 1.3 | NA | 2.8 ± 4.8 | NA | NA | NA | 1.9 ± 1.4 | 8.5 ± 2.4 |
| (2.5) | (3.3) | (4.1) | (5.2) | (1.4) | (1.5) | (8.9) | ||||||
| 1.0–3.6 | 1.4–6.4 | 2.7–5.6 | 3.3–6.9 | 0.02–18 | 0.81–6.8 | 0.85–10 | ||||||
| Stipe | 2.5 ± 0.3 | 1.4 ± 0.8 | 3.0 ± 0.9 | 3.8 ± 1.1 | NA | 1.5 ± 1.2 | NA | NA | NA | 0.89 ± 0.49 | 5.2 ± 2.1 | |
| (2.4) | (1.2) | (3.1) | (3.9) | (1.2) | (0.84) | (5.2) | ||||||
| 2.1–3.1 | 0.66–3.2 | 1.8–4.2 | 2.1–5.1 | 0.49–4.7 | 0.33–1.9 | 0.10–8.0 | ||||||
| Soil | 13 ± 3 | 8.3 ± 2.1 | 6.8 ± 1.4 | 8.6 ± 3.3 | 7.1 ± 1.4 | 12 ± 4 | 10 ± 1 | 7.9 ± 1.6 | 16 ± 6 | NA | NA | |
| (12) | (7.4) | (6.1) | (7.6) | (7.4) | (10) | (10) | (7.8) | (17) | ||||
| 11–16 | 6.9–12 | 6.9–9.3 | 5.4–16 | 5.0–8.4 | 7.9–16 | 8.9–11 | 5.9–10 | 11–21 | ||||
| Rb | Cap | 300 ± 150 | 120 ± 48 | 130 ± 69 | 190 ± 55 | 41± ± 16 | 110 ± 46 | 180 ± 67 | 26 ± 21 | NA | NA | NA |
| (230) | (120) | (110) | (210) | (38) | (100) | (180) | (18) | |||||
| 150–660 | 41–200 | 56–290 | 100–280 | 26–71 | 41–220 | 40–280 | 5.5–62 | |||||
| Stipe | 140 ± 37 | 69 ± 39 | 76 ± 48 | 110 ± 46 | 24 ± 8 | 37 ± 14 | 80 ± 24 | 14 ± 10 | NA | NA | NA | |
| (150) | (59) | (66) | (120) | (22) | (35) | (85) | (12) | |||||
| 79–200 | 19–180 | 37–180 | 49–200 | 14–38 | 16–71 | 23–120 | 5.2–42 | |||||
| Soil | 1.4 ± 0.2 | 1.9 ± 0.2 | 3.0 ± 0.6 | 3.5 ± 1.3 | 4.1 ± 1.1 | 5.9 ± 2.6 | 3.3 ± 0.8 | 2.9 ± 0.9 | 3.0 ± 3.2 | NA | NA | |
| (1.4) | (1.8) | (2.9) | (2.8) | (4.5) | (5.4) | (3.4) | (2.9) | (1.6) | ||||
| 1.2–1.7 | 1.8–2.2 | 2.5–4.0 | 2.2–5.9 | 2.3–5.1 | 2.9–9.2 | 2.1–4.3 | 1.7–3.9 | 1.3–10 | ||||
| Sr | Cap | 0.44 ± 0.47 | 0.37 ± 0.19 | 0.80 ± 0.36 | 1.3 ± 1.1 | 0.59 ± 0.25 | 0.67 ± 0.12 | 0.61 ± 0.14 | 0.23 ± 0.23 | 0.25 ± 0.18 | 0.49 ± 0.25 | 0.30 ± 0.07 |
| (0.20) | (0.32) | (0.73) | (0.89) | (0.56) | (0.69) | (0.56) | (0.19) | (0.18) | (0.45) | (0.38) | ||
| 0.05–1.5 | 0.17–0.83 | 0.39–1.6 | 0.30–3.4 | 0.23–0.90 | 0.50–0.88 | 0.50–0.99 | 0.058–0.96 | 0.10–0.58 | 0.23–1.1 | 0.11–0.41 | ||
| Stipe | 0.65 ± 0.40 | 0.30 ± 0.16 | 1.5 ± 1.0 | 2.9 ± 1.6 | 0.72 ± 0.25 | 0.66 ± 0.14 | 0.72 ± 0.15 | 1.2 ± 0.6 | 0.29 ± 0.20 | 0.68 ± 0.83 | 0.29 ± 0.14 | |
| (0.43) | (0.27) | (1.3) | (2.4) | (0.79) | (0.63) | (0.67) | (1.1) | (0.20) | (0.43) | (0.23) | ||
| 0.26–1.4 | 0.11–0.61 | 0.55–3.0 | 0.70–4.9 | 0.38–1.2 | 0.48–0.99 | 0.56–1.1 | 0.46–2.5 | 0.11–0.61 | 0.17–3.6 | 0.12–0.55 | ||
| Soil | 4.5 ± 1.3 | 0.96 ± 0.20 | 2.3 ± 0.8 | 3.7 ± 1.1 | 2.0 ± 0.3 | 4.2 ± 4.2 | 1.1 ± 0.6 | 2.5 ± 1.1 | 4.8 ± 1.7 | NA | NA | |
| (4.2) | (0.83) | (2.2) | (6.1) | (1.9) | (1.5) | (0.90) | (2.8) | (5.1) | ||||
| 3.2–6.0 | 0.80–1.2 | 1.4–3.4 | 3.6–6.7 | 1.7–2.4 | 0.76–9.0 | 0.82–2.3 | 1.1–3.8 | 2.8–6.7 | ||||
| Zn | Cap | 190 ± 45 | 93 ± 24 | 100 ± 11 | 120 ± 22 | 140 ± 57 | 92 ± 20 | 120 ± 20 | 74 ± 6 | 110 ± 35 | 92 ± 21 | 75 ± 13 |
| (200) | (90) | (100) | (120) | (150) | (95) | (120) | (73) | (100) | (98) | (77) | ||
| 73–240 | 67–140 | 93–120 | 85–170 | 51–220 | 64–120 | 91–170 | 64–85 | 83–180 | 54–120 | 70–100 | ||
| Stipe | 110 ± 17 | 57 ± 12 | 65 ± 12 | 67 ± 16 | 87 ± 24 | 48 ± 12 | 58 ± 11 | 49 ± 8 | 54 ± 7 | 67 ± 20 | 46 ± 12 | |
| (110) | (57) | (60) | (64) | (100) | (47) | (55) | (52) | (54) | (60) | (49) | ||
| 71–140 | 29–75 | 53–80 | 43–96 | 44–100 | 31–69 | 42–79 | 34–65 | 43–62 | 45–100 | 22–62 | ||
| Soil | 13 ± 5 | 6.7 ± 0.9 | 10 ± 2 | 46 ± 28 | 12 ± 2 | 17 ± 10 | 7.3 ± 1.0 | 10 ± 4 | 18 ± 8 | NA | NA | |
| (13) | (6.4) | (9.6) | (45) | (12) | (11) | (7.1) | (10) | (16) | ||||
| 7.9–18 | 5.9–7.7 | 9.0–14 | 15–100 | 10–15 | 8.0–30 | 6.0–8.7 | 5.4–Q17 | 11–30 | ||||
aNot analyzed
Mineral constituent uptake rate from the soil to fruiting bodies was assessed using the common concept of transfer factor that is commonly also expressed as an enrichment factor or BCF, which is simply the mushroom’s constituent concentration divided by the soil (or other substratum media) mineral constituent concentration.
