Abstract
The efficacy of adenovirus (Ad)-based gene therapy of solid tumors, such as prostate cancer, is limited. One of the many problems is that the virus infects many different cell types in the body, resulting in high toxicity, whereas the target cancer cells are often less prone to wild-type Ad infection. Our aim was to develop genetically de- and retargeted Ad vectors to reduce off-target effects and increase target infection for prostate cancer. We have previously reported an Ad5 vector specific for the cancer-associated receptor Her2/neu, created by inserting Her2/neu-reactive Affibody® molecules (ZH) into the HI loop of a coxsackievirus and adenovirus receptor binding-ablated fiber (Ad[ZH/1]). In addition to virus retargeting to Her2/neu, this virus was further modified from wild-type Ad by changing the RGD motif in the penton base to EGD and by substitution of the KKTK motif in the third shaft repeat to RKSK, resulting in the vector Ad[ZH/3]. The ZH-containing vectors could be produced to high titers and were specific for their target, resulting in efficient infection and killing of Her2/neu-positive androgen-dependent PC346C prostate cancer cells in vitro. Here we show that the oncolytic Ad[ZH/3] vector significantly prolonged survival time and reduced serum prostate-specific antigen levels in an orthotopic prostate tumor model in nude mice to the same extent as wild-type Ad5. Our results show that Her2/neu targeting using Ad-based vectors for prostate cancer is feasible and may serve as a basis for the development of gene therapy of human prostate cancer as well as other Her2/neu-expressing cancers.
Magnusson and colleagues have developed an Ad5 vector specific for the cancer-associated receptor Her2/neu and show that it can efficiently infect and kill Her2/neu-positive androgen-dependent PC346C prostate cancer cells in vitro. They also show that this vector can significantly prolong survival time and reduce serum prostate-specific antigen levels in an orthotopic prostate tumor mouse model.
Introduction
Prostate cancer (PCa) is one of the most common types of cancer in humans and is often fatal. The only potential cures for localized disease are complete surgical resection and local radiotherapy. However, about 35% of patients relapse due to metastatic disease (Auclerc et al., 2000). For advanced PCa androgen ablation is the treatment of choice, but after a variable period of time castration-resistant (CR) disease emerges, which lacks curative options. For castration-resistant disease, a survival benefit is afforded by docetaxel in combination with prednisone or mitoxantrone or next-generation drugs to manipulate the residual hormone response (Reid et al., 2010). The prognosis for CR PCa remains poor, with a mean survival of less than 20 months (Tannock et al., 2004). Effective systemic therapies for CR and metastatic PCa are required to augment the current agents. During the last decade gene therapy for PCa, using adenoviral (Ad) vectors, has emerged as a treatment option and several clinical studies have been conducted or are ongoing (reviewed in Schenk et al., 2010). Several problems have been identified, for example, inability of the vector to reach metastases and poor vector spread in the tumors. Another general problem is that the coxsackievirus and adenovirus receptor (CAR) has been shown to be expressed to a variable and often low degree in both prostate cancer specimens and prostate tumor cell lines (Rauen et al., 2002; Pandha et al., 2003). Moreover, CAR is associated mainly with the tight junctions, making it inaccessible from the apical side of a complex epithelial tissue. To improve infection of tumor cells by Ad, retargeting of Ad to other cancer surface antigens than CAR is an appealing approach. For PCa one such target is prostate-specific membrane antigen (PSMA) (Elsasser-Beile et al., 2009). More recently, Her2/neu (also named c-erbB2) has emerged as a potential target. Her2/neu is overexpressed in 25–30% of diagnosed breast cancers and is a therapeutic target for these patients, using trastuzumab (Herceptin), a humanized monoclonal antibody, in combination with conventional chemotherapy and surgery (Slamon et al., 1989; Nahta and Esteva, 2003; Piccart-Gebhart et al., 2005). In PCa assessment of Her2/neu expression has been problematic, but data have shown that Her2/neu overexpression is detected in approximately 25% of patients with untreated primary tumors, 59% of patients with localized tumors after treatment with total androgen ablation, and 78% of patients with metastatic disease who fail to respond to total androgen ablation therapy (Signoretti et al., 2000). These data suggest a gradual increase in Her2/neu expression in the progression of PCa. As a result, several phase I studies have been carried out, but so far no clinical data in support of the use of trastuzumab for patients with PCa have been obtained (Small et al., 2001; Morris et al., 2002). Nevertheless, Her2/neu remains a functional molecule for the targeting of new agents such as gene therapy vectors.
To retarget Ad one need to (1) genetically introduce a new binding specificity and (2) abolish the binding to native receptors and normal tissues. It has been difficult to find effective ligand scaffolds for retargeting of Ad, but a small and robust binding scaffold that functions well is the Affibody® molecule, based on which binders to a variety of targets, including Her2/neu, have been selected and evaluated (Nilsson et al., 1987; Nord et al., 1997; Henning et al., 2002; Wikman et al., 2004; Orlova et al., 2007). The Ad entry pathway in vitro has been described in detail and starts with the binding of the “knob” domain of the Ad5 fiber protein to CAR followed by internalization via interaction of Arg-Gly-Asp (RGD) sequences in the penton base with cellular integrins αvβ3 and αvβ5 (Wickham et al., 1993; Bergelson et al., 1997). Besides CAR, other surface molecules have also been described as Ad receptors such as heparan sulfate proteoglycans (HSPGs), which binds to the KKTK motif in the third shaft repeat (Dechecchi et al., 2000). In addition to these, a multitude of factors in vivo including blood cells, blood factors, endothelial cells, hepatocytes, and the innate and adaptive immune responses affect Ad transduction after systemic delivery (Barry et al., 2009). These barriers must be taken into account in order to fully detarget Ad vectors for systemic delivery.
We have previously reported the construction of a virus with specificity for Her2/neu, using the Affibody ZH and ablation of the binding sites to CAR and αv integrins (Magnusson et al., 2007). The ZH-liganded virus was shown to be specific for its new target, devoid of binding to CAR, and showed close to wild-type growth characteristics (Magnusson et al., 2007). In this study we have further investigated the properties of the ZH-targeted vectors with regard to in vitro and in vivo infection efficiency and specificity. We show that the Her2/neu-retargeted vectors are functional; specifically infect Her2/neu-expressing prostate cancer cells, both in two and three dimensions; and are able to kill these cells in a dose-dependent manner. Also, for the Ad[ZH/3] vector, viral oncolysis in vivo was almost as potent as wild-type Ad and resulted in reduced serum prostate-specific antigen (PSA) levels and significantly prolonged survival in a human prostate cancer xenograft model in nude mice.
Materials and Methods
Cell lines
HEK-293 cells (293 cells) were purchased from Microbix (Toronto, ON, Canada). 293Her2/neu cells were produced within the project and are HEK-293 cells stably transfected for surface expression of Her2/neu (Magnusson et al., 2007). MM39 cells were kindly supplied by P. Boulanger (Lyon, France). The human prostate cancer cell line PC346C was developed from a PC346 xenograft originating from transurethral resection material of the tumor of a patient with nonprogressive prostate cancer. Both xenograft and cell line are androgen responsive, secrete PSA, and harbor the wild-type androgen receptor (van Weerden et al., 1996). ShMac5 was generated as previously described (Maitland et al., 2004) and is androgen receptor negative (Lang et al., 2006), and the BPH-1 benign prostate epithelial cell line was a gift from S. Hayward (Vanderbilt University, Nashville, TN). LNCaP and the androgen receptor-positive (AR+)/PSA-expressing breast carcinoma cell line T47D were purchased from the American Type Culture Collection (ATCC, Manassas, VA).
293, 293Her2/neu, and MM39 cell lines were cultivated in Iscove's medium (Gibco-BRL/Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Sigma-Aldrich, Stockholm, Sweden) and gentamicin (50 μg/ml; Gibco-BRL/Invitrogen) (complete Iscove's medium). Human PC346C cells were cultured in Dulbecco's modified Eagle's medium (DMEM)–F12 medium (Cambrex BioWhittaker, Verviers, Belgium) supplemented with 2% fetal calf serum (FCS; PAN Biotech, Aidenbach, Germany), 1% insulin–transferrin–selenium (Gibco-BRL/Invitrogen), 0.01% bovine serum albumin (Boehringer Mannheim, Mannheim, Germany), epidermal growth factor (10 ng/ml; Sigma-Aldrich), penicillin–streptomycin antibiotics (penicillin at 100 U/ml, streptomycin at 100 mg/ml; Cambrex BioWhittaker), plus the following additions: fibronectin (100 ng/ml; Harbor Bio-Products/Tebu-bio, Heerhugowaard, The Netherlands); fetuin (20 mg/ml; ICN Biomedicals, Amsterdam, The Netherlands); cholera toxin (50 ng/ml), 0.1 mM phosphoethanolamine, triiodothyronine (0.6 ng/ml), and dexamethasone (500 ng/ml) (all from Sigma-Aldrich); and 0.1 nM 17-methyltrienolone (R1881; New England Nuclear/PerkinElmer, Boston, MA). Cells were grown in T-25 Primaria tissue culture flasks (BD Biosciences, Benelux). The other prostate cancer cell lines were cultivated in a variety of specialty media as previously described (Swift et al., 2010). All cells were maintained at 37°C and 5% CO2.
