Abstract
One of the applications of electroporation/electropulsation in biomedicine is gene electrotransfer, the wider use of which is hindered by low transfection efficiency in vivo compared with viral vectors. The aim of our study was to determine whether modulation of the extracellular matrix in solid tumors, using collagenase and hyaluronidase, could increase the transfection efficiency of gene electrotransfer in histologically different solid subcutaneous tumors in mice. Tumors were treated with enzymes before electrotransfer of plasmid DNA encoding either green fluorescent protein or luciferase. Transfection efficiency was determined 3, 9, and 15 days posttransfection. We demonstrated that pretreatment of tumors with a combination of enzymes significantly increased the transfection efficiency of electrotransfer in tumors with a high extracellular matrix area (LPB fibrosarcoma). In tumors with a smaller extracellular matrix area and less organized collagen lattice, the increase was not so pronounced (SA-1 fibrosarcoma and EAT carcinoma), whereas in B16 melanoma, in which only traces of collagen are present, pretreatment of tumors with hyaluronidase alone was more efficient than pretreatment with both enzymes. In conclusion, our results suggest that modification of the extracellular matrix could improve distribution of plasmid DNA in solid subcutaneous tumors, demonstrated by an increase in transfection efficiency, and thus have important clinical implications for electrogene therapy.
Cemazar and colleagues evaluate the effect of collagenase and hyaluronidase pretreatment of solid mouse subcutaneous tumors in the transfection efficiency of subsequent gene electrotransfer. Enzymatic pretreatment increases transfection efficiency, particularly in tumors with a greater extracellular matrix area.
Introduction
Electroporation/electropulsation has many biomedical applications. In human medicine, its most advanced use is electrochemotherapy, in which electroporation is combined with injection of the chemotherapeutic drugs bleomycin and cisplatin to locally potentiate the antitumor effectiveness of these chemotherapeutic drugs that are used in many clinical protocols. Results of published clinical studies demonstrate a high complete response rate of this therapy (up to 100%) and in 2010 approximately 80 oncology centers around the world routinely use this therapy for treatment of various subcutaneous tumors regardless of their histological type (Sersa et al., 2008). Another application of electroporation/electropulsation in medicine is gene electrotransfer. It can be used either for vaccination or treatment of various diseases, such as cancer, for which therapies are targeted either directly to tumor cells or aim to increase the immune response of the organism against cancer cells (Cemazar et al., 2006; Bodles-Brakhop and Draghia-Akli, 2008; Mir, 2009; Gothelf and Gehl, 2010). After the first encouraging reports of gene electrotransfer in skin, liver, tumors, and muscle in mice (Titomirov et al., 1991; Heller et al., 1996; Nishi et al., 1996; Aihara and Miyazaki, 1998; Rols et al., 1998; Mir et al., 1999), electrotransfer of plasmid DNA was also tested in several other tissues (Cemazar et al., 2006). Electrogene therapy can offer a systemic component to electrochemotherapy, which is effective but lacks a systemic effect, as other local treatments. Specifically, the first clinical trial of gene electrotransfer has been successfully completed. Electrotransfer of interleukin (IL)-12 to melanoma tumor nodules resulted in a pronounced antitumor effect, including on distant metastases, and demonstrated the feasibility of this approach in a clinical setting (Daud et al., 2008).
The mechanisms of electrotransfer of plasmid DNA into cells are not yet fully understood (Escoffre et al., 2010). Experiments in vitro and in vivo demonstrated that both electropermeabilization of the cell membrane and electrophoretic drift of nucleic acids are needed for effective transport of plasmid DNA into the cytoplasm (Golzio et al., 2002; Cemazar et al., 2009). In vivo, tissue organization provides hindrance to the movement of plasmid DNA and the resulting biodistribution (Zaharoff et al., 2002; Mesojednik et al., 2007). As a result, the level of transgene expression is low in tumors, never reaching more than 5% (Rols et al., 1998; Bettan et al., 2000; Cemazar et al., 2002, 2009).
The organization of specific tissues is determined by the type of cells and the presence of extracellular matrix. This network contains various components including such structures as collagen fibers, proteoglycans, and glycosaminoglycans, which together form a structured gel that fills the space between the cells and fibers (Pluen et al., 2001; Kumar et al., 2007). To improve the distribution of plasmid DNA before electropulsation in the muscle and its electrophoretically driven drift to the target cells, pretreatment with hyaluronidase (an enzyme that breaks down hyaluronan, a component of the extracellular matrix) was tested. The results of these studies demonstrated that an approximately 5-fold increase in expression of the gene of interest could be achieved after hyaluronidase muscle pretreatment (McMahon et al., 2001; Mennuni et al., 2002; Molnar et al., 2004). However, hyaluronidase did not improve transfection in skin (Vandermeulen et al., 2009). In tumors, where interstitial pressure is uniformly elevated and convection in the tumor tissue is negligible, the distribution of molecules relies on passive diffusion, which is mostly ineffective. Hyaluronidase led to improved distribution and therapeutic efficacy of chemotherapeutic drugs (Eikenes et al., 2005). Another possible way to increase the distribution of plasmid DNA in the muscle or tumors is by using collagenase, because it was demonstrated that diffusion in tumors correlated with collagen content, but not with sulfated glycosaminoglycan or hyaluronan content (Alexandrakis et al., 2004). Indeed, pretreatment of glioma tumors with collagenase led to increased efficiency of adenovirus-mediated gene therapy and oncolytic herpes simplex virus vector (Kuriyama et al., 2001; McKee et al., 2006).
To our knowledge, there are no data available on pretreatment of tumors with either of the extracellular matrix degradation enzymes and gene electrotransfer in tumors. Because the therapeutic use of gene therapy depends considerably on effective delivery of genetic material to target cells, the aim of our study was to determine whether alteration of the extracellular matrix in tumors, using collagenase and hyaluronidase, can increase the transfection efficiency of gene electrotransfer in histologically different solid subcutaneous tumors in mice.
Materials and Methods
Tumors and mice
In this study, four different murine tumor cell lines were used: SA-1 fibrosarcoma syngeneic to A/J mice, LPB fibrosarcoma and B16F1 melanoma syngeneic to C57BL/6 mice, and EAT mammary carcinoma syngeneic to CBA mice. LPB and B16F1 cells were routinely maintained in Eagle's minimal essential medium (EMEM; Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal calf serum (FCS; Sigma-Aldrich) and antibiotics in a humidified atmosphere at 37°C, containing 5% CO2. SA-1 and EAT cells were obtained by peritoneal lavage of ascitic tumor-bearing donor mice with 3–5 ml of 0.9% NaCl solution. Ascitic tumors were induced with 5.0×105 viable SA-1 cells or EAT cells injected intraperitoneally and harvested 4–5 days later. Because of the various generation times for the selected tumor cells, various amounts of cells (1.3×106 LPB, 5.0×105 SA-1, 3.0×106 EAT, and 1.0×106 B16F1 cells in 0.1 ml of 0.9% NaCl) were injected subcutaneously in order to obtain tumors 6 mm in diameter in approximately 8–12 days. During this period of time, no tumor necrosis was observed. After tumors reached the desired size (approximately 6 mm in diameter), the animals were randomly distributed into experimental groups and subjected to specific experimental protocols.
