Abstract
To address how low titer, variable expression, and gene silencing affect gene therapy vectors for hemoglobinopathies, in a previous study we successfully used the HPFH (hereditary persistence of fetal hemoglobin)-2 enhancer in a series of oncoretroviral vectors. On the basis of these data, we generated a novel insulated self-inactivating (SIN) lentiviral vector, termed GGHI, carrying the Aγ-globin gene with the −117 HPFH point mutation and the HPFH-2 enhancer and exhibiting a pancellular pattern of Aγ-globin gene expression in MEL-585 clones. To assess the eventual clinical feasibility of this vector, GGHI was tested on CD34+ hematopoietic stem cells from nonmobilized peripheral blood or bone marrow from 20 patients with β-thalassemia. Our results show that GGHI increased the production of γ-globin by 32.9% as measured by high-performance liquid chromatography (p=0.001), with a mean vector copy number per cell of 1.1 and a mean transduction efficiency of 40.3%. Transduced populations also exhibited a lower rate of apoptosis and resulted in improvement of erythropoiesis with a higher percentage of orthochromatic erythroblasts. This is the first report of a locus control region (LCR)-free SIN insulated lentiviral vector that can be used to efficiently produce the anticipated therapeutic levels of γ-globin protein in the erythroid progeny of primary human thalassemic hematopoietic stem cells in vitro.
Papanikolaou and colleagues describe a novel locus control region (LCR)-free self-inactivating (SIN) γ-globin-insulated lentiviral vector for gene therapy in β-thalassemia. The authors show that this vector has a mean transduction efficiency of 40.3% and is able to increase the production of γ-globin by 32.9% in CD34+ hematopoietic stem cells isolated from patients with β-thalassemia.
Introduction
The β-thalassemias constitute inherited anemias caused by mutations that reduce or abolish production of the β-globin chain of hemoglobin, and are most prevalent in the Mediterranean region, the Middle East, the Indian subcontinent, and Southeast Asia, representing a serious global health problem. The severity of β-thalassemia is directly linked to the degree of imbalance in the production of α and β-like chains. The excess of α-globin chains that are not incorporated into adult hemoglobin (α2β2) precipitate in red blood cell precursors, impairing erythroid maturation and causing mechanical damage, oxidative membrane destruction, and eventually apoptosis (Orkin and Nathan, 1998; Weatherall, 1998, 2001; Mathias et al., 2000). However, the β-thalassemic phenotype is heterogeneous depending on the genotype as well as on the degree of γ-globin chain expression. The beneficial effects of elevated fetal hemoglobin (HbF) in β-thalassemia have long been recognized because of the amelioration of the clinical course of β-thalassemia syndromes due to the ability of γ-globin chains to bind free α-globin chains (Bunn, 2001). Direct experimental evidence indicating a beneficial effect of γ-globin expression has been obtained in both β-thalassemia and sickle cell disease (SCD) mouse models by mating diseased mice with transgenic mice that expressed different amounts of γ-globin mRNA and protein (Persons et al., 2001) and also in the gene therapy context (Perumbeti et al., 2009; Pestina et al., 2009). Most commonly, hereditary persistence of fetal hemoglobin (HPFH) is caused by a number of naturally occurring deletions at the 3′ end of the globin locus (Tuan et al., 1983; Anagnou et al., 1995). As a result of these deletions, distal enhancers such as the HPFH-2 enhancer (Fig. 1), located at the 3′ end of the globin gene locus, juxtapose next to the γ-globin genes, leading to their activation (Katsantoni et al., 2003). HPFH can also result from point mutations in the promoters of Aγ or Gγ genes. Such a mutation is the so-called −117 Greek mutation found in high frequency among the Greek population (Collins et al., 1985). Patients who carry a β-thalassemia gene and an HPFH genotype are either asymptomatic or have a milder clinical course. Hence, gene therapy of β-thalassemia based on γ-globin gene vectors displays a considerable advantage.
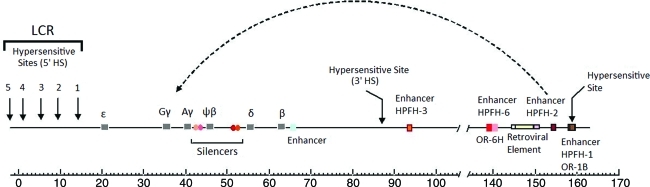
FIG. 1.
The human β-globin locus on chromosome 11 with regulatory elements. The positions of globin genes as well as the relevant enhancer and silencer (Gazouli et al., 2009) elements are illustrated. The HPFH-1 and HPFH-6 enhancers are located within the two olfactory receptor genes, OR-1B (Feingold et al., 1999) and OR-H6 (Kosteas et al., 1997), respectively. In the deletional HPFH phenotype, one of the hypotheses proposes that distal enhancer elements (such as HPFH-2) juxtapose next to the γ-globin genes, leading to their activation during adult life. Color images available online at www.liebertonline.com/hum
However, the development of vectors for globin genes as an efficient tool for the gene therapy of β-chain hemoglobinopathies still faces major challenges regarding the efficacy and therapeutic potential of the vectors, and has also been hampered by low titers, variable expression, and gene silencing (Papanikolaou and Anagnou, 2010). Incorporation of the locus control region (LCR) element, derived from the 5′ end of the β-globin locus, in these vectors has resulted in significant disease amelioration in mouse models (Imren et al., 2002; Rivella et al., 2003; Hanawa et al., 2004, Miccio et al., 2008). However, globin was inconsistently expressed by these vectors because of chromosomal position effects, despite the curative effect they were capable to demonstrate (Persons et al., 2003; Hanawa et al., 2004), a situation that eventually led to the use of insulators (Puthenveetil et al., 2004; Arumugam et al., 2007).
Previous work by our group clearly documented the ability of the HPFH-2 enhancer to maintain expression of the γ-globin gene in adult mice (Katsantoni et al., 2003). Moreover, when the HPFH-2 enhancer was combined with the −117 activating mutation in the context of oncoretroviral vectors, it demonstrated high levels of γ-globin mRNA/mouse α-globin mRNA and virtually no silencing of the transgene in MEL-585 cells (Fragkos et al., 2005).
On the basis of the previous data and in order to (1) improve the viral titers by exclusion of LCR, (2) reduce the rate of transgene silencing, and (3) further exploit the superior transducing efficiency of hematopoietic stem cells by lentiviral vectors, in the present study we generated a novel self-inactivating (SIN) LCR-free γ-globin-insulated lentiviral vector, containing the HS40 enhancer from the α-globin locus, the −117 activating HPFH mutation in the Aγ promoter, and the HPFH-2 enhancer element at the 3′ end of the transgene. This vector was tested in terms of efficacy and therapeutic potential in MEL-585 cells and CD34+ hematopoietic stem cells isolated from patients with β-thalassemia.
Materials and Methods
Construction of vector GGHI
Vector GGHI (Fig. 2) was constructed using the backbone of pCCL.sin.hPGKdeltaNGFR.WPRE.cHS4sense (a kind gift from L. Naldini, San Raffaele Telethon Institute for Gene Therapy, Milan, Italy), which is an SIN lentiviral vector carrying a gene encoding a truncated form of the nerve growth factor receptor, deltaNGFR, under the control of the human phosphoglycerate kinase (PGK) promoter. This vector also contains the full-length (1200-bp) cHS4 (chicken hypersensitive site-4) insulator in the deleted U3 region, the polypurine tract (PPT) and the woodchuck hepatitis posttranscriptional regulatory element (WPRE). To generate vector GGHI, the 1700-bp HS40-117Aγ cassette containing the Aγ-globin gene under the control of the Aγ promoter carrying the −117 activating HPFH mutation from vector Aγ117HS40-HPFH2 (Fragkos et al., 2005) was isolated as a HindIII fragment and cloned in the HindIII site of plasmid pBluescript II SK(+), creating plasmid pBlue/117/Aγ. The γ-globin gene in this setting has a 713-bp deletion in the second intron compared with the original genomic sequence. The 700-bp ScaI–NruI core element of HPFH-2 (Katsantoni et al., 2003) was cloned in the EcoRV site of pBlue/117/Aγ immediately downstream of the HS40-117Aγ cassette, creating plasmid pBlue/117/Aγ/HPFH2. Then the 2400-bp SalI–SmaI fragment from pBlue/117/Aγ/HPFH2 containing the HS40-117Aγ-HPFH2 cassette was cloned into the SalI–EcoRV sites of pCCL.sin.hPGKdeltaNGFR.WPRE.cHS4sense after simultaneous removal of the hPGK-deltaNGFR cassette with the same enzymes. Thus, the resulting vector GGHI contains the Aγ-globin gene with the −117 HPFH point mutation, both the HS-40 and HPFH-2 enhancers, and the cHS4 insulator in the deleted U3 region of the 3′ long terminal repeat (LTR).
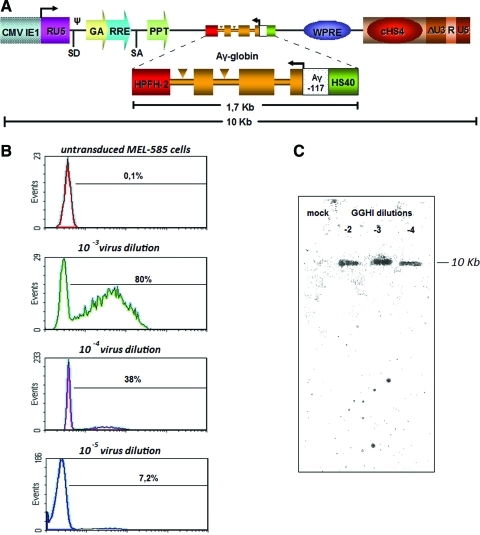
FIG. 2.