All data produced were statistically treated to find possible statistically significant differences between the variables with the aid of the nonparametric tests: Kruskal–Wallis and Mann–Whitney U, principal component analysis (PCA), and cluster analysis (CA) (Figs. 2, 3 and 4; online resource, Figs. A.2–A.5). The computer software package Statistica version 8.0 used was for statistical analysis of data.
Fig. 2.
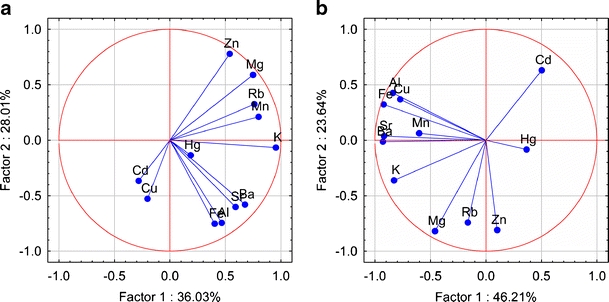
Plot of loadings (unrotated) based on the concentration of metallic elements in caps (a) and stipes (b) of Parasol Mushrooms in the space of the first and second factors
Fig. 3.
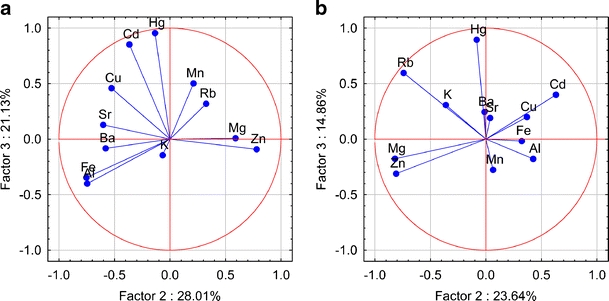
Plot of loadings (unrotated) based on the concentration of metallic elements in caps (a) and stipes (b) of Parasol Mushrooms in the space of the second and third factors
Fig. 4.
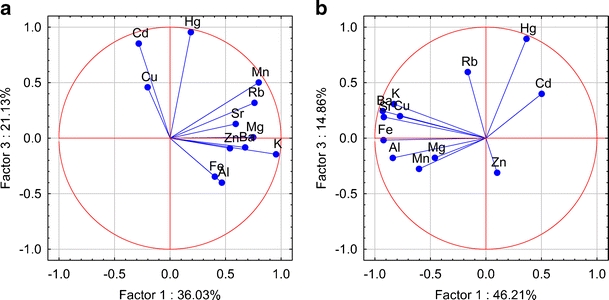
Plot of loadings (unrotated) based on the concentration of metallic elements in caps (a) and stipes (b) of Parasol Mushrooms in the space of the first and third factors
Results and discussion
Macroelements (K, P, Mg, Ca, Na)
Potassium
Both the caps and stipes of Parasol Mushrooms can be considered rich in K. Good sources of K for biota are the caps of Parasol Mushrooms. The median concentrations varied in the caps between 25 and 47 mg g−1 dw for the sites surveyed. These values show a certain degree of variability (p < 0.01; Mann–Whitney U test) in the supplementation of caps with K, when considering their use as food. Caps are characterized by a greater content of K compared to stipes. The median values of cap to stipe K concentration quotient (Q C/S) for these sites were between 1.1 and 2.1, and the maximum value in a single specimen was 5.2. Nevertheless, both the caps and stipes of Parasol Mushrooms are highly enriched with K in relation to its content in soil, where the median values for the 11 sites varied between 0.074 and 0.25 mg g−1 dw (Table 1). The BCF median values of K for the caps in this study were between 120 and 500, and up to 1,100 in a single cap and up to 650 in a single stipe (online resource, Table A.1).
Phosphorous
In soil, the median values of P were between 0.13 and 0.28 mg g−1 dw. An absolute concentration value between the stands per site usually varied twofold and maximally up to threefold for one site. For all stands, this variation was around fourfold, i.e., were 0.093–0.41 mg g−1 dw. P occurred in the caps of Parasol Mushrooms at six sites between 8.4 and 18 mg g−1 dw, and the median values were between 10 and 16 mg g−1 dw. These caps, which are nearly twice more abundant in P compared to stipes and medians of Q C/S, were between 1.7 and 2.3 and having a total range from 1.1 to 3.1. This mushroom assimilates P well from the surrounding substratum. The values of BCF of P and single caps were between 23 and 150, while medians varied significantly (45–100; p < 0.01) between the sites (online resource, Table A.1).
Magnesium
Soil Mg was found at the median concentrations, varying between 0.13 and 0.36 mg g−1 dw, and for single stands, usually <0.50 mg g−1 dw. Caps contained Mg at a relatively narrow concentration range with the median values varying between 1.0 and 2.0 mg g−1 dw (total, 0.75–2.3 mg g−1 dw). Mg in caps was from 1.3 to 2.1 in excess (median values), when compared to stipes (total Q C/S range from 0.99 to 5.5). This, essentially, to fungi and animal metal, is well taken up by Parasol Mushrooms and is sequestered in its caps (median BCF values per site varied between 3.3 and 11; total 0.86–14) and stipes (median BCF values per site varied between 0.92 and 6.3; online resource, Table A.1).
Calcium
Soils varied in its content of Ca more than the other macroelements determined. The median values of Ca concentrations varied between 620 and 2,900 μg g−1 dw soil at six sites and were much less, i.e., between 200 and 360 μg g−1 dw, at three other sites (Table 1). The caps and stipes of this species are relatively abundant in Ca, and the median concentration values varied between 54 and 230 μg g−1 dw and 70 and 860 μg g−1 dw, respectively. For most of the sites surveyed, the stipes were richer in Ca compared to the caps, and the median values of Q C/S were between 0.19 and 1.3. In terms of bioconcentration concept, Ca is excluded in Parasol Mushrooms. The median BCF values of this element in fruiting bodies at the sites surveyed varied between 0.036 and 0.36 for the caps and between 0.047 and 0.62 for the stipes (online resource, Table A.1).