Female C57BL/6, CBA, and A/J mice were purchased from the Institute of Pathology (Medical Faculty, University of Ljubljana, Ljubljana, Slovenia). For some of the experiments female C57BL/6 mice were obtained from Charles River (L'Arbresle, France) for certain experiments with LPB tumors. At the start of the experiments, animals were 10–12 weeks old. Mice were kept in a conventional animal colony at a constant room temperature (21°C) and a natural day/night light cycle. Food and water were provided ad libitum. Animals were subjected to an adaptation period of 7–10 days before experiments. The mice were 11–14 weeks old at the beginning of the experiments, and weighed 20–25 g.
Procedures were performed according to approved protocols, in accordance with the French Centre National de la Recherche Scientifique (CNRS), Slovenian, and EU commission regulations for care of laboratory animals.
Plasmids and enzymes
The commercial plasmid pEGFP-N1 (Clontech, Basingstoke, UK), encoding the enhanced green fluorescent protein (GFP), and pCMVLuc, encoding luciferase (kind gift from L.M. Mir, Institute Gustave Roussy, Villejuif, France), both controlled by the cytomegalovirus promoter, were used throughout to assess transfection efficiency. Plasmids were isolated with an EndoFree mega kit (Qiagen, Hilden, Germany). Collagenase was obtained from Roche Diagnostics (Mannheim, Germany). It was dissolved in 0.9% NaCl and injected intratumorally at 30 μg/50 μl per tumor. Hyaluronidase was obtained from Sigma-Aldrich. It was dissolved in 0.9% NaCl and injected at 500 IU/50 μl per tumor.
Treatment protocol
Plasmid DNA (50 μg/50 μl) was slowly injected intratumorally with or without pretreatment with collagenase or hyaluronidase and with or without subsequent electroporation (EP) of tumors. Tumors that served as negative controls were injected with, instead of enzyme solutions, 50 μl of 0.9% NaCl. Exact experimental conditions (time intervals between collagenase, hyaluronidase, and DNA injections) are specified in the figure legends. Time intervals between injection of collagenase, hyaluronidase, and plasmid DNA and application of electric pulses were chosen according to literature results in order to comply with the ethical principles for animal research (3 R's: reduction, refinement, and replacement). The intervals between injection of the enzymes (24 hr for collagenase and 2 hr for hyaluronidase) and gene electrotransfer were selected according to the literature and should lead to degradation of extracellular matrix, allowing more efficient distribution of plasmid DNA (Netti et al., 2000; McMahon et al., 2001). Electric pulses were delivered immediately after DNA injection. Briefly, the applicator consisted of two flat parallel electrodes 7 mm apart (two stainless-steel strips [width, 7 mm], with rounded corners). Electrodes were placed percutaneously at the opposite margins of the tumor. Good contact between the electrodes and the overlying skin was ensured by means of a conductive gel (Parker Laboratories, Fairfield, NJ). Eight square-wave pulses (voltage-to-distance ratio, 600 V/cm; pulse duration, 5 msec; repetition frequency, 1 Hz) were generated with a GHT 1287 electropulsator (Jouan, St. Herblain, France).
Tumor growth was monitored by measuring three mutually orthogonal tumor diameters (e1, e2, and e3) with a Vernier caliper. Tumor volumes were calculated by the formula V=π×e1×e2×e3/6. The arithmetic mean of tumor volumes and areas and standard error of the mean (SE) were calculated for each experimental group (Hill and Bristow, 2004; Mundt et al., 2006). Tumor doubling time was determined for each individual tumor from the growth curves. At the end of tumor growth measurement, an autopsy was performed on each animal to determine the presence of possible metastases.
Assessment of transfection efficiency
GFP expression in the tumors was measured on days 2, 9, and 15 posttransfection. To reliably assess GFP expression within a tumor, tumors were carefully excised, removed from the overlying skin, and cut in half along the longest diameter. A fluorescence stereomicroscope (MZFL III; Leica Microsystems, Wetzlar, Germany) was used for visualization of GFP fluorescence of two tumor halves at ×0.8 magnification (see Fig. 1A). The image was recorded as a 12-bit, 1.3M-pixel file with a cooled charge-coupled device (CCD) camera (CoolSNAP fx; Photometrics/Roper Scientific, Tucson, AZ). The camera was driven by MetaVue software (Universal/Molecular Devices, Sunnyvale, CA). The exposure time was set at 1 sec with no binning. The fluorescence excitation was obtained with a mercury arc lamp (HBO; Osram, Munich, Germany) and the GFP filter (Leica Microsystems) was used for emission. Images were analyzed with MetaVue 5.7 software (Universal/Molecular Devices). The fluorescent area of the tumor was defined by threshold analysis as the region of interest (ROI), and the surface and mean fluorescence in the gated area (transfected area) were quantitatively estimated (measure/region measurement). Numerical values obtained by MetaVue analysis software were transferred to Excel 97 for Windows (Microsoft, Redmond, WA). They were then used for data analysis.
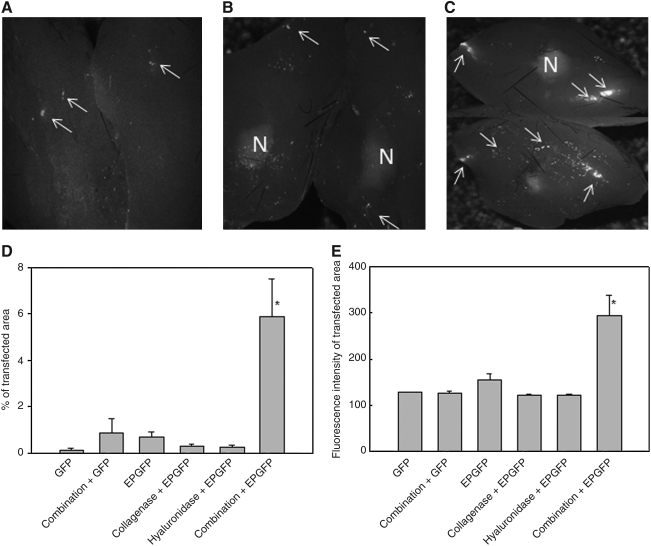
FIG. 1.