Vector design, titer, and stability. (A) The LCR-free SIN and insulated γ-globin lentiviral vector GGHI contains the HS40 enhancer from the α-globin locus, the −117 activating HPFH mutation in the Aγ promoter, and the HPFH-2 enhancer sequence at the 3′ end of the Aγ transgene. GGHI also carries the 1.2-kb cHS4 insulator, cloned in the forward orientation into the 3′ LTR. (B) Determination of viral titer by MEL-585 transduction. For determination of viral titer serial dilutions of the virus were made. Then 105 MEL-585 cells were transduced for 24 hr. Two days after removing the virus (3 days posttransduction), the cells were induced with 10 μM hemin and 3 mM HMBA. Flow cytometry was performed after 4 days of induction, using an anti-γ-globin monoclonal antibody. The percentage of γ-globin-expressing cells is indicated relative to the viral dilutions. (C) Stability of the GGHI vector, using Southern hybridization in MEL-585 cells transduced with GGHI at serial dilutions. Color images available online at www.liebertonline.com/hum
Virus production and titration
Virus was produced by transient cotransfection of 293T cells, using a third-generation lentiviral system (Dull et al., 1998; Zufferey et al., 1998) comprising the following plasmids: vector (GGHI), packaging plasmids (pMDLg/pRRE and pRSV-Rev), and envelope plasmid encoding the vesicular stomatitis virus glycoprotein VSVG (pMD2.VSVG). All accessory plasmids were kind gifts from L. Naldini (additional information about these plasmids can also be found at www.addgene.com). Briefly, 150-cm2 flasks were seeded with 107 cells and transfection took place after the flasks were approximately 80% confluent, using calcium phosphate precipitation with 18 μg of pMD2.VSVG, 30 μg of pMDLg/pRRE, 15.6 μg of pRSV-Rev, and 60 μg of vector GGHI. Virus-containing supernatant was collected 60 hr after transfection, centrifuged at 1200 rpm for 5 min, filtered through a 0.45-μm pore size cellulose acetate low protein binding filter (Sarstedt, Nümbrecht, Germany), and concentrated 100-fold by ultracentrifugation (30,000 rpm for 180 min). The virus was resuspended in StemSpan serum-free expansion medium (SFEM) (Stemcell Technologies, Vancouver, BC, Canada) containing 0.5% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO), incubated overnight at 4°C, and then aliquoted and stored at −80°C. To generate viruses used for mock transductions, cotransfections were carried out under exactly the same conditions described previously, but instead of GGHI vector, plasmid pCCL.sin.hPGKdeltaNGFR.WPRE.cHS4sense was used. Viral titers were determined by transducing 5×105 mouse erythroleukemia cells (MEL-585), using serial dilutions of concentrated virus. Differentiation was achieved with 10 μM hemin (Sigma-Aldrich) and 3 mM HMBA (N,N′-hexamethylene bisacetamide; Sigma-Aldrich) for 3–4 days and documented by analyzing them for HbF expression by fluorescence-activated cell sorting (FACS), using a fluorescein isothiocyanate (FITC)-conjugated anti-HbF monoclonal antibody (BD Biosciences, San Jose, CA). Both mock and GGHI vectors were titrated by p24 antigen assay, using an HIV1/2 combi kit (Roche Diagnostics, Indianapolis, IN) on a COBAS CORE analyzer (Roche Diagnostics). For both GGHI and mock transductions the same multiplicity of infection (MOI) was maintained.
Human CD34+ isolation and transduction: Sample collection and processing
We used CD34+ hematopoietic stem cells isolated from 20 patients with β-thalassemia. Diagnosis of β-thalassemia was based on family history, complete transfusion-dependence treatment, and hemoglobin electrophoresis. Two of 20 samples were derived from bone marrow, and the rest were obtained from nonmobilized peripheral blood. All samples were harvested from volunteer donors according to protocols approved by the local institutional review board at Saint Sophia Children's Hospital (Athens, Greece) after obtaining written informed consent in accordance with the Declaration of Helsinki. Thalassemia bone marrow was obtained when patients were under general anesthesia for another clinically indicated surgical operation. Peripheral blood was drawn from patients right before transfusion. CD34+ cells were isolated from mononuclear cells, using Midi-MACS columns (Miltenyi Biotec, Bergisch Gladbach, Germany) after two rounds of selection, as described by the manufacturer. Samples obtained in this way were >90% enriched in CD34+ cells (data not shown). CD34+ cells were resuspended overnight in pretransduction serum-free medium (StemSpan SFEM; Stemcell Technologies) at a density of 104–105 cells/ml and containing the following recombinant human cytokines: interleukin (IL)-6 (100 ng/ml), Flt-3 ligand (100 ng/ml), thrombopoietin (50 ng/ml), and stem cell factor (100 ng/ml). The next day, as indicated in Supplementary Fig. S1 (supplementary data are available online at www.liebertonline.com/hum), half the cells were transduced in the same medium with vector GGHI at an MOI of 100 for 18 hr and half the cells were mock-transduced and maintained as controls. Cells were then washed and portions of transduced and mock-transduced cells were either plated in methylcellulose medium, that is, 103 cells/ml per 35-mm2 Petri dish of MethoCult H4434 methylcellulose medium (StemCell Technologies) for colony-forming assays, or placed in liquid erythroid cultures for 18 days in StemSpan SFEM containing 20% FBS, human stem cell factor (50 ng/ml; R&D Systems, Minneapolis, MN), 10–6 M β-estradiol (Sigma-Aldrich), IL-3 (1 ng/ml; R&D Systems), erythropoietin (1 U/ml; Roche Diagnostics), and 10–6 M dexamethasone (Sigma-Aldrich). Colonies were picked at 2 weeks and were further subjected to FACS analysis and RNA isolation in order to measure the percent fold increase in γ-globin mRNA per sample, and to DNA isolation in order to determine the vector copy number per cell and the vector transduction efficiency. Liquid cultures were analyzed for erythroid cell expansion, differentiation using Wright-Giemsa stains on Cytospin slides for measurement of orthochromatic erythroblasts, HbF production by high-performance liquid chromatography (HPLC), FACS analysis, apoptosis using annexin-V labeling on day 18, and were further processed for RNA isolation, as described in the Supplementary Data.
HPLC for HbF quantitation
Erythroid cells (1–2×106 total cells) from in vitro erythroid cultures were resuspended in double-distilled H2O (HPLC grade) at a concentration of 2×107 cells/ml and resolved by ion-exchange HPLC (Chromsystems, Munich, Germany) with continuous gradient elution by increased ionic strength of elution buffer. During this procedure, a preprogrammed binary pump increases the percentage of buffer B in the elution buffer mixture that passes through the column. Detection of the absorbance of the eluate is done by a dual-wavelength photometer at 415 nm (with reference at 690 nm). Changes in absorbance are monitored and displayed as a chromatogram of absorbance versus time. Data from the detector are integrated and printed on a sample report. The percentage for every peak represents the ratio of the integration for this peak to the integration of total hemoglobins. The retention time (RT) of various hemoglobins was identified with control samples and the final results were interpreted against calibrator samples according to World Health Organization standards (NIBSC Code 85/616, version 02 for HbF).
Statistical analysis
The Student two-tailed t test was used to determine statistically significant differences between mean values of different data sets, using SPSS software (SPSS, Chicago, IL).
Results
Vector design, titer, and stability
The novel LCR-free, SIN γ-globin lentiviral vector, termed GGHI (Fig. 2A), carries an Aγ-globin gene driven by the Aγ promoter with the −117 activating HPFH mutation in reverse orientation relative to the HIV transcription initiation site located in the R region. The HPFH-2 (Katsantoni et al., 2003) and HS40 enhancer elements are located at the 3′ and 5′ ends of the transgene, respectively. To reduce repressive chromosomal position effects on vector expression and to diminish variegation in transgene expression, GGHI also carries the 1.2-kb cHS4 insulator (Chung et al., 1997) cloned in the forward orientation into the 3′ LTR in place of a 400-bp U3 deletion, so that on integration in the host genome, the insulated U3 region is copied in the 5′ LTR, thereby flanking the Aγ-globin cassette at both ends. GGHI was titrated after transducing MEL-585 cells with serial dilutions of the virus and exhibited a high titer that was calculated to be 2×108 transducing units (TU)/ml after 100-fold concentration (Fig. 2B). A more detailed presentation of the viral titers obtained from several batches is shown in Supplementary Table S1. Because it is well known that inclusion of large elements in the LTR might lead to molecular instability of the vector, we have also performed Southern hybridization experiments in MEL-585 cells, using pools of clones. The results documented that proviral integration is intact, with no detectable rearrangements (Fig. 2C). Because in our case a large insertion in the U3 region had no apparent effect on the vector's stability and titer, it is conceivable that the combination of both insulators incorporated in the U3 region along with large expression cassettes (e.g., LCR-cassettes) could be the probable cause for the reported LCR-vector titer reduction (Puthenveetil et al., 2004). To clarify this issue, we made a head-to-head comparison of the titers obtained both by GGHI and by another γ-globin lentiviral vector designated V5m3-400, that comprises the LCR region and a 400-bp fragment of the cHS4 insulator within the U3 region (Wilber et al., 2011). For these specific experiments, the V5m3-400 vector batches were made in exactly the same way as the GGHI vector batches and the titration procedures were performed in parallel for both vectors. The results documented high titers for both vectors, that is, 2×108 TU/ml for GGHI and 1×109 TU/ml for V5m3-400, with the latter vector displaying a comparative 5-fold higher titer.
Vector transduction and expression in MEL-585 cells
Because CD34+ hematopoietic stem cells are not 100% transducible by lentiviral vectors (Santoni de Sio et al., 2006; Santoni de Sio and Naldini, 2009), and to test whether γ-globin production from GGHI exhibits a pancellular or heterocellular pattern of expression, we used the mouse erythroleukemia cell line MEL-585. MEL cells were transduced at various multiplicities of infection (1–10) and were plated at limiting dilution in 96-well flat-bottomed dishes without selection. After inspection, wells containing one cell only were marked and left to expand in order to generate clones. Twenty-four clones were analyzed by PCR and FACS analysis and a total of 9 clones were tested positive for GGHI, based on PCR analysis and γ-globin production. These clones were induced to differentiate, using hemin and HMBA, and the level of expression for the Aγ-globin transgene was determined by real-time PCR and FACS analysis. Also, these clones were maintained in culture for 2 months and were inspected periodically for production of γ-globin. All nine positive clones (Fig. 3A and Table 1) expressed γ-globin in a pancellular manner and continued to express γ-globin throughout the 2-month inspection period, indicating that GGHI is capable of maintaining stable expression without detectable signs of silencing. Quantification of the histograms documented that clones transduced with GGHI exhibited an overall net mean fluorescence intensity (MFI) of 697.1±347.9 (Table 1). Levels of γ-globin expression were also determined at the RNA level by real-time PCR analysis in the same clones after induction. Our results show (Fig. 3B) that GGHI was able to express γ-globin at 282.1±491.2% per copy of mouse α-globin.
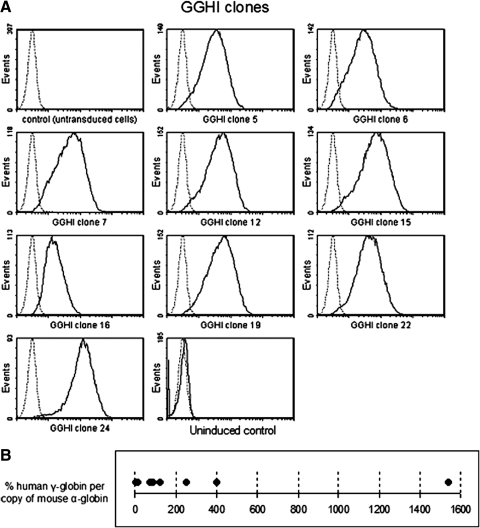
FIG. 3.
Expression of γ-globin from GGHI in MEL-585 clones. (A) MEL-585 cells were transduced with GGHI vector at an MOI of 1–10. Individual clones were isolated, induced with hemin and HMBA, and subjected to FACS analysis. The clones were monitored for at least 2 months and showed no signs of silencing. Note that the uninduced control cells do not express γ-globin, suggesting that the expression from GGHI is tissue specific. (B) The production of human γ-globin mRNA, expressed as the percent ratio of mouse α-globin mRNA per vector copy number.
Table 1.
