Abstract
Orexins A and B are newly discovered neuropeptides with pleiotropic activity. They signal through two G protein-coupled receptors: OX1 and OX2. In this study, we examined the expression of orexin receptors and effects of the receptors’ activation on cyclic AMP formation in the primary neuronal cell cultures from rat cerebral cortex. Both types of orexin receptors were expressed in rat cortical neurons; the level of OX2R was markedly higher compared to OX1R. Orexin A (an agonist of OX1R and OX2R) and [Ala11-D-Leu15]orexin B (a selective agonist of OX2R) did not affect basal cyclic AMP formation in the primary neuronal cell cultures. Both peptides (0.001–1 μM) inhibited, in a concentration-dependent manner and IC50 values in low nanomolar range, the increase in the nucleotide production evoked by forskolin (1 μM; a direct activator of adenylyl cyclase), pituitary adenylate cyclase-activating polypeptide (PACAP27; 0.1 μM), and vasoactive intestinal peptide (VIP; 3 μM). Effects of orexin A on forskolin-, PACAP27-, and VIP-stimulated cyclic AMP synthesis were blocked by TCS OX2 29 (a selective antagonist of OX2R), and unaffected by SB 408124 (a selective antagonist of OX1R). Pretreatment of neuronal cell cultures with pertussis toxin (PTX) abolished the inhibitory action of orexin A on forskolin- and PACAP-stimulated cyclic AMP accumulation. It is suggested that in cultured rat cortical neurons orexins, acting at OX2 receptors coupled to PTX-sensitive Gi protein, inhibit cyclic AMP synthesis.
Keywords: Orexin, Hypocretin, Orexin receptors, Cyclic AMP, PACAP, VIP, Neuronal cell culture, Cerebral cortex, Rat
Introduction
Orexins (orexins A and B), also known as hypocretins (hypocretins 1 and 2), are multifunctional neuropeptides discovered by two independent research groups in 1998 using orphan receptor technologies (Sakurai et al. 1998) and subtractive cDNA cloning (de Lecea et al. 1998). Both orexins are derived from a common precursor, preproorexin, by proteolytic cleavage (Sakurai et al. 1998, 1999), and share 46% aminoacid identity in humans (de Lecea and Sutcliffe 1999). Although production of the peptides is restricted to a discrete population of neurons in the lateral and posterior hypothalamus, the projection fields of these neurons have been identified in numerous brain regions, including the cortex, thalamus, hypothalamus, brain stem, and spinal cord (Matsuki and Sakurai 2008). This diffuse projection pattern suggests that the orexin system regulates multiple complex physiological functions. Indeed, accumulated experimental evidence indicate an involvement of orexins in the regulation of vigilance and sleep/wake cycle, feeding, appetite, reward seeking, and energy homeostasis (Kukkonen et al. 2002; Matsuki and Sakurai 2008; Carter et al. 2009; Kodatek and Cai 2010). The peptides also control hypothalamo–pituitary–adrenal axis and functions of miscellaneous peripheral organs, including heart, kidney, thyroid, lung, testis, ovaries, and adipose tissues (Voisin et al. 2003; Spinazzi et al. 2006; Heinonen et al. 2008; Okumura and Takakusaki 2008; Kagerer and Jöhren 2010). The loss or dysfunction of orexin neurons has been shown to cause human and animal narcolepsy [e.g., (Chemelli et al. 1999; Lin et al. 1999; Geraschenko et al. 2001; Thannickal et al. 2003; Mieda et al. 2004)].
Orexins orchestrate their diverse central and peripheral effects via two membrane-bound G protein-coupled receptors, OX1R and OX2R (Sakurai et al. 1998). These receptors share a 64% identity to each other in their amino acid sequences. Studies in heterologous expression systems have demonstrated that OX2R has an equal affinity for both orexins A and B, whereas OX1R has approximately tenfold greater affinity for orexin A than for orexin B (Sakurai et al. 1998; Ammoun et al. 2003). Signal coupling of orexin receptors has been rigorously investigated in few studies, the vast majority of them was performed on cell lines recombinantly expressing OX1R or OX2R. OX1R primarily couples via the Gq/11 subclass of G protein, while OX2R couples to Gq/11, Gs, and Gi/o subclasses (Sakurai et al. 1998; van den Pol et al. 1998; Randeva et al. 2001; Karteris and Randeva 2003; Kukkonen and Åkerman 2005; Ramanjaneya et al. 2009). One of the most marked responses to stimulation of orexin receptors is a robust increase in intracellular Ca2+ concentration, [Ca2+]i, resulting from activation of two pathways: receptor-operated Ca2+ influx and phospholipase C—inositol-(1,4,5)-trisphosphate/diacylglycerol (Sakurai et al. 1998; Zhu et al. 2003; Holmqvist et al. 2005; Ekholm et al. 2007; Gorojankina et al. 2007; Tang et al. 2008; reviewed by Kukkonen and Åkerman 2005). A few reports suggest a link between orexin receptors and the cyclic AMP pathway (Zhu et al. 2003; Holmqvist et al. 2005; Magga et al. 2006; Tang et al. 2008).
