Introduction
Chronic graft versus host disease (cGVHD) is a common cause of morbidity and mortality following allogeneic stem cell transplant (HCT), with approximately 50-60% of long-term HCT survivors developing one or more manifestations of the disorder. While acute GVHD is typically limited to skin, liver and gastrointestinal involvement, virtually every organ is at risk for the development of cGVHD. Though the pathophysiology of cGVHD remains poorly understood, some of the most severe organ manifestations are linked by end organ fibrosis. In particular, fibrotic cutaneous and bronchiolar changes, resulting in scleroderma-like changes and bronchiolitis obliterans syndrome (BOS), respectively, are two of the most devastating outcomes for these patients. Both sclerotic GVHD (ScGVHD) and BOS have been reported in 5 to 15% of patients with cGVHD (1, 2)ENREF 1.
Unfortunately, standard treatment for cGVHD has not changed significantly in the past 30 years, and relies heavily on the use of agents that reduce the activity of alloreactive T-cells. Many of the manifestations of cGVHD however, share clinical characteristics seen in non-transplant conditions, including systemic sclerosis or pulmonary fibrosis. Thus, understanding the pathophysiology underlying these related conditions may help identify potential mechanisms and ultimately new therapeutic options for patients with cGVHD.
Tyrosine kinase inhibitors (TKIs) have been shown to inhibit two different profibrotic pathways (transforming growth factor β [TGF-β] and platelet-derived growth factor [PDGF]) in various mouse models of fibrotic disease, and offer a possible novel treatment approach for cGVHD patients suffering from severe sclerosis (3). Likewise, overexpression of tumor necrosis factor (TNF)-α has been shown to induce fibrogenesis in experimental hepatocellular disease (4), and has been linked with human scleroderma-associated interstitial pulmonary fibrosis (5) and profibrotic responses in human osteoarthritic hip joint fibroblasts (6). The use of TNF antagonists has been examined in some clinical situations associated with fibrosis (7, 8), suggesting they may also be of some benefit to patients with cGVHD; however, this must first be prospectively tested.
Lung Complications in Long-Term HCT Survivors
HCT patients are particularly vulnerable to both non-GVHD-related and GVHD-related pulmonary complications post-HCT, with rates of 40-60% in adults (2) and as high as 70% in pediatric recipients (9). These complications can account for as much as 50% of transplant-related mortality. cGVHD of the lung, manifesting as BOS, is a clinical syndrome defined as a new fixed airflow obstruction of the small airways that typically occurs late after allogeneic HCT. Rates of BOS in patients that develop cGVHD range from 7 to 16%, with a median time to diagnosis > 400 days post-HCT (10, 11). Therefore, HCT physicians must remain vigilant and investigate all signs and symptoms of lung dysfunction beyond the first year post-HCT, particularly in patients with already established cGVHD.
The gold standard for diagnosis of bronchiolitis obliterans relies on lung biopsy, which typically reveals obliteration of the lumen of the terminal bronchioles with relative sparing of the distal alveolar structures. The bronchiolar lumen is characterized as being filled with fibrinous tissue that may contain an inflammatory cellular infiltrate (12). Cellular infiltrates are more commonly seen early in the course of the disease and usually contain neutrophils and mononuclear cells which ultimately leads to progressive circumferential fibrosis of the terminal airways. Clinically, this is often accompanied by fixed airway obstruction noted on pulmonary function testing (PFT), leading to the diagnosis of clinical BOS. Despite the need to identify histologic abnormalities, it is uncommon in clinical practice to pursue surgical lung biopsies due to an unacceptably high complication rate in these patients. Recognizing this limitation, the NIH published guidelines for the clinical diagnosis of BOS without histologic confirmation in 2005 (13); further refinement of these criteria were recently proposed by Williams et al. (14) (See Table 1). These refinements in the diagnosis allow for correction of poor PFTs prior to transplant, and for identification of patients with airway obstruction that might otherwise be missed secondary to concomitant restrictive defects from ScGVHD or myositis.
Table 1.