Features of MEL-585 Clone Expression After GGHI Vector Transduction
| MEL clone number | Vector copy number/cell | Percent human γ-globin/mouse α-globin | Mean fluorescence intensity |
|---|---|---|---|
| 5 | 1.44 | 14.73 | 469 |
| 6 | 0.90 | 128.07 | 417 |
| 7 | 1.99 | 76.66 | 681 |
| 12 | 3.11 | 408.30 | 1040 |
| 15 | 1.09 | 1543.92 | 865 |
| 16 | 0.65 | 3.92 | 196 |
| 19 | 1.52 | 19.08 | 677 |
| 22 | 1.15 | 89.27 | 579 |
| 24 | 1.86 | 254.84 | 1350 |
| Mean | 1.52 | 282.09 | 697.11 |
These results corroborate our previous findings (Fragkos et al., 2005) using oncoretroviral vectors, where γ-globin from vector Aγ117HS40-HPFH2 was expressed at 248±99% per copy of mouse α-globin. Last, as shown in Table 1, there was a relatively good correlation between γ-globin expression from GGHI and the viral copy number per cell, as clones with higher copy number tend to produce higher levels of γ-globin. However, this fact was not universal, because the clone with the highest copy number (clone 12) did not exhibit the highest ratio of human γ-globin to murine α-globin, or MFI value, suggesting that expression from GGHI is not entirely dependent on the vector per se but may also be influenced by the site of integration. Another point of interest is the absence of γ-globin production in the uninduced MEL sample (Fig. 3A), suggesting that transgene expression from vector GGHI is erythroid specific, a desirable feature for the gene therapy of thalassemia.
Thalassemic sample collection
Human thalassemia major bone marrow CD34+ cells from 2 patients and peripheral blood CD34+ cells from 18 nonmobilized patients were isolated and processed. Ten of the patients were homozygotes for β0-thalassemia mutations, 5 patients were compound heterozygotes for two β+ mutations, and 5 patients were compound heterozygotes for a β0 mutation and a β+ mutation. All 20 patients had four intact α-globin genes and all of them were completely transfusion dependent since infancy. Typical yield from bone marrow contained 1–2×106 CD34+ cells, while the yield from an initial volume of 20 ml of peripheral blood ranged from 104 to 4×105 CD34+ cells. The ideal approach for γ-globin-based gene therapy assessment is to include patients with a low percentage of HbF in order to permit accurate measurements of the amount of vector-encoded γ-globin. Unfortunately, this was not feasible in our case, because the vast majority of patients with thalassemia major in Greece exhibit high percentages of HbF (Kattamis et al., 1982). For that reason, our cohort of patients expressed on average 67.52±41.56% of HbF (Supplementary Table S2) with a range of 24–90% (data from HPLC analysis of total blood of the aforementioned patients).
Differentiation of erythroid progenitors and HbF production
We first sought to detect the amount of HbF produced in our in vitro model of human erythropoiesis and then to test this model for its ability to recapitulate the pattern of hemoglobin expression associated with the developmental stage of the originating primary cell source. For this purpose, purified CD34+ cells isolated from bone marrow (BM) of normal adult donors (NDs), and CD34+ cells isolated from cord blood, were established in liquid culture under conditions designed to mimic erythroid maturation (see Materials and Methods). Briefly, CD34+ cells were placed overnight in pretransduction serum-free medium containing only IL-6, Flt-3 ligand, thrombopoietin, and stem cell factor. The next day, cells were washed and placed in liquid erythroid cultures for 18 days in medium containing FBS, human stem cell factor, β-estradiol, IL-3, erythropoietin, and dexamethasone. Erythroid maturation was monitored by flow cytometry on day 18, based on glycophorin A expression (Migliaccio et al., 2002). As shown in Fig. 4A, the majority of the cells were erythroid cells and more specifically late-stage erythroblasts, as reflected by the nearly complete enrichment for expression of glycophorin A. Morphological evaluation after Cytospin preparation and Giemsa staining indicated that the majority of cells in all cultures were terminally maturing erythroblasts (Fig. 4B).
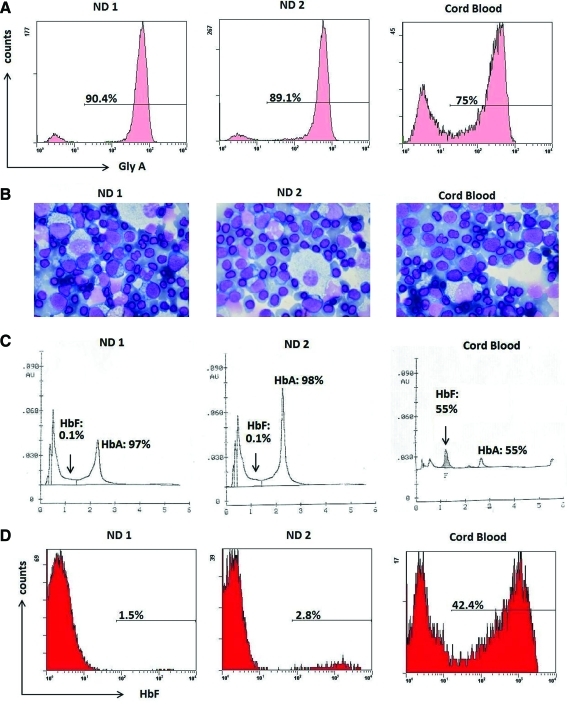
FIG. 4.
Differentiation of erythroid progenitors and HbF production derived from normal and cord blood CD34+ cells. (A) FACS analysis for erythroid maturation based on glycophorin A expression on day 18. (B) Cytospin preparations stained with Wright–Giemsa from erythroid cultures on day 18. (C) HPLC analysis for HbF quantification, demonstrating varying production of HbF in erythroblasts according to the developmental stage of the originating primary cell source. Vertical arrows in the ND1 and ND2 samples indicate the position of the minimally eluted HbF in trace amounts (0.1%). (D) FACS analysis for the detection of HbF-positive cells (F cells). ND, normal donor. Color images available online at www.liebertonline.com/hum
HPLC analysis demonstrated that virtually all of the hemoglobin in erythroblasts derived from adult bone marrow CD34+ cells was adult hemoglobin A (Fig. 4C). Accordingly, erythroblasts derived from cord blood CD34+ cells had approximately equal proportions of HbF (55%) and HbA (45%). These results were further corroborated by flow cytometry, where cultures derived from adult bone marrow had low percentages of F cells (<3%; Fig. 4D) in contrast to the cord blood-derived cultures, which exhibited high percentage of F cells (42.4%). Overall, these results suggest that this system permits reliable evaluation of the percentage of HbF and that production of HbF is associated with the developmental stage of the originating primary cell source.
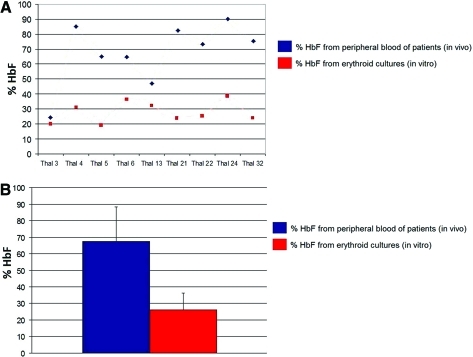
On the basis of the above-described findings, by employing this culture system, we were able to reduce the in vitro percentage of HbF produced from thalassemic samples relative to the in vivo values, a strategy that has been successfully used in assessments of HbF production via gene transfer approaches (Wilber et al., 2011). As shown in Fig. 5A, the in vitro percentage of HbF was always lower compared with the in vivo counts from several thalassemic patients. Furthermore, the mean in vitro percentage of HbF derived from all thalassemic patients was 26.7±19.9% (Table 2) ranging from 13.8 to 48.8%, and was three times lower relative to the in vivo percentage (Fig. 5B).
FIG. 5.
HbF percentage in thalassemic patients in vivo and in vitro. (A) Head-to-head comparison between the HbF percentages of thalassemia patients derived in vivo or in vitro after cultivating CD34+ cells for 18 days. HbF quantification was performed by HPLC. (B) Mean HbF percentage derived from thalassemia patients in vivo and in vitro. Color images available online at www.liebertonline.com/hum
Table 2.