Although the expression pattern of the orexinergic system in the CNS has been well characterized, little is known about the presence of native orexin receptors and their signaling system(s) in the primary neuronal cultures. In this study, we investigated the expression of orexin receptors and the intracellular mechanism followed the receptors’ activation that can affect cyclic AMP formation in neuronal cell cultures derived from rat cerebral cortex. We also determined which type of orexin receptors is involved in this action. Our results demonstrated that stimulation of OX2R inhibits cyclic AMP production in a pertussin-toxin sensitive manner. This suggests an involvement of Gi protein in coupling of OX2R to adenylyl cyclase.
Materials and Methods
Animals and Cell Culture
Experiments were performed on primary neuronal cell cultures prepared from Wistar rat embryos on day 16 of gestation. Animal procedures were in strict accordance with the Polish governmental regulations concerning experiments on animals (Dz.U.05.33.289), and the experimental protocol was approved by the Local Ethical Commission for Experimentation on Animals.
Primary neuronal cell cultures were prepared according to the method of Brewer (1995), described in detail by Nowak and coworkers (2007). Briefly, pregnant females were anesthetized with ether vapor, killed by decapitation, and subjected to cesarean section in order to remove fetuses. After brain’s dissection, the cerebral cortex was isolated, incubated for 15 min in trypsin/EDTA (0.05%) at 37°C, triturated in a solution of DNase I (0.05 mg/ml) and fetal bovine serum (20%), and finally centrifuged at 210 × g for 5 min at 21°C. The cells were suspended in neurobasal medium supplemented with B27, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin and plated at a density of 300,000–350,000 cells/well onto poly-l-ornithine (0.01 mg/ml) coated multi-well plates. Cells were grown in humidified atmosphere of 95% air and 5% CO2 at 37°C for 6–7 days prior to experimentation. Seventy-two hours after plating the solution of 1-β-d-arabinofuranosylcytosine (a final concentration—5 μM) was added to the neurobasal medium to stop the glial proliferation. The purity of neuronal cultures was verified by using antibodies against microtubule associating protein-2 for neurons, and against glial fibrillary acidic protein (GFAP), for astrocytes. The latter analysis revealed the presence of approximately 6–10% of GFAP-positive cells, which indicated that the primary neuronal cultures represented in fact neuron-enriched preparations.
Real-Time Quantitative RT-PCR
Total RNA was extracted from neuronal cells by using TRI Pure Isolation Reagent (Roche, Meylan, France) according to the manufacturer’s instruction. For each sample, total RNA (1 μg) was subjected to reverse transcription (RevertAid H Minus First Strand cDNA Synthesis Kit, Fermentas, Burlington, Canada) according to the manufacturer’s specifications.
Specific sense and antisense oligonucleotide primers for amplification of mRNAs of rat OX1R and OX2R were obtained from Invitrogen (Karlsruhe, Germany). The sequences of specific primers and the procedure of real-time quantitative PCR (qPCR) was published previously (Jöhren et al. 2001). Shortly, 2 μl of first strand cDNA reaction was incubated in the presence of 3 mM MgCl2; 200 μM of dGTP, dATP, dCTP, and dUTP; Platinum Taq DNA Polymerase; the fluorescence dye SYBR green I; and the appropriate sense and antisense primers in a final volume of 25 μl (Platinum® SYBR® Green qPCR SuperMix, Invitrogen) on the 7000 Sequence Detection System of Applied Biosystems (Darmstadt, Germany). Each sample was analyzed in duplicate along with standards and no template controls. Product purity was regularly confirmed for each sample by dissociation curve analysis.
Copy number calculations were based on the cycle threshold method (Higuchi et al. 1993). Serial dilutions of known amounts of specific cDNA fragments were used to generate standard curves. The threshold cycle number (C T) for each sample was calculated using the 7000 Sequence Detection System software with an automatic baseline setting and a fluorescence threshold (R n) of 0.2.
Assay of Cyclic AMP Formation
On the day of experiment, the culture medium was removed, fresh serum-free culture medium was added, and cells were incubated in the presence of [3H]adenine for 30 min at 37°C. The formation of [3H]cyclic AMP in [3H]adenine prelabeled cells was assayed according to Shimizu et al. (1969), and the formed [3H]cyclic AMP was isolated by a sequential Dowex-alumina chromatography according to Salomon et al. (1974). The results were individually corrected for percentage recovery with the aid of [14C]cyclic AMP added to each column system prior to the nucleotide extraction. The mean recovery was in the range of 38–46%.The accumulation of cyclic AMP during a 15-min stimulation period was assessed as a percentage of the conversion of [3H]adenine to [3H]cyclic AMP. Antagonists of orexin receptors were added 10-min prior to addition of orexin A and [Ala11-D-Leu15]orexin B. All experiments were carried out in the presence of 0.1 mM 3-isobuthyl-1-methyxanthine (IBMX, an inhibitor of cyclic nucleotides’ phosphodiesterase). Pertussis toxin (PTX; 100 ng/ml) was added 18 h prior to addition of orexin A. A day after addition of pertussis toxin, the culture medium was removed and fresh serum-free culture medium was added.