Green fluorescent protein (GFP) expression in LPB tumors 9 days postelectrotransfection. Shown are representative images of excised tumors cut into two halves, which were treated with plasmid DNA expressing GFP (A), pretreated with a combination of both enzymes followed by plasmid DNA injection alone (B) or pretreated with a combination of both enzymes followed by electrotransfer of plasmid DNA (C), and visualized under a digitalized fluorescence stereomicroscope. Original magnification, ×0.8. N, necrotic part; arrows, GFP fluorescent spots. (D) Percentage of transfected (GFP-positive) areas and (E) fluorescence intensity of transfected area. Data represent the mean±SEM of 3 or 4 tumors from 3 independent experiments (total, 9–12).*p<0.05 compared with all other groups. GFP, injection of plasmid DNA encoding GFP alone; Combination+GFP, injection of collagenase 24 hr and hyaluronidase 2 hr before injection of plasmid DNA; EPGFP, injection of plasmid DNA followed by application of EP; Collagenase+EPGFP, injection of collagenase 24 hr before electrotransfection of tumors; Hyaluronidase+EPGFP, injection of hyaluronidase 2 hr before electrotransfection of tumors; Combination+EPGFP, injection of collagenase 24 hr and hyaluronidase 2 hr before electrotransfection of tumors.
Luciferase expression in tumors was determined on day 2 posttransfection. Tumors were excised, weighed, immediately frozen in liquid nitrogen, and stored at −80°C until further procedures. Thawed tumors were homogenized in 1 ml of Glo lysis reagent (Promega, Madison, WI), using a UP200H homogenizer (Hielscher Ultrasonics, Teltow, Germany). Thereafter, the samples were centrifuged at 10,000×g for 10 min and the supernatant was stored at −80°C. Luciferase activity was measured in thawed supernatants, using a Genios luminometer (Tecan, Zurich, Switzerland). Photoemission was measured during a 5-sec period after a 5-min incubation at room temperature of 100 μl of tumor cell lysate and 100 μl of luciferase assay substrate (Promega). Luciferase activity was quantified as relative light units and then converted to picograms of luciferase per milligram of tumor tissue, using the calibration curve.
Histology of tumors
Histological analyses for proteoglycan and collagen content and cell density were performed on six subcutaneous LPB tumors, 6 mm in diameter, after treatment with the enzymes at the time when electrotransfer was performed in other experiments of the study. Tumors were excised, cut into two pieces along the largest diameter, and fixed in 10% buffered formalin. The tumors were then embedded in paraffin, cut into 5-μm sections, and stained with Masson's trichrome for collagen, periodic acid–Schiff reagent (PAS) for proteoglycans, and hematoxylin–eosin (H&E) for cell density estimation (Bancroft and Cook, 1994). Tumor slides were observed by transmission microscopy with a×60 objective, and images were taken with a CCD camera (DP70; Olympus, Hamburg, Germany). In H&E-stained tumor sections, the number of cells in the microscopic field was determined with DP-Soft software (Olympus). Three microscopic fields without necrosis were scored per section. Cell density was determined as the average number of cells per field and expressed as the number of tumor cells per square millimeter. To determine proteoglycan and collagen content in the tumors, the M-100 multipurpose test system was used, which is a standard stereological grid (Howard and Reed, 1998). Areal density of proteoglycans and collagen was estimated in each tumor slide by the pint counting morphometric method. At least 10 microscopic fields per slide were analyzed.
Statistical analysis
Data were tested for normality of distribution, using the Kolmogorov–Smirnov test. Differences between experimental groups were statistically evaluated by one-way analysis of variance (ANOVA) followed by the Holm–Sidak test for multiple comparison. A p value less than 0.05 was considered to be statistically significant. Statistical analysis was done with SigmaStat (Systat Software, London, UK) software. Data shown are the mean of three to six mice per group, unless stated otherwise.
Results
GFP expression after electrotransfer in LPB tumors after pretreatment of tumors with collagenase and hyaluronidase
Pretreatment of tumors with an intratumoral injection of a combination of both collagenase and hyaluronidase greatly increased the transfection efficiency of electrotransfer of plasmid DNA encoding GFP. The expression of GFP was scattered throughout the whole tumor and was not confined to the track of the needle. In addition, the necrotic part could be seen as a diffuse autofluorescence signal in the middle of the tumors and could be easily distinguished from the GFP fluorescence-positive cells (Fig. 1A–C).
Quantification of GFP expression demonstrated an ∼10 times higher percentage of the transfected area in the tumor pretreated with a combination of enzymes compared with the transfected area in tumors treated according to other transfection protocols (Fig. 1D). Electrotransfer of plasmid DNA alone or pretreatment of the tumors with either of the enzymes or a combination of enzymes and subsequent plasmid DNA injection did not significantly increase expression of GFP compared with tumors that were injected with plasmid DNA alone (Fig. 1D). In addition, the fluorescence intensity of the transfected area was significantly increased in electrotransfected tumors pretreated with a combination of enzymes, compared with the fluorescence intensity in tumors treated according to other transfection protocols (Fig. 1E). This indicates that in addition to a larger transfection area, a higher amount of plasmid DNA was also introduced into tumor cells by pretreatment of tumors with a combination of both enzymes.
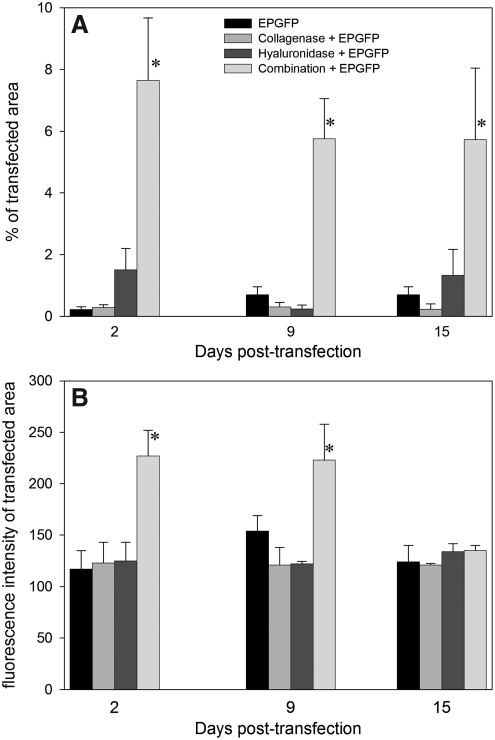
Further experiments were conducted to determine the duration of GFP expression in tumors. GFP expression was monitored for 15 days in LPB tumors, until their size required that the mice be killed. The percentage of transfected tumor area did not change during the observation period, regardless of the transfection protocol (Fig. 2A). The fluorescence intensity of the transfected area was highest in tumors that were pretreated with both enzymes before electrotransfer; in other groups the fluorescence intensity was only minimally above the background fluorescence intensity (Fig. 2B). The decrease in fluorescence intensity with time could be detected only in the combination group, as in the other conditions it remained similar to the background level.