Detailed Results from All Patients with Thalassemia
| |
|
|
|
In vitro erythroid culture |
HPLC |
Apoptosis |
|
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
|
|
Non transduced |
Transduced |
Non transduced |
Transduced |
Non transduced |
Transduced |
|
|
|||||||||||
| Sample | Phenotype | Mutation | Source | MFI | % HbF cells | MFI | % HbF cells | increase in protein level | % increase in protein level | fold increase in RNA level | %HbF | %HbF | increase from HPLC | % increase from HPLC | MFI AnnV | % apoptotic cells | MFI AnnV | % apoptotic cells | decrease | % decrease | TSD efficiency | VCN/cell |
| Thal 3 | β+ | cd39/IVS1 n6 | BM | 100,0 | 40,0 | 130,0 | 47,0 | 0,3 | 30,0 | 75,0 | 20,0 | 32,0 | 0,6 | 60,0 | 145,0 | 56,0 | 72,0 | 50,0 | 0,1 | 10,7 | 30,0 | 2,2 |
| Thal 4 | β0 | IVS1 n1/IVS1 n1 | PB | 125,0 | 35,0 | 147,0 | 40,0 | 0,2 | 17,6 | 25,0 | 31,2 | 33,0 | 0,1 | 5,8 | 87,0 | 12,2 | 45,0 | 8,9 | 0,3 | 27,0 | 10,0 | 3,3 |
| Thal 5 | β+ | cd8/IVS1 n6 | PB | 525,0 | 53,0 | 610,0 | 57,0 | 0,2 | 16,2 | 22,0 | 19,2 | 22,0 | 0,1 | 14,6 | 223,0 | 87,9 | 14,0 | 48,1 | 0,5 | 45,3 | 60,0 | 1,1 |
| Thal 6 | β0 | cd39/cd5 | PB | 39,6 | 42,3 | 42,0 | 41,8 | 0,1 | 6,1 | 12,5 | 36,4 | 39,7 | 0,1 | 9,1 | 75,0 | 34,2 | 62,0 | 33,6 | 0,0 | 1,8 | 40,0 | 1,5 |
| Thal 11 | β+ | IVS1 n110/IVS1 n 6 | BM | 74,0 | 14,3 | 112,0 | 25,2 | 0,5 | 51,4 | 65,0 | 42,8 | 77,9 | 0,8 | 82,0 | 197,0 | 82,6 | 27,0 | 11,2 | 0,9 | 86,5 | 70,0 | 1,3 |
| Thal 12 | β0 | cd39/IVS2 n745 | PB | 110,0 | 27,8 | 113,0 | 31,1 | 0,0 | 2,7 | 24,8 | 18,8 | 24,5 | 0,3 | 30,3 | 16,3 | 21,5 | 13,5 | 19,6 | 0,1 | 8,8 | 10,0 | 0,6 |
| Thal 13 | β0 | IVS2 n1/IVS1 n1 | PB | 106,0 | 38,5 | 111,0 | 42,4 | 0,0 | 4,7 | 11,3 | 32,2 | 32,4 | 0,0 | 0,6 | 12,5 | 15,1 | 12,2 | 17,0 | 0,0 | 0,0 | 10,0 | 0,6 |
| Thal 17 | β+ | IVSI n110/ IVS1 n110 | PB | 299,0 | 31,8 | 312,0 | 35,0 | 0,0 | 4,3 | 45,0 | 13,8 | 19,5 | 0,4 | 41,3 | 202,0 | 51,3 | 95,5 | 41,1 | 0,2 | 19,9 | 85,0 | 2,1 |
| Thal 18 | β+ | IVS1 n110/cd39 | PB | 784,0 | 45,7 | 860,0 | 49,4 | 0,1 | 9,7 | 62,3 | 23,2 | 39,6 | 0,7 | 70,7 | 62,0 | 19,8 | 58,0 | 15,0 | 0,2 | 24,2 | 46,0 | 2,7 |
| Thal 19 | β0 | cd39/cd44 | PB | 469,0 | 47,6 | 480,0 | 49,1 | 0,0 | 2,3 | 28,7 | 48,8 | 69,7 | 0,4 | 42,8 | 69,8 | 34,4 | 38,7 | 16,4 | 0,5 | 52,3 | 26,6 | 1,1 |
| Thal 20 | β0 | IVS21-/cd39 | PB | - | - | - | - | - | - | - | 16,4 | 36,0 | 1,2 | 119,5 | 110,0 | 62,2 | 52,0 | 33,0 | 0,5 | 46,9 | 93,0 | 0,8 |
| Thal 21 | β0 | cd39/cd39 | PB | 691,0 | 41,0 | 902,0 | 42,0 | 0,3 | 30,5 | 32,0 | 23,9 | 30,7 | 0,3 | 28,5 | 54,4 | 22,5 | 53,0 | 21,0 | 0,1 | 6,7 | 21,4 | 0,3 |
| Thal 22 | β0 | IVS2 n745/ IVS2 n87 | PB | 85,5 | 43,6 | 86,0 | 56,0 | 0,0 | 0,6 | 12,5 | 25,4 | 37,2 | 0,5 | 46,5 | 58,0 | 17,0 | 43,6 | 11,8 | 0,3 | 30,6 | 53,3 | 1,5 |
| Thal 24 | β+ | IVS1 n110/cd39 | PB | 67,3 | 71,4 | 70,0 | 70,9 | 0,0 | 4,0 | 5,1 | 38,6 | 40,0 | 0,0 | 3,6 | 68,6 | 75,6 | 50,5 | 68,1 | 0,1 | 9,9 | 30,0 | 0,5 |
| Thal 28 | β0 | cd39/cd28 | PB | 48,1 | 76,2 | 49,0 | 79,3 | 0,0 | 1,9 | 12,3 | 14,8 | 15,7 | 0,1 | 6,1 | 57,8 | 67,2 | 57,6 | 62,2 | 0,1 | 7,4 | 40,0 | 0,5 |
| Thal 31 | β0 | cd39/δβ sic | PB | 54,2 | 36,5 | 75,0 | 62,1 | 0,4 | 38,4 | 42,0 | - | - | - | - | 334,0 | 72,3 | 18,3 | 9,0 | 0,9 | 87,6 | 66,7 | 0,9 |
| Thal 32 | β+ | IVSI n110/IVS1 n110 | PB | 52,4 | 68,0 | 71,2 | 73,0 | 0,4 | 35,9 | 40,0 | 24,1 | 25,8 | 0,1 | 7,1 | 88,3 | 17,3 | 62,9 | 7,0 | 0,6 | 59,5 | 34,3 | 0,5 |
| Thal 33 | β+ | IVSI n110/IVS1 n44 | PB | 2300,0 | 50,4 | 2390,0 | 51,4 | 0,0 | 3,9 | 15,5 | 32,0 | 35,5 | 0,1 | 10,9 | 87,0 | 44,0 | 81,0 | 42,0 | 0,0 | 4,5 | 25,0 | 0,4 |
| Thal 34 | β+ | cd39/IVS1 n6 | PB | 1750,0 | 58,0 | 1870,0 | 54,5 | 0,1 | 6,9 | 16,0 | 19,5 | 22,1 | 0,1 | 13,3 | 91,0 | 44,3 | 64,4 | 43,0 | 0,0 | 2,9 | 30,0 | 0,5 |
| Thal 35 | β+ | IVSI n1/IVSI n110 | PB | 98,3 | 77,0 | 101,0 | 74,0 | 0,0 | 2,7 | 5,7 | - | - | - | - | 94,1 | 19,0 | 83,0 | 17,0 | 0,1 | 10,5 | 24,0 | 0,4 |
| Average | 409,4 | 47,3 | 449,0 | 51,6 | 0,1 | 14,2 | 29,1 | 26,7 | 35,2 | 0,3 | 32,9 | 106,6 | 42,8 | 50,2 | 28,7 | 0,3 | 27,2 | 40,3 | 1,1 | |||
Note: Dashes indicate that the relevant assays were not done because of limited number of cells. BM, bone marrow; PB, peripheral blood; MFI, mean fluorescence intensity; AnnV, annexin-V; TSD, transduction; VCN, vector copy number.
Gene transfer efficiency and vector copy number in transduced thalassemic erythroid burst-forming units
To test the efficacy of the new vector GGHI, we transduced CD34+ cells derived from thalassemic patients. The experimental design is depicted in Supplementary Fig. S1. Briefly, after CD34+ isolation, the cells were cultured under serum-free conditions for 18 hr and then they were divided into two subpopulations; one subpopulation was transduced with vector GGHI at an MOI of 100 for an additional 18 hr, whereas the second subpopulation of the cells were mock-transduced and served as a control. The next day, cells were washed twice with PBS and resuspended in erythroid specific medium (see Materials and Methods).
To determine the gene transfer efficiency, CD34+ cells from thalassemic patients that were either transduced with GGHI or mock-transduced were plated in methylcellulose semiliquid medium for colony-forming assays for 15 days. About 15 to 20 individual erythroid burst-forming units (BFUe) per patient in each experiment were analyzed by semiquantitative PCR using vector-specific primers. Gene transfer efficiency of the lentiviral vector in the CD34+ progenitors was determined by assessing the proportion of BFUe colonies that tested positive for vector sequence. As shown in Table 2, the mean transduction (TSD) efficiency for GGHI was 40.3% (median, 32.2%) with a range of 10–93%. The clonogenicity of mock- or GGHI-transduced CD34+ cells was also similar (data not shown). Furthermore, the vector copy number per cell was calculated for each BFUe tested positive for vector sequence from each patient and then a mean value was extracted from all positive BFUe for each patient. The mean vector copy number per cell was 1.1 (median, 0.8) with a range of 0.3–3.3, as shown in Table 2. These results were also evaluated regarding β+- and β0-thalassemia patients. Thus, the mean transduction efficiency observed in patients with β0-thalassemia was 37.1% (median, 33.3%) with a range of 10–93% (Table 3), whereas for patients with β+-thalassemia the mean transduction efficiency was 43.4% (median, 32.2%) with a range of 24–85% (Table 4). The vector copy number was slightly higher in patients with β+-thalassemia (1.2) relative to patients with β0-thalassemia (1.1). However, these differences between the two sets of patients were not statistically significant.
Table 3.
Detailed Results from Patients with β0-Thalassemia
| |
|
In vitro erythroid culture |
HPLC |
Apoptosis |
|
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
Non transduced |
Transduced |
Non transduced |
Transduced |
Non transduced |
Transduced |
|
|
|||||||||||
| Sample | Phenotype | MFI | %HbF cells | MFI | %HbF cells | increase in protein level | %increase in protein level | fold increase in RNA level | %HbF | %HbF | increase from HPLC | %increase from HPLC | MFI AnnV | % apoptotic cells | MFI AnnV | % apoptotic cells | decrease | % decrease | TSD efficiency | VCN/cell |
| Thal 4 | β0 | 125,0 | 35,0 | 147,0 | 40,0 | 0,2 | 17,6 | 25,0 | 31,2 | 33,0 | 0,1 | 5,8 | 87,0 | 12,2 | 45,0 | 8,9 | 0,3 | 27,0 | 10,0 | 3,3 |
| Thal 6 | β0 | 39,6 | 42,3 | 42,0 | 41,8 | 0,1 | 6,1 | 12,5 | 36,4 | 39,7 | 0,1 | 9,1 | 75,0 | 34,2 | 62,0 | 33,6 | 0,0 | 1,8 | 40,0 | 1,5 |
| Thal 12 | β0 | 110,0 | 27,8 | 113,0 | 31,1 | 0,0 | 2,7 | 24,8 | 18,8 | 24,5 | 0,3 | 30,3 | 16,3 | 21,5 | 13,5 | 19,6 | 0,1 | 8,8 | 10,0 | 0,6 |
| Thal 13 | β0 | 106,0 | 38,5 | 111,0 | 42,4 | 0,0 | 4,7 | 11,3 | 32,2 | 32,4 | 0,0 | 0,6 | 12,5 | 15,1 | 12,2 | 17,0 | 0,0 | 0,0 | 10,0 | 0,6 |
| Thal 19 | β0 | 469,0 | 47,6 | 480,0 | 49,1 | 0,0 | 2,3 | 28,7 | 48,8 | 69,7 | 0,4 | 42,8 | 69,8 | 34,4 | 38,7 | 16,4 | 0,5 | 52,3 | 26,6 | 1,1 |
| Thal 20 | β0 | - | - | - | - | - | - | - | 16,4 | 36,0 | 1,2 | 119,5 | 110,0 | 62,2 | 52,0 | 33,0 | 0,5 | 46,9 | 93,0 | 0,8 |
| Thal 21 | β0 | 691,0 | 41,0 | 902,0 | 42,0 | 0,3 | 30,5 | 32,0 | 23,9 | 30,7 | 0,3 | 28,5 | 54,4 | 22,5 | 53,0 | 21,0 | 0,1 | 6,7 | 21,4 | 0,3 |
| Thal 22 | β0 | 85,5 | 43,6 | 86,0 | 56,0 | 0,0 | 0,6 | 12,5 | 25,4 | 37,2 | 0,5 | 46,5 | 58,0 | 17,0 | 43,6 | 11,8 | 0,3 | 30,6 | 53,3 | 1,5 |
| Thal 28 | β0 | 48,1 | 76,2 | 49,0 | 79,3 | 0,0 | 1,9 | 12,3 | 14,8 | 15,7 | 0,1 | 6,1 | 57,8 | 67,2 | 57,6 | 62,2 | 0,1 | 7,4 | 40,0 | 0,5 |
| Thal 31 | β0 | 54,2 | 36,5 | 75,0 | 62,1 | 0,4 | 38,4 | 42,0 | - | - | - | - | 334,0 | 72,3 | 18,3 | 9,0 | 0,9 | 87,6 | 66,7 | 0,9 |
| Average | 192,0 | 43,2 | 222,8 | 49,3 | 0,1 | 11,6 | 22,3 | 27,5 | 35,4 | 0,3 | 32,1 | 87,5 | 35,9 | 39,6 | 23,3 | 0,3 | 26,9 | 37,1 | 1,1 | |
Notes: Dashes indicate that the relevant assays were not done because of limited number of cells. MFI, mean fluorescence intensity; AnnV, annexin-V; TSD, transduction; VCN, vector copy number.
Table 4.