Chemicals
The peptides orexin A, [Ala11-D-Leu15]orexin B, PACAP27 (human, ovine, and rat), and vasoactive intestinal peptide (VIP; human, porcine, and rat) were from NeoMPS (Strasbourg, France). N-(6,8-difluoro-2-methyl-4-quinolinyl)-N′-[4-(dimethylamino)phenyl]urea (SB 408124) and (2S)-1-(3,4-dihydro-6,7-dimethoxy-2(1H)-isoquinolinyl)-3,3-dimethyl-2-[(4-pyridinylmethyl)amino]-1-butanone hydrochloride (TCS OX2 29), selective antagonists of OX1R and OX2R, respectively, were purchased from Tocris Bioscience (Bristol, UK). Forskolin, IBMX, poly-l-ornithine, DNase I, trypsin, glutamine, penicillin, and streptomycin were from Sigma-Aldrich (Poznan, Poland). Neurobasal medium, B27, and fetal bovine serum were from Gibco (Paisley, Scotland, UK). dGTP, dATP, dCTP, and dUTP, and Platinum Taq DNA Polymerase were from Invitrogen (Karlsruhe, Germany). Anti-neuronal class III β-tubulin mouse monoclonal antibody, anti-glial fibrillary acidic protein rabbit polyclonal antibody, Texas Red® dye-conjugated goat anti-mouse antibody, and fluorescein (FITC)-conjugated goat anti-rabbit antibody were purchased from STEMCELL Technologies, Inc. (Vancouver, Canada). Petri dishes and multi-well plates for cell cultures were from Nunc (Wiesbaden, Germany). Radioactive compounds: [3H]adenine (sp. activity 24 Ci/mmol) and [14C]cyclic AMP (sp. activity 53 mCi/mmol) were from Hartmann-Analytic GmbH (Braunschweig, Germany). Other chemicals were of analytical purity and were obtained mainly from Sigma-Aldrich (Poznan, Poland).
Data Analysis
Data are expressed as mean ± standard error of the mean (SEM) values and were analyzed for statistical significance by one-way ANOVA followed by post hoc Student–Newman–Keul’s test, using InStat version 3.05 for Windows 95 (GraphPad, San Diego, CA, USA).
Results
Expression of Orexin Receptors in the Primary Neuronal Cell Cultures
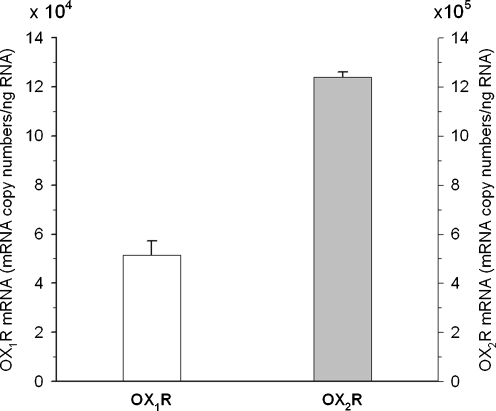
Quantitative RT-PCR analysis with the use of specific couples of primers demonstrated the expression of both types of orexin receptors, OX1R and OX2R, in the primary neuronal cell cultures derived from rat cerebral cortex. Interestingly, primary neuronal cells express about 24-fold more OX2R than OX1R at the mRNA level (Fig. 1).
Fig. 1.