Sodium
This metal occurred in the soil substratum of Parasol Mushrooms with a median concentration varying between 3.2 and 15 μg g−1 dw. Apart from one site, the soil in the other sites contained Na in median concentration ≥5.0 μg g−1 dw (Table 1). The stipes were usually more abundant in Na than the caps, and the median concentration values were between 55 and 670 μg g−1 dw and 20 and 290 μg g−1 dw, respectively. For the Morąg and Łukta (4) sites, the caps and stipes had the same Na content on average. In other sites, the median values of Q C/S were between 0.14 and 0.49. Accordingly, due to highly variable soil and mushroom Na content, the assessed potential of Parasol Mushrooms to bioconcentrate this element varied between the sites. The median values of BCF were between 1.4 and 44 for caps and between 5.3 and 110 for stipes (online resource, Table A.1).
Essential trace metals (Cu, Fe, Mn, Zn)
Copper
Soil Cu content was around 1.0 μg g−1 dw in most of the sites examined, and in the Szczecinek (9) and Mierzeja Wiślana (3) sites, the median values were 5.8 and 5.6 μg g−1 dw. Both the caps and stipes of Parasol Mushrooms in forested sites of Northern Poland are rich in Cu—the median values were between 94 and 200 μg g−1 dw in caps and between 58 and 150 μg g−1 dw in stipes. Depending on the site surveyed, the median values of Q C/S were between 0.81 and 1.8. Cu is highly enriched in Parasol Mushrooms. The median values of Cu BCF for caps at the sites with relatively small soil Cu concentration were between 70 and 220, while 22 and 27 were in two sites mentioned, where soil is relatively rich in this element (Table 1; online resource, Table A.1). These differences suggest good and active regulation of Cu uptake and sequestration in maturating fruiting bodies, and this happens according to the physiological needs of Parasol Mushrooms, when emerging at the background (uncontaminated with Cu and other metals) areas.
Iron
This metal is abundant in the soil substratum to Parasol Mushrooms. Its median concentration values varied for the sites between 1,100 and 2,900 μg g−1 dw. These absolute concentration data noted for Fe in the top layer of the soil are of the same order of magnitude as Ca, while the caps and stipes of the mushroom are more abundant in Ca than Fe, on average. In caps and stipes, Fe occurred in the median concentrations between 36 and 200 μg g−1 dw and 29 and 200 μg g−1 dw, respectively. These concentrations varied largely between the specimens—in caps, between 41 and 660 μg g−1 dw and in stipes, between 19 and 600 μg g−1 dw. For some sampling sites, an abundance of Fe in Parasol Mushrooms was similar (p > 0.05) but for some varied highly (p < 0.01) (Table 1). Fe was excluded in this species (BCF < 1). The caps of Parasol Mushrooms usually contained Fe in greater concentration when compared to stipes, and the median value of Q C/S for most of the sites was from 1.2 to 1.6 and at two sites was 0.34 and 0.64 (online resource, Table A.1).
Manganese
The soil substratum Mn concentrations varied between 94 and 250 μg g−1 dw (median values), and the total range was from 67 to 310 μg g−1 dw. Mn, in terms of the bioconcentration/exclusion concept, is bio-excluded in Parasol Mushrooms. This is because the median BCF values, as calculated for caps and stipes of fruiting bodies from all the sites surveyed in this study, were <0.2 and <0.3, and the maximum values for two single samples were 0.89 and 0.86, respectively (online resource, Table A.1). This mushroom is moderately abundant in Mn. The median values of Mn in Parasol Mushroom from the sites in Northern Poland varied between 11 and 24 μg g−1 dw (total, 7.2–66 μg g−1 dw) in caps and 9.3 and 46 μg g−1 dw (4.4–260 μg g−1 dw) in stipes (Table 1).
Zinc
Soil Zn concentrations varied significantly (p < 0.01; Mann–Whitney U test) between some of the sites. The median values for Zn in soil in the eight sites varied between 6.4 and 16 μg g−1 dw (total range, 5.9–30 μg g−1 dw). For the stands of Parasol Mushrooms in the Mierzeja Wiślana (3) site, the median value in soil was 45 μg Zn g−1 dw, which is significantly greater (p < 0.001; Mann–Whitney U test) than elsewhere in this survey. The Parasol Mushroom contains Zn in the caps in greater concentration than in the stipes, and the Q C/S values were between 1.5 and 2.0 per site (total range, 0.62–4.2). The median concentration values were between 73 and 200 μg Zn g−1 dw (total, 64–240 μg Zn g−1 dw) for caps and 47 and 110 μg g−1 dw (total 22–140 μg g−1 dw) for stipes. This metal is moderately bioconcentrated in fruiting bodies, and the median BCF values were between 3.7 and 15 for caps and 1.8 and 7.7 for stipes (online resource, Table A.1).
These macro and trace metals of nutritional value occurred in the Parasol Mushroom’s fresh caps (assuming 10% dry weight) at the sites surveyed at the median concentrations (micrograms per gram wet weight (ww)) varying between 2,500 and 4,700 (K), 13 and 28 (P), 13 and 36 (Mg), 62 and 290 (Ca), 0.32 and 1.5 (Na), 9.4 and 20 (Cu), 3.6 and 20 (Fe), 1.1 and 2.4 (Mn), and 4.7 and 10 (Zn). Estimated, the essential mineral intake, resulting from the consumption of a 300- or 500-g portion of cap from a particular site varied between 750 and 2,300 mg K, 3.9–14 mg P, 3.9–15 mg Mg, 19–140 mg Ca, 2.8–10 mg Cu, 1.1–10 mg Fe, 0.33–1.2 mg Mn, 1.4–5.0 mg Zn, and 96–750 μg Na.
Some other trace metals (Ag, Al, Ba, Co, Cr, Ni, Rb, Sr)
Silver
This metal occurred in very small concentrations in the top layer of soil substrates to Parasol Mushroom but was very efficiently taken up and sequestered in fruiting bodies. The median values of Ag concentrations in soil varied among the sites between 0.0076 and 0.038 μg g−1 dw. At no one single stand did the Ag content of soil exceed 0.1 μg g−1 dw (Table 1). On the other hand, the fruiting bodies were relatively abundant in this metal, and the median values of Ag concentrations in caps varied between 0.065 and 4.8 μg g−1 dw and in stipes between 1.0 and 3.9 μg g−1 dw. This efficient accumulation of Ag in relation to its very small abundance in soil resulted in high BCF, with the median values varying between 67 and 420 for caps and 85 and 440 for stipes. Ag was not uniformly distributed between the caps and stipes of fruiting bodies. At two sites, evidently, caps were more enriched in Ag when compared to stipes, and an opposite can be noted for some other sites (online resource, Table A.1).