Detailed Results from Patients with β+-Thalassemia
| |
|
In vitro erythroid culture |
HPLC |
Apoptosis |
|
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
Non transduced |
Transduced |
Non transduced |
Transduced |
Non transduced |
Transduced |
|
|
|||||||||||
| Sample | Phenotype | MFI | % HbF cells | MFI | % HbF cells | increase in protein level | % increase in protein level | fold increase in RNA level | %HbF | %HbF | increase from HPLC | %increase from HPLC | MFI AnnV | % apoptotic cells | MFI AnnV | % apoptotic cells | decrease | % decrease | TSD efficiency | VCN/cell |
| Thal 3 | β+ | 100,0 | 40,0 | 130,0 | 47,0 | 0,3 | 30,0 | 75,0 | 20,0 | 32,0 | 0,6 | 60,0 | 145,0 | 56,0 | 72,0 | 50,0 | 0,1 | 10,7 | 30,0 | 2,2 |
| Thal 5 | β+ | 525,0 | 53,0 | 610,0 | 57,0 | 0,2 | 16,2 | 22,0 | 19,2 | 22,0 | 0,1 | 14,6 | 223,0 | 87,9 | 14,0 | 48,1 | 0,5 | 45,3 | 60,0 | 1,1 |
| Thal 11 | β+ | 74,0 | 14,3 | 112,0 | 25,2 | 0,5 | 51,4 | 65,0 | 42,8 | 77,9 | 0,8 | 82,0 | 197,0 | 82,6 | 27,0 | 11,2 | 0,9 | 86,5 | 70,0 | 1,3 |
| Thal 17 | β+ | 299,0 | 31,8 | 312,0 | 35,0 | 0,0 | 4,3 | 45,0 | 13,8 | 19,5 | 0,4 | 41,3 | 202,0 | 51,3 | 95,5 | 41,1 | 0,2 | 19,9 | 85,0 | 2,1 |
| Thal 18 | β+ | 784,0 | 45,7 | 860,0 | 49,4 | 0,1 | 9,7 | 62,3 | 23,2 | 39,6 | 0,7 | 70,7 | 62,0 | 19,8 | 58,0 | 15,0 | 0,2 | 24,2 | 46,0 | 2,7 |
| Thal 24 | β+ | 67,3 | 71,4 | 70,0 | 70,9 | 0,0 | 4,0 | 5,1 | 38,6 | 40,0 | 0,0 | 3,6 | 68,6 | 75,6 | 50,5 | 68,1 | 0,1 | 9,9 | 30,0 | 0,5 |
| Thal 32 | β+ | 52,4 | 68,0 | 71,2 | 73,0 | 0,4 | 35,9 | 40,0 | 24,1 | 25,8 | 0,1 | 7,1 | 88,3 | 17,3 | 62,9 | 7,0 | 0,6 | 59,5 | 34,3 | 0,5 |
| Thal 33 | β+ | 2300,0 | 50,4 | 2390,0 | 51,4 | 0,0 | 3,9 | 15,5 | 32,0 | 35,5 | 0,1 | 10,9 | 87,0 | 44,0 | 81,0 | 42,0 | 0,0 | 4,5 | 25,0 | 0,4 |
| Thal 34 | β+ | 1750,0 | 58,0 | 1870,0 | 54,5 | 0,1 | 6,9 | 16,0 | 19,5 | 22,1 | 0,1 | 13,3 | 91,0 | 44,3 | 64,4 | 43,0 | 0,0 | 2,9 | 30,0 | 0,5 |
| Thal 35 | β+ | 98,3 | 77,0 | 101,0 | 74,0 | 0,0 | 2,7 | 5,7 | - | - | - | - | 94,1 | 19,0 | 83,0 | 17,0 | 0,1 | 10,5 | 24,0 | 0,4 |
| Average | 605,0 | 51,0 | 652,6 | 53,7 | 0,2 | 16,5 | 35,2 | 25,9 | 34,9 | 0,3 | 33,7 | 125,8 | 49,8 | 60,8 | 34,2 | 0,3 | 27,4 | 43,4 | 1,2 | |
Note: Dashes indicate that the relevant assays were not done because of limited number of cells.
Effective erythropoiesis from vector-transduced human thalassemia major CD34+ cells in vitro
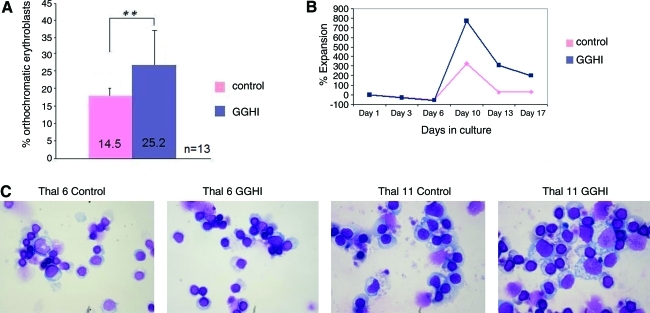
To test the effects of GGHI transduction on the restoration of thalassemic erythropoiesis, CD34+ cells were placed in liquid cultures that promote erythroid differentiation, thus recapitulating in vivo erythropoiesis. The progression of differentiation in these cultures was documented on day 18 by employing FACS analysis, using an anti-glycophorin A monoclonal antibody, because mature erythroid cells express glycophorin A (Migliaccio et al., 2002). Erythroid maturation was further quantified by performing differential counts of the percentage of orthochromatic erythroblasts on Cytospin preparations of liquid cultures, also on day 18 (Fig. 6C). A central feature of thalassemic erythropoiesis is that erythroid cells are arrested at the polychromatophilic normoblast stage with little progression to the orthochromatic normoblast stage (Mathias et al., 2000). However, GGHI-transduced CD34+ cells exhibited higher levels of erythroid differentiation because the mean percentage of orthochromatic erythroblasts was 25.2% versus mock-transduced cells, for which the relevant percentage was 14.5% (p=0.00077; Fig. 6A and Table 5). This accounts for a 78.4% increase in the percentage of orthochromatic erythroblasts because of the transduction with GGHI. These data suggest that GGHI is able to promote erythroid differentiation efficiently in thalassemic cells. A detailed description for several patients is illustrated in Table 5. In some cases, because of the limited number of cells, determining the percentage of orthochromatic erythroblasts was not applicable.
FIG. 6.
Effective erythropoiesis from vector-transduced human thalassemia major CD34+ cells in vitro. (A) Mean percentage of orthochromatic erythroblasts from GGHI-transduced and mock-transduced cells. Differentiated cells from erythroid cultures were picked on day 18 and the percentage of orthochromatic erythroblasts per sample was determined. The difference was statistically significant, **p<0.001. The mean fold increase in the percentage of orthochromatic erythroblasts was 78.4% for all patients. (B) Cell expansion of GGHI-transduced and mock-transduced erythroid cultures expressed as the percent increase in cell number. Results are shown from one representative experiment. (C) Cytospin preparations stained with Wright–Giemsa from erythroid cultures on day 18 were used in the determination of orthochromatic erythroblasts. Color images available online at www.liebertonline.com/hum
Table 5.
Percentage of Orthochromatic Erythroblasts from Erythroid Cultures
| Control | GGHI | Percent increase | |
|---|---|---|---|
| Thal 3 | 19.3 | 27.5 | 42.5 |
| Thal 6 | 13.2 | 20.8 | 57.6 |
| Thal 11 | 19.2 | 44.0 | 129.2 |
| Thal 12 | 12.2 | 27.2 | 123.0 |
| Thal 13 | 9.8 | 18.6 | 89.8 |
| Thal 20 | 7.3 | 13.6 | 85.1 |
| Thal 22 | 21.8 | 36.2 | 66.1 |
| Thal 24 | 10.2 | 11.7 | 14.4 |
| Thal 28 | 12.0 | 16.8 | 40.0 |
| Thal 32 | 9.4 | 17.0 | 80.9 |
| Thal 33 | 9.7 | 30.7 | 217.2 |
| Thal 34 | 32.4 | 49.4 | 52.5 |
| Thal 35 | 12.0 | 14.6 | 21.7 |
| Average | 14.5 | 25.2 | 78.4 |
The aforementioned results were also corroborated by the total cell expansion that either mock- or GGHI-transduced CD34+ cells exhibited in erythroid cultures. Specifically, both mock- and GGHI-transduced CD34+ cells were plated for 18 hr after transduction in erythroid medium and were examined relative to cell expansion biweekly. As a general feature, the cell number in both populations declined during the first week of culture but increased thereafter to reach its maximal value between day 10 and day 13. As shown from one representative experiment (Fig. 6B), the magnitude of the amplification obtained in the transduced populations was higher than that observed in mock-transduced populations. More specifically, despite the slower proliferation rate at the beginning of the culture, transduced cells started to show a growth advantage relative to control cells on day 6 that went on until day 18, when the cultures were discontinued in order to perform FACS analysis, RNA analysis, and HPLC. GGHI-transduced cells exhibited a 200% increase in cell number on day 17, relative to control cells that exhibited only a 32% increase (p=0.07) on the same day. The number of cells as well as the magnitude of the cell expansion between the transduced and control populations is shown in further detail in Supplementary Table S3 and Supplementary Fig. S2.
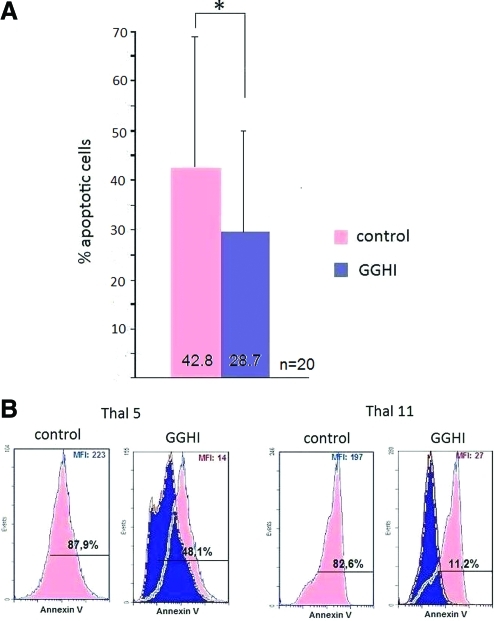
Furthermore, cultures were also assessed on day 18 for effects on apoptosis, a feature characteristic of thalassemia major erythropoiesis (Mathias et al., 2000), by employing FACS analysis and an annexin-V monoclonal antibody. The proportion of cells undergoing apoptosis was significant in the thalassemic mock-transduced populations; however, transduction with GGHI was able to ameliorate the apoptotic rate (Fig. 7A). More specifically, our results show that transduction with GGHI resulted in a 50% reduction of apoptosis, because the mean MFI in control cells was 106.6 versus 50.2 for transduced cells (p=0.007; Table 2) and a mean 27.2% reduction in the percentage of apoptotic cells (p=0.007) as shown in Table 2. Furthermore, the percentage of annexin-V-positive cells of β0-thalassemic patients (Table 3) was 35.9% for the mock-transduced populations and 23.3% for the GGHI-transduced populations, accounting for a 35% decrease in the apoptotic rate for patients with β0-thalassemia (p=0.07). Last, patients with β+-thalassemia (Table 4) exhibited a 31.3% decrease in apoptotic rate because the mean percentage of annexin-V-positive cells in control populations was 49.8% versus 34.2% in GGHI-transduced populations (p=0.05). Representative FACS histograms from both mock- and GGHI-transduced populations are shown in Fig. 7B.