FIG. 2.
Time dependence of transfection efficiency in LPB tumors. (A) Percentage of transfected (GFP positive) areas and (B) fluorescence intensity of transfected area on days 2, 9, and 15. Data represent the mean±SEM of 3 or 4 tumors from 3 independent experiments (total, 9–12). *p<0.05 compared with all other groups. EPGFP, injection of plasmid DNA followed by application of EP; Collagenase+EPGFP, injection of collagenase 24 hr before electrotransfection of tumors; Hyaluronidase+EPGFP, injection of hyaluronidase 2 hr before electrotransfection of tumors; Combination+EPGFP, injection of collagenase 24 hr and hyaluronidase 2 hr before electrotransfection of tumors.
Extracellular matrix content in LPB tumors after collagenase and hyaluronidase treatment
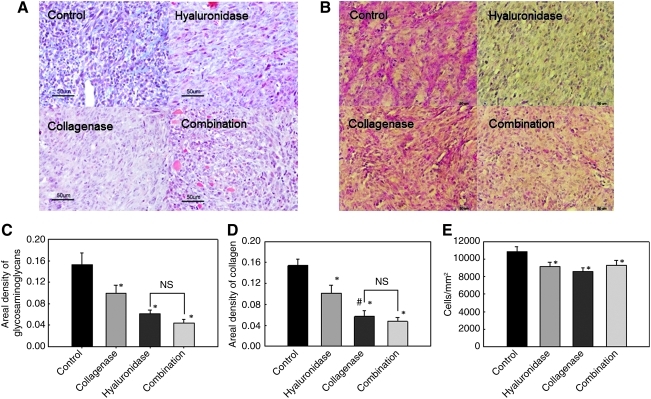
To determine the effect of pretreatment of tumors with collagenase and hyaluronidase on the composition of the extracellular matrix, histological analysis was performed. Both enzymes injected either alone or in combination significantly affected the composition of the extracellular matrix, by reducing the areal density of collagen or proteoglycans (Fig. 3). The most reduced areal density of the extracellular matrix (both proteoglycans and collagen) was determined after treatment of tumors with a combination of both enzymes.
FIG. 3.
Collagen content (A) and glycosaminoglycan/proteoglycan content (B) in LPB tumors after collagenase and hyaluronidase treatment. Shown are representative histological images of LPB tumors stained for collagen (light blue) and glycosaminoglycans/proteoglycans (pink) in control tumors and after treatment with collagenase, hyaluronidase, or a combination of both enzymes. Extracellular matrix content: (C) glycosaminoglycans/proteoglycans; (D) collagen; and (E) cell density of LPB tumors after collagenase and/or hyaluronidase treatment. Data represent the mean±SEM of six tumors. *p<0.05 compared with the control group. #p<0.05 compared with treatment of tumors with hyaluronidase. NS, not significant.
As expected, the treatment of tumors with both enzymes significantly reduced the areal density of glycosaminoglycans compared with the control and collagenase-treated tumors, but not when compared with the areal density of tumors treated with hyaluronidase (Fig. 3C). In addition, collagenase alone also significantly reduced the areal density of glycosaminoglycans compared with control, but to a much lesser extent than treatment with hyaluronidase and both enzymes (Fig. 3C).
Furthermore, the treatment of tumors with both enzymes significantly reduced the areal density of collagen compared with the control and hyaluronidase-pretreated tumors, but not when compared with the areal density of tumors pretreated with collagenase (Fig. 3D). Moreover, treatment with collagenase also significantly reduced the areal density of collagen compared with hyaluronidase (Fig. 3D). On the other hand, treatment with hyaluronidase alone significantly reduced the areal density of collagen compared with control, but this reduction was not as pronounced as with other treatments.
The degradation of extracellular matrix compounds observed after the treatment of tumors with each enzyme alone or in combination was associated with a decreased cell density in LPB tumors, indicating expansion of the extracellular matrix volume. It was significantly lower compared with the cell density in control tumors (Fig. 3E), which were injected with 0.9% NaCl instead of enzymes.
Tumor growth of LPB tumors after electrotransfer following pretreatment of tumors with collagenase and hyaluronidase
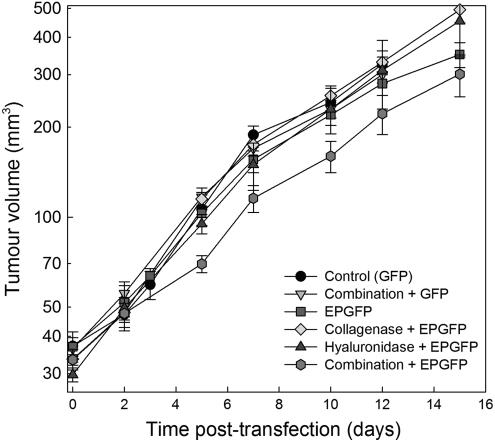
The tumor doubling time of control tumors was 4.0 days. No significant change in tumor growth was observed after the treatments. Only treatment including electrotransfer and both enzymes had a minor effect on tumor growth that was nonsignificant at the doses of enzymes used (Fig. 4). Autopsy of the animals was performed after the tumor diameter measurements were concluded. All visceral organs were free of macroscopic metastases. In addition, lungs were fixed in Bouin's fixative and examination of the lungs by stereomicroscopy was performed, demonstrating no metastases (data not shown).
FIG. 4.
Growth curves of LPB tumors after electrotransfection. Combination+GFP, injection of collagenase 24 hr and hyaluronidase 2 hr before injection of plasmid DNA; EPGFP, injection of plasmid DNA followed by application of EP; Collagenase+EPGFP, injection of collagenase 24 hr before electrotransfection of tumors; Hyaluronidase+EPGFP, injection of hyaluronidase 2 hr before electrotransfection of tumors; Combination+EPGFP, injection of collagenase 24 hr and hyaluronidase 2 hr before electrotransfection of tumors. Data represent the mean±SEM of six tumors.
Electrotransfer efficiency after pretreatment with collagenase and hyaluronidase in four tumor models assayed by luciferase expression
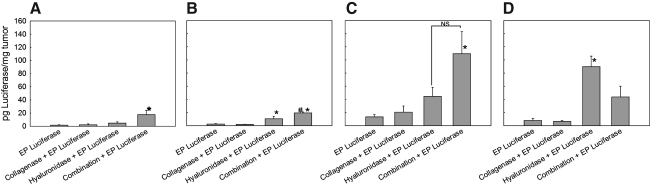
To demonstrate that pretreatment of tumors with a combination of enzymes led to increased transfection efficiency in other tumor models, three murine tumors with different histological properties, together with LPB tumors, were electrotransfected with plasmid DNA encoding luciferase (Fig. 5). Pretreatment of tumors with both enzymes significantly increased luciferase expression in LPB tumors compared with tumors that were only electrotransfected or pretreated with each of the enzymes, confirming the results obtained with GFP (Fig. 5A).