Aluminum
Soils from the Parasol Mushroom stands at the eight sites contained in the top layer Al in concentrations varying between 1,100 and 2,700 μg g−1 dw (median values), and much less, i.e., 570 μg g−1 dw, was in the Mierzeja Wiślana (3) site which is localized near the Baltic Sea coast. This metal is bio-excluded in fruiting bodies by the Parasol Mushroom. Nevertheless, the median BCF value of Al for caps (0.62) and stipes (1.4) of the fruiting bodies at the Mierzeja Wiślana (3) site are evidently higher, when compared to the sites with soil more abundant in Al—the medians of BCF < 0.3 (online resource, Table A.1). This observation implies the better availability of Al to Parasol Mushroom, when Al is a less abundant component of the soil substratum, while there is no evidence on its essentiality to mushrooms. Al, on average, is equally distributed between the caps and stipes of fruiting bodies, but some variations between specimens and sites can be noted (Table 1).
Barium
The median values of Ba concentration of soil substrates varied in the sites between 8.9 and 23 μg g−1 dw (total range from 7.2 to 42 μg g−1 dw). These concentrations are greater compared to that determined to closely related element strontium. The caps and stipes of fruiting bodies of Parasol Mushrooms, depending on the site of collection, varied in Ba content (p < 0.01). The median concentration values of Ba were between 0.30 and 1.3 μg g−1 dw (total, 0.10 and 11 μg g−1 dw) in caps and between 0.41 and 7.0 μg g−1 dw (total, 0.21 and 14 μg g−1 dw) in stipes. These data show that Ba, which is more or less equally distributed between the cap and stipe of fruiting bodies, is bio-excluded by Parasol Mushroom in fruiting bodies, and for both caps and stipes BCF was <1 (online resource, Table A.1).
Cobalt
Soils in the sites surveyed contained Co in concentrations varying between 0.39 and 1.0 μg g−1 dw (median values). Cobalt in fruiting bodies of Parasol Mushroom is a minor trace metal, and its median concentration values varied between 0.06 and 0.23 in caps and 0.06 and 0.37 μg g−1 dw in stipes. In light of these data, Co was bio-excluded by Parasol Mushroom, and a value of BCF for this metal was <0.5 both in caps and stipes. The distribution of Co between the cap and stipe of the fruiting bodies was equal for mushrooms from most of the sites, but the range of median values of Q C/S was between 0.61 and 1.4 (online resource, Table A.1).
Chromium
This metal occurred as trace compound in all soil substrates sampled. The median values of its concentrations varied for the sites between 0.95 and 2.6 μg g−1 dw, and the maximum concentration in a single stand was up to 3.3 μg g−1 dw. Cr was a minor trace metal in Parasol Mushroom in this study. The fruiting bodies at the sites surveyed contained Cr in the caps and stipes in concentrations between 0.14 and 0.70 μg g−1 dw and 0.10 and 0.80 μg g−1 dw, respectively (median values). The median values of Q C/S in the case of Cr varied per site between 0.31 and 2.1. Since the BCF values of Cr were <1 for caps and stipes, this metal can be considered as bio-excluded in fruiting bodies by the Parasol Mushroom.
Nickel
This element is one of the minor metallic elements determined in Parasol Mushrooms. Specimens collected at the Kętrzyn (5), Szczecinek (9), and Trójmiejski Park Krajobrazowy (2) sites from the northern region of Poland were more abundant (p < 0.05; Mann–Whitney U test) in nickel (1.1 ± 0.3, 0.43 ± 0.50, and 0.39 ± 0.40 μg g−1 dw in caps and 1.3 ± 0.5, 0.32 ± 0.27, and 0.11 ± 0.18 μg g−1 dw in stipes, respectively) when compared to other sites surveyed (Table 1). Both the caps and stipes of Parasol Mushrooms from the Trójmiejski Park Krajobrazowy (2), Mierzeja Wiślana (3), and Kętrzyn (5) sites contain nickel in comparable concentrations, and the Q C/S value for this metal was ∼1 (online resource, Table A.1). Nickel in the background areas is among the metallic elements that are bio-excluded by Parasol Mushroom (BCF < 1; online resource, Table A.1).
Rubidium
Soil Rb content differed between some of the sites (p < 0.01; Mann–Whitney U test), and the median concentration values varied between 1.4 and 5.4 μg g−1 dw (total range, 1.2–10 μg g−1 dw). The fruiting bodies contained Rb in the caps in concentrations around twice greater than in stipes (range of the median Q C/S values was from 1.4 to 3.1). The median values of Rb concentration in caps varied between 100 and 230 μg g−1 dw for six sites and in stipes for these sites was between 35 and 150 μg g−1 dw. At the Morąg/Łukta (4) and Rafa (11) sites, Rb abundance of fruiting bodies was much lower than elsewhere in this survey, i.e., in these two sites they contained 18 and 38 μg g−1 dw in caps and 12 and 22 μg g−1 dw in stipes, respectively. Rb is relatively well bioconcentrated by the Parasol Mushroom. The BCF median values in the fruiting bodies varied between 10 and 170 for the caps and 5.1 and 97 for the stipes (online resource, Table A.1).
Strontium
The sites varied in Sr content in the soil substratum to Parasol Mushroom (p < 0.01), and the median values were between 0.83 and 5.1 μg g−1 dw (total range, 0.76–6.1 μg g−1 dw). Sr is bio-excluded by this mushroom in fruiting bodies, and the median values of BCF of this metal for caps and stipes were <1. Hence, Sr content in fruiting bodies of Parasol Mushroom was low. The median concentration values in caps varied between 0.18 and 0.89 (0.05–3.4) and in stipes between 0.23 and 2.4 (0.11–4.9) μg g−1 dw.
Toxic metals (Cd, Pb, Hg)
Cadmium
The median values of Cd concentration in soil substrates to Parasol Mushroom at the sites surveyed varied between 0.068 and 0.16 μg g−1 dw, and the maximum value per stand was 0.28 μg g−1 dw. This mushroom is able to efficiently uptake Cd from the surrounding environment and sequester in fruiting bodies. The BCF for caps was between 3.3 and 36 and up to 140 per single cap (online resource, Table A.1). The median concentrations of Cd in caps were between 0.49 and 3.7 μg g−1 dw and in stipes between 0.10 and 1.2 μg g−1 dw. Caps were at least twice more abundant in cadmium than stipes, on average, and up to 22 per single cap (Table 1).