Proposed Modifications to NIH BOS Clinical Definition (adapted from (14))
| Absence of infection (No Change) |
| Another cGVHD manifestation in another organ (No Change) |
| FEV1 < 75% predicted (No Change) or > 10% decline from pre-HCT value (Modification) |
| Signs of Obstruction |
|
NIH: National Institutes of Health; BOS: bronchiolitis obliterans syndrome; cGVHD: chronic graft-versus-host disease; FEV1: forced expiratory volume in 1 second; ; SVC: slow vital capacity; RV: residual volume; TLC, total lung capacity; HRCT: high-resolution computed tomography.
The pathophysiology of BOS remains elusive. BOS is not observed in recipients of autologous transplant (11) and is rarely the sole manifestation of cGVHD in allogeneic HCT recipients. Therefore, BOS is presumed to be the result of donor T-cell recognition of alloantigens present in the recipient airways. This presumption is further supported by clinical and pathologic findings of chronic rejection in lung transplant recipients, in whom the clinical manifestations and pathologic findings are essentially interchangeable with cGVHD patients. Of course, in lung transplant recipients the allorecognition occurs in the opposite direction, with the host recognizing the graft as non-self. Therefore, collaborations between physicians and scientists familiar with the development of BOS, regardless of the antecedent clinical condition, may prove critical in advancing treatment, helping elucidate the pathophysiology of this post-HCT complication, and offering insight into future targeted therapies.
Infectious complications are common in HCT recipients, and the frequency increases in the setting of cGVHD and subsequent increases in immunosuppression. Hence one of the diagnostic criteria for confirming BOS is the absence of infection, primarily because untreated infections can also lead to obstructive airflow defects detected on PFT. In the absence of overt signs of infection, many physicians may be hesitant to request bronchoscopy with bronchoalveolar lavage (BAL) due to concerns over procedural complications. At the University of Michigan, we reviewed outcomes following BAL performed from 2001 to 2008 for pulmonary dysfunction following allogeneic transplant (Greg Yanik, personal communication). During the study period, 897 allogeneic transplants were performed, and in 327 cases (36%) a BAL was required for further evaluation of pulmonary dysfunction, which was defined as 1) clinical symptoms (cough, hypoxia, or dyspnea) plus 2) radiographic abnormalities (chest radiograph or CT). We found that BAL identified a pathogenic organism only 22% of the time in patients < 100 days (“early”) post-HCT, whereas pathogenic organisms were identified in 41% of patients > 100 days (“late”) post-HCT. The most common organisms identified in BAL procedures performed late post-HCT were Aspergillus species (n=39, median time to diagnosis 362 days) and Pseudomonas species (n=8, median time to diagnosis 700 days). The overall rate of intra- or post-procedure complications such as hypoxia leading to intubation, hemorrhage or hypotension was low, at only 3% (unpublished observations). These results support the need to bronchoscopically assess new pulmonary symptoms, radiographic changes, and/or PFT decline, since establishing an infectious process has significant impact on subsequent therapy recommendations.
Effective therapy for established BOS remains limited, with augmented immunosuppression being the primary mode of treatment, and results are suboptimal. The dismal overall survival for BOS patients is approximately 15% (11, 15). Though augmentation of immunosuppression has been shown to help slow the progression of BOS in lung transplant recipients, the results in HCT recipients have been less encouraging, with response rates to high-dose systemic corticosteroids (prednisone 1 mg/kg/day or equivalent) resulting in improvement in only 20 % of BOS patients (16). The poor response to standard cGVHD therapy further highlights the need for rigorous clinical trials to identify potential beneficial therapeutic inventions for at-risk patients. The hope is that improved screening and early intervention for BOS in HCT patients may prevent further decline prior to the patient meeting the full criteria for BOS. As an example, inhaled corticosteroids (and additional immune modifiers such as montelukast and azithromycin) are currently being studied for efficacy in slowing the progression of the disease (17).