FIG. 5.
Transfection efficiency in LPB (A), SA-1 (B), EAT (C), and B16 (D) tumors after pretreatment of tumors with collagenase and hyaluronidase. EP Luciferase, injection of plasmid DNA followed by application of EP; Collagenase+EP Luciferase, injection of collagenase 24 hr before electrotransfection of tumors; Hyaluronidase+EP Luciferase, injection of hyaluronidase 2 hr before electrotransfection of tumors; Combination+EP Luciferase, injection of collagenase 24 hr and hyaluronidase 2 hr before electrotransfection of tumors. Data represent the mean±SEM of 3 or 4 tumors from 3 independent experiments (total, 9–12). *p<0.05 compared with other treatment groups; #p<0.05 compared with the Hyaluronidase+EP Luciferase group.
In SA-1 tumors, pretreatment of tumors with both enzymes also significantly increased luciferase expression compared with the other treatment groups. In addition, pretreatment of tumors with hyaluronidase increased luciferase expression in SA-1 tumors (Fig. 5B), whereas collagenase alone had no effect.
In EAT tumors, pretreatment of tumors with both enzymes resulted in significantly increased expression of luciferase compared with the control and collagenase-pretreated tumors. In addition, a trend toward higher transfection efficiency in tumors pretreated with collagenase or hyaluronidase alone was present (Fig. 5C).
In B16 tumors, the highest increase in luciferase expression was obtained after pretreatment of tumors with hyaluronidase. Pretreatment of tumors with collagenase alone did not result in increased transfection efficiency, whereas pretreatment of tumors with both enzymes resulted in a pronounced, but not significant, increase in transfection efficiency compared with control and collagenase-pretreated tumors (Fig. 5D).
The relative increase in transfection efficiency in LPB tumors was higher compared with SA-1, EAT, and B16 tumors, in line with the histological properties of these tumors (Mesojednik et al., 2007).
Discussion
Our results demonstrate that treatment of tumors with collagenase and hyaluronidase before gene electrotransfer greatly increases expression of the transgene. Specifically, the increase in transfection efficiency was higher in tumors with a high content of extracellular matrix (fibrosarcoma LPB) than in tumors with less extracellular matrix component (fibrosarcoma SA-1, carcinoma EAT, and melanoma B16) (Mesojednik et al., 2007).
The feasibility of electropulsation as a nucleic acid delivery system has already been demonstrated with various reporter genes as well as therapeutic genes. The most commonly used reporter genes were those encoding GFP, β-galactosidase, and luciferase, the first two enabling visualization of overall transfection efficiency as well as spatial distribution of expression. Reporter genes were used for proof of concept. For preclinical application, numerous therapeutic genes have also been used in electrotransfection protocols (Cemazar and Sersa, 2007; Mir, 2009). However, the main barrier to the further success of electrogene therapy still remains its fairly poor transfection efficiency. Therefore, new approaches leading to increased transfection efficiency, comparable to that of viral vectors, are needed (Cemazar et al., 2006).
In our study, one such approach was evaluated, namely modification of the tumor extracellular matrix with the matrix-degrading enzymes collagenase and hyaluronidase. The extracellular matrix in tumors is more abundant than in normal tissues. In all studies published so far, only a single enzyme was used to assess changes in the distribution of various molecules and therapeutic efficacy. In our study, in addition to treatment of tumors with a single enzyme, we tested whether a combination of both enzymes would further improve the distribution of plasmid DNA in tumors. We demonstrated that a combination of enzymes significantly increased the transfection efficiency in tumors with a high extracellular matrix area (i.e., LPB tumors). In tumors with a smaller extracellular matrix area and less organized collagen lattice, the increase was not so pronounced (i.e., SA-1 and EAT tumors), whereas in B16 tumors, in which only traces of collagen are present, tumor pretreatment with hyaluronidase was more efficient than pretreatment with both enzymes. We can presume that the pretreatment of tumor with enzymes led to degradation of a dense network of extracellular molecules and consequently to decreased interstitial fluid pressure, which is normally elevated in tumors. Degradation of the extracellular matrix in tumors by treatment with collagenase or hyaluronidase has been described in several studies together with changes in microvascular pressure, interstitial fluid pressure, diffusion, compound distribution, and so on (Eikenes et al., 2004, 2010). It was demonstrated that both collagenase and hyaluronidase reduce interstitial fluid pressure to a similar extent, whereas collagenase also reduces microvascular pressure, probably by reducing vascular resistance through the degradation of collagen associated with vascular vessels. It resulted in improved distribution of various molecules, such as antibodies, liposomal doxorubicin, and viruses. Furthermore, it has also been shown in clinical studies that hyaluronidase can improve the therapeutic effectiveness of chemotherapeutic drugs (Spruss et al., 1995; Baumgartner et al., 1998). Collagenase improved the distribution and efficacy of an oncolytic herpes simplex virus vector injected intratumorally in a human melanoma xenograft (McKee et al., 2006).
First, we evaluated the transfection efficiency and duration of reporter gene expression after gene electrotransfer in tumors pretreated with the enzymes. On the basis of our previous study, we selected LPB fibrosarcoma tumors with a high content of extracellular matrix. In addition, the level of transfected cells, compared with other tumor models, was low in that study (Mesojednik et al., 2007). In the present study, we found that only the combination of both enzymes increased transfection efficiency (Figs. 1 and 2). Neither collagenase alone nor hyaluronidase alone increased the transfection efficiency compared with control tumors that were exposed to electric pulses after plasmid DNA injection. Transfection efficiency was increased ∼30-fold and the transfected relative area of tumor did not change during the observation period of 15 days. In addition, the fluorescence intensity of the transfected area also increased, indicating that not only a larger number of cells was transfected, but also a larger number of plasmid DNA molecules was introduced into these cells. The level of fluorescence intensity decreased to control levels on day 15. The main reason for this observation is the dilution of plasmid DNA with cell proliferation; however, silencing of plasmid expression and clearance of plasmid could also contribute to the decreased fluorescence intensity (Kamenšek et al., 2011).