Expression of orexin receptor subtypes OX1R and OX2R by qPCR in rat cultured neurons. Shown are the specific RNA copy numbers per nanograms of total RNA
Effects of Orexin A and [Ala11-D-Leu15]Orexin B on Cyclic AMP Formation in the Primary Neuronal Cells
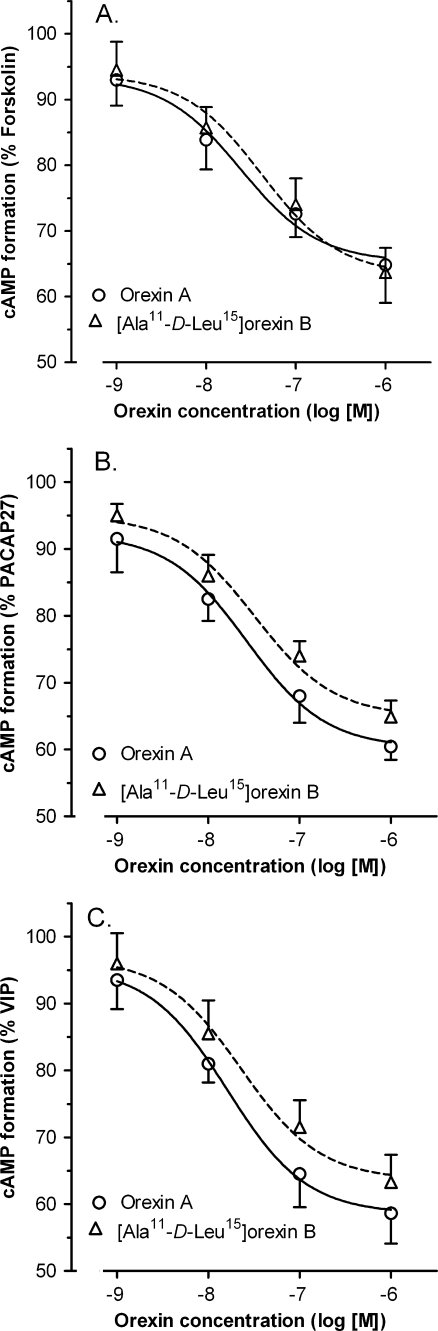
No significant effects of orexin A (an agonist of OX1R and OX2R) and [Ala11-D-Leu15]orexin B (a selective agonist of OX2R), both applied at 0.001–1 μM concentrations, on basal cyclic AMP accumulation in cultured rat cortical neurons were observed (data not shown). In line with previous demonstrations (Jozwiak-Bebenista et al. 2007), incubation of rat neuronal cell cultures with forskolin (a direct activator of adenylyl cyclase; 1 μM), PACAP27 (0.1 μM), or VIP (3 μM) resulted in a potent (three- to fivefold over the basal level) increase in cyclic AMP production (Figs. 2, 3 and 4). Orexin A and [Ala11-D-Leu15]orexin B (0.001–1 μM) inhibited, in a concentration-dependent manner, the stimulatory action of forskolin, PACAP27, and VIP on cyclic AMP formation in cultured rat cortical neurons (Fig. 2). The calculated IC50 values for orexin A were: 24.5 ± 1.8 nM (experiments with forskolin), 26.3 ± 1.4 nM (experiments with PACAP27), and 16.2 ± 1.2 nM (experiments with VIP); and for [Ala11-D-Leu15]orexin B: 42.0 ± 2.0 nM (experiments with forskolin), 32.3 ± 1.9 nM (experiments with PACAP27), and 22.9 ± 1.6 nM (experiments with VIP).
Fig. 2.
Effects of orexin A (an agonist of OX1R and OX2R) and [Ala11-D-Leu15]orexin B (a selective agonist of OX2R) on cyclic AMP formation in primary neuronal cell cultures from rat cerebral cortex stimulated by forskolin (a), PACAP27 (b), and VIP (c). Values are expressed as percent of the response to forskolin, PACAP27, and VIP, and are means ± SEM (n = 8–16). Cyclic AMP accumulation: experiments with forskolin—control cultures, 0.86 ± 0.12% conversion (n = 18); forskolin (1 μM) stimulated cultures, 3.39 ± 0.22% conversion (n = 20); experiments with PACAP27—control cultures, 0.83 ± 0.14% conversion (n = 16); PACAP27 (0.1 μM) stimulated cultures, 5.09 ± 0.42% conversion (n = 24); experiments with VIP—control cultures, 0.88 ± 0.15% conversion (n = 16); VIP (3 μM) stimulated cultures, 4.52 ± 0.26% conversion (n = 20)
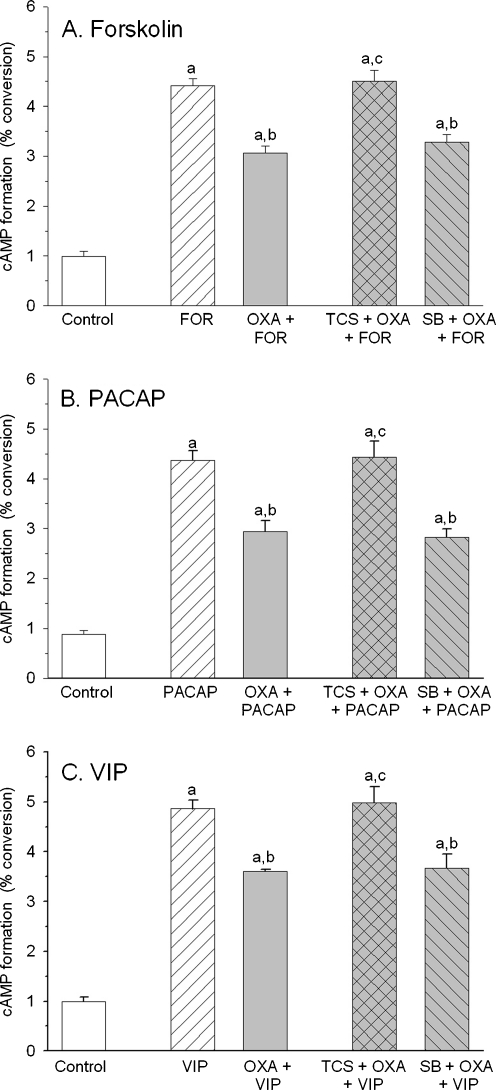
Fig. 3.