TaqMan assay for Her2/neu expression
Normal prostate and prostate cancer tissue samples were obtained from the frozen tissue bank of Erasmus MC (Rotterdam, The Netherlands). Samples were collected between 1984 and 2001, snap-frozen, and stored in liquid nitrogen. Samples were collected according to national legislation concerning ethical requirements. All samples contained at least 70% tumor cells (van der Heul-Nieuwenhuijsen et al., 2009). The Erasmus MC Medical Ethics Committee, according to the Medical Research Involving Human Subjects Act, approved the use of these samples. Her2/neu messenger RNA expression was determined relative to the porphobilinogen deaminase (PBGD) housekeeping gene, using real-time RT-PCR as described previously (Marques et al., 2010) (Her2/neu primers/probe set: Hs 00170433–a1 ERBB2; Applied Biosystems, Foster City, CA).
Recombinant adenoviruses and vector production
The ZH-targeted adenoviruses were created as described earlier, in which a tandem repeat of ZH was inserted into the HI loop, using linkers between the knob and ZH (PSIGSGGGS), between ZH and ZH (GGGGSGGGGS), and between ZH and knob (SGGSGGPSI) (Magnusson et al., 2007). Ablation of the CAR-binding site was accomplished through deletion of residues Leu-485 and Thr-486 in the fiber knob. The KKTK motif in the third shaft repeat was changed to RKSK, using splicing by overlap extension. For deletion of αv integrin binding, the RGD motif in the penton base was changed to EGD as described earlier (Magnusson et al., 2007).
The fibers and the penton base were cloned into the Ad5 genome, using a previously described procedure based on homologous recombination and cosmid cloning, and the wild-type Ad5 fiber was inserted in the same way as a control (Magnusson et al., 2001). Recombinant fibers and penton base were first cloned into a genome including a cytomegalovirus–enhanced green fluorescent protein (CMV–EGFP) cassette instead of the E1 region for convenient detection of expression. From these vectors the right arm sequence from PmeI to PacI was cloned together with the wild-type Ad5 left arm sequence from PacI to PmeI to yield replication-competent vectors. In this procedure we ensured that the fiber and penton sequences were identical in corresponding wild-type E1 and ΔE1 constructs.
Recombinant plasmids were linearized with PacI and transfected into 293Her2/neu cells, using FuGENE 6 (Roche Applied Science, Indianapolis, IN) to generate virus. Large quantities of viruses were produced in the same cell line and purified by CsCl density gradient centrifugation followed by dialysis against NNKS buffer (140 mM NaCl, 5 mM Na2HPO4·2H2O, 1.5 mM KH2PO4, 5% sucrose). The amount of physical particles (PP) was determined by measuring absorption at 260 nm. The amount of functional particles (plaque-forming units, PFU) was measured by titration on 293Her2/neu cells by end-point dilution.
Ad[mock] and Ad[WT] were gifts from M. Essand (Uppsala University, Uppsala, Sweden) (Cheng et al., 2006).
Adenovirus-mediated gene transfer assays
For growth rate analysis 293Her2/neu cells were infected in triplicate in 24-well plates with recombinant and wild-type viruses (20 PFU/cell) at 37°C in 100 μl of Iscove's medium. After 1 hr the cells were washed and 1 ml of complete Iscove's medium was added. Cells and medium was harvested 24, 48, and 72 hr postinfection. After cell lysis by freeze–thawing, the number of infectious virions in the lysates was determined on 293Her2/neu cells by end-point dilution, and the recombinant virus yield was expressed as plaque-forming units per cell.
For heparin blocking experiments on MM39 cells, heparin at 0, 50, or 100 ng/ml was added to the virus, incubated for 1 hr at 37°C, and thereafter used to infect cells. Infections were performed in 24-well plates for 1 hr at 37°C in 200 μl of Iscove's. After infection, cells were washed once in complete Iscove's and kept in medium with the same supplements overnight. After approximately 15–20 hr, the cells were harvested, fixed with 1.5% paraformaldehyde for 15 min, and washed with phosphate-buffered saline (PBS). GFP-expressing cells were counted by fluorescence-activated cell-sorting (FACS) analysis (FACSort; BD Biosciences). For cytopathogen effect (CPE) analysis on PC346C, LNCaP, and 293Her2/neu cells, virus was added to 90% confluent wells (n=4) and the plates were incubated at 37°C with 5% CO2 for 7–12 days. On the final day the viability of the cells was measured with a cell proliferation kit II (XTT) (Roche Applied Science) at 450 nm.
For two-dimensional (2D) infections of prostate cancer cell lines, medium was removed from 24-well plates, cells were washed with 0.5 ml of PBS, and 400 μl of fresh growth medium supplemented with the appropriate amount of HER2Ad. All cells were infected with 100 μl of the viral inoculum in triplicate with one additional (reserve) well, and incubated for the required time (48 hr) at 37°C in a 5% CO2 incubator.
For three-dimensional (3D) infections, 1×105 cells were added to 24-well round-bottom plates with poly(2-hydroxyethyl methacrylate) (poly-HEMA) (P3932; Sigma-Aldrich)-coated wells in a final volume of 200 μl. Spheroids were allowed to form for 3 days, after which medium was carefully removed and viral inoculum was added to the wells, again in triplicate, before harvesting for FACS analysis as follows.
For 2D infections: Cells were washed with PBS and incubated with 0.5 ml of trypsin–EDTA at 37°C until the cells detached. Trypsin was inhibited with high-serum medium and the cells were pelleted by low-speed centrifugation, before further washing in ice-cold PBS and dispersal as a single-cell suspension. GFP fluorescence was measured with a CyAn FACS analyzer (Dako, Heverlee, Belgium) at a low flow rate, counting 104 cells in the R1 channel.
For 3D spheroids: Recovered and washed samples were disaggregated with Accutase (Invitrogen) at 37°C for 20–30 min, followed by washing in cold PBS as described previously. Spheroid samples were further disaggregated by gentle syringing before analysis as described previously, although lower cell numbers were collectable (103–5×103).
For live cell confocal imaging, the collected spheroids were washed, stained with Hoechst 33258, and viewed directly with a Zeiss LSM 510 meta confocal system (Carl Zeiss, Oberkochen, Germany).
In vivo antitumoral efficacy studies
Six- to 7-week-old male NMRI nu/nu mice (Taconic, Ry, Denmark) were inoculated with 106 PC346C cells via the dorsolateral prostate (Rembrink et al., 1997). Mice were kept in individually ventilated cages (Tecniplast, Buguggiate, Varese, Italy) under a 12-hr light–dark cycle and received irradiated chow and acidified drinking water ad libitum. Orthotopic tumor growth was monitored over time by transrectal ultrasonography, using an adapted intravascular imaging system (Kraaij et al., 2002). Animal experiments were performed under the national Experiments on Animals Act, which serves the implementation of guidelines on the protection of experimental animals by the Council of Europe (1986), Directive 86/609/EEC, and only after a positive recommendation by the Animal Experiments Committee. No alternatives (in relation to Replacement, Reduction, or Refinement) were available.
When tumors reached a size of 50–100 mm3 (15–20 days after inoculation), vectors were administered at a dose of 1010 PP in a total volume of 20 μl of PBS. Tumor volumes were measured once per week by transrectal ultrasonography and mice were killed when tumor volumes exceeded 1000 mm3 or after 91 days. Long-term survival was statistically analyzed by logrank tests, using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA) as described previously (Limpens et al., 2006). Differences were considered statistically significant when p values were less than 0.05.
Blood (0.1 ml) was obtained by retro-orbital puncture and collected in a heparin tube (Sarstedt, Nümbrecht, Germany). Plasma was obtained after centrifugation for 5 min at 1500×g and plasma PSA levels were determined at the Department of Clinical Chemistry of Erasmus MC (Rotterdam, The Netherlands), using an automated ELISA (Elecsys total PSA immunoassay; lower detection limit, 2 ng/liter; Roche Diagnostics, Indianapolis, IN).
Necrosis was assessed on hematoxylin and eosin-stained, histological sections of each tumor, where purple staining represented viable areas and pink staining necrotic areas. Necrosis was expressed as necrotic surface per total tumor surface of the respective section. Surfaces were determined with an Olympus BX41 microscope (Olympus Nederland, Zoeterwoude, The Netherlands) using cellB analysis software (Olympus Soft Imaging Solutions). Three sections per tumor were measured.