FIG. 7.
Correction of thalassemic phenotype through rescue from apoptosis. Differentiated cells from erythroid cultures were picked on day 18 and analyzed for apoptosis by FACS analysis with annexin-V labeling. (A) The diagram shows the mean percentage of apoptotic cells in transduced and control cell populations. The difference was statistically significant, *p<0.01. The mean fold decrease observed for all patients was 27.2%. (B) Results from FACS analysis are shown from two representative experiments. Color images available online at www.liebertonline.com/hum
Taken together, these data document a more efficient erythroid expansion and maturation of GGHI-transduced thalassemic progenitor cells, with significant reduction of apoptosis and effective erythroid production.
Production of γ-globin in transduced thalassemic hematopoietic stem cells in erythroid liquid cultures
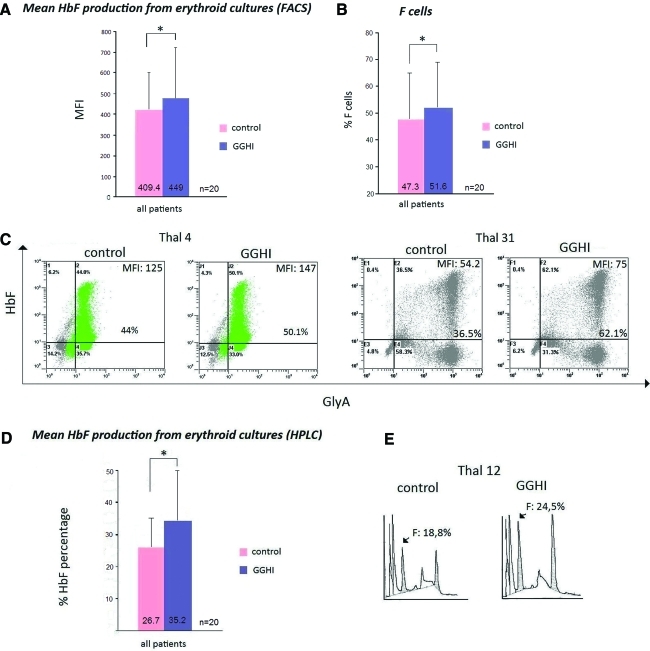
HbF and glycophorin A determination by FACS analysis in liquid cultures was performed on day 18. Representative FACS analyses (Fig. 8C), and summarized results depicted in Table 2, indicate that in the vast majority of the cases there was a net increase in the MFI of transduced versus mock-transduced cells, suggesting that transduced populations produce higher levels of HbF. The mean MFI from all patients was 409.4 for untransduced cells and 449 for transduced cells (p=0.005; Fig. 8A). Transduced cells also exhibited a statistically significant (p=0.009) higher percentage of F cells (51.6%) versus controls (47.3%), as shown in Fig. 8B. This finding is consistent with the results retrieved from the apoptosis analysis. Also, the relative increase in HbF RNA levels was estimated to be 29.1-fold from these experiments. The results again varied between the two groups of patients; β0-thalassemia CD34+ cells transduced with GGHI (Table 3) exhibited a 16% increase in the production of γ-globin (p=0.21), whereas β+-thalassemia CD34+ cells (Table 4) exhibited a 7% increase (p=0.005).
FIG. 8.
Production of HbF in transduced thalassemic CD34+ hematopoietic stem cells in erythroid liquid cultures. (A) Mean production of HbF from GGHI-transduced and mock-transduced cells, determined by FACS analysis. Differentiated cells from erythroid cultures were picked on day 18 and subjected to FACS analysis using an anti-HbF monoclonal antibody. The diagram shows the mean production of HbF in transduced and control cell populations, expressed as MFI. (B) Mean percentage of F cells from GGHI-transduced and mock-transduced cells. (C) Results from FACS analysis shown from two representative experiments. (D) Mean production of HbF from GGHI-transduced and mock-transduced cells, using HPLC analysis. Differentiated cells from erythroid cultures were picked on day 18 and subjected to HPLC analysis for quantification of HbF. The diagram shows the mean production of HbF in transduced and control cell populations. The mean fold increase in HbF production was 32.9% for all patients. (E) Results from HPLC analysis shown from one representative experiment. The asterisk (*) denotes statistical significance (p<0.01). Color images available online at www.liebertonline.com/hum
To quantify γ-globin produced from vector GGHI, cell lysates from liquid cultures from all experiments were subjected to HPLC analysis. Under the specified experimental conditions, both mock- and GGHI-transduced cells produced measurable amounts of HbF (Table 2). The results from these experiments (Fig. 8D and Table 2) clearly indicate that GGHI-transduced CD34+ cell populations produce higher levels of HbF compared with mock-transduced cells. The mean increase in HbF from all patients was 32.9% (p=0.001), with 32.1% for patients with β0-thalassemia (p=0.01; Table 3) and 33.7% for patients with β+-thalassemia (p=0.03; Table 4). A representative HPLC histogram is shown in Fig. 8E, and more HPLC charts are shown in Supplementary Fig. S3.
Overall, these results suggest that the novel LCR-free SIN lentiviral vector GGHI can efficiently transduce thalassemic CD34+ hematopoietic stem cells and that the γ-globin production from the GGHI vector is significantly expressed, leading to the restoration of thalassemic hematopoiesis, and suggesting that eventual production of γ-globin from this vector at the clinical level can achieve therapeutic levels.
Discussion
Hematopoietic stem cell (HSC)-targeted gene therapy using autologous cells holds promise for the cure of a variety of lympho-hematopoietic disorders due to single-gene defects. The gene therapy approaches that are currently being pursued for the treatment of β-chain hemoglobinopathies rely on the use of recombinant viral vectors to stably transfer expression cassettes either for human β-globin or γ-globin into reconstituting hematopoietic stem cells of thalassemic patients. Vectors for human γ-globin have been pursued because of (1) the antisickling properties of γ-globin for the treatment of SCD, (2) the fact that γ-globin is not immunogenic, as well as (3) the therapeutic potential of γ-globin for the treatment of patients with β-thalassemia, based on the large number of clinical studies done over the past 40–50 years, which strongly indicated that a gene therapy approach based on γ-globin should prove effective for the treatment of β-thalassemia. For example, compound heterozygotes for β-thalassemia and deletional HPFH exhibit a mild phenotype (Weatherall, 2001); compound heterozygotes for β-thalassemia and a nondeletional HPFH allele exhibit a β-thalassemia intermedia phenotype (Fessas and Stamatoyannopoulos, 1964; Tate et al., 1986); and compound δβ-thalassemia/β-thalassemia heterozygotes exhibit a β-thalassemia intermedia to mild phenotype (Stamatoyannopoulos et al., 1969). Homozygotes for δβ-thalassemia produce only HbF and present a phenotype of thalassemia intermedia (Weatherall, 2001), and homozygotes for deletional HPFH display only HbF in their blood and are clinically normal, although they have a slightly elevated hematocrit and dysmorphic erythrocytic indices, presumably secondary to the increased oxygen affinity of HbF (Wheeler and Krevans, 1961; Charache et al., 1976).
However, the development of gene therapy approaches for SCD and β-thalassemia has been plagued by problems inherent to the design of stable vectors such as low titers, variable expression, and gene silencing, and more recently, the risks of insertional mutagenesis have highlighted the need to achieve these goals with minimal copies of proviral integrants per cell (Hacein-Bey-Abina et al., 2003; Hargrove et al., 2008; Cavazzana-Calvo et al., 2010).
In the present study we sought to develop a novel lentiviral vector for effective globin gene therapy that would be able to drive the expression of γ-globin at therapeutic levels; and exhibit high titer, reduced positional effects, absence of silencing, and improved vector biosafety features with the use of cHS4 insulator. We designed and tested this LCR-free lentiviral vector, termed GGHI, which expresses the human γ-globin gene under the control of regulatory elements that promote the continuous expression of γ-globin in the adult environment and therefore prevent silencing of the γ-globin gene, namely the –117 HPFH mutation in the Aγ promoter (Collins et al., 1985) and the HPFH-2 enhancer (Anagnou et al., 1995; Katsantoni et al., 2003; Fragkos et al., 2005). In the present study, we show that this improved vector exhibits high-level transduction of bone marrow progenitor cells from 20 patients with transfusion-dependent thalassemia major and leads to phenotypic as well as functional correction of transduced cells in the model of human thalassemia erythropoiesis in vitro. To the best of our knowledge, this is the first report of an LCR-free, SIN, and insulated lentiviral vector that can be used to efficiently produce the anticipated therapeutic levels of γ-globin protein in the erythroid progeny of primary human hematopoietic stem cells in vitro.
In terms of viral titer and stability, GGHI exhibited high functional titers of approximately 2×108 TU/ml as measured by FACS analysis on MEL-585 cells and was also stable without any detectable rearrangements, corroborating our previous findings in the context of oncoretroviral vectors (Fragkos et al., 2005). These facts render GGHI an excellent candidate for clinical use, because the aforementioned titers were achievable by only 100-fold concentration. Previously reported globin insulated vectors (Puthenveetil et al., 2004) exhibited low titers and had to be concentrated 10,000-fold, probably because of the coexistence of parts of the LCR and the 1.2-kb cHS4 in the 3′ LTR that altogether result in a rather large genomic size for packaging into a lentivirus. To address this specific point, we proceeded to a head-to-head comparison of the titer obtained both by GGHI and by vector V5m3-400, which is another γ-globin lentiviral vector that comprises the LCR region and a 400-bp fragment of the cHS4 insulator within the U3 region (Wilber et al., 2011). In accordance with procedures done in parallel for both vectors, the generated titers were 2×108 TU/ml for GGHI and 1×109 TU/ml for V5m3-400. On the basis of these data, the 5-fold higher titer of vector V5m3-400 implies that the size of cHS4, incorporated in the U3 region, is probably a critical parameter in defining the titer of the vector, because GGHI combines a large insulator and a relatively small expression cassette (1.7 kb), whereas V5m3-400 combines a small core insulator (400 bp) and a large expression cassette. The majority of globin vectors used in the globin gene therapy field do not contain insulators (Imren et al., 2002; Rivella et al., 2003; Hanawa et al., 2004; Lisowski and Sadelain, 2007; Miccio et al., 2008), although the use of the latter is considered almost necessary because not only do they increase expression of β-globin (Arumugam et al., 2007) but they also have been proven in a large number of studies (Desprat and Bouhassira, 2009; Hanawa et al., 2009) to act more efficiently in reducing the risk of insertional mutagenesis. In addition, Arumugam and colleagues (2009) showed that the core sequence of cHS4 alone does not insulate viral vectors effectively and that both 3′ as well as 5′ sequences are needed for full insulator activity. Overall, these data demonstrate that more experiments should be performed in order to obtain a definitive conclusion concerning the effects of insulators. Furthermore, another key point would be the assessment of the efficacy of fully insulated vectors with shorter expression cassettes versus noninsulated vectors with larger globin cassettes in terms of titer, transgene expression, and safety, in view of the upcoming clinical trials.