Second, to determine whether treatment of tumors with enzymes indeed affected the composition of the extracellular matrix, histological analyses were performed (Fig. 3). Both enzymes significantly reduced the areal density of extracellular matrix components as well as the cell density in LPB tumors. Hyaluronidase cleaves the β-N-acetyl-hexosamine glycosidic bonds in hyaluronan and consequently the function of hyaluronan in the extracellular matrix is impeded. Intact hyaluronan binds water and forms a viscous, gelatin-like matrix that provides structural support for the surrounding tissue. Therefore, hyaluronidase-induced short hyaluronan fragments can still bind water molecules, but cannot form a functional viscous gel and consequently the areal density of the glycosaminoglycans was reduced (Eikenes et al., 2005; Shiftan et al., 2005). On the other hand, collagenase cleaves collagen and the collagen lattice in the LPB tumors was disrupted, leading to reduced areal density of collagen (Kumar et al., 2007). We found that treatment of tumors with a single enzyme or both enzymes led to the same reduction of the specific extracellular component. In addition, a small reduction of the areal density of collagen was also obtained after hyaluronidase treatment and, vice versa, a small reduction of the areal density of glycosaminoglycans was obtained after collagenase treatment. We can presume that the reason for the observed results lies in the interconnection of collagen and hyaluronan in the extracellular matrix; they support each other and are necessary for the proper functioning of the extracellular matrix. If one of the components is degraded, it cannot support the other extracellular matrix components, resulting in reduced areal density of the latter. In addition, degradation of one extracellular matrix component can lead to increased water content in the extracellular matrix, which in turn may result in expansion of the extracellular matrix volume; this was reflected in our study by decreased cell density and may be one reason for the reduced areal density of specific extracellular matrix components. However, although degradation of one matrix component results in the reduced areal density of another, it is not degraded and can still represent an obstacle to plasmid DNA distribution. Therefore, the increase in transfection efficiency in tumors treated with single enzymes is not the same as the increase in transfection efficiency in tumors treated with a combination of the enzymes, demonstrating that degradation of both components of the extracellular matrix is needed for increased transfection efficiency.
Third, concerns related to collagenase treatment and metastatic spread have been raised in previous studies (Bouzin and Feron, 2007). Treatment with enzymes before electrotransfer of plasmid DNA encoding GFP had a minor effect on tumor growth at the doses we used (Fig. 4), although in another study it was demonstrated that electrotransfer of a reporter gene encoding luciferase can lead even to regression of B16 melanoma tumors (Heller and Coppola, 2002). Furthermore, at the end of the experiments, autopsy of the animals was performed to check for the presence of metastases. We did not observe any macroscopically visible metastases in any of the treatment groups. Although 15 days for LBP tumors is not an optimal time to assess the presence of metastases, because 14 days are needed for the growth of lung metastases after intravenous injection (Tevz et al., 2009), indications were that neither collagenase treatment, nor the collagenase treatment with gene electrotransfer, induces the spread of metastases. In addition, our more recent results demonstrate that electric pulses used in gene electrotransfer did not alter gene expression responsible for the metastatic process (Mlakar et al., 2009; Todorovic et al., 2011).
Fourth, to validate and extend our results to other tumor types, gene electrotransfer of the tumor was combined with enzyme pretreatment in another three histologically different tumor models in comparison with LPB tumors (Fig. 5). These tumor models differ in their collagen and proteoglycan content (Mesojednik et al., 2007). As already demonstrated, tumors with a well-organized collagen lattice and high proteoglycan content are more difficult to transfect by gene electrotransfer compared with tumors with less collagen and a small amount of proteoglycans (Mesojednik et al., 2007). In line with this, the degradation of the extracellular matrix resulted in high potentiation of the transfection efficiency in LPB tumors with a well-organized collagen lattice. Potentiation was also pronounced in SA-1 tumors, which also have well-organized collagen lattices but a smaller area of extracellular matrix compared with LPB tumors. Specifically, disruption of collagen by collagenase alone was not enough to increase the transfection efficiency. In both tumors, pretreatment of tumors with both enzymes was necessary to increase the transfection efficiency. Therefore, we can speculate that besides disruption of collagen, which forms the support and stabilizes hyaluronan, degradation of hyaluronan is also needed to increase the mobility and distribution of plasmid DNA in the tissue (Netti et al., 2000). In addition, application of electric pulses increases the mobility of DNA in tissues, as shown by Zaharoff and colleagues (2002). Furthermore, pretreatment of EAT tumors with both enzymes also led to increased transfection efficiency of gene electrotransfer. The level of transfection was higher compared with SA-1 and LPB tumors. However, besides degradation of the extracellular matrix, this can be attributed to the larger cell size and smaller cell density, which are both factors that are important in gene electrotransfer (Mesojednik et al., 2007). Namely, our data as well as mathematical models and in vitro studies demonstrated that increased cell membrane permeability due to the application of electric pulses is dependent not only on electric field intensity and cell size, but also on cell shape, orientation, and cell density and organization in multicellular structures (Susil et al., 1998; Gimsa and Wachner, 2001; Canatella et al., 2004; Valic et al., 2004; Pucihar et al., 2007; Miklavcic and Towhidi, 2010). The electrotransfection efficiency was also increased by pretreatment in B16 tumors, which are soft tumors with a low cell density and large cells. In addition, these tumors have a small content of proteoglycan and collagen and numerous adipocytes, which occupy 2% of the tumor area. Furthermore, these tumors are well vascularized. Therefore, the presence of fluids in these tumors is high (Mesojednik et al., 2007). In this tumor model, the degradation of hyaluronan yielded high expression of luciferase that was not enhanced by pretreatment of tumors with collagenase. The reason for such a high increase in transfection efficiency probably lies in the low level of collagen, which cannot support and stabilize proteoglycans. Hence, the degradation of hyaluronan enables better mobility and distribution of plasmid DNA and consequently results in high expression of the reporter luciferase gene.
In other gene electrotransfer studies, only hyaluronidase was used to enhance the mobility and distribution of plasmid DNA. These studies were done in muscle and skin (McMahon et al., 2001; Mennuni et al., 2002; Vandermeulen et al., 2009). The transfection efficiency was increased only in muscle. Here, we demonstrate for the first time that hyaluronidase alone increases transfection efficiency in soft, well-vascularized B16 tumors, whereas in other tumor models, which possess a higher fraction of extracellular matrix, pretreatment of tumors with both collagenase and hyaluronidase is compulsory to obtain an increase in electrotransfer efficiency. In addition, treatment with enzymes did not promote the spreading of metastases during the time of the experiment (15 days).
In conclusion, our results show that modification of the extracellular matrix can improve the distribution of plasmid DNA in tumors, demonstrated by increased transfection efficiency, and thus have important clinical implications for electrogene therapy.
Acknowledgments
This study was supported by program grant P3-0003 and project grants from the CNRS CEA IPA Network and the Region Midi-Pyrénées (Réseau Thérapie Génique), and was conducted in the scope of the EBAM European Associated Laboratory (LEA).