a–c TCS OX2 29 (TCS, 10 μM), a selective antagonist of OX2R, but not SB 408124 (SB, 10 μM), a selective antagonist of OX1R, blocked the inhibitory action of orexin A (1 μM) on forskolin (1 μM), PACA27 (0.1 μM), and VIP (3 μM) induced increase in cyclic AMP formation in primary neuronal cell cultures from rat cerebral cortex. Values shown are means ± SEM (n = 8–15). a P < 0.05 versus control, b P < 0.05 versus stimulatory agent (Forskolin, PACAP27, or VIP); c P < 0.05 versus stimulatory agent and orexin
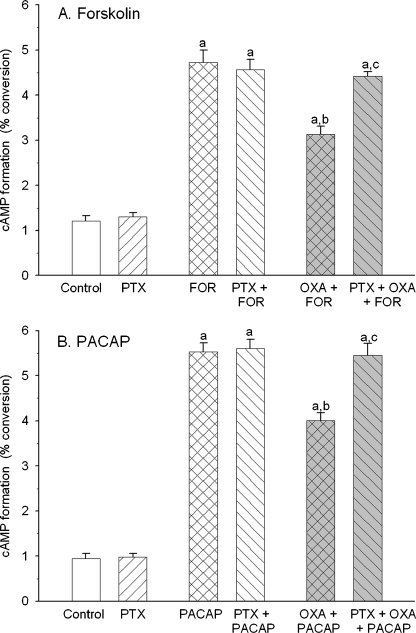
Fig. 4.
Pretreatment of rat cortical neuron cultures with pertussis toxin (PTX; 18 h, 100 ng/ml) abolished the inhibitory action of orexin A (1 μM) on forskolin (1 μM) and PACAP27 (0.1 μM) induced increase in cyclic AMP formation. Values shown are means ± SEM (n = 9–12). a P < 0.05 versus control, b P < 0.05 versus Forskolin or PACAP27, c P < 0.05 versus Forskolin or PACAP27 and orexin A
Effects of OX1R and OX2R Antagonists on the Orexin A-Induced Decrease in Cyclic AMP Formation in the Primary Neuronal Cell Cultures
The observed by us similar potency of orexin A and [Ala11-D-Leu15]orexin B suggested an involvement of OX2R in the regulation of cyclic AMP synthesis in cultured rat cortical neurons. In order to verify this hypothesis, in the next set of experiments selective antagonists of OX1R (SB 408124) and OX2R (TCS 0X2 29) were used. TCS 0X2 29 (10 μM), but not SB 408124 (10 μM), blocked the inhibitory actions of orexin A (1 μM) on increases of cyclic AMP formation produced by forskolin, PACAP27, and VIP (Fig. 3).
Effects of Pertussis Toxin on the Orexin A-Induced Decrease in Cyclic AMP Formation in the Primary Neuronal Cell Cultures
Pretreatment of rat neuronal cell cultures with pertussis toxin (18 h, 100 ng/ml) did not affect the basal as well as forskolin- and PACAP27-stimulated cyclic AMP accumulation. Pertussis toxin abolished the inhibitory action of orexin A on rises in the nucleotide formation produced by forskolin and PACAP27 (Fig. 4).
Discussion
A number of studies, performed predominantly on transfected cell lines, have demonstrated that orexin receptors could interact with three families of G proteins, i.e., Gq/11, Gs, and Gi/o, with different efficacies, and activate several signaling pathways (e.g., Zhu et al. 2003; Holmqvist et al. 2005; Tang et al. 2008). The increase in [Ca2+]i is the most typical response seen in many cell types (de Lecea et al. 1998; Zhu et al. 2003; Ammoun et al. 2006; Magga et al. 2006; Näsman et al 2006; Gorojankina et al. 2007). Regulation of adenylyl cyclase activity is also considered as an important component of orexin receptors signaling (Zhu et al. 2003; Holmqvist et al. 2005; Gorojankina et al. 2007; Tang et al. 2008), although this pathway has seldom been investigated.
The results obtained in the present study provide evidence that in the primary neuronal cell cultures from rat cerebral cortex expression of OX2R is considerably higher than that of OX1R. Our further studies on the primary astrocyte cultures from rat brain identified similar expressions of both types of orexin receptors at the mRNA level; however, they were markedly lower compared to neuronal cell cultures (unpublished data). This suggests that in the rat cerebral cortex the expression of orexin receptors is closely related to the cell type, and may trigger distinct cellular responses. To test this hypothesis we evaluated a potential role of the orexin receptors’ activation in the regulation of cyclic AMP production in neurons. Treatment of cortical neuronal cultures with orexin A (the nonselective agonists of OX1R and OX2R) and [Ala11-D-Leu15]orexin B (the selective OX2R agonist; Asahi et al. 2003) failed to significantly affect the basal cyclic AMP accumulation suggesting that orexin receptors expressed in this cell type do not couple to the Gs subclass of G protein. On the other hand, orexin A and [Ala11-D-Leu15]orexin B potently inhibited, in a concentration-dependent manner, cyclic AMP production stimulated by the diterpene forskolin, the direct activator of adenylyl cyclase, and by two neuropeptides, i.e., PACAP and VIP, well-known activators of Gs proteins (Vaudry et al. 2009). The calculated IC50 values for both peptides were in low, nanomolar range, suggesting that this inhibition is of physiological relevance. Two lines of evidence point to the involvement of OX2R in the studied phenomenon. Firstly, [Ala11-D-Leu15]orexin B was almost equipotent to orexin A. Secondly, the inhibitory action of orexin A was blocked by TCS 0X2 29, the selective OX2R antagonist (Hirose et al. 2003), and not affected by SB 408124, the selective OX1R antagonist (Porter et al. 2001). Pertussis toxin, which deactivates Gi/Go through ADP ribosylation, abolished the inhibitory effect of orexin A on stimulated cyclic AMP production. Hence, it would appear that this effect is mediated by OX2R-coupling to Gi protein. A functional coupling between Gi/o proteins and OX2R has been previously demonstrated in HEK293 and BIM cell lines heterologously expressing human OX2R (Hoang et al. 2003; Zhu et al. 2003; Tang et al. 2008).