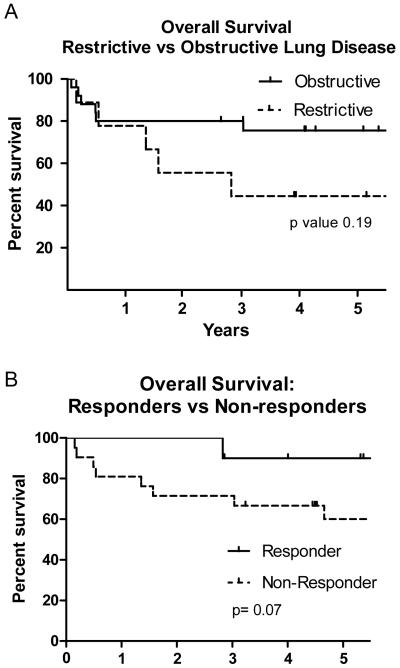
At the University of Michigan, we observed an approximate 25% incidence of both restrictive and obstructive lung defects on pulmonary function testing post-HCT (Yanik, GA et al. Biol Blood Marrow Transplant, in press). The emergence of restrictive lung defects occurred within 3-6 months post-HCT and was likely related to regimen-associated toxicity. Obstructive defects continued to slowly increase post-HCT with rates of 14% and 23% at one and two years post-HCT, respectively, and were more likely to be associated with cGVHD. Based on the high rate of sub-acute lung injury post-HCT, our group at University of Michigan developed a clinical trial investigating the use of etanercept for TNF-α inhibition for this patient population (ClinicalTrials.gov NCT00141726). Eligible patients needed to demonstrate >15% decline in PFTs from pre-HCT values (defined as FVC < 80% predicted for a restrictive defect or an FEV1/FVC < 75% predicted for an obstructive defect). Additionally, all patients underwent BAL prior to receiving study treatment. Fifty-seven patients were enrolled, but 23 were excluded for infectious (predominantly fungal) findings on BAL. The remainder of the patients (n=34) received etanercept and serial PFTs to evaluate for response, which was defined as a 10% improvement in the target PFT parameter. The median FEV1 at study entry was 51% of predicted; the median FVC was 62% of predicted. The overall response rate was approximately 33% in both the restrictive and obstructive patient populations, and the 5-year overall survival trended toward being higher in the patients with obstructive, compared to restrictive, defects (67% vs. 44%, p=0.19), and in those patients who responded to therapy compared to those that did not (90% vs. 55%, p=0.07) (Figure 1). An additional important observation was that most of the responders had been diagnosed with their sub-acute lung injury less than 2 years prior to study enrollment, highlighting the importance of early identification of BOS and the need for rapid intervention (Yanik, GA et al. Biol Blood Marrow Transplant, in press).
Figure 1.
Effect of etanercept on survival in post-HCT patients with subacute lung injury. A) Overall 5-year survival by pulmonary function testing defect. Patients with an obstructive defect (solid line) had a 5-year survival of 67% compared to 44% in those with a restrictive lung defect (dashed line) (p=0.19). B) Overall 5-year survival by response to therapy. Patients who responded to etanercept therapy (solid line) had a 5-year survival of 90% compared to 55% in patients who failed to respond (dashed line) (p=0.07). (Figures re-printed with permission, Biol Blood and Marrow Trans).
Lessons Learned from Laboratory Models of Lung Fibrosis
Fibrosis is the end result of numerous inflammatory and infectious insults to the lung. While a great deal of information regarding the contributions of soluble mediators and cellular components to lung fibrogenesis has been elucidated, other contributors are less well-studied. One such area under intense current investigation is the lung extracellular matrix (ECM). Long regarded as an inert scaffolding upon which cells sit, the ECM is now recognized as a dynamic tissue that imparts contextual, mechanical, and spatial clues to cells, thereby driving phenotypic behaviors (18). In fibrotic disorders, deposition of ECM is considered an endpoint; however, recent evidence suggests that ECM also plays an active role in lung fibrogenesis. Given the critically important role of ECM in development (as evidenced by the observations that transgenic animals lacking many key ECM genes are embryonically lethal) and tissue homeostasis (as evidenced by the development of disease in patients with known ECM gene mutations, for example Ehlers-Danlos syndrome in patients with mutations in various collagen or collagen-modulating genes), it follows that delineation of the mechanisms by which ECM influences cell behavior and function will be critical to understanding fibrotic disease pathogenesis. Reductionist studies using cells grown on planar ECM-coated plastic dishes have been informative in elucidating the mechanistic role of ECM in cell biology. Yet this approach does not account for the compositional or spatial complexity of a native microenvironment in which cells simultaneously encounter multiple different proteins in a three-dimensional (3D) spatial orientation.