Immunohistochemistry for adenovirus was performed for 10 min on paraffin-embedded sections after antigen retrieval in 10 mM citrate buffer (pH 6.0) according to standard procedures, staining with mouse anti-adenovirus antibody [M58+M73] (ab3648; Abcam, Cambridge, UK) at 1000 times dilution and detection with an EnVision detection system (Dako) and REAL hematoxylin (Dako) counterstaining.
Immunohistochemistry for Her2/neu was performed on paraffin-embedded sections, after deparaffination, using 100 μl of Pathway HER2 4B5 rabbit monoclonal antibody for 32 min at 37°C (790-2991; Ventana Medical Systems, Tucson, AZ). Detection and counterstaining were done as described previously.
Results
Her2/neu is expressed by prostate tumor cells
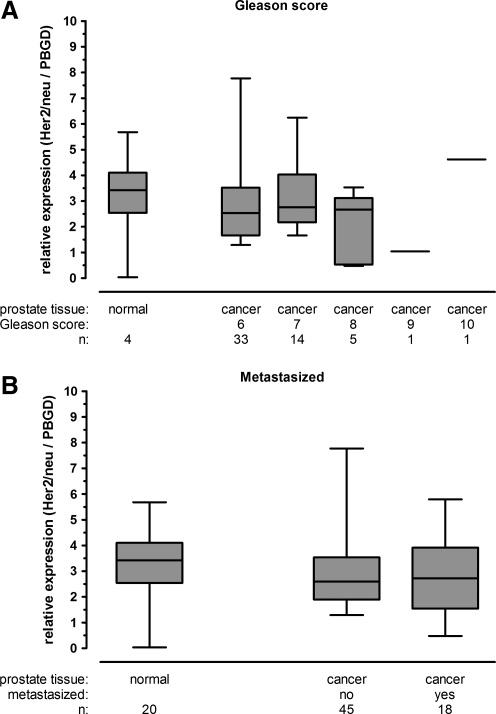
Because of reports of variable expression levels of Her2/neu in PCa we analyzed primary human prostate tumor samples at various progression stages in comparison with normal prostate tissue. The results indicated that Her2/neu is expressed, but not overexpressed, in prostate cancer with no relationship to Gleason score (Fig. 1A). The prostate cancer patient material was derived mostly from tumors with Gleason score 6–8; only one patient each had scores of 9 and 10. Her2/neu evaluation was also performed on normal prostate tissue and nonmetastasized or metastasized tumor specimens, which showed similar Her2/neu expression levels in all groups (Fig. 1B). Taken together, the results demonstrated that Her2/neu was expressed in all stages of PCa and can be used as a receptor for Ad gene therapy.
FIG. 1.
Her2/neu expression levels in primary human prostate specimens. Total RNA was extracted from prostate tissue and mRNA levels was determined by RT-PCR and TaqMan assay and normalized to porphobilinogen deaminase (PBGD). Her2/neu levels in normal prostate were compared with prostate cancer specimens based on Gleason score (A) and on metastasis status (B).
Re- and detargeted virions are constructed by genetic modifications
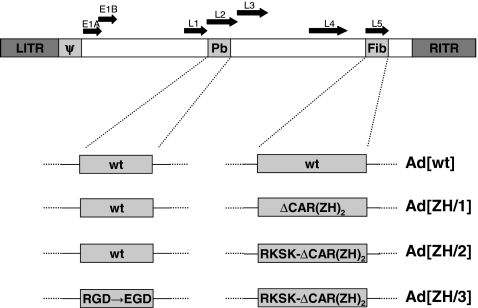
In a previous study we constructed a vector retargeted to Her2/neu by insertion of a tandem repeat of the Affibody ZH in the HI loop flanked by flexible linkers and detargeted from CAR by amino acid deletions in the fiber knob (Magnusson et al., 2007). The virus was shown to be specific for Her2/neu and detargeted from CAR. It could also be detargeted from αv integrin binding by exchanging the RGD motif in the penton base for EGD (RGD→EGD). To further detarget this vector we now endeavored to remove the HSPG-binding region in the third shaft repeat by altering the KKTK motif to RKSK. Modified fibers and penton base were cloned into an Ad5 genome deleted for E1, with a CMV-EGFP cassette in E1 giving rise to single-, double-, or triple-mutated retargeted viruses. Previously the Her2/neu-targeted and CAR-detargeted virus was named Ad5/FibΔCAR-HI-Link-ZHZH (Magnusson et al., 2007), but is here designated Ad[ZH/1]. Ablation of CAR binding and mutation of RKSK yielded Ad[ZH/2] and mutation of all three binding structures yielded Ad[ZH/3]. A description of the viruses used in this study is found in Fig. 2. For analysis of growth rates, HSPG binding and 2D/3D infections, GFP-expressing E1-deleted vectors were used whereas replication-competent vectors (wild-type E1) were used for cell killing in vitro and in vivo. To differentiate the replication-incompetent vectors from their replicating equivalents, ΔE1 was inserted into their names, for example, AdΔE1[ZH/1] and Ad[ZH/1].
FIG. 2.
Schematic diagram showing the genetic modifications of the Her2/neu-targeted vectors. Ad[ZH/1] is deleted for CAR binding and has a tandem repeat of ZH in the HI loop. Ad[ZH/2] contains the modifications from Ad[ZH/1] together with an RKSK motif in the third shaft repeat instead of KKTK. Ad[ZH/3] contains the modifications from Ad[ZH/2] together with a mutation in the penton base yielding EGD instead of RGD. For in vitro studies the vectors were deleted for E1 with insertion of a CMV-EGFP cassette. For in vivo studies and the cell-killing assay, vectors with wild-type E1 were used. (Sizes are not proportional.) CAR, coxsackievirus–adenovirus receptor; Fib, fiber; LITR and RITR, left and right inverted terminal repeat; Pb, penton base; wt, wild type; ZH, Her2/neu-reactive Affibody molecule.
Recombinant ZH vectors are functional, can be produced to high titers, and kill PC346C cells in vitro
To ascertain the functionality and specificity of the various Ad[ZH] viruses in vitro we studied viral purification yields, infectivity ratio (physical particles per functional particles, PP/PFU), HSPG binding, growth rate, and killing of PC346C cells, 293Her2/neu cells, and LNCaP cells as indicated by cytopathogen effect (CPE). Viral yield and infectivity ratios are highly relevant as minor capsid modifications can have a negative impact on Ad production and quality. Two or three different purifications of each Ad[ZH] virus were made and the viruses could be produced to almost Ad wild-type levels (Table 1). The average infectivity ratio for the Ad wild-type virus was 50 and ranged from 140 to 805 for the Ad[ZH] vectors (Table 1), with Ad[ZH/2] and Ad[ZH/3] having PFU/PP ratios more than 10 times higher than that of Ad[WT]. Despite this difference, it was shown that the genetic modifications did not have detrimental effects on particle yield or particle quality, a critical aspect when approaching the upscaling of virus production.
Table 1.
Viral Yield and Infectivity Ratio of Wild-Type and Recombinant Viruses
| Virus | PP/mla(× 1012) | PP/PFUa |
|---|---|---|
| Ad[WT] | 2.9±0.7 | 50±31 |
| Ad[ZH/1] | 1.8±0.8 | 140±23 |
| Ad[ZH/2] | 2.1±0.2 | 805±162 |
| Ad[ZH/3] | 1.6±1.4 | 620±202 |
Ad[ZH/1], Her2/neu-targeted and CAR-detargeted virus; Ad[ZH/2], virus with ablation of CAR binding and mutation of RKSK; Ad[ZH/3], virus with mutation of all three binding structures; PFU, plaque-forming unit; PP, physical particles; WT, wild type.
Measurements from two or three different CsCℓ purifications of 10 infected 175-cm2 flasks, displayed as the average±SD.
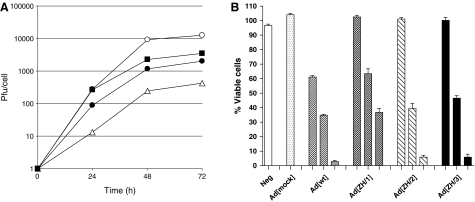
The growth rate of AdΔE1[WT] and ΔE1 virus recombinants was determined by measurement of the infectious progeny yields titered by plaque assay on 293Her2/neu cells. The 293Her2/neu cell line was used to measure plaque-forming units, both for the growth assays and viral purifications, because it was the only cell line that could be used uniformly for comparison of wild-type vectors and ZH-retargeted vectors with and without the E1 gene. As reported previously, the growth rate of AdΔE1[ZH/1] was more than 1 log less than that of AdΔE1[WT]. However, the AdΔE1[ZH/2] and AdΔE1[ZH/3] vectors containing RKSK grew to titers about 0.5–1 log less than that of AdΔE1[WT] (Fig. 3A). Similar growth rates were detected for wild-type E1 vectors (data not shown).