The expression assays on MEL cells displayed efficient levels of HbF production, comparable to those published previously (Fragkos et al., 2005). In our previous study we used the exact same regulatory elements in oncoretroviral vectors and documented the robust synergistic capacity of the HS-40 and HPFH-2 elements in enhancing transcription as well as the ability of the HPFH-2 enhancer to reduce the rate of gene silencing in concert with the ability of the −117 activating HPFH mutation to support Aγ-globin gene expression in the adult erythroid environment. Although the expression of γ-globin from the MEL-585 clones correlated relatively well with viral copy, there was one clone (clone 12) that, although having the highest copy number, did not exhibit the highest ratio of human γ-globin to murine α-globin. This suggests that expression from GGHI is not entirely dependent on the vector per se but may also be influenced by the site of integration and implies that further experiments are needed in order to address the function of the insulator. In terms of tissue specificity of the GGHI vector, we tested uninduced MEL-585 clones for γ-globin expression. Results from these experiments demonstrated lack of expression of γ-globin, therefore suggesting that expression of γ-globin from GGHI vector is erythroid specific.
To move forward to clinical translation, the therapeutic efficacy of GGHI was tested in the context of the eventual cell target of gene therapy trials, namely human CD34+ hematopoietic stem cells isolated from patients with thalassemia major. For that reason, we first focused on the use of human primitive hematopoietic cells from various developmental stages to derive erythroid cultures of maturing erythroblasts that can be used to efficiently evaluate the molecular mechanisms of switching. In addition, we established a culture system with minimal production of HbF in vitro, a feature that permits accurate assessment of vector-derived γ-globin production as previously described (Wilber et al., 2011). This culture system actually resulted in low levels of HbF production in cells derived from adult bone marrow and in high levels of HbF in cells derived from cord blood, suggesting that it represents a reliable recapitulation of the in vivo status. Indeed, when CD34+ cells from thalassemic patients were cultured under the same conditions, the average percentage of HbF in vitro was much lower than the actual percentage of HbF that the same patients expressed in vivo. Moreover, in a relevant study (Wilber et al., 2011) that also included cultivation of thalassemic CD34+ cells in vitro, HbF production from three thalassemic patients was significantly low, that is, 38.3, 26.7, and 24%, respectively, thus corroborating our data.
The average vector copy number (VCN) per cell for GGHI was calculated as 1.1, reflecting the desired value for a clinical setting. Some patients, however, exhibited low VCN per cell. It is not unusual to observe a vector copy number per cell of less than one (VCN,<1) in gene therapy experiments involving thalassemia. In a study that included transduction of CD34+ cells isolated from thalassemic patients with a β-globin lentiviral vector, the average VCN per cell was 1.6 (Roselli et al., 2010). However, in the same study, 4 of 22 patients had a VCN per cell of less than one (ranging from 0.4 to 0.9 in colony-forming units), whereas there was no correlation between transduction rate and VCN per cell (Roselli et al., 2010), a finding consistent with our data. Moreover, in another study involving transduction of CD34+ cells derived from three thalassemic donors by a γ-globin lentiviral vector (Wilber et al., 2011) the average VCN per cell was 0.73. Last, in the thalassemia clinical trial organized by the group of P. Leboulch, VCN was calculated by qPCR on pooled in vitro colony-forming cells (CFCs), 27 months posttransplantation, and gave an average readout of 0.14 copy/cell (Cavazzana-Calvo et al., 2010).
Our results show that of 20 patients, 9 patients (45%) displayed a more than 20% increase in HbF production. The therapeutic level of globin gene expression required in the red blood cells derived from genetically modified HSCs has been estimated from experiments performed in murine models. Direct experimental evidence indicating a beneficial effect of γ-globin expression in both β-thalassemia and SCD mouse models has been obtained by mating diseased mice with transgenic mice that expressed various amounts of γ-globin mRNA and protein (Persons et al., 2001). This experiment showed that γ-globin mRNA levels of 7% of the endogenous α-globin level, resulting in a total HbF protein level of 13% of the endogenous level, significantly improved murine β-thalassemia intermedia under circumstances of a high degree of donor cell chimerism, and that mRNA levels of about 13% of the endogenous α-globin level, resulting in a total HbF protein level of 27% of the endogenous level, yielded nearly complete phenotypic correction. Similarly, in a study using an SCD mouse model, γ-globin mRNA expression in the majority of reticulocytes at 19 to 24% of the levels of the endogenous globin genes resulted in corresponding levels of HbF protein of 16 to 25%, significantly improving the disease phenotype and life span of the animals (Blouin et al., 2000). Using a different SCD mouse model, Perumbeti and colleagues (2009) corroborated the aforementioned findings. Together, these observations suggest that a significant therapeutic benefit may occur in patients with SCD if a level of gene transfer efficiency into stem cells of 20% is achieved with a vector capable of expressing γ-globin mRNA in erythroid cells at a level of 15 to 20% of that of the α-globin genes. For transfusion-dependent β-thalassemia, in which an individual expresses low levels of endogenous β-globin, a gene transfer efficiency of 10 to 20% and a γ-globin mRNA level of 20% of the α-globin level is likely to have a therapeutic impact. Higher levels would be required for the more severe form of β0-thalassemia, in which no endogenous β-globin is present (Lebensburger and Persons, 2008).
Our results indicate that the mean increase in HbF from all patients was 32.9% (p=0.001), which accounts for almost one-third of total hemoglobin; specifically, 32.1% for patients with β0-thalassemia (p=0.01) and 33.7% for patients with β+-thalassemia (p=0.03). Similarly, in the paper describing the first clinical trial for thalassemia gene therapy by the Leboulch group (Cavazzana-Calvo et al., 2010) it is documented that exogenous β-globin accounts for one-third of total hemoglobin in a patient who had been transfusion independent for more than 1 year. Also, in a relevant study involving a β-globin vector to treat thalassemia major (Roselli et al., 2010) it is stated that the β/α ratio in control normal cells (ND1 and ND2) is 0.97 and 0.79, respectively, whereas vector transduction results in a β/α ratio of 0.30 in patients with β0-thalassemia and 0.35 in patients with β+-thalassemia. This ratio in vector-transduced cells again accounts for approximately one-third of the normal value. On the basis of the data retrieved from the literature, it seems that expression from GGHI can reach the anticipated therapeutic levels, because our data from human CD34+ hematopoietic stem cells clearly show that the production of HbF from GGHI is increased by more than 20% in transduced cells whereas the average gene transfer efficiency was 40.3% (Table 2). Last, in a study that included evaluation of a γ-globin lentiviral vector in the context of thalassemic patients (Wilber et al., 2011) an average increase in HbF percentage of more than 100% in transduced cell populations from three patients was documented. On the basis of our findings, we also documented an analogous increase in transduced cells of three thalassemic patients.
GGHI conferred phenotypic as well as functional correction in transduced cells in the model of human thalassemia erythropoiesis in vitro, because we observed a significant decrease in the apoptotic rate and a related increase in the percentage of orthochromatic erythroblasts. The gene transfer efficiency from GGHI was lower than that previously published for CD34+ cells (Puthenveetil et al., 2004); however, the relevant study included only four patients, with transduction efficiencies of 75, 100, 90, and 80%, respectively.
Last, another point of interest is the correlation of thalassemic patients with minimal (<10%) or no increase in HbF with their respective thalassemia mutations. Our results show that six patients (approximately 30% of all patients) showed only marginal increases in the percentage of HbF after gene transfer. Of these, four were patients with β0-thalassemia (thal 4, thal 6, thal 13, and thal 28) and two were patients with β+-thalassemia (thal 24 and thal 32). More specifically, the relevant mutations for each patient were as follows: thal 4 (IVS1 n1/IVS1 n1), thal 6 (cd39/cd5), thal 13 (IVS2 n1/IVS1 n1), and thal 28 (cd39/ cd28) for patients with β0-thalassemia and thal 24 (IVS1 n110/cd39) and thal 32 (IVS1 n110/IVS1 n110) for patients with β+-thalassemia. This is an important clinical point to consider for future studies in order to address putative dominant negative effects of these genotypes as previously observed (Roselli et al., 2010).
In conclusion, in the present study our rationale was to combine the −117 HPFH activating mutation and the HPFH-2 enhancer in an SIN γ-globin lentiviral vector for gene therapy of β-thalassemia and to validate its therapeutic potential in transduced thalassemic hematopoietic stem cells. Overall, our results demonstrate that (1) thalassemic CD34+ hematopoietic stem cells can be efficiently transduced with vector GGHI and (2) this transduction leads to elevated HbF levels, resulting in efficient erythropoiesis, as evidenced by the increased percentage of orthochromatic erythroblasts and decreased apoptosis. The therapeutic dynamic of GGHI was established with a mean vector copy number per cell of 1.1 and a mean transduction rate of 40.3%, both of which constitute clinically achievable goals. These data support GGHI as the first LCR-free globin vector that is able to produce γ-globin at therapeutic levels and therefore provide new perspectives to the field of globin gene therapy.
Supplementary Material
Acknowledgments
Special appreciation goes to Luigi Naldini and Giuliana Ferrari for providing plasmids pCCL.sin.hPGKdeltaNGFR.WPRE.cHS4sense, pMDLg/pRRE, pRSV-Rev, and pMD2.VSVG. Also, the authors thank Derek Persons for sharing the V5m3-400 vector and for granting permission to publish the relevant data. Last, the authors thank David W. Emery for providing part of Fig. S1. This research was supported by the European Union Integrated Project Concerted Safety and Efficiency Evaluation of Retroviral Transgenesis for Gene Therapy of Inherited Diseases (CONSERT), grant no. 005242, and by EPAN Project Network for the Development of Technology and Application of Gene Therapy in Hematopoietic Disorders, grant no. YB/90 from the Greek General Secretariat of Research and Technology and the European Commission to N.P.A.
Author Disclosure Statement
The authors have no conflict of interest and have nothing to disclose.