Cadmium in the flesh of edible wild mushrooms is among the toxic substances of concern. In the European Union, the maximum level of cadmium in cultivated mushrooms (Oyster Mushroom Pleurotus ostreatus, Champignon Mushroom Agaricus bisporus, and Shiitake Lentinus edodes) is 0.20 μg g−1 fresh weight (fw) (equivalent to 2.0 μg g−1 fw, assuming 90% moisture) and for other fungi is 1.0 μg g−1 fw (10 μg g−1 dw) (EU 2008). In light of the European Commission regulations on Cd, the median values of cadmium concentrations in caps were well below 10 μg g−1 dw. In three of 141 caps examined, Cd concentration was between 9.0 and 11 μg g−1 dw (Table 1). The provisional tolerable weekly intake (PTWI) for cadmium is 7 μg kg−1 body weight (bw) (equivalent to 1 μg kg−1 bw per day), and a tolerable weekly intake (TWI) of 2.5 μg kg−1 bw (equivalent to 0.36 μg kg−1 bw per day) (European Food Safety Authority EFSA 2009; World Health Organization WHO 1989). Estimated cadmium intake resulting from the consumption of a 300- or 500-g portion of caps is 4.7–111 and 24.5–185 μg (median Cd content between 0.49 and 0.37 μg g−1 wet weight, assuming the water content is 90%), and what is equivalent to 0.073–1.85 and 0.41–3.1 μg kg−1 body weight (an adult of 60 kg bw). As mentioned earlier, caps of Parasol Mushrooms can be large in size and are highly valued by the mushrooming fanciers, and all specimens found are collected. No doubt, some individuals from the local countryside populations and the cottage house owners or the camp tourists, temporarily, eat them even several times in a week in the summer time. Hence, in a good “mushroom” year an abundance of this species will lead to a short time (2 weeks to up to 2 months) exposure of fanciers to elevated Cd doses that exceed the PTWI and TWI recommendations.
The assessed doses show that a single meal composed of 300- or 500-g caps at some sites surveyed will result in exceeding the PTWI and revised TWI rates for Cd. A few studies on bioavailability of Cd from the mushroom meals gave results leading to an opposite conclusions. In two studies (Diehl and Schlemmer 1984; Schellmann et al. 1980) absorption of Cd was below 10%, while much more effective in three other studies (Lind et al. 1995; Mitra et al. 1995; Seeger et al. 1986). Hence, Cd in caps of Parasol Mushrooms at some of the sites surveyed might be of concern for consumers, if eaten frequently by an individual in the mushrooming season.
Lead
Pb is bio-excluded by Parasol Mushroom (BCF <1). Soil contained Pb in concentrations varying between 6.1 and 17 μg g−1 dw (medians), while up to 21 μg g−1 dw was per a single stand (online resource, Table A.1). For caps, Pb concentrations varied between 1.4 and 8.9 μg g−1 dw (medians) per site. Caps were from 0.86 to 3.1 (Q C/S) more abundant in Pb than stipes, which contained between 0.84 and 5.2 μg g−1 dw (medians). In the case of Pb, the maximum level permitted by EU regulation in cultivated mushrooms (Oyster Mushroom, Champignon Mushroom, and Shiitake) is 0.3 μg g−1 fw (3.0 μg g−1 dw; assuming 90% moisture) (EU 2008). Pb content in other fungi (assuming that other than the mentioned three species in the case of cultivated mushrooms as well as all edible species of wild-grown mushrooms) is not regulated by the EU law. The acceptable daily intake of lead for adults is between 210 and 250 μg per day and 1,500 and 1,750 μg per week or 25 and 29 μg per kg bw weekly for an individual of 60 kg bw (World Health Organization WHO 1993). Estimated Pb intake from consumption of a 300- or 500-g cap was 42–267 and 70–445 μg per meal, respectively. These values are based on the median concentrations of Pb found in caps in this survey. Hence, the Pb intake rates show a cause for concern associated with Pb resulting from the consumption of between 300- and 500-g caps daily, on a frequent basis in the mushrooming season but at some of the sites surveyed only.
Mercury
The BCF values of mercury in the literature have been reported to be in the range from 16 ± 6 to 220 ± 110 (total range from 0.52 to 470) for the caps and from 7.6 ± 2.6 to 130 ± 96 (total range from 0.52 to 340) for the stipes (Falandysz et al. 2007a; Falandysz and Gucia 2008). The PTWI for Hg is 300 μg (equivalent to 5 or 0.7 μg kg−1 bw per day), and a reference dose is 0.3 μg kg−1 bw per day (JECFA 1978; US EPA 2005). Parasol Mushrooms contained Hg in caps in median concentration varying for the sites surveyed between 1.3 and 7.0 μg g−1 dw (0.13 and 0.7 μg g−1 ww; assuming water content is 90%). Estimated Hg intake resulting from the consumption of 300- or 500-g portions of caps is 39–210 and 65–350 μg and what is equivalent to 0.65–3.5 and 1.1–5.8 μg kg−1 bw (an adult of 60 kg bw). Also, Hg in caps of Parasol Mushrooms for some of the sites surveyed might be of concern, if eaten by fanciers frequently in the mushrooming season.
Principal component analysis and cluster analysis of data
PCA enables a reduction in data and description of a given multidimensional system by means of a small number of new variables. PCA with no rotation was applied to data set. In this respect, loadings >0.90 are typically regarded as excellent and <0.70 very poor. The number of significant components, initial eigenvalues, total variance (percent), and factor loadings were explained by using an unrotate method and are tabulated in Table A.2 (online resource). The results of PCA for data sets indicate three factors explaining approximately 85% of the total variance for caps and 84% for stipes. For caps, the first principal component (PC1) comprised K, Mg, Mn, and Rb with high positive loadings (> 0.75) and explains 36% of the variance. In the case of stipes, PC1 is strongly influenced by negatively correlated variables describing K, Al, Ba, Cu, Fe, Sr and explains 46% of the variance. For caps and stipes, the component PC2 explained 28% and 24% of the variance, respectively, and it was made up of Al, Fe, Zn for caps and Mg, Rb, Zn for stipes. With the third principal component (PC3), associated are positively correlated Cd and Hg for caps, and for stipes associated is positively correlated Hg. In the case of caps, the first principal component—spanning the greater amount of variance, includes K, Mg, Mn, and Rb. This association may suggest that the source of these metals is natural, i.e., not related to human activities in the studied areas. The second factor groups were Al, Fe, and Zn. The positive loading for Zn might indicate that this metal is controlled by a different geochemical mechanism or it has a different source in analyzed regions. The concentrations of Cd and Hg in caps of Parasol Mushrooms appear in the PC3 and could be related to the anthropogenic sources such as industry, traffic, or agriculture. In Figs. 2, 3, and 4 the interdependences among the elements in the factor space as a PCA plot are shown graphically. As can be deduced from these figures, some metallic elements tend to cluster together. For example, in caps, the pair Mn–Rb cluster together (associated with PC1), and in stipes, negatively correlated pairs Sr–Ba and Al–Fe–Cu cluster together. In caps, the pair Al–Fe is separated, and in stipes, Mg–Zn tends to cluster together and is associated with PC2. In caps, the pair Cd–Hg tends to cluster together when associated with PC3. Metals such as K, Mg, Zn for caps and K, Rb for stipes are separated from other metals. A projection of Parasol Mushroom trace metals data set on the principal components plane allowed to visualize contribution of the particular groups of metallic elements to the specimens spread (online resource, Figs. A.1–A.3). In spite of that, the elements such as K, Mg, Mn, Rb in the caps and K, Al, Ba, Cu, Fe, Sr in the stipes introduced the greatest variance in mineral composition; only the samples from the MW site could be separated based on these criteria (online resource, Figs. A.1 and A.3). Parasol Mushroom at the SZ site clearly overwhelmed others due to the Zn content in caps and stipes, and Mg, Rb content in stipes. A great variability of Al and Fe content is noted for caps from the MW area (Fig. 7). The principal components 2 and 3 planes (online resource, Fig. A.2) did indicate specimens from the SZ site as more contaminated with Hg.