Lung fibrogenesis is often studied in rodent models utilizing intratracheal or subcutaneous exposure to agents known to result in lung damage (such as bleomycin) that induce vigorous inflammation followed by a fibrotic response (19). However, these models do not recapitulate all aspects of human disease with a primary difference being the self-limited nature of experimental lung fibrosis. Indeed, the literature abounds with reports of various therapeutic agents that appear effective against fibrosis in rodents but that are not successful when assessed in humans with clinical disease (20). Because the bleomycin model of lung fibrosis does not recapitulate all aspects of human lung fibrosis, our research group at the University of Michigan now utilizes primary human tissues to begin to address some of the important mechanistic questions about the role of ECM in the development of lung fibrosis. The lung tissue model utilizes a series of detergent and nuclease washes, resulting in a structure devoid of all cellular and nuclear material (21, 22), consisting of only the ECM “skeleton” within which cells may be cultured and assayed.
This in vitro lung tissue model has the clear advantage of being clinically relevant, since the tissues are derived from humans with the disease under study, although a significant disadvantage is the current inability to study effects that occur in a breathing, perfusing lung. It is, however, more likely to closely resemble the biologic conditions encountered by cells in vivo rather than cultured in vitro on tissue culture plastic. As evidence of clinical relevance, some investigators have begun to utilize these decellularized matrices as a structure into which new cells may be seeded, resulting in the regeneration of functional organ tissue (23, 24). Indeed, this approach has already been adopted in humans as well, with the development of bio-engineered airways derived from cadaveric donors but re-seeded with recipient cellular material (22). In our lab, decellularized human normal and fibrotic lungs are cored using a standard punch biopsy, fixed in agarose, and then cut with a vibratome to generate uniform-thickness slices that are utilized to support in vitro cell culture.
Using this approach, our research group at University of Michigan has found that human lung ECM retains a normal 3D spatial orientation and composition following decellularization, with normal and fibrotic human lungs being easily discernible on the microscopic and ultramicroscopic levels. Additionally, fibrotic lung matrices are measurably stiffer than normal lung matrices using atomic force microscopy, suggesting that the decellularization process does not result in significant alteration of the structural makeup of the tissue. Finally, preliminary data show that fibrotic lung matrix substantially alters normal cellular phenotype compared to normal lung matrix; for example, fibrotic lung matrix was observed to impart a myofibroblastic, pro-fibrotic phenotype to normal lung fibroblasts, whereas the same cells cultured on normal lung matrix retained quiescent fibroblast features. In total, these data suggest that decellularized normal and fibrotic lung matrices can feasibly be used as a culture substrate to investigate the role of ECM in driving cellular functional and secretory phenotypes, and suggest that this approach may be extrapolated to study other fibrotic disease states in the lung, such as cGVHD.
Challenges of Clinical Trial Design and Implementation in ScGVHD
Extensive, sclerotic skin changes with superficial or deep subcutaneous or fascial involvement are seen in approximately 4-13% of patients with cGVHD and can be a life-threatening manifestation (25). ScGVHD of the skin includes several cutaneous presentations characterized by inflammation and progressive fibrosis of the dermis and subcutaneous tissues. These changes can resemble morphea, systemic sclerosis, or eosinophilic fasciitis and may or may not occur in the setting of concurrent overlying epidermal GVHD. When severe, ScGVHD can result in contractures, severe wasting, and chest wall restriction. Additionally, skin ulceration and poor wound healing associated with skin fibrosis can cause significant morbidity and increases the risk of infection. The mean onset of sclerotic skin changes following transplant is late (529 days in one study (1)), the natural history of this process is unknown, and there are no large, published series of patients that describe the clinical, histological, and evolutionary aspects of ScGVHD.