FIG. 3.
Growth rate and cell-killing assays for wild-type and recombinant viruses. (A) Viral growth rates after infection of 293Her2/neu cells. Cells were infected with 20 PFU/cell for 1 hr at 37°C and harvested 24, 48, and 72 hr postinfection. The number of plaque-forming units per cell was determined by end-point dilution. Symbols: AdΔE1[WT] (open circles), AdΔE1[ZH/1] (open triangles), AdΔE1[ZH/2] (solid squares), and AdΔE1[ZH/3] (solid circles). (B) Viability of infected PC346C cellular monolayers 7 days postinfection. Cells were incubated with 1000, 100 or 10 multiplicities of infection (Ad[mock] only MOI 1000) and a viability test (XTT) was performed after 7 days. Each value represents the mean±SD of three or four separate experiments.
As shown earlier, AdΔE1[ZH/1] has been proven to infect cells via Her2/neu instead of CAR (Magnusson et al., 2007), and vectors with EGD in the penton base have lost their binding to αv integrins (Henning et al., 2005). To investigate the specificity for HSPG of viral vectors with the RKSK mutation in the third shaft repeat, we examined blocking of infection with heparin, which is an analog competitor for HSPG. Infection rates of these modified adenoviruses were similar to that of adenoviruses with fibers with the KKTK motif, and showed that the mutation had only a minor impact on HSPG-mediated transduction (Supplementary Fig. S1; supplementary data are available online at www.liebertonline.com/hum). Despite this, the RKSK-mutated viruses were further investigated because of their increased growth rate abilities shown in Fig. 3A and as described subsequently.
To evaluate the functional attachment of Ad[ZH]-liganded vectors on a relevant target cell line, the Her2/neu-expressing prostate cancer cell line PC364C was infected with various multiplicities of infection (MOIs) and cell survival was determined 7 days postinfection (Fig. 3B). Results showed clear dose-dependent cell killing, with Ad[ZH/1] 1 log less efficient than Ad[WT], and Ad[ZH/2] and Ad[ZH/3] closer in efficiency to Ad[WT] (Fig. 3B). To further compare the efficiency of viral spread between the various recombinants and Ad[WT], low-titer infections were performed on the prostate cancer cell line LNCaP and on 293Her2/neu cells (Supplementary Fig. S2A and B). Cytotoxicity was evaluated on day 12 after several rounds of replication and revealed a similar pattern as the growth rate assay, with Ad[ZH/2] and Ad[ZH/3] being the most efficient recombinant vectors. In addition to the vectors described in this study, the Ad5/EGD/FibΔCAR-HI-Link-ZHZH vector described previously (Magnusson et al., 2007) was included, showing that the KKTK mutation is essential for the increased growth efficiency (Supplementary Fig. S2A and B). As shown in Fig. 3A and Supplementary Fig. S2A and B, Ad[ZH/2] is slightly more efficient than Ad[ZH/3]. However, on the basis of specificity data and our main objective to make a vector detargeted from wild-type Ad5 receptors, the Ad[ZH/3] vector was chosen as the candidate for further study.
AdΔE1[ZH/3] infects Her2/neu-expressing prostate cancer cells in 2D and 3D in vitro systems
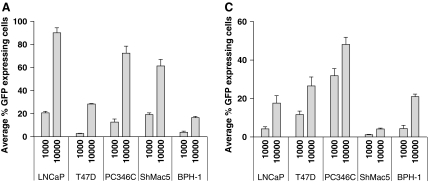
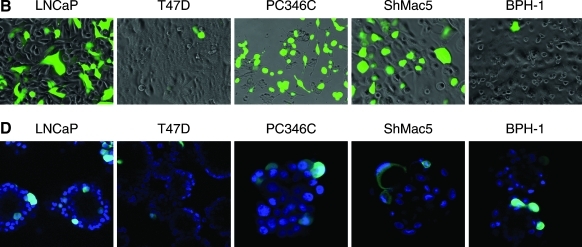
Infection of epithelial monolayers with retargeted viruses takes little account of the polarized nature of cells within epithelial cell structures in vivo. As other potential therapeutic viruses have shown a disappointing ability to spread within tissues, the ability of the AdΔE1[ZH/3] vector to infect a range of prostate epithelial cells was further investigated in 2D and 3D culture systems. As shown in Fig. 4A and B, all tested cell lines were readily infectable with AdΔE1[ZH/3], especially PC346C, LNCaP, and ShMac5, with a clear dose response indicating that the receptor density was not limiting. The lowest infectivity was seen in the androgen receptor (AR)–/Her2/neu– benign epithelial cell line BPH-1, and maximal saturation infection was 90% at an MOI of 104 in LNCaP cells at 48 hr postinfection. In these subconfluent 2D cultures it is likely that the presence of cell–cell junctions is limited, and masking of receptors would probably not be relevant.
FIG. 4.
Comparison of attachment/penetrative ability of AdΔE1[ZH/3] in two and three dimensions. (A) GFP (% cells) expression in 2D (monolayer) cultures 48 hr postinfection with the indicated MOI of AdΔE1[ZH/3]. (B) Images of AdΔE1[ZH/3]-infected cells in monolayer (left to right, highest to lowest infectivity). Adherent cells were infected at an MOI of 10,000 for 48 hr, and then viewed with a fluorescence microscope equipped with a×20 objective. (C) GFP (% cells after spheroid disaggregation) expression in 3D spheroid cultures 48 hr postinfection with the indicated MOI of AdΔE1[ZH/3]. (D) Images of AdΔE1[ZH/3]-infected cells in spheroids (left to right, highest to lowest infectivity). Spheroids were infected at an MOI of 10,000 for 48 hr, stained with Hoechst 33258, and viewed by confocal microscopy with a×40 objective. Note: Confocal slicing does not represent the true infectivity, but does indicate the restriction of infection to the peripheral layers of cells.
In 3D cultures, however, tight junctions have been formed (Maitland et al., 2010) and the ability of adenoviruses to penetrate structures (and possibly to access cell receptors such as Her2/neu) may therefore be restricted. Although a dose response was observed, the percentage of infected cells was dramatically reduced in 3D spheroids (Fig. 4C), with the most notable exception being PC346C cells with an infection rate of 48% versus less than 25% in the other cell lines. As shown in the confocal sections (Fig. 4D), the strongest GFP marker expression was restricted to the outer layers of the spheroids, especially for the LNCaP spheroids and less so for the PC346C spheroids. This pattern has also been observed previously with untargeted Ad[WT] binding to CAR (Maitland et al., 2010). This effect may be determined by Her2/neu receptor accessibility of the virus; clearly PC346C formed spheroids with the least structure and potentially also the lowest number of tight junctions. Moreover, in 3D, viral infectivity was highest in cells expressing the androgen receptor (PC346C and LNCaP), whereas BPH-1 cells express very little AR and ShMac5 cells are AR negative (Table 2).
Table 2.
Summary of Ad[ZH/3] Viral Infectivity of Cells in Monolayer and Three-Dimensional Culture
| Cell line | Infection efficiency, 2D | Infection efficiency, 3D | Her2/neu expression | AR status |
|---|---|---|---|---|
| PC346C | High | High | Higha | +++(van Weerden et al., 1996) |
| LNCaP | High | Low | High (El Sheikh et al., 2004) | +++(Maitland et al., 2010) |
| ShMac5 | High | Low | ND | – (Lang et al., 2006) |
| T47D | Low | Moderate | Moderate (Millson et al., 2003) | ++b |
| BPH-1 | Low | Low | Low (El Sheikh et al., 2004) | – (Maitland et al., 2010) |
2D, two-dimensional; 3D, three-dimensional; AR, androgen receptor; ND, not determined.