References
- Anagnou N.P. Perez-Stable C. Gelinas R., et al. Sequences located 3′ to the breakpoint of the hereditary persistence of fetal hemoglobin-3 deletion exhibit enhancer activity and can modify the developmental expression of the human fetal A γ-globin gene in transgenic mice. J. Biol. Chem. 1995;270:10256–10263. doi: 10.1074/jbc.270.17.10256. [DOI] [PubMed] [Google Scholar]
- Arumugam P.I. Scholes J. Perelman N., et al. Improved human β-globin expression from self-inactivating lentiviral vectors carrying the chicken hypersensitive site-4 (cHS4) insulator element. Mol. Ther. 2007;15:1863–1871. doi: 10.1038/sj.mt.6300259. [DOI] [PubMed] [Google Scholar]
- Arumugam P.I. Urbinati F. Velu C.S., et al. The 3′ region of the chicken hypersensitive site-4 insulator has properties similar to its core and is required for full insulator activity. PLoS One. 2009;4:e6995. doi: 10.1371/journal.pone.0006995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin M.J. Beauchemin H. Wright A., et al. Genetic correction of sickle cell disease: Insights using transgenic mouse models. Nat. Med. 2000;6:177–182. doi: 10.1038/72279. [DOI] [PubMed] [Google Scholar]
- Bunn F.H. Human hemoglobins: Sickle hemoglobin and other mutants. In: Stamatoyannopoulos G., editor; Majerus P.W., editor; Pelmutter R.M., editor; Varmus H., editor. Molecular Basis of Blood Diseases. 2nd. Saunders; Philadelphia: 2001. pp. 183–226. [Google Scholar]
- Cavazzana-Calvo M. Payen E. Negre O., et al. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467:318–323. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charache S. Clegg J.B. Weatherall D.J. The negro variety of hereditary persistence of fetal haemoglobin is a mild form of thalassaemia. Br. J. Haematol. 1976;34:527–534. doi: 10.1111/j.1365-2141.1976.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chung J.H. Bell A.C. Felsenfeld G. Characterization of the chicken β-globin insulator. Proc. Natl. Acad. Sci. U.S.A. 1997;94:575–580. doi: 10.1073/pnas.94.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F.S. Metherall J.E. Yamakawa M., et al. A point mutation in the A gamma-globin gene promoter in Greek hereditary persistence of fetal haemoglobin. Nature. 1985;313:325–326. doi: 10.1038/313325a0. [DOI] [PubMed] [Google Scholar]
- Desprat R. Bouhassira E.E. Gene specificity of suppression of transgene-mediated insertional transcriptional activation by the chicken HS4 insulator. PLoS One. 2009;4:e5956. doi: 10.1371/journal.pone.0005956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dull T. Zufferey R. Kelly M., et al. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold E.A. Penny L.A. Nienhuis A.W. Forget B.G. An olfactory receptor gene is located in the extended human β-globin gene cluster and is expressed in erythroid cells. Genomics. 1999;61:15–23. doi: 10.1006/geno.1999.5935. [DOI] [PubMed] [Google Scholar]
- Fessas P. Stamatoyannopoulos G. Hereditary persistence of fetal hemoglobin in Greece: A study and a comparison. Blood. 1964;24:223–240. [PubMed] [Google Scholar]
- Fragkos M. Anagnou N.P. Tubb J. Emery D. Use of hereditary persistence of fetal hemoglobin 2 enhancer to increase the expression of oncoretrovirus vectors for human γ-globin. Gene Ther. 2005;12:1591–1600. doi: 10.1038/sj.gt.3302566. [DOI] [PubMed] [Google Scholar]
- Gazouli M. Katsantoni E.Z. Kosteas T. Anagnou N.P. Persistent fetal γ-globin expression in adult transgenic mice following deletion of two silencer elements located 3′ to the human Aγ-globin gene. Mol. Med. 2009;15:415–424. doi: 10.2119/molmed.2009.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S. Von Kalle C. Schmidt M., et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Hanawa H. Hargrove P.W. Kepes S., et al. Extended β-globin locus control region elements promote consistent therapeutic expression of a γ-globin lentiviral vector in murine β-thalassemia. Blood. 2004;104:2281–2290. doi: 10.1182/blood-2004-03-0863. [DOI] [PubMed] [Google Scholar]
- Hanawa H. Yamamoto M. Zhao H., et al. Optimized lentiviral vector design improves titer and transgene expression of vectors containing the chicken β-globin locus HS4 insulator element. Mol. Ther. 2009;17:667–674. doi: 10.1038/mt.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove P.W. Kepes S. Hanawa H., et al. Globin lentiviral vector insertions can perturb the expression of endogenous genes in β-thalassemic hematopoietic cells. Mol. Ther. 2008;16:525–533. doi: 10.1038/sj.mt.6300394. [DOI] [PubMed] [Google Scholar]
- Imren S. Payen E. Westerman K.A., et al. Permanent and panerythroid correction of murine β thalassemia by multiple lentiviral integration in hematopoietic stem cells. Proc. Natl. Acad. Sci. U.S.A. 2002;99:14380–14385. doi: 10.1073/pnas.212507099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsantoni E.Z. Langeveld A. Wai A.W.K., et al. Persistent γ-globin expression in adult transgenic mice is mediated by HPFH-2, HPFH-3, and HPFH-6 breakpoint sequences. Blood. 2003;102:3412–3419. doi: 10.1182/blood-2003-05-1681. [DOI] [PubMed] [Google Scholar]
- Kattamis C. Metaxotou-Mavromati A. Ladis V., et al. The clinical phenotype of β and δβ thalassemias in Greece. Eur. J. Pediatr. 1982;139:135–138. doi: 10.1007/BF00441497. [DOI] [PubMed] [Google Scholar]
- Kosteas T. Palena A. Anagnou N.P. Molecular cloning of the breakpoints of the hereditary persistence of fetal hemoglobin type-6 (HPFH-6) deletion and sequence analysis of the novel juxtaposed region from the 3′ end of the β-globin gene cluster. Hum. Genet. 1997;100:441–445. doi: 10.1007/s004390050530. [DOI] [PubMed] [Google Scholar]
- Lebensburger J. Persons D.A. Progress toward safe and effective gene therapy for β-thalassemia and sickle cell disease. Curr. Opin. Drug Discov. Dev. 2008;11:225–232. [PubMed] [Google Scholar]
- Lisowski L. Sadelain M. Locus control region elements HS1 and HS4 enhance the therapeutic efficacy of globin gene transfer in β-thalassemic mice. Blood. 2007;110:4175–4178. doi: 10.1182/blood-2007-08-108647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias L.A. Fisher T.C. Zeng L., et al. Ineffective erythropoiesis in β-thalassemia major is due to apoptosis at the polychromatophilic normoblast stage. Exp. Hematol. 2000;28:1343–1353. doi: 10.1016/s0301-472x(00)00555-5. [DOI] [PubMed] [Google Scholar]
- Miccio A. Cesari R. Lotti F., et al. In vivo selection of genetically modified erythroblastic progenitors leads to long-term correction of β-thalassemia. Proc. Natl. Acad. Sci. U.S.A. 2008;105:10547–10552. doi: 10.1073/pnas.0711666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio G. Di Pietro R. Di Giacomo V., et al. In vitro mass production of human erythroid cells from the blood of normal donors and of thalassemic patients. Blood Cells Mol. Dis. 2002;28:169–180. doi: 10.1006/bcmd.2002.0502. [DOI] [PubMed] [Google Scholar]
- Orkin S.H. Nathan D. The thalassemias. In: Orkin S.H., editor; Nathan D., editor. Nathan and Oski's Hematology of Infancy and Childhood. 5th. Saunders; Philadelphia: 1998. pp. 811–886. [Google Scholar]
- Papanikolaou E. Anagnou N.P. Major challenges for gene therapy of thalassemia and sickle cell disease. Curr. Gene Ther. 2010;10:404–412. doi: 10.2174/156652310793180724. [DOI] [PubMed] [Google Scholar]
- Persons D.A. Allay E.R. Sabatino D.E., et al. Functional requirements for phenotypic correction of murine β-thalassemia: Implications for human gene therapy. Blood. 2001;97:3275–3282. doi: 10.1182/blood.v97.10.3275. [DOI] [PubMed] [Google Scholar]
- Persons D.A. Hargrove P.W. Allay E.R., et al. The degree of phenotypic correction of murine β-thalassemia intermedia following lentiviral-mediated transfer of a human γ-globin gene is influenced by chromosomal position effects and vector copy number. Blood. 2003;101:2175–2183. doi: 10.1182/blood-2002-07-2211. [DOI] [PubMed] [Google Scholar]
- Perumbeti A. Higashimoto T. Urbinati F., et al. A novel human gamma-globin gene vector for genetic correction of sickle cell anemia in a humanized sickle mouse model: Critical determinants for successful correction. Blood. 2009;114:1174–1185. doi: 10.1182/blood-2009-01-201863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestina T.I. Hargrove P.W. Jay D., et al. Correction of murine sickle cell disease using gamma-globin lentiviral vectors to mediate high-level expression of fetal hemoglobin. Mol. Ther. 2009;17:245–252. doi: 10.1038/mt.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthenveetil G. Scholes J. Carbonell D., et al. Successful correction of the human β-thalassemia major phenotype using a lentiviral vector. Blood. 2004;104:3445–3453. doi: 10.1182/blood-2004-04-1427. [DOI] [PubMed] [Google Scholar]
- Rivella S. May C. Chadburn A., et al. A novel murine model for Cooley anemia and its rescue by lentiviral-mediated human β-globin gene transfer. Blood. 2003;101:2932–2939. doi: 10.1182/blood-2002-10-3305. [DOI] [PubMed] [Google Scholar]
- Roselli E.A. Mezzadra R. Frittoli M.C., et al. Correction of β-thalassemia major by gene transfer in haematopoietic progenitors of pediatric patients. EMBO Mol. Med. 2010;2:1–14. doi: 10.1002/emmm.201000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni de Sio F.R. Naldini L. Short-term culture of human CD34+ cells for lentiviral gene transfer. Methods Mol. Biol. 2009;506:59–70. doi: 10.1007/978-1-59745-409-4_5. [DOI] [PubMed] [Google Scholar]
- Santoni de Sio F.R. Cascio P. Zingale A., et al. Proteasome activity restricts lentiviral gene transfer into hematopoietic stem cells and is down-regulated by cytokines that enhance transduction. Blood. 2006;107:4257–4265. doi: 10.1182/blood-2005-10-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatoyannopoulos G. Fessas P. Papayannopoulou T. F-thalassemia: A study of thirty-one families with simple heterozygotes and combinations of F-thalassemia with A2-thalassemia. Am. J. Med. 1969;47:194–208. doi: 10.1016/0002-9343(69)90146-6. [DOI] [PubMed] [Google Scholar]
- Tate V.E. Wood W.G. Weatherall D.J. The British form of hereditary persistence of fetal hemoglobin results from a single base mutation adjacent to an S1 hypersensitive site 5′ of the Aγ globin gene. Blood. 1986;68:1389–1393. [PubMed] [Google Scholar]
- Tuan D. Feingold E. Newman M., et al. Different 3′ end points of deletions causing delta beta-thalassemia and hereditary persistence of fetal hemoglobin: Implications for the control of gamma-globin gene expression in man. Proc. Natl. Acad. Sci. U.S.A. 1983;80:6937–6941. doi: 10.1073/pnas.80.22.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherall D.J. Pathophysiology of thalassemia. Baillieres Clin. Haematol. 1998;11:127–146. doi: 10.1016/s0950-3536(98)80072-3. [DOI] [PubMed] [Google Scholar]
- Weatherall D.J. The thalassemias. In: Stamatoyannopoulos G., editor; Nienhuis A.W., editor; Majerus P.W., editor; Varmus H., editor. Molecular Basis of Blood Diseases. 3rd. Saunders; Philadelphia: 2001. pp. 185–229. [Google Scholar]
- Wheeler J.T. Krevans J.R. The homozygous state of persistent fetal hemoglobin and the interaction of persistent fetal hemoglobin with thalassemia. Bull. Johns Hopkins Hosp. 1961;109:217–233. [PubMed] [Google Scholar]
- Wilber A. Hargrove P.W. Kim Y.S., et al. Therapeutic levels of fetal hemoglobin in erythroid progeny of β-thalassemic CD34+ cells following lentiviral vector-mediated gene transfer. Blood. 2011;117:2817–2826. doi: 10.1182/blood-2010-08-300723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R. Dull T. Mandel R.J., et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J. Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.