To examine trace element clustering and to evaluate similarities in their accumulation patterns in Parasol Mushrooms between the sampling sites, the CA was applied to the data set by using the Ward’s method combined with 1-Pearson r method (Yetimoĝlu and Ercan 2008). The results of CA are shown in Figs. A.4 and A.5 (online resource). A cluster analysis dendrogram of the element concentration similarities for caps show two main clusters (online resource, Fig. A.4). The first cluster contains K, Mn, Rb, Mg, and Zn. The second cluster contains Al, Fe, Ba, Sr, Cd, Hg, and Cu. In the case of stipes, a dendrogram also indicates two main clusters (online resource, Figs. A.4). The first fraction separated elements such as K, Ba, Sr, Mn, Al, Fe, and Cu while the second fraction such as Mg, Rb, Zn, Cd, and Hg. The dendrogram of the data set for caps of Parasol Mushrooms shows how sampling sites are clearly divided into two clusters with different characteristics regarding the measured concentrations of metals (online resource, Figs. A.5). In the first fraction, the strongest similarity occurred between the SZ–ML sites and TPK–MW sites. In the second case, up to two subfractions could be recognized, which described the CZ–RA and OL–ST sites. A cluster analysis diagram of the data for stipes of Parasol Mushroom (online resource, Figs. A.5) shows that the sampling sites such as SZ–ML, CZ–OL–ST and TPK–MW–RA are grouped separately.
Conclusions
A potential of Parasol Mushrooms to bioconcentrate in fruiting bodies certain metals contained in soil can be very high. The Parasol Mushroom is a species harvested in the wild, and its caps are of unique taste and can contain a spectrum of essential and hazardous mineral compounds accumulated to elevated concentrations, even if grown at the background (pristine) areas. The estimated intake rates of Cd, Hg, and Pb with the Parasol Mushroom’s caps show a cause for concern associated with these metals resulting from the consumption of between 300- and 500-g caps daily, on a frequent basis in the mushrooming season.
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Acknowledgments
Technical support by Krzysztof Lipka and Ewa Łukaszewicz is acknowledged. This study in part has been supported by the Ministry of Science and Higher Education of Poland under grant no. DS-8025-4-0092-11.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Alonso J, García MÁ, Pérez-López M, Melgar MJ. The concentrations and bioconcentration factors of copper and zinc in edible mushrooms. Arch Environ Contam Toxicol. 2003;44:180–188. doi: 10.1007/s00244-002-2051-0. [DOI] [PubMed] [Google Scholar]
- Baptista P, Ferreira S, Soares E, Coelho V, de Lourdes BM. Tolerance and stress response of Macrolepiota procera to nickel. J Agric Food Chem. 2009;57:7145–7152. doi: 10.1021/jf902075b. [DOI] [PubMed] [Google Scholar]
- Borovička J, Řanda Z, Jelínek E. Gold content of ectomycorrhizal and saprobic macrofungi from non-auriferous and unpolluted areas. Mycol Res. 2005;109:951–955. doi: 10.1017/S095375620500328X. [DOI] [PubMed] [Google Scholar]
- Borovička J, Řanda Z, Jelínek E, Kotrba P, Dunn CE. Hyperaccumulation of silver by Amanita strobiliformis and related species of the section Lepidella. Mycol Res. 2007;111:1339–1344. doi: 10.1016/j.mycres.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Borovička J, Kotrba P, Gryndler M, Mihaljevič M, Řanda Z, Rohovec J, Cajthaml T, Stijve T, Dunn CE. Bioaccumulation of silver in ectomycorrhizal and saprobic macrofungi from pristine and polluted areas. Sci Total Environ. 2009;408:2733–2744. doi: 10.1016/j.scitotenv.2010.02.031. [DOI] [PubMed] [Google Scholar]
- Borovička J, Dunn CE, Gryndler M, Mihaljevič M, Jelínek E, Rohovec J, Rohošková M, Řanda Z. Bioaccumulation of gold in macrofungi and ectomycorrhizae from the vicinity of the Mokrsko gold deposit Czech Republic. Soil Biol Biochem. 2010;42:83–91. doi: 10.1016/j.soilbio.2009.10.003. [DOI] [Google Scholar]
- Brzostowski A, Bielawski L, Orlikowska A, Plichta S, Falandysz J. Instrumental analysis of metals profile in Poison Pax (Paxillus involutus) collected at two sites in Bory Tucholskie. Chem Anal (Warsaw) 2009;54:907–920. [Google Scholar]
- Brzostowski A, Falandysz J, Jarzyńska G, Zhang D (2011a) Bioconcentration potential of metallic elements by Poison Pax (Paxillus involutus) mushroom. J Environ Sci Health A 46:378–393 [DOI] [PubMed]
- Brzostowski A, Jarzyńska G, Kojta AK, Wydmańska D, Falandysz J. Variations in metal levels accumulated in Poison Pax (Paxillus involutus) mushroom collected at one site over four years. J Environ Sci Health A. 2011;46:581–588. doi: 10.1080/10934529.2011.562827. [DOI] [PubMed] [Google Scholar]
- Carvalho MI, Pimentel AC, Fernandes B. Study of heavy metals in wild edible mushrooms under different pollution conditions by X-ray fluorescence spectrometry. Anal Sci. 2005;21:747–750. doi: 10.2116/analsci.21.747. [DOI] [PubMed] [Google Scholar]
- Cenci RM, Cocchi L, Petrini O, Sena F, Siniscalco C, Vescovi L (2010) Elementi chimici nei funghi superiori I funghi di riferimento come strumento di lavoro per la bioindicazione e la biodiversità. JRC Sci Tech Rep EUR 24415 IT 2010 (in Italian)
- Chang ST. Composition of foods mushrooms as food. Food Lab News. 1990;21:7–8. [Google Scholar]
- Chudzyński K, Falandysz J. Multivariate analysis of elements content of Larch Bolete (Suillus grevillei) mushroom. Chemosphere. 2008;78:1230–1239. doi: 10.1016/j.chemosphere.2008.07.055. [DOI] [PubMed] [Google Scholar]
- Chudzyński K, Bielawski L, Falandysz J. Mercury bio-concentration potential of Larch Bolete Suillus grevillei mushroom. Bull Environ Contam Toxicol. 2009;83:275–279. doi: 10.1007/s00128-009-9723-7. [DOI] [PubMed] [Google Scholar]