Described at www.atcc.org
Time course experiments in vivo show growth suppression of tumors treated with replication-competent vectors
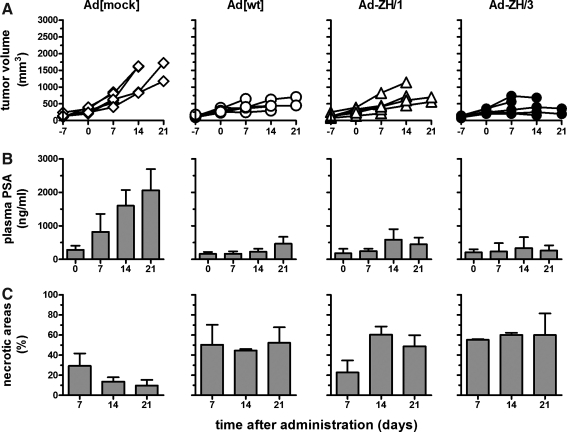
To assess the therapeutic potential of Ad[ZH/3], a time course experiment was set up for treatment of an androgen-dependent orthotopic human prostate cancer model in nude mice. Tumor cells (PC346C) were implanted into the prostate and at tumor sizes of approximately 100 mm3 (2 to 3 weeks postimplantation) the mice were treated by a single intratumoral viral injection of 1×1010 PP (day 0). Ad[ZH/3] potential was tested against Ad[WT] and Ad[ZH/1] with a replication-deficient Ad5 vector (Ad[mock]) as control. Animals treated with replication-competent vectors showed reduced tumor growth and lower serum PSA levels as compared with animals that were treated with Ad[mock], all of whom showed a rapid increase in both tumor volume and serum PSA levels (Fig. 5A and B). For detection of tumor necrosis, mice were killed on days 7, 14, and 21 and necrotic areas were estimated by histological examination. In concordance with tumor growth and PSA levels, results revealed induction of large necrotic areas in the tumors treated with replication-competent vectors as compared with the Ad[mock]-treated tumors. Concerning the ZH recombinants and Ad[WT], 40–60% of the tumor mass was necrotic on days 14 and 21 postinjection, whereas less than 20% of the tumor mass was necrotic in the Ad[mock]-treated tumors (Fig. 5C). Hence, replication-competent vectors induced cell killing in the PC346C tumors whereas Ad[mock]-treated mice had progressive tumors. Interestingly, and in contrast to the other replication-competent vectors, Ad[ZH/1] tumors had much less necrosis at 7 days posttreatment. This slight delay in viral spread correlated well with the slower growth and spread detected for this vector observed in the in vitro studies.
FIG. 5.
Effects of wild-type and Her2/neu-targeted viruses in vivo in a time course study using a PC346C xenograft model. Male NMRI nu/nu mice were injected with 106 PC346C cells via the dorsolateral prostate. Two to 3 weeks after inoculation, at tumor sizes of approximately 100 mm3, Ad[mock], [Ad[WT], Ad[ZH/1], or Ad[ZH/3] (1×1010 PP in 20 μl) was injected into each prostate tumor. There were 6 mice per group, and 2 mice from each group were killed 7, 14 and 21 days posttreatment. (A) Tumor growth was monitored every week by transrectal ultrasonography, using an adapted intravascular ultrasound system. (B) Plasma PSA levels were determined by ELISA. (C) The levels of necrotic areas were estimated in multiple hematoxylin and eosin-stained samples.
Intratumoral injection of Ad[ZH/3] and Ad[WT] prolongs survival time
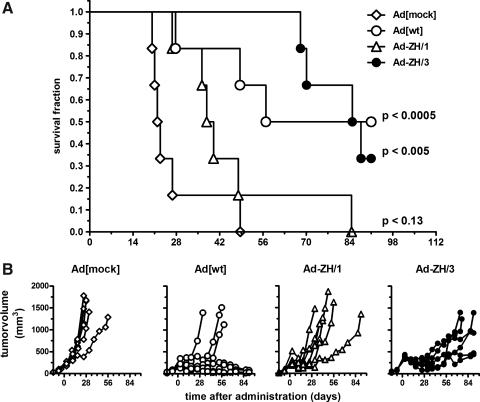
Antitumor efficiency over time was determined in the same human prostate orthotopic xenograft model as described previously, and survival and tumor growth were monitored for up to 90 days after virus injection. Ad[ZH/3] treatment resulted in significantly prolonged survival (p<0.0005) of mice, with a median survival of 84 days as compared with 21 days for the Ad[mock]-treated animals (Fig. 6A). Ad[WT] also showed significantly prolonged survival time (p<0.005), whereas Ad[ZH/1] showed only moderate activity with a median survival of 36 days (Fig. 6A). In the Ad[ZH/3]-treated group there were two survivors of six at the end of the experiment, whereas three of six mice survived in the Ad[WT] group. There were no survivors in the Ad[ZH/1] and Ad[mock] groups.
FIG. 6.
Survival and tumor growth in vivo in tumor-bearing mice treated with wild-type and Her2/neu-targeted viruses. PC346C cells were injected into the dorsolateral prostate of male NMRI nu/nu mice and, when tumor size reached approximately 100 mm3, were treated with 1×1010 PP of Ad vector on day 0 (n=6). (A) Kaplan–Meier plot demonstrating survival after viral treatment. Mice were killed when tumor volume exceeded 1000 mm3 or after 91 days. The exact time points at which the tumors reached a volume of 1000 mm3 were determined by nonlinear regression and used for survival analysis, using GraphPad Prism version 4.00 for Windows. Differences were statistically analyzed by logrank tests and considered significant when p values were lower than 0.05. (B) Tumor growth monitored by transrectal ultrasonography.
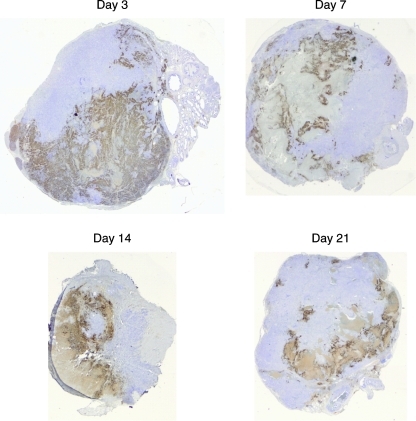
As determined by tumor volume changes, Ad[ZH/3] showed the most pronounced initial reduction in tumor sizes, but after approximately 40 days all tumors escaped and only in the Ad[WT] group did some mice retain stable disease without regrowth of tumors (Fig. 6B). This demonstrated that in the long run the Her2/neu-targeted Ad[ZH] viruses was less efficient than Ad[WT] even though there seemed to be more efficient tumor reduction at early time points. To analyze tumor escape in the Ad[ZH/3] group, histological samples from the time course experiments were used and stained for adenovirus hexon protein to reveal replicating virus. Results showed that there were extensive areas of adenovirus infection (brown staining) at early time points (3 and 7 days), but at later time points (21 days) spread of the vector throughout the tumor was much less pronounced, potentially causing tumor escape (Fig. 7). Also, evaluation of Her2/neu expression in tumors 21 days postinjection revealed weak to moderate expression in 40–70% of cells treated with Ad[WT] virus whereas tumors treated with Ad[ZH/3] were negative for Her2/neu, suggesting selection for Her2/neu-negative tumor cells. Tumors of mice treated with Ad[ZH/1] showed Her2/neu expression more comparable to that of Ad [WT]-treated tumors. Surprisingly, mock virus-treated tumors showed heterogeneous weak Her2/neu expression in 5% of cells (Supplementary Fig. S3).
FIG. 7.
Cross-sections of Ad[ZH/3]-treated tumors for detection of viral spread. Representative histological samples from days 3, 7, 14, and 21 posttreatment, stained for Ad hexon protein (dark brown), necrosis (light brown/gray), and hematoxylin (purple).
Taken together, these results showed that the Ad[ZH/3] vector had a therapeutic effect, but a single-dose treatment regimen of a vector targeted to a single receptor seems not enough to eradicate tumors in the experimental system used.
Discussion
In this study, we evaluated transductionally retargeted Ad vectors and the potential use of Her2/neu as a target receptor for virotherapy or gene therapy treatment of prostate cancers expressing Her2/neu. We also studied properties of vectors detargeted to different degrees and the two vectors with RKSK modifications were found most successful. Ad[ZH/3] was chosen for further in vivo evaluation because the differences between this vector and Ad[ZH/2] were only minor.
The expression status of Her2/neu in PCa is uncertain and the apparent degree of expression has been suggested to be due to the methodology used for Her2/neu detection (Simon et al., 2001). A meta-analysis concluded that Her2/neu may be a predictor of clinically severe outcome in patients with PCa (Neto et al., 2010). This supports its potential use as a prostate cancer marker for imaging and treatment. Because of the divergent reports we also evaluated Her2/neu expression in clinical prostate tissue. Although we could detect Her2/neu expression in tumor tissues in all stages of disease as well as in normal prostate, we were unable to show differences in Her2/neu expression when patient groups were stratified on the basis of Gleason score or metastasis status. Undoubtedly, the question remains concerning whether patients with prostate cancer would benefit from Her2/neu-targeted treatments, but the association of low overexpression of Her2/neu and poor prognosis argues in favor of the use of Her2/neu as a prostate cancer target. In contrast to treatment of high Her2/neu-overexpressing breast and ovarian cancers, which show a greater difference between tumor and normal cell expression, a different dosage could be applied or, as in the case of cytolytic adenoviral vectors, transductional targeting in conjunction with transcriptional targeting should to be used to ensure safety and efficacy. Indeed, this combination would most likely need to be used for safety reasons even for cytolytic Ad treatment of breast and ovarian cancers, because Her2/neu expression has been identified on epithelial cells in the gastrointestinal, respiratory, reproductive, and urinary tract as well as in the skin, breast, and placenta (Press et al., 1990). In all these tissues Her2/neu expression was similar to the levels found in nonamplified, non-overexpressing breast cancers (Press et al., 1990). Another argument in favor of adenovirus retargeting to any other target than CAR is that CAR has been shown to be downregulated in many different human cancers, probably regulated by hypoxia (Kuster et al., 2010). For prostate cancer, it has been reported that, compared with normal prostate, CAR expression in tumors with increasing Gleason score decreased in both intensity and percentage of cells stained (Rauen et al., 2002). Only metastatic prostate cancers showed high expression of membrane CAR (Rauen et al., 2002).