ScGVHD responds poorly to topical interventions and is often resistant to systemic therapy (25). Many therapies have been employed, including calcineurin inhibitors, steroids, antimetabolites, biologic agents, hydroxychloroquine, and extracorporeal photopheresis. Responses are typically mixed and slow to occur if seen. The risk factors, clinical markers, and mechanisms of ScGVHD development remain largely unknown despite the major impact on long-term morbidity. CD3+ T-cell dose in the graft, eosinophilia, positive ANA, and antecedent non-sclerotic cGVHD skin involvement have been proposed as markers of ScGVHD (25), however, most studies do not distinguish between sclerotic and non-sclerotic cutaneous manifestations of cGVHD (25, 26) making conclusions specific to ScGVHD difficult to generalize.
Challenges in Clinical Trial Design for ScGVHD
Development of clinical trials for patients with cGVHD is difficult due to the complexity and heterogeneity of disease, variable approaches to treatment, and the lack of standardized assessments of disease (27). In particular, the study of ScGVHD lacks universally accepted measures of disease burden and response. Investigators have employed several measures to assess ScGVHD involvement including body surface area (BSA), magnetic resonance imaging (MRI), ultrasound, and range-of-motion (ROM) measurements. Additionally, investigators have tried to apply the Rodnan score, the standard measure for skin involvement in scleroderma. Thus far, none of these measures has proven to be completely reliable in the setting of ScGVHD and it is likely that multiple measures will need to be integrated into the assessment of ScGVHD.
TKIs for ScGVHD
Imatinib mesylate (Gleevec® in the US; Glivec® in Europe, Australia, and Latin America, marketed by Novartis) is a TKI that has biological activity against both PDGF and TGF-β signaling pathways. Both cytokines have been implicated in the pathogenesis of several fibrosing diseases, including hepatic, renal, and lung, as well as in scleroderma, a disease which closely resembles ScGVHD (28-31). In addition, stimulatory antibodies specific for the PDGF receptor (PDGFR) were identified in a series of 39 patients with extensive cGVHD with higher levels detected in those patients with skin involvement (28). Similar stimulatory antibodies targeting PDGFR have been reported in patients with scleroderma, suggesting an important therapeutic target for these fibrosing conditions (29). Imatinib mesylate has particularly potent activity against PDGF and is FDA-approved in the United States for the treatment of several disorders associated with aberrant PDGFR signaling (32-34). The side effect profile of the drug is well established in non-HCT patients, which is helpful in the setting of a therapy for allo-HSCT patients, many of whom have multi-organ system symptoms and possible dysfunction, and who will require ongoing immunosuppressive therapy (33, 34).
Imatinib mesylate has demonstrated pre-clinical and clinical activity in several non-malignant fibrosing conditions such as rheumatoid arthritis, renal fibrosis, and dermal sclerosis (30, 35), although a recent trial of imatinib in patients with pulmonary fibrosis showed no efficacy in the primary endpoint of time to progression of pulmonary dysfunction(20). However, there is a report of improved pulmonary function in an allogeneic HCT recipient with BOS treated with imatinib mesylate for recurrent CML (36) as well as a case series reporting lower GVHD incidence in post-HCT patients receiving imatinib mesylate (37). In 2009, two separate pilot studies of imatinib mesylate reported promising results in ScGVHD (38, 39). Several larger studies are currently ongoing and nearing completion in the US. At the NIH, we enrolled 24 patients into a trial of imatinib mesylate for severe ScGVHD. Though final analysis is ongoing, in general imatinib was poorly tolerated in this patient population and all patients required dose reduction in order to remain on-study. No patients had complete resolution of sclerosis, but many patients improved their ROM; imatinib proved to be steroid-sparing for many patients as well.