Author Disclosure Statement
There are no competing or financial interests to disclose.
References
- Aihara H. Miyazaki J. Gene transfer into muscle by electroporation in vivo. Nat. Biotechnol. 1998;16:867–870. doi: 10.1038/nbt0998-867. [DOI] [PubMed] [Google Scholar]
- Alexandrakis G. Brown E.B. Tong R.T., et al. Two-photon fluorescence correlation microscopy reveals the two-phase nature of transport in tumors. Nat. Med. 2004;10:203–207. doi: 10.1038/nm981. [DOI] [PubMed] [Google Scholar]
- Bancroft J.D. Cook H.C. Manual of Histological Techniques and Their Diagnostic Application. Churchill Livingstone; New York: 1994. [Google Scholar]
- Baumgartner G. Gomar-Hoss C. Sakr L., et al. The impact of extracellular matrix on the chemoresistance of solid tumors: Experimental and clinical results of hyaluronidase as additive to cytostatic chemotherapy. Cancer Lett. 1998;131:85–99. [PubMed] [Google Scholar]
- Bettan M. Ivanov M.A. Mir L.M., et al. Efficient DNA electrotransfer into tumors. Bioelectrochemistry. 2000;52:83–90. doi: 10.1016/s0302-4598(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Bodles-Brakhop A.M. Draghia-Akli R. DNA vaccination and gene therapy: Optimization and delivery for cancer therapy. Expert Rev. Vaccines. 2008;7:1085–1101. doi: 10.1586/14760584.7.7.1085. [DOI] [PubMed] [Google Scholar]
- Bouzin C. Feron O. Targeting tumor stroma and exploiting mature tumor vasculature to improve anti-cancer drug delivery. Drug Resist. Updat. 2007;10:109–120. doi: 10.1016/j.drup.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Canatella P.J. Black M.M. Bonnichsen D.M., et al. Tissue electroporation: Quantification and analysis of heterogeneous transport in multicellular environments. Biophys. J. 2004;86:3260–3268. doi: 10.1016/S0006-3495(04)74374-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cemazar M. Sersa G. Electrotransfer of therapeutic molecules into tissues. Curr. Opin. Mol. Ther. 2007;9:554–562. [PubMed] [Google Scholar]
- Cemazar M. Sersa G. Wilson J., et al. Effective gene transfer to solid tumors using different nonviral gene delivery techniques: Electroporation, liposomes, and integrin-targeted vector. Cancer Gene Ther. 2002;9:399–406. doi: 10.1038/sj.cgt.7700454. [DOI] [PubMed] [Google Scholar]
- Cemazar M. Golzio M. Sersa G., et al. Electrically-assisted nucleic acids delivery to tissues in vivo: Where do we stand? Curr. Pharm. Des. 2006;12:3817–3825. doi: 10.2174/138161206778559740. [DOI] [PubMed] [Google Scholar]
- Cemazar M. Golzio M. Sersa G., et al. Control by pulse parameters of DNA electrotransfer into solid tumors in mice. Gene Ther. 2009;16:635–644. doi: 10.1038/gt.2009.10. [DOI] [PubMed] [Google Scholar]
- Daud A.I. DeConti R.C. Andrews S., et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J. Clin. Oncol. 2008;26:5896–5903. doi: 10.1200/JCO.2007.15.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikenes L. Bruland O.S. Brekken C. de Lange Davies C. Collagenase increases the transcapillary pressure gradient and improves the uptake and distribution of monoclonal antibodies in human osteosarcoma xenografts. Cancer Res. 2004;64:4768–4773. doi: 10.1158/0008-5472.CAN-03-1472. [DOI] [PubMed] [Google Scholar]
- Eikenes L. Tari M. Tufto I., et al. Hyaluronidase induces a transcapillary pressure gradient and improves the distribution and uptake of liposomal doxorubicin (Caelyx) in human osteosarcoma xenografts. Br. J. Cancer. 2005;93:81–88. doi: 10.1038/sj.bjc.6602626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikenes L. Tufto I. Schnell E.A., et al. Effect of collagenase and hyaluronidase on free and anomalous diffusion in multicellular spheroids and xenografts. Anticancer Res. 2010;30:359–368. [PubMed] [Google Scholar]
- Escoffre J.M. Teissie J. Rols M.P. Gene transfer: How can the biological barriers be overcome? J. Membr. Biol. 2010;236:61–74. doi: 10.1007/s00232-010-9275-0. [DOI] [PubMed] [Google Scholar]
- Gimsa J. Wachner D. Analytical description of the transmembrane voltage induced on arbitrarily oriented ellipsoidal and cylindrical cells. Biophys. J. 2001;81:1888–1896. doi: 10.1016/S0006-3495(01)75840-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golzio M. Teissie J. Rols M.P. Direct visualization at the single-cell level of electrically mediated gene delivery. Proc. Natl. Acad. Sci. U.S.A. 2002;99:1292–1297. doi: 10.1073/pnas.022646499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf A. Gehl J. Gene electrotransfer to skin: Review of existing literature and clinical perspectives. Curr. Gene Ther. 2010;10:287–299. doi: 10.2174/156652310791823443. [DOI] [PubMed] [Google Scholar]
- Heller L. Coppola D. Electrically mediated delivery of vector plasmid DNA elicits an antitumor effect. Gene Ther. 2002;9:1321–1325. doi: 10.1038/sj.gt.3301802. [DOI] [PubMed] [Google Scholar]
- Heller R. Jaroszeski M. Atkin A., et al. In vivo gene electroinjection and expression in rat liver. FEBS Lett. 1996;389:225–228. doi: 10.1016/0014-5793(96)00590-x. [DOI] [PubMed] [Google Scholar]
- Hill R.P. Bristow R.G. The scientific basis of radiotherapy. In: Tannock I.F., editor; Hill R.P., editor; Bristow R.G., editor; Harrington L., editor. The Basic Science of Oncology. McGraw-Hill Medical Publishing Division; New York: 2004. pp. 289–321. [Google Scholar]
- Howard C.V. Reed M.G. Unbiased stereology. Bioscientific Publishers; Oxford, United Kingdom: 1998. [Google Scholar]
- Kamenšek U. Sersa G. Vidic S., et al. Irradiation, cisplatin and 5-azacytidine up-regulate cytomegalovirus promoter in tumors and muscles: Implementation of noninvasive fluorescence imaging. Mol. Imaging Biol. 2011;13:43–52. doi: 10.1007/s11307-010-0300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V. Abbas A.K. Fausto N. Mitchell R.N. Robbins Basic Pathology. 8th. Saunders Elsevier; Philadelphia: 2007. [Google Scholar]