After demonstrating the presence of Her2/neu in all stages of PCa, we evaluated Her2/neu-targeted Ad vectors for prostate cancer treatment. We have already proved the functionality and specificity of the AdΔE1[ZH/1] vector with and without modifications in the penton base (Magnusson et al., 2007). Here we further evaluated these viruses and also made a triple-mutated vector by altering the KKTK motif in the third shaft repeat to RKSK. Others have successfully exchanged KKTK for GATK, GAGA, or RGDK to confer new binding, or nonbinding abilities to Ad vectors (Smith et al., 2003b; Bayo-Puxan et al., 2006, 2009). Also, it was postulated that the HSPG-binding KKTK motif in the third shaft repeat mediated liver transduction and a substitution to a GAGA motif dramatically reduced liver transduction in mice, rats, and nonhuman primates (Smith et al., 2003a,b). In contrast to this, Di Paolo and colleagues reported that the KKTK motif within the Ad5 fiber played a minimal role in hepatic cell transduction in vivo (Di Paolo et al., 2007). In our study we found that the RKSK mutation did not affect heparin inhibition and was thus not a true triple-ablated vector. This is probably because the new RKSK motif still fully matches the typical linear heparin-binding domain consensus sequence [-X-B-B-X-B-X-] and [-X-B-B-B-X-X-B-X-], in which B is a basic residue and X a hydropathic residue (Cardin and Weintraub, 1989; Hileman et al., 1998; Vives et al., 2006). Surprisingly, however, the RKSK modification did result in increased growth rates and more efficient cell killing both in vitro and in vivo. The reason for this is still unknown but may be due to altered intracellular trafficking, or it may be caused by a possible change of the hinge in the fiber resulting in improved Her2/neu binding and internalization rates. The increased growth rate was observed in all the different purifications of viruses with RKSK in the third shaft repeat, even when based on different ligations into the Ad genome, showing that the increase in viral growth was not caused by single mutations in other genes. Further studies on this are on-going with construction and evaluation of a wild-type fiber containing RKSK as a first step.
When producing genetically re- and detargeted Ad vectors it is essential to study the quality and quantity of the amplified virus stocks. The assessment of this is crucial, because genetic modifications can have detrimental effects on viral yields and quality (Magnusson et al., 2001; Henning et al., 2005). In this study we confirmed the functionality of the recombinant viruses and showed that production yields were normal and infectivity ratios were about 10-fold higher than with Ad[WT]. However, it should be realized that ZH and wild-type Ad vector activities rely on different receptors and possibly on different internalization routes that may affect the PP/PFU ratios.
The in vitro cell-killing potency of Ad[ZH/2] and Ad[ZH/3] was the most efficient among the recombinants and was in concordance with the growth rate curves. Because of the more efficient spread of these vectors and our objection to make a vector detargeted from wild-type Ad receptors, Ad[ZH/3], which does not bind to αv integrins, was chosen as the prime candidate. However, Ad[ZH/2] remains an interesting vector and should be further evaluated in future studies. After choosing the vector candidate, Ad[ZH/3] infectivity was further tested in two- and three-dimensional cell cultures. In both cell systems there was a clear dose response with highest infectivity rates in Her2/neuhigh/AR+ cells, and low infectivity in the benign (Her2/neu–/AR–) cell line BPH-1. These data confirm the high specificity of the ZH Affibody molecule for Her2/neu and consequently confirm its highest efficacy in Her2/neu-positive prostate tumors. In addition, our data suggest that a Her2/neu-targeted virus would show greatest efficacy as a first-line therapy against Her2/neu+, wild-type AR+ tumors before hormone therapy.
To evaluate in vivo efficacy, an orthotopic hormone-dependent prostate cancer model was used. After treatment an initial tumor regression was detected together with a concomitant and pronounced drop in serum PSA levels. Treatment of mice with Ad[ZH/3] and Ad[WT] significantly prolonged survival of the animals, with two and three of six surviving animals, respectively. However, the tumors were not cured and histology showed that the spread of the vector was, in fact, rather poor. It is likely that intratumoral barriers such as those observed in the 3D in vitro assays were responsible for this as it has been shown by others that the extracellular matrix forms a physical barrier that prevents viral spread (Kuppen et al., 2001; Li et al., 2004). Possible ways to circumvent this may include, for example, dose escalation, multiple injections, repeated treatments, or addition of transgenes with potent bystander effects or matrix metalloproteinases. Another approach is to use dual targeting with two different specificities of Affibody molecules in the HI loop (Myhre et al., 2009), such as Her2/neu and epidermal growth factor receptor (Nordberg et al., 2008). Tumors are generally rather heterogeneous with respect to receptor expression. Hence, it may be necessary to target more than one receptor in order to minimize tumor escape caused by receptor downregulation. Indeed, in our prostate cancer model, we observed heterogeneous expression, showing tumor areas that lacked Her2/neu expression and that as a consequence may have halted the spread of the Ad[ZH/3] virus. The observation that tumors that progressed after Ad[ZH/3] treatment were largely negative for Her2/neu suggests that, indeed, selection of Her2/neu-negative tumor cells occurred.
For metastatic prostate cancer systemic injection is essential and requires Ad vectors shielded from binding to blood cells and blood factors. The detargeting steps taken here will not diminish liver uptake or prolong the circulation time in blood because tail vein injection of Ad[ZH/3] virus resulted in similar accumulation in the liver as Ad[WT] (our unpublished data). A possible solution to get around this problem is to coat the vector directly with polymers, such as polyethylene glycol (PEG) or poly[N-(2-hydroxypropyl)-methacrylamide] (Fisher et al., 2001; Wortmann et al., 2008) or to confer site-specific PEGylation by addition of cysteines in the capsid (Kreppel et al., 2005). In such a setting the use of recombinantly retargeted vectors such as Ad[ZH/3] will be of greater importance once the virus has reached the target, because the viral progeny will spread within the tumor, using the ZH motif. Also, detargeting from CAR, integrins, and HSPGs, that is, normal Ad5 tissue entrance mechanisms, will thus be of importance for viral specificity and patient safety.
Virotherapy of tumors was explored even in the 1950s to 1970s (Kelly and Russell, 2007). Trials using different Ad vectors have shown promise (Schenk et al., 2010) but further improvements are needed regarding delivery, therapeutic transgenes, and targeted replication abilities (de Vrij et al., 2010). In particular, a high safety profile is required before virotherapy can be used in routine practice, both regarding the treated patients and possible leakage of the virus to the environment.
Concerning target efficacy and safety, Her2/neu-specific treatment has in most cases proven to be safe and thousands of patients with metastatic breast cancer have shown clinical benefit from trastuzumab treatment (Hudis, 2007). Also, multiple studies immunizing against epitopes of Her2/neu have been performed and adoptive transfer of autologous anti-Her2/neu cytotoxic T lymphocyte clones in the setting of breast cancer have also been used clinically (Bernhard et al., 2008). However, a case report described the death of a patient with colon cancer treated with highly active anti-Her2/neu-directed T cells (Morgan et al., 2010). Thus, Her2/neu expression in normal human cells, as described by us previously for prostate cells, may be an obstacle to clinical use of Her2/neu-targeted vectors because this will most likely result in viral spread and killing of normal cells. This is a situation not seen in experimental mice models because normal mouse cells do not express Her2/neu. To increase safety, Ad vectors can be designed to carry tumor-specific promoters and/or suicide genes, which hopefully will restrict or, if needed, eliminate viral replication. This safety net is not obtainable when using cytotoxic T cells as described by Morgan and colleagues (2010).