- Chudzyński K, Jarzyńska G, Stefańska A, Falandysz J. Mercury content and bio-concentration potential of Slippery Jack Suillus luteus mushroom. Food Chem. 2011;125:986–990. doi: 10.1016/j.foodchem.2010.09.102. [DOI] [Google Scholar]
- Cocchi L, Vescovi L, Petrini LE, Petrini O. Heavy metals in edible mushrooms in Italy. Food Chem. 2006;98:277–284. doi: 10.1016/j.foodchem.2005.05.068. [DOI] [Google Scholar]
- Diehl JF, Schlemmer U. Bestimmung der Bioverfügharkeit von Cadmium in der Pilzen durh fütterungsversuche. Zeit für Ernährungswissensschaft. 1984;23:126–135. doi: 10.1007/BF02021687. [DOI] [PubMed] [Google Scholar]
- EU (2008) Commission Regulation (EC) No 629/2008 of 2 July 2008 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Office J European Union 372008L173/6–9
- European Food Safety Authority (EFSA) (2009) Cadmium in food. EFSA J 980:1–139. http://wwwefsaeuropaeu/cs/BlobServer/Scientific_Opinion/contam_op_ej980_cadmium_en_rev1pdf?ssbinary=true/. Cited 12 Dec 2009
- Falandysz J. Mercury content of squid Loligo opalescence. Food Chem. 1990;38:171–177. doi: 10.1016/0308-8146(90)90191-6. [DOI] [Google Scholar]
- Falandysz J. Mercury in mushrooms and soil of the Tarnobrzeska Plain southeastern Poland. J Environ Sci Health A. 2002;37:343–352. doi: 10.1081/ESE-120002833. [DOI] [PubMed] [Google Scholar]
- Falandysz J. Selenium in edible mushrooms. J Environ Sci Health C. 2008;26:256–299. doi: 10.1080/10590500802350086. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Brzostowski A. Mercury and its bioconcentration factors in Poison Pax (Paxillus involutus) from various sites in Poland. J Environ Sci Health A. 2007;42:1095–1100. doi: 10.1080/10934520701418599. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Chwir A. The concentrations and bioconcentration factors of mercury in mushrooms from the Mierzeja Wiślana sand-bar Northern Poland. Sci Total Environ. 1997;203:221–228. doi: 10.1016/S0048-9697(97)00150-2. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Danisiewicz D. Bioconcentration factors (BCF) of silver in wild Agaricus campestris. Bull Environ Contam Toxicol. 1995;55:122–129. doi: 10.1007/BF00212398. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Gucia M. Bioconcentration factors of mercury by Parasol Mushroom (Macrolepiota procera) Environ Geochem Health. 2008;30:121–125. doi: 10.1007/s10653-008-9133-5. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Bona H, Danisiewicz D. Silver content of wild-grown mushrooms from northern Poland. Z Lebensm Unters Forsch. 1994;199:223–224. doi: 10.1007/BF01193449. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Bona H, Danisiewicz D. Silver uptake by Agaricus bisporus from an artificially enriched substrate. Z Lebensm Unters Forsch. 1994;199:225–228. doi: 10.1007/BF01193450. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Szymczyk K, Ichihashi H, Bielawski L, Gucia M, Frankowska A, Yamasaki S. ICP/MS and ICP/AES elemental analysis (38 elements) of edible wild mushrooms growing in Poland. Food Addit Contam. 2001;18:503–513. doi: 10.1080/02652030119625. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Bielawski L, Kannan K, Gucia M, Lipka K, Brzostowski A. Mercury in wild mushrooms and underlying soil substrate from the great lakes land in Poland. J Environ Monit. 2002;4:473–476. doi: 10.1039/b202946d. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Bielawski L, Kawano M, Brzostowski A, Chudzyński K. Mercury in mushrooms and soil from the Wieluńska Upland in south-central Poland. J Environ Sci Health A. 2002;37:1409–1420. doi: 10.1081/ESE-120013266. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Gucia M, Skwarzec B, Frankowska A, Klawikowska K. Total mercury in mushrooms and underlying soil from the Borecka Forest Northeastern Poland. Arch Environ Contam Toxicol. 2002;42:145–154. doi: 10.1007/s00244-001-0026-1. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Lipka K, Gucia M, Kawano M, Strumnik K, Kannan K. Accumulation factors of mercury in mushrooms from Zaborski Lndscape Park Poland. Environ Int. 2002;28:421–427. doi: 10.1016/S0160-4120(02)00067-3. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Brzostowski A, Kawano M, Kannan K, Puzyn T, Lipka K. Concentrations of mercury in wild growing higher fungi and underlying substrate near Lake Wdzydze Poland. Water Air Soil Pollut. 2003;148:127–137. doi: 10.1023/A:1025422017868. [DOI] [Google Scholar]
- Falandysz J, Kawano M, Świeczkowski A, Brzostowski A, Dadej M. Total mercury in wild-grown higher mushrooms and underlying soil from Wdzydze Landscape Park Northern Poland. Food Chem. 2003;81:21–26. doi: 10.1016/S0308-8146(02)00344-8. [DOI] [Google Scholar]
- Falandysz J, Lipka K, Kawano M, Brzostowski A, Dadej M, Jędrusiak A, Puzyn T. Mercury content and its bioconcentration factors in wild mushrooms at Łukta Northeastern Poland. J Agric Food Chem. 2003;51:2832–2836. doi: 10.1021/jf026016l. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Jędrusiak A, Lipka K, Kurunthachalam K, Kawano M, Gucia M, Brzostowski A, Dadej M. Mercury in wild mushrooms and underlying soil substrate from Koszalin North-central Poland. Chemosphere. 2004;54:461–466. doi: 10.1016/S0045-6535(03)00700-8. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Gucia M, Mazur A. Content and bioconcentration factors of mercury by Parasol Mushroom Macrolepiota procera. J Environ Sci Health B. 2007;42:735–740. doi: 10.1080/03601230701466005. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Kunito T, Kubota R, Lipka K, Mazur A, Falandysz JJ, Tanabe S. Selected elements in Fly Agaric Amanita muscaria. J Environ Sci Health A. 2007;42:1615–1623. doi: 10.1080/10934520701517853. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Kunito T, Kubota R, Bielawski L, Frankowska A, Falandysz JJ, Tanabe S. Multivariate characterization of elements accumulated in King Bolete Boletus edulis mushroom at lowland and high mountain regions. J Environ Sci Health A. 2008;43:1692–1699. doi: 10.1080/10934520802330206. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Kunito T, Kubota R, Gucia M, Mazur A, Falandysz JJ, Tanabe S. Some mineral constituents of Parasol Mushroom Macrolepiota procera. J Environ Sci Health B. 2008;43:187–192. doi: 10.1080/03601230701795247. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Frankowska A, Jarzyńska G, Dryżałowska A, Kojta AK, Zhang D. Survey on composition and bioconcentration potential of 12 metallic elements in King Bolete (Boletus edulis) mushroom that emerged at 11 spatially distant sites. J Environ Sci Health B. 2011;46:231–246. doi: 10.1080/03601234.2011.540528. [DOI] [PubMed] [Google Scholar]