In the context of the present study it is interesting to note that our previous results in astrocyte cultures from rat cerebral cortex demonstrated a stimulatory effect of orexin A, but not [Ala11-D-Leu15]orexin B, on cyclic AMP synthesis indicating a predominant OX1R involvement in this action (Woldan-Tambor et al. 2011). Thus, it could be hypothesized that depending on a cell type in the rat cerebral cortex the biochemical responses to the tested peptides are mediated via different types of receptors interacting with distinct families of G proteins. It would be highly interesting to know whether the orexinergic system in astrocyte compartment in the rat brain can affect neuronal functions.
A physiological significance of the OX2R-trigerred intracellular signaling, including cyclic AMP pathway, in rat cortical neurons is yet to be evaluated. Studies on HEK293-OX2R cells suggested that adenylyl cyclase–cyclic AMP pathway is, in part, functionally linked to the activation by orexins of extracellular signal-regulated kinase (ERK1/2) and p38 mitogen-activated protein kinase (p38 MAPK) (Tang et al. 2008). This hypothesis appears particularly interesting in light of the well-known involvement of MAPK signaling pathways in the regulation of cell growth, differentiation, and gene expression.
In conclusion, the results presented here demonstrate that in cultured rat cortical neurons orexins, acting at OX2 receptors coupled to PTX-sensitive Gi protein, inhibit cyclic AMP synthesis.
Acknowledgments
This work was supported by the grants from the Ministry of Science and Higher Education, Warsaw, Poland (P01/2010/38), and the Medical University of Lodz (503/3-011-01/503-01). We thank Mrs. Teresa Kwapisz for excellent technical assistance.
Conflict of Interest
The authors state no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- FOR
Forskolin
- GFAP
Glial fibrillary acidic protein
- IBMX
3-Isobuthyl-1-methyxanthine
- OX1R
Type 1 orexin receptor
- OX2R
Type 2 orexin receptor
- PACAP
Pituitary adenylate cyclase-activating polypeptide
- PTX
Pertussis toxin
- SB 408124
N-(6,8-difluoro-2-methyl-4-quinolinyl)-N′-[4-(dimethylamino)phenyl]urea
- TCS OX2 29
(2S)-1-(3,4-dihydro-6,7-dimethoxy-2(1H)-isoquinolinyl)-3,3-dimethyl-2-[(4-pyridinylmethyl)amino]-1-butanone hydrochloride
- VIP
Vasoactive intestinal peptide
References
- Ammoun S, Holmqvist T, Shariatmadari R, Oonk HB, Detheux M, Parmentier M, Åkerman KE, Kukkonen JP. Distinct recognition of OX1 and OX2 receptors by orexin peptides. J Pharmacol Exp Ther. 2003;305:507–514. doi: 10.1124/jpet.102.048025. [DOI] [PubMed] [Google Scholar]
- Ammoun S, Johansson L, Ekholm ME, Holmqvist T, Danis AS, Korhonen L, Sergeeva OA, Haas HL, Åkerman KE, Kukkonen JP. OX1 orexin receptors activate extracellular signal-regulated kinase in Chinese hamster ovary cells via multiple mechanisms: the role of Ca2+ influx in OX1 receptor signaling. Mol Endocrinol. 2006;20:80–99. doi: 10.1210/me.2004-0389. [DOI] [PubMed] [Google Scholar]
- Asahi S, Egashira S-I, Matsuda M, Iwaasa H, Kanatani A, Ohkubo M, Ihara M, Morishima H. Development of an orexin-2 receptor selective agonist, [Ala11, D-Leu15]orexin-B. Bioorg Med Chem Lett. 2003;13:111–113. doi: 10.1016/S0960-894X(02)00851-X. [DOI] [PubMed] [Google Scholar]
- Brewer GJ. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J Neurosci Res. 1995;42:674–483. doi: 10.1002/jnr.490420510. [DOI] [PubMed] [Google Scholar]
- Carter ME, Borg JS, de Lecea L. The brain hypocretins and their receptors: mediators of allostatic arousal. Curr Opin Pharmacol. 2009;9:39–45. doi: 10.1016/j.coph.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/S0092-8674(00)81973-X. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Sutcliffe JG. The hypocretins/orexins: novel hypothalamic neuropeptides involved in different physiological systems. Cell Mol Life Sci. 1999;56:473–480. doi: 10.1007/s000180050446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm ME, Johansson L, Kukkonen JP. IP3-independent signalling of OX1 orexin/hypocretin receptors to Ca2+ influx and ERK. Biochem Biophys Res Commun. 2007;353:475–480. doi: 10.1016/j.bbrc.2006.12.045. [DOI] [PubMed] [Google Scholar]