In summary, our findings show that adenovirus can successfully be retargeted to Her2/neu, using Her2/neu-specific Affibody molecules; detargeted from wild-type Ad receptors; and effectively reduce the growth of human prostate cancer xenografts in mice. This retargeted and detargeted Ad vector has potential for local treatment of Her2/neu-positive prostate cancer, possibly as a multitargeted vector directed to several antigens, to reduce the risk of tumor escape, and combined with translational targeting, to increase patient safety. To use this vector for metastatic disease, however, further developments should address the issues of non–tumor-related viral uptake and replication in liver and other nontarget organs on the one hand and the issue of prolonged stability of the virus in circulation on the other hand. An Ad vector with a wild-type capsid is already in clinical trials (Freytag et al., 2003) and a Her2/neu-targeted approach could imply increased efficacy and safety for patients with defined Her2/neu overexpression. Use of the Ad[ZH/3] vector for targeting prostate cancer as described and suggested here could also provide a basis for further development toward tumor-targeted Ad gene therapy for many different Her2/neu-expressing cancers including breast, ovary, and kidney.
Supplementary Material
Acknowledgments
This work was supported by the European Community on behalf of GIANT (grant LSHB-CT-2004-512087), the Johan and Jakob Söderberg Foundation, Socialstyrelsen's Foundation, and Åke Wiberg's Foundation. The authors thank Theo van der Kwast and Arno van Leenders for histopathological examination of the prostate samples, and Natasja Dits and Guido Jenster for preparing and providing the RNA patient samples. The authors also thank Affibody AB for permission to use the Her2/neu-directed Affibody molecule in this study.
Author Disclosure Statement
No competing financial interests exist.
References
- Auclerc G. Antoine E.C. Cajfinger F., et al. Management of advanced prostate cancer. Oncologist. 2000;5:36–44. doi: 10.1634/theoncologist.5-1-36. [DOI] [PubMed] [Google Scholar]
- Barry M.A. Hofherr S.E. Chen C.Y., et al. Systemic delivery of therapeutic viruses. Curr. Opin. Mol. Ther. 2009;11:411–420. [PubMed] [Google Scholar]
- Bayo-Puxan N. Cascallo M. Gros A., et al. Role of the putative heparan sulfate glycosaminoglycan-binding site of the adenovirus type 5 fiber shaft on liver detargeting and knob-mediated retargeting. J. Gen. Virol. 2006;87:2487–2495. doi: 10.1099/vir.0.81889-0. [DOI] [PubMed] [Google Scholar]
- Bayo-Puxan N. Gimenez-Alejandre M. Lavilla-AlonSO S., et al. Replacement of adenovirus type 5 fiber shaft heparan sulfate proteoglycan-binding domain with RGD for improved tumor infectivity and targeting. Hum. Gene Ther. 2009;20:1214–1221. doi: 10.1089/hum.2009.038. [DOI] [PubMed] [Google Scholar]
- Bergelson J.M. Cunningham J.A. Droguett G., et al. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- Bernhard H. Neudorfer J. Gebhard K., et al. Adoptive transfer of autologous, HER2-specific, cytotoxic T lymphocytes for the treatment of HER2-overexpressing breast cancer. Cancer Immunol. Immunother. 2008;57:271–280. doi: 10.1007/s00262-007-0355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin A.D. Weintraub H.J. Molecular modeling of protein–glycosaminoglycan interactions. Arteriosclerosis. 1989;9:21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- Cheng W.S. Dzojic H. Nilsson B., et al. An oncolytic conditionally replicating adenovirus for hormone-dependent and hormone-independent prostate cancer. Cancer Gene Ther. 2006;13:13–20. doi: 10.1038/sj.cgt.7700881. [DOI] [PubMed] [Google Scholar]
- Dechecchi M.C. Tamanini A. Bonizzato A. Cabrini G. Heparan sulfate glycosaminoglycans are involved in adenovirus type 5 and 2-host cell interactions. Virology. 2000;268:382–390. doi: 10.1006/viro.1999.0171. [DOI] [PubMed] [Google Scholar]
- de Vrij J. Willemsen R.A. Lindholm L., et al. Adenovirus-derived vectors for prostate cancer gene therapy. Hum. Gene Ther. 2010;21:795–805. doi: 10.1089/hum.2009.203. [DOI] [PubMed] [Google Scholar]
- Di Paolo N.C. Kalyuzhniy O. Shayakhmetov D.M. Fiber shaft-chimeric adenovirus vectors lacking the KKTK-motif efficiently infect liver cells in vivo. J. Virol. 2007;81:12249–2259. doi: 10.1128/JVI.01584-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsasser-Beile U. Buhler P. Wolf P. Targeted therapies for prostate cancer against the prostate specific membrane antigen. Curr. Drug Targets. 2009;10:118–125. doi: 10.2174/138945009787354601. [DOI] [PubMed] [Google Scholar]
- El Sheikh S.S. Domin J. Abel P., et al. Phosphorylation of both EGFR and ErbB2 is a reliable predictor of prostate cancer cell proliferation in response to EGF. Neoplasia. 2004;6:846–853. doi: 10.1593/neo.04379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K.D. Stallwood Y. Green N.K., et al. Polymer-coated adenovirus permits efficient retargeting and evades neutralising antibodies. Gene Ther. 2001;8:341–348. doi: 10.1038/sj.gt.3301389. [DOI] [PubMed] [Google Scholar]
- Freytag S.O. Stricker H. Pegg J., et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three-dimensional conformal radiation therapy for the treatment of newly diagnosed, intermediate- to high-risk prostate cancer. Cancer Res. 2003;63:7497–7506. [PubMed] [Google Scholar]
- Henning P. Magnusson M.K. Gunneriusson E., et al. Genetic modification of adenovirus 5 tropism by a novel class of ligands based on a three-helix bundle scaffold derived from staphylococcal protein A. Hum. Gene Ther. 2002;13:1427–1439. doi: 10.1089/10430340260185067. [DOI] [PubMed] [Google Scholar]
- Henning P. Andersson K.M. Frykholm K., et al. Tumor cell targeted gene delivery by adenovirus 5 vectors carrying knobless fibers with antibody-binding domains. Gene Ther. 2005;12:211–224. doi: 10.1038/sj.gt.3302408. [DOI] [PubMed] [Google Scholar]
- Hileman R.E. Fromm J.R. Weiler J.M. Linhardt R.J. Glycosaminoglycan–protein interactions: Definition of consensus sites in glycosaminoglycan binding proteins. Bioessays. 1998;20:156–167. doi: 10.1002/(SICI)1521-1878(199802)20:2<156::AID-BIES8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Hudis C.A. Trastuzumab: Mechanism of action and use in clinical practice. N. Engl. J. Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- Kelly E. Russell S.J. History of oncolytic viruses: Genesis to genetic engineering. Mol. Ther. 2007;15:651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- Kraaij R. van Weerden W.M. De Ridder C.M., et al. Validation of transrectal ultrasonographic volumetry for orthotopic prostate tumours in mice. Lab. Anim. 2002;36:165–172. doi: 10.1258/0023677021912451. [DOI] [PubMed] [Google Scholar]
- Kreppel F. Gackowski J. Schmidt E. Kochanek S. Combined genetic and chemical capsid modifications enable flexible and efficient de- and retargeting of adenovirus vectors. Mol. Ther. 2005;12:107–117. doi: 10.1016/j.ymthe.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Kuppen P.J. Van der Eb M.M. Jonges L.E., et al. Tumor structure and extracellular matrix as a possible barrier for therapeutic approaches using immune cells or adenoviruses in colorectal cancer. Histochem. Cell Biol. 2001;115:67–72. doi: 10.1007/s004180000224. [DOI] [PubMed] [Google Scholar]
- Kuster K. Koschel A. Rohwer N., et al. Downregulation of the coxsackie and adenovirus receptor in cancer cells by hypoxia depends on HIF-1α. Cancer Gene Ther. 2010;17:141–146. doi: 10.1038/cgt.2009.49. [DOI] [PubMed] [Google Scholar]
- Lang S.H. Smith J. Hyde C., et al. Differentiation of prostate epithelial cell cultures by Matrigel/stromal cell glandular reconstruction. In Vitro Cell Dev. Biol. Anim. 2006;42:273–280. doi: 10.1290/0511080.1. [DOI] [PubMed] [Google Scholar]
- Li Z.Y. Ni S. Yang X., et al. Xenograft models for liver metastasis: Relationship between tumor morphology and adenovirus vector transduction. Mol. Ther. 2004;9:650–657. doi: 10.1016/j.ymthe.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Limpens J. Schroder F.H. De Ridder C.M., et al. Combined lycopene and vitamin E treatment suppresses the growth of PC-346C human prostate cancer cells in nude mice. J. Nutr. 2006;136:1287–1293. doi: 10.1093/jn/136.5.1287. [DOI] [PubMed] [Google Scholar]