Due to early enthusiasm of TKI therapy, industry-funded studies of another TKI, nilotinib, are currently underway at various institutions (e.g. ClinicalTrials.gov NCT01155817). If these results look promising, there is the potential for expansion within a larger network such as the Chronic GVHD Consortium or the BMT Clinical Trials Network (CTN).
Other Anti-Fibrotic Agents
Future therapeutic interventions for cGVHD may be discovered among experimental agents currently being evaluated and showing preliminary success in experimental models of hepatic and renal fibrosis. As mentioned above, TGF-β plays a central role in fibrogenesis and investigators are evaluating the use of agents that block or inhibit TGF-β signaling, such as the small molecule decorin, halofuginone, and pirfenidone (40-42). Pirfenidone is a novel anti-fibrotic, anti-inflammatory, antioxidant drug which inhibits fibroblast growth and collagen synthesis. The mechanism of action of pirfenidone is thought to occur primarily through its inhibition of pro-inflammatory cascades induced by members of the TNF-α cytokine superfamily. Pirfenidone has been successfully used in patients with various autoimmune disorders and fibrotic states; however, despite encouraging initial results in patients with idiopathic pulmonary fibrosis (42), larger prospective trials have been unable to replicate the results (43). That fact that pirfenidone is well-tolerated and lacks significant immunosuppressive side effects could make it an especially attractive agent for evaluation in patients with cGVHD.
Currently, the likelihood of reversing deep-seated sclerotic or fibrotic manifestations of cGVHD is quite low and prevention and early detection is key. Better prospective studies are needed to help identify the risk factors for the development of sclerosis and hopefully identify the causes in order to prevent this difficult complication.
Conclusions
The pathophysiology of the various manifestations of cGVHD remains elusive, but improvements in the diagnostic and assessment tools as proposed by the NIH Consensus Criteria in 2005 have helped facilitate clinical trials in assessing the natural history of the disease, as well as determine meaningful response to therapy. Through the efforts of the Chronic GVHD Consortium, led by Stephanie Lee at the Fred Hutchinson Cancer Research Center, there is a multicenter, ongoing prospective evaluation of the NIH diagnostic and assessment tools. This effort has already resulted in several publications (44-46) which have further refined essential criteria for cGVHD evaluation, including organ-specific manifestations such as BOS and ScGVHD. Currently, the Consortium is conducting a multicenter prospective clinical trial of fluticasone propionate, azithromycin, and montelukast for the treatment of BOS (ClinicalTrials.gov NCT01307462); a separate trial of imatinib vs. rituximab for treatment of ScGVHD is also enrolling subjects (ClinicalTrials.gov NCT01309997).
Although cGVHD remains a significant problem for many long-term survivors of HCT, critical advances in cGVHD research and treatment can be achieved by cooperative group efforts such as those put forth by the Chronic GVHD Consortium and the Clinical Trials Network.