- Kuriyama N. Kuriyama H. Julin C.M., et al. Protease pretreatment increases the efficacy of adenovirus-mediated gene therapy for the treatment of an experimental glioblastoma model. Cancer Res. 2001;61:1805–1809. [PubMed] [Google Scholar]
- McKee T.D. Grandi P. Mok W., et al. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006;66:2509–2513. doi: 10.1158/0008-5472.CAN-05-2242. [DOI] [PubMed] [Google Scholar]
- McMahon J.M. Signori E. Wells K.E., et al. Optimisation of electrotransfer of plasmid into skeletal muscle by pretreatment with hyaluronidase: Increased expression with reduced muscle damage. Gene Ther. 2001;8:1264–1270. doi: 10.1038/sj.gt.3301522. [DOI] [PubMed] [Google Scholar]
- Mennuni C. Calvaruso F. Zampaglione I., et al. Hyaluronidase increases electrogene transfer efficiency in skeletal muscle. Hum. Gene Ther. 2002;13:355–365. doi: 10.1089/10430340252792495. [DOI] [PubMed] [Google Scholar]
- Mesojednik S. Pavlin D. Sersa G., et al. The effect of the histological properties of tumors on transfection efficiency of electrically assisted gene delivery to solid tumors in mice. Gene Ther. 2007;14:1261–1269. doi: 10.1038/sj.gt.3302989. [DOI] [PubMed] [Google Scholar]
- Miklavcic D. Towhidi L. Numerical study of the electroporation pulse shape effect on molecular uptake of biological cells. Radiol. Oncol. 2010;44:34–41. doi: 10.2478/v10019-010-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir L.M. Nucleic acids electrotransfer-based gene therapy (electrogenetherapy): Past, current, and future. Mol. Biotechnol. 2009;43:167–176. doi: 10.1007/s12033-009-9192-6. [DOI] [PubMed] [Google Scholar]
- Mir L.M. Bureau M.F. Gehl J., et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc. Natl. Acad. Sci. U.S.A. 1999;96:4262–4267. doi: 10.1073/pnas.96.8.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlakar V. Todorovic V. Cemazar M., et al. Electric pulses used in electrochemotherapy and electrogene therapy do not significantly change the expression profile of genes involved in the development of cancer in malignant melanoma cells. BMC Cancer. 2009;9:299. doi: 10.1186/1471-2407-9-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar M.J. Gilbert R. Lu Y., et al. Factors influencing the efficacy, longevity, and safety of electroporation-assisted plasmid-based gene transfer into mouse muscles. Mol. Ther. 2004;10:447–455. doi: 10.1016/j.ymthe.2004.06.642. [DOI] [PubMed] [Google Scholar]
- Mundt A.J. Roeske J.C. Chung T.D. Weichselbaum R.R. Principles of radiation oncology. In: Holland J.F., editor; Frei E., editor. Cancer Medicine. BC Decker Hamilton; London: 2006. pp. 517–536. [Google Scholar]
- Netti P.A. Berk D.A. Swartz M.A., et al. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–2503. [PubMed] [Google Scholar]
- Nishi T. Yoshizato K. Yamashiro S., et al. High-efficiency in vivo gene transfer using intraarterial plasmid DNA injection following in vivo electroporation. Cancer Res. 1996;56:1050–1055. [PubMed] [Google Scholar]
- Pluen A. Boucher Y. Ramanujan S., et al. Role of tumor–host interactions in interstitial diffusion of macromolecules: Cranial vs. subcutaneous tumors. Proc. Natl. Acad. Sci. U.S.A. 2001;98:4628–4633. doi: 10.1073/pnas.081626898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucihar G. Kotnik T. Teissie J. Miklavcic D. Electropermeabilization of dense cell suspensions. Eur. Biophys. J. 2007;36:173–185. doi: 10.1007/s00249-006-0115-1. [DOI] [PubMed] [Google Scholar]
- Rols M.P. Delteil C. Golzio M., et al. In vivo electrically mediated protein and gene transfer in murine melanoma. Nat. Biotechnol. 1998;16:168–171. doi: 10.1038/nbt0298-168. [DOI] [PubMed] [Google Scholar]
- Sersa G. Miklavcic D. Cemazar M., et al. Electrochemotherapy in treatment of tumours. Eur. J. Surg. Oncol. 2008;34:232–240. doi: 10.1016/j.ejso.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Shiftan L. Israely T. Cohen M., et al. Magnetic resonance imaging visualization of hyaluronidase in ovarian carcinoma. Cancer Res. 2005;65:10316–10323. doi: 10.1158/0008-5472.CAN-04-3947. [DOI] [PubMed] [Google Scholar]
- Spruss T. Bernhardt G. Schonenberger H. Schiess W. Hyaluronidase significantly enhances the efficacy of regional vinblastine chemotherapy of malignant melanoma. J. Cancer Res. Clin. Oncol. 1995;121:193–202. doi: 10.1007/BF01366962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susil R. Semrov D. Miklavcic D. Electric field-induced transmembrane potential depends on cell density and organization. Electro Magnetobiol. 1998;17:391–399. [Google Scholar]
- Tevz G. Kranjc S. Cemazar M., et al. Controlled systemic release of interleukin-12 after gene electrotransfer to muscle for cancer gene therapy alone or in combination with ionizing radiation in murine sarcomas. J. Gene Med. 2009;11:1125–1137. doi: 10.1002/jgm.1403. [DOI] [PubMed] [Google Scholar]
- Titomirov A.V. Sukharev S. Kistanova E. In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA. Biochim. Biophys. Acta. 1991;1088:131–134. doi: 10.1016/0167-4781(91)90162-f. [DOI] [PubMed] [Google Scholar]
- Todorovic V. Sersa G. Mlakar V., et al. Metastatic potential of melanoma cells is not affected by electrochemotherapy. Melanoma Res. 2011;21:196–205. doi: 10.1097/CMR.0b013e328337abd7. [DOI] [PubMed] [Google Scholar]
- Valic B. Pavlin M. Miklavcic D. The effect of resting transmembrane voltage on cell electropermeabilization: A numerical analysis. Bioelectrochemistry. 2004;63:311–315. doi: 10.1016/j.bioelechem.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Vandermeulen G. Daugimont L. Richiardi H., et al. Effect of tape stripping and adjuvants on immune response after intradermal DNA electroporation. Pharm. Res. 2009;26:1745–1751. doi: 10.1007/s11095-009-9885-3. [DOI] [PubMed] [Google Scholar]
- Zaharoff D.A. Barr R.C. Li C.Y. Yuan F. Electromobility of plasmid DNA in tumor tissues during electric field-mediated gene delivery. Gene Ther. 2002;9:1286–1290. doi: 10.1038/sj.gt.3301799. [DOI] [PubMed] [Google Scholar]