- Frankowska A, Ziółkowska J, Bielawski L, Falandysz J. Profile and bioconcentration of minerals by King Bolete (Boletes edulis) from the Płocka Dale in Poland. Food Addit Contam B. 2010;3:1–6. doi: 10.1080/19440040903505232. [DOI] [PubMed] [Google Scholar]
- Gadd GM. Geomycology: biogeochemical transformations of rocks minerals metals and radionuclides by fungi bioweathering and bioremediation. Mycol Res. 2007;111:3–49. doi: 10.1016/j.mycres.2006.12.001. [DOI] [PubMed] [Google Scholar]
- García MÁ, Alonso J, Melgar MJ. Lead in edible mushrooms. Levels and bioconcentration factors. J Haz Mat. 2009;167:777–783. doi: 10.1016/j.jhazmat.2009.01.058. [DOI] [PubMed] [Google Scholar]
- Gumińska B, Wojewoda W. Grzyby i ich oznaczanie. Warszawa: PWRiL; 1988. [Google Scholar]
- Jarzyńska G, Falandysz J (2011a) The determination of mercury in mushrooms by CV–AAS and ICP–AES techniques. J Environ Sci Health A 46:569–573 [DOI] [PubMed]
- Jarzyńska G, Falandysz J (2011b) Selenium and 17 other largely essential and toxic metals in muscle and organ meats of Red Deer (Cervus elaphus)–Consequences to human health. Environ Intern 37:882–888 [DOI] [PubMed]
- JECFA (1978) Evaluation of certain food additives and contaminants Twenty-second report of the Joint FAO/WHO Expert Committee on Food Additives WHO Technical Report Series 631 [PubMed]
- Lind Y, Glynn AW, Engmam J, Jorhem L. Bioavailability of cadmium from crab hepatopancreas and mushroom in relation to inorganic cadmium: a 9-week feeding study in mice. Food Chem Toxicol. 1995;33:667–673. doi: 10.1016/0278-6915(95)00036-2. [DOI] [PubMed] [Google Scholar]
- Malinowska E, Szefer P, Falandysz J. Metals bioaccumulation by bay bolete Xerocomus badius from selected sites in Poland. Food Chem. 2004;84:405–416. doi: 10.1016/S0308-8146(03)00250-4. [DOI] [Google Scholar]
- Melgar MJ, Alonso J, Pérez-López M, García MÁ. Influence of some factors in toxicity and accumulation of cadmium from edible wild macrofungi in NW Spain. J Environ Sci Health B. 1998;33:439–455. doi: 10.1080/03601239809373156. [DOI] [PubMed] [Google Scholar]
- Melgar MJ, Alonso J, García MÁ. Mercury in edible mushrooms and soil. Bioconcentration factors and toxicological risk. Sci Total Environ. 2009;407:328–5334. doi: 10.1016/j.scitotenv.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Mitra AK, Purkayastha RP, Chantterjee NB, Bhattacharyya R. Uptake and tissue distribution of cadmium in albino rat after oral exposure to cadmium-contaminated edible mushroom and its effect on blood. Curr Sci. 1995;68:1050–1053. [Google Scholar]
- Schellmann B, Hilz MJ, Optiz O. Cadmium–und Kupferausscheidung Nach Aufnahme Von Champignon-Mahizeiten. Zeitschr Lebensm Unters Forsch. 1980;171:189–192. doi: 10.1007/BF01042648. [DOI] [PubMed] [Google Scholar]
- Seeger R, Schiefelbein F, Senffert R, Zant W. Absorption of cadmum ingested with mushrooms. Naunyn-Schmiedeberg’s Arch Pharmacol. 1986;332:110. [Google Scholar]
- Singer R. New and interesting species of Basidiomycetes. Pap Mich Aca Sci Arts Lett. 1946;32:141. [Google Scholar]
- Stijve T, Besson R. Mercury cadmium lead and selenium content of mushroom species belonging to the genus Agaricus. Chemosphere. 1976;7:151–158. doi: 10.1016/0045-6535(76)90036-9. [DOI] [Google Scholar]
- Stijve T, Roschnik R. Mercury and methyl mercury content of different species of fungi. Trav Chim Aliment Hyg. 1974;65:209–220. [Google Scholar]
- Stijve T, Vellinga EC, Herrmann A. Arsenic accumulation in some higher fungi. Personia. 1990;14:161–166. [Google Scholar]
- Stijve T, Noorloos T, Byrne AR, Slejkovec Z, Goessler H. High selenium levels in edible Albatrellus mushrooms. Deutsch Lebensm-Runds. 1998;94:275–279. [Google Scholar]
- US EPA . Toxicological review of zinc and compounds. In support of summary information on the integrated risk information system. Washington DC: US Environmental Protection Agency; 2005. [Google Scholar]
- Vetter J, Siller I. Ásványi anyagok mennyiségének alakulása a gomba termőtestben (Macrolepiota procera) Mikolódiai Közlemények. 1997;36:33–39. [Google Scholar]
- World Health Organization (WHO) (1989) Toxicological evaluation of certain food additives and contaminants 33rd Report of the Joint FAO/WHO Expert Committee on Food Additives Food Additives Series No 24 Geneva (Switzerland)
- World Health Organization (WHO) (1993) Evaluation of certain food additives and contaminants 41st Report of the Joint FAO/WHO Expert Committee on Food Additives Technical Report Series No 837 Geneva (Switzerland)
- Yetimoĝlu EK, Ercan Ö. Multivariate analysis of metal contamination in street dusts of Istanbul D-100 highway. J Braz Chem Soc. 2008;19:1399–1404. [Google Scholar]
- Zhang D, Frankowska A, Jarzyńska G, Kojta AK, Drewnowska M, Wydmańska D, Bielawski L, Wang J, Falandysz J. Metals of King Bolete (Boletus edulis) Bull: Fr collected at the same site over two years. Afr J Agric Res. 2010;5:3050–3055. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