- Magnusson M.K. Hong S.S. Boulanger P. Lindholm L. Genetic retargeting of adenovirus: Novel strategy employing “deknobbing” of the fiber. J. Virol. 2001;75:7280–7289. doi: 10.1128/JVI.75.16.7280-7289.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson M.K. Henning P. Myhre S., et al. Adenovirus 5 vector genetically re-targeted by an Affibody molecule with specificity for tumor antigen HER2/neu. Cancer Gene Ther. 2007;14:468–479. doi: 10.1038/sj.cgt.7701027. [DOI] [PubMed] [Google Scholar]
- Maitland N. Chambers K. Georgopoulos L., et al. Gene transfer vectors targeted to human prostate cancer: Do we need better preclinical testing systems? Hum. Gene Ther. 2010;21:815–827. doi: 10.1089/hum.2009.210. [DOI] [PubMed] [Google Scholar]
- Maitland N.J. Macintosh C.A. Schmitz C. Lang S.H. Immortalization of human prostate cells with the human papillomavirus type 16 E6 gene. Methods Mol. Med. 2004;88:275–285. doi: 10.1385/1-59259-406-9:275. [DOI] [PubMed] [Google Scholar]
- Marques R.B. Dits N.F. Erkens-Schulze S., et al. Bypass mechanisms of the androgen receptor pathway in therapy-resistant prostate cancer cell models. PloS One. 2010;5:e13500. doi: 10.1371/journal.pone.0013500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millson A. Suli A. Hartung L., et al. Comparison of two quantitative polymerase chain reaction methods for detecting HER2/neu amplification. J. Mol. Diagn. 2003;5:184–190. doi: 10.1016/S1525-1578(10)60471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R.A. Yang J.C. Kitano M., et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M.J. Reuter V.E. Kelly W.K., et al. HER-2 profiling and targeting in prostate carcinoma. Cancer. 2002;94:980–986. [PubMed] [Google Scholar]
- Myhre S. Henning P. Friedman M., et al. Re-targeted adenovirus vectors with dual specificity: Binding specificities conferred by two different Affibody molecules in the fiber. Gene Ther. 2009;16:252–261. doi: 10.1038/gt.2008.160. [DOI] [PubMed] [Google Scholar]
- Nahta R. Esteva F.J. HER-2-targeted therapy: Lessons learned and future directions. Clin. Cancer Res. 2003;9:5078–5084. [PubMed] [Google Scholar]
- Neto A.S. Tobias-Machado M. Wroclawski M.L., et al. Her-2/neu expression in prostate adenocarcinoma: A systematic review and meta-analysis. J. Urol. 2010;184:842–850. doi: 10.1016/j.juro.2010.04.077. [DOI] [PubMed] [Google Scholar]
- Nilsson B. Moks T. Jansson B., et al. A synthetic IgG-binding domain based on staphylococcal protein A. Protein Eng. 1987;1:107–113. doi: 10.1093/protein/1.2.107. [DOI] [PubMed] [Google Scholar]
- Nord K. Gunneriusson E. Ringdahl J., et al. Binding proteins selected from combinatorial libraries of an α-helical bacterial receptor domain. Nat. Biotechnol. 1997;15:772–777. doi: 10.1038/nbt0897-772. [DOI] [PubMed] [Google Scholar]
- Nordberg E. Orlova A. Friedman M., et al. In vivo and in vitro uptake of 111In, delivered with the Affibody molecule (ZEGFR:955)2, in EGFR expressing tumour cells. Oncol. Rep. 2008;19:853–857. doi: 10.3892/or.19.4.853. [DOI] [PubMed] [Google Scholar]
- Orlova A. Rosik D. Sandstrom M., et al. Evaluation of [111/114mIn]CHX-A′′-DTPA-ZHER2:342, an Affibody ligand conjugate for targeting of HER2-expressing malignant tumors. Q. J. Nucl. Med. Mol. Imaging. 2007 [PubMed] [Google Scholar]
- Pandha H.S. Stockwin L.H. Eaton J., et al. Coxsackie B and adenovirus receptor, integrin and major histocompatibility complex class I expression in human prostate cancer cell lines: Implications for gene therapy strategies. Prostate Cancer Prostatic Dis. 2003;6:6–11. doi: 10.1038/sj.pcan.4500611. [DOI] [PubMed] [Google Scholar]
- Piccart-Gebhart M.J. Procter M. Leyland-Jones B., et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- Press M.F. Cordon-Cardo C. Slamon D.J. Expression of Her2-/Neu proto-oncogene in normal human adult and fetal tissues. Oncogene. 1990;7:953–962. [PubMed] [Google Scholar]
- Rauen K.A. Sudilovsky D. Le J.L., et al. Expression of the coxsackie adenovirus receptor in normal prostate and in primary and metastatic prostate carcinoma: Potential relevance to gene therapy. Cancer Res. 2002;62:3812–3818. [PubMed] [Google Scholar]
- Reid A.H. Attard G. Danila D.C., et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J. Clin. Oncol. 2010;28:1489–1495. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rembrink K. Romijn J.C. Van der Kwast T.H., et al. Orthotopic implantation of human prostate cancer cell lines: A clinically relevant animal model for metastatic prostate cancer. Prostate. 1997;31:168–174. doi: 10.1002/(sici)1097-0045(19970515)31:3<168::aid-pros4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Schenk E. Essand M. Bangma C.H., et al. Clinical adenoviral gene therapy for prostate cancer. Hum. Gene Ther. 2010;21:807–813. doi: 10.1089/hum.2009.206. [DOI] [PubMed] [Google Scholar]
- Signoretti S. Montironi R. Manola J., et al. Her-2-neu expression and progression toward androgen independence in human prostate cancer. J. Natl. Cancer Inst. 2000;92:1918–1925. doi: 10.1093/jnci/92.23.1918. [DOI] [PubMed] [Google Scholar]
- Simon R. Nocito A. Hubscher T., et al. Patterns of her-2/neu amplification and overexpression in primary and metastatic breast cancer. J. Natl. Cancer Inst. 2001;93:1141–1146. doi: 10.1093/jnci/93.15.1141. [DOI] [PubMed] [Google Scholar]
- Slamon D.J. Godolphin W. Jones L.A., et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Small E.J. Bok R. Reese D.M., et al. Docetaxel, estramustine, plus trastuzumab in patients with metastatic androgen-independent prostate cancer. Semin. Oncol. 2001;28:71–76. doi: 10.1016/s0093-7754(01)90159-9. [DOI] [PubMed] [Google Scholar]
- Smith T.A. Idamakanti N. Marshall-Neff J., et al. Receptor interactions involved in adenoviral-mediated gene delivery after systemic administration in non-human primates. Hum. Gene Ther. 2003a;14:1595–1604. doi: 10.1089/104303403322542248. [DOI] [PubMed] [Google Scholar]
- Smith T.A. Idamakanti N. Rollence M.L., et al. Adenovirus serotype 5 fiber shaft influences in vivo gene transfer in mice. Hum. Gene Ther. 2003b;14:777–787. doi: 10.1089/104303403765255165. [DOI] [PubMed] [Google Scholar]
- Swift S.L. Burns J.E. Maitland N.J. Altered expression of neurotensin receptors is associated with the differentiation state of prostate cancer. Cancer Res. 2010;70:347–356. doi: 10.1158/0008-5472.CAN-09-1252. [DOI] [PubMed] [Google Scholar]
- Tannock I.F. De Wit R. Berry W.R., et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- van der Heul-Nieuwenhuijsen L. Dits N.F. Jenster G. Gene expression of forkhead transcription factors in the normal and diseased human prostate. BJU Int. 2009;103:1574–1580. doi: 10.1111/j.1464-410X.2009.08351.x. [DOI] [PubMed] [Google Scholar]
- van Weerden W.M. De Ridder C.M. Verdaasdonk C.L., et al. Development of seven new human prostate tumor xenograft models and their histopathological characterization. Am. J. Pathol. 1996;149:1055–1062. [PMC free article] [PubMed] [Google Scholar]
- Vives R.R. Lortat-Jacob H. Fender P. Heparan sulphate proteoglycans and viral vectors: Ally or foe? Curr. Gene Ther. 2006;6:35–44. doi: 10.2174/156652306775515565. [DOI] [PubMed] [Google Scholar]
- Wickham T.J. Mathias P. Cheresh D.A. Nemerow G.R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- Wikman M. Steffen A.C. Gunneriusson E., et al. Selection and characterization of HER2/neu-binding affibody ligands. Protein Eng. Des. Sel. 2004;18:18. doi: 10.1093/protein/gzh053. [DOI] [PubMed] [Google Scholar]
- Wortmann A. Vohringer S. Engler T., et al. Fully detargeted polyethylene glycol-coated adenovirus vectors are potent genetic vaccines and escape from pre-existing anti-adenovirus antibodies. Mol. Ther. 2008;16:154–162. doi: 10.1038/sj.mt.6300306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.