- Geraschenko D, Kohls MD, Greco M, Waleh NS, Salin-Pascual R, Kilduff TS, Lappi DA, Shiromani PJ. Hypocretin-2-saporin lesions of the lateral hypothalamus produce narcoleptic-like sleep behavior in the rat. J Neurosci. 2001;21:7273–7283. doi: 10.1523/JNEUROSCI.21-18-07273.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorojankina T, Grébert D, Salesse R, Tanfin Z, Caillol M. Study of orexin signal transduction pathways in rat olfactory mucosa and in olfactory sensory neurons-derived cell line Odora: multiple orexin signalling pathways. Reg Peptides. 2007;141:361–369. doi: 10.1016/j.regpep.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Heinonen MV, Purhonen AK, Mäkelä KA, Herzig KH. Functions of orexins in peripheral tissues. Acta Physiol (Oxf.) 2008;192:471–485. doi: 10.1111/j.1748-1716.2008.01836.x. [DOI] [PubMed] [Google Scholar]
- Higuchi R, Fockler C, Dollinger G, Watson R. Kinetic PCR analysis: real-time monitoring of DNA amplification reactions. Biotechnology (N.Y.) 1993;11:1026–1030. doi: 10.1038/nbt0993-1026. [DOI] [PubMed] [Google Scholar]
- Hirose M, Egashira S, Goto Y, Hashihayata T, Ohtake N, Iwaasa H, Hata M, Fukami T, Kanatani A, Yamada K. N-acyl 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline: the first orexin-2 receptor selective non-peptidic antagonist. Bioorg Med Chem Lett. 2003;13:4497–4499. doi: 10.1016/j.bmcl.2003.08.038. [DOI] [PubMed] [Google Scholar]
- Hoang QV, Bajic D, Yanagisawa M, Nakajima S, Nakajima Y. Effects of orexin (hypocretin) on GIRK channels. J Neurophysiol. 2003;90:693–702. doi: 10.1152/jn.00001.2003. [DOI] [PubMed] [Google Scholar]
- Holmqvist T, Johansson L, Ostman M, Ammoun S, Åkerman KE, Kukkonen JP. OX1 orexin receptors couple to adenylyl cyclase regulation via multiple mechanisms. J Biol Chem. 2005;280:6570–6579. doi: 10.1074/jbc.M407397200. [DOI] [PubMed] [Google Scholar]
- Jöhren O, Neidert SJ, Kummer M, Dendorfer A, Dominiak P. Prepro-orexin and orexin receptor mRNAs are differentially expressed in peripheral tissues of male and female rats. Endocrinology. 2001;142:3324–3331. doi: 10.1210/en.142.8.3324. [DOI] [PubMed] [Google Scholar]
- Jozwiak-Bebenista M, Dejda A, Nowak JZ. Effects of PACAP, VIP and related peptides on cyclic AMP formation in rat neuronal and astrocyte cultures and cerebral cortical slices. Pharmacol Rep. 2007;59:414–420. [PubMed] [Google Scholar]
- Kagerer SM, Jöhren O. Interaction of orexins/hypocretins with adrenocortical functions. Acta Physiol (Oxf.) 2010;198:361–371. doi: 10.1111/j.1748-1716.2009.02034.x. [DOI] [PubMed] [Google Scholar]
- Karteris E, Randeva HS. Orexin receptors and G-protein coupling: evidence for another “promiscuous” seven transmembrane domain receptor. J Pharmacol Sci. 2003;93:126–128. doi: 10.1254/jphs.93.126. [DOI] [PubMed] [Google Scholar]
- Kodatek T, Cai D. Chemistry and biology of orexin signaling. Mol Biosyst. 2010;6:1366–1375. doi: 10.1039/c003468a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukkonen JP, Åkerman KE. Intracellular signal pathways utilized by the hypocretin/orexin receptors. In: de Lecea L, Sutcliffe JG, editors. Hypocretins as integrators of physiological signals. Berlin: Springer Science Business Media; 2005. pp. 221–231. [Google Scholar]
- Kukkonen JP, Holmqvist T, Ammoun S, Åkerman KE. Functions of the orexinergic/hypocretinergic system. Am J Physiol Cell Physiol. 2002;283:C1567–1591. doi: 10.1152/ajpcell.00055.2002. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/S0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Magga J, Bart G, Oker-Blom C, Kukkonen JP, Åkerman KE, Näsman J. Agonist potency differentiates G protein activation and Ca2+ signalling by the orexin receptor type 1. Biochem Pharmacol. 2006;71:827–836. doi: 10.1016/j.bcp.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Matsuki T, Sakurai T. Orexins and orexin receptors: from molecules to integrative physiology. Results Probl Cell Differ. 2008;46:27–55. doi: 10.1007/400_2007_047. [DOI] [PubMed] [Google Scholar]
- Mieda M, Willie JT, Hara J, Sinton CM, Sakurai T, Yanagisawa M. Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc Natl Acad Sci U S A. 2004;101:4649–4654. doi: 10.1073/pnas.0400590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näsman J, Bart G, Larsson K, Louhivuori L, Peltonen H, Åkerman KE. The orexin OX1 receptor regulates Ca2+ entry via diacylglycerol-activated channels in differentiated neuroblastoma cells. J Neurosci. 2006;26:10658–10666. doi: 10.1523/JNEUROSCI.2609-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak JZ, Jozwiak-Bebenista M, Bednarek K. Effects of PACAP and VIP on cyclic AMP formation in rat neuronal and astrocyte cultures under normoxic and hypoxic condition. Peptides. 2007;28:1706–1712. doi: 10.1016/j.peptides.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Okumura T, Takakusaki K. Role of orexin in central regulation of gastrointestinal functions. J Gastroenterol. 2008;43:652–660. doi: 10.1007/s00535-008-2218-1. [DOI] [PubMed] [Google Scholar]
- Porter RA, Chan WN, Coulton S, Johns A, Hadley MS, Widdowson K, Jerman JC, Brough SJ, Coldwell M, Smart D, Jewitt F, Jeffrey P, Austin N. 1,3-Biarylureas as selective non-peptide antagonists of the orexin-1 receptor. Bioorg Med Chem Lett. 2001;11:1907–1910. doi: 10.1016/S0960-894X(01)00343-2. [DOI] [PubMed] [Google Scholar]
- Ramanjaneya M, Conner AC, Chen J, Kumar P, Brown JE, Jöhren O, Lehnert H, Stanfield PR, Randeva HS. Orexin-stimulated MAP kinase cascades are activated through multiple G-protein signalling pathways in human H295R adrenocortical cells: diverse roles for orexins A and B. J Endocrinol. 2009;202:249–261. doi: 10.1677/JOE-08-0536. [DOI] [PubMed] [Google Scholar]
- Randeva HS, Karteris E, Grammatopoulos D, Hillhouse EW. Expression of orexin-A and functional orexin type 2 receptors in the human adult adrenals: implications for adrenal function and energy homeostasis. J Clin Endocrinol Metab. 2001;86:4808–4813. doi: 10.1210/jc.86.10.4808. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/S0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Moriguchi T, Furuya K, Kajiwara N, Nakamura T, Yanagisawa M, Goto K. Structure and function of human prepro-orexin gene. J Biol Chem. 1999;274:17771–17776. doi: 10.1074/jbc.274.25.17771. [DOI] [PubMed] [Google Scholar]
- Salomon Y, Londos C, Rodbell M. A highly sensitive adenylate cyclase assay. Analyt Biochem. 1974;58:541–548. doi: 10.1016/0003-2697(74)90222-X. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Daly JW, Creveling CR. A radioisotopic method for measuring the formation of adenosine 3′,5′-monophosphate in incubated slices of brain. J Neurochem. 1969;16:1609–1619. doi: 10.1111/j.1471-4159.1969.tb10360.x. [DOI] [PubMed] [Google Scholar]
- Spinazzi R, Andreis PG, Rossi GP, Nussdorfer GG. Orexins in the regulation of the hypothalamic–pituitary–adrenal axis. Pharmacol Rev. 2006;58:46–57. doi: 10.1124/pr.58.1.4. [DOI] [PubMed] [Google Scholar]
- Tang J, Chen J, Ramanjaneya M, Punn A, Conner AC, Randeva HS. The signaling profile of recombinant human orexin-2 receptor. Cell Sign. 2008;20:1651–1661. doi: 10.1016/j.cellsig.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Siegel JM, Nienhuis R, Moore RY. Pattern of hypocretin (orexin) soma and axon loss, and gliosis, in human narcolepsy. Brain Pathol. 2003;13:340–351. doi: 10.1111/j.1750-3639.2003.tb00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, Fournier A, Chow BK, Hashimoto H, Galas L, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;6:1283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- Voisin T, Rouet-Benzineb P, Reuter N, Laburthe M. Orexins and their receptors: structural aspects and role in peripheral tissues. Cell Mol Life Sci. 2003;60:72–87. doi: 10.1007/s000180300005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldan-Tambor A, Biegańska K, Wiktorowska-Owczarek A, Zawilska JB (2011) Activation of orexin/hypocretin type 1-like receptors stimulates cAMP synthesis in primary cultures of rat astrocytes. Pharmacol Rep 63 (in press) [DOI] [PubMed]
- Zhu Y, Miwa Y, Yamanaka A, Yada T, Shibahara M, Abe Y, Samurai T, Goto K. Orexin receptor type-1 couples exclusively to pertussis toxin-insensitive G-proteins, while orexin receptor type-2 couples to both pertussis toxin-sensitive and -insensitive G-proteins. J Pharmacol Sci. 2003;92:259–266. doi: 10.1254/jphs.92.259. [DOI] [PubMed] [Google Scholar]