ACKNOWLEDGMENTS
Financial disclosure: The authors have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Penas PF, Jones-Caballero M, Aragues M, Fernandez-Herrera J, Fraga J, Garcia-Diez A. Sclerodermatous graft-vs-host disease: clinical and pathological study of 17 patients. Arch Dermatol. 2002;138:924–934. doi: 10.1001/archderm.138.7.924. [DOI] [PubMed] [Google Scholar]
- 2.Chien JW, Duncan S, Williams KM, Pavletic SZ. Bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation-an increasingly recognized manifestation of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16:S106–114. doi: 10.1016/j.bbmt.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Distler JH, Distler O. Tyrosine kinase inhibitors for the treatment of fibrotic diseases such as systemic sclerosis: towards molecular targeted therapies. Ann Rheum Dis. 2010;69(Suppl 1):i48–51. doi: 10.1136/ard.2009.120196. [DOI] [PubMed] [Google Scholar]
- 4.Tarrats N, Moles A, Morales A, Garcia-Ruiz C, Fernandez-Checa JC, Mari M. Critical role of tumor necrosis factor receptor 1, but not 2, in hepatic stellate cell proliferation, extracellular matrix remodeling, and liver fibrogenesis. Hepatology. 2011;54:319–327. doi: 10.1002/hep.24388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathai SK, Gulati M, Peng X, et al. Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab Invest. 2010;90:812–823. doi: 10.1038/labinvest.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattyasovszky SG, Hofmann A, Brochhausen C, et al. The effect of the pro-inflammatory cytokine tumor necrosis factor-alpha on human joint capsule myofibroblasts. Arthritis Res Ther. 2010;12:R4. doi: 10.1186/ar2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antoniou KM, Mamoulaki M, Malagari K, et al. Infliximab therapy in pulmonary fibrosis associated with collagen vascular disease. Clin Exp Rheumatol. 2007;25:23–28. [PubMed] [Google Scholar]
- 8.Vassallo R, Matteson E, Thomas CF., Jr. Clinical response of rheumatoid arthritis-associated pulmonary fibrosis to tumor necrosis factor-alpha inhibition. Chest. 2002;122:1093–1096. doi: 10.1378/chest.122.3.1093. [DOI] [PubMed] [Google Scholar]
- 9.Kaya Z, Weiner DJ, Yilmaz D, Rowan J, Goyal RK. Lung function, pulmonary complications, and mortality after allogeneic blood and marrow transplantation in children. Biol Blood Marrow Transplant. 2009;15:817–826. doi: 10.1016/j.bbmt.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17:1072–1078. doi: 10.1016/j.bbmt.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudek A, Mahaseth H, DeFor T, Weisdorf D. Bronchiolitis obliterans in chronic graft-versus-host disease: analysis of risk factors and treatment outcomes. Biology of Blood and Marrow Transplantation. 2003;9:657–666. doi: 10.1016/s1083-8791(03)00242-8. [DOI] [PubMed] [Google Scholar]
- 12.Shulman HM, Kleiner D, Lee SJ, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant. 2006;12:31–47. doi: 10.1016/j.bbmt.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 13.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Williams KM, Chien JW, Gladwin MT, Pavletic SZ. Bronchiolitis obliterans after allogeneic hematopoietic stem cell transplantation. JAMA. 2009;302:306–314. doi: 10.1001/jama.2009.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chien JW, Martin PJ, Gooley TA, et al. Airflow obstruction after myeloablative allogeneic hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2003;168:208–214. doi: 10.1164/rccm.200212-1468OC. [DOI] [PubMed] [Google Scholar]
- 16.Hildebrandt GC, Fazekas T, Lawitschka A, et al. Diagnosis and treatment of pulmonary chronic GVHD: report from the consensus conference on clinical practice in chronic GVHD. Bone Marrow Transplant. 2011 doi: 10.1038/bmt.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norman BC, Jacobsohn DA, Williams KM, et al. Fluticasone, azithromycin and montelukast therapy in reducing corticosteroid exposure in bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: a case series of eight patients. Bone Marrow Transplant. 2010 doi: 10.1038/bmt.2010.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White ES, Baralle FE, Muro AF. New insights into form and function of fibronectin splice variants. J Pathol. 2008;216:1–14. doi: 10.1002/path.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L152–160. doi: 10.1152/ajplung.00313.2007. [DOI] [PubMed] [Google Scholar]
- 20.Daniels CE, Lasky JA, Limper AH, Mieras K, Gabor E, Schroeder DR. Imatinib treatment for idiopathic pulmonary fibrosis: Randomized placebo-controlled trial results. Am J Respir Crit Care Med. 2010;181:604–610. doi: 10.1164/rccm.200906-0964OC. [DOI] [PubMed] [Google Scholar]
- 21.Price AP, England KA, Matson AM, Blazar BR, Panoskaltsis-Mortari A. Development of a Decellularized Lung Bioreactor System for Bioengineering the Lung: The Matrix Reloaded. Tissue Eng Part A. 2010 doi: 10.1089/ten.tea.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 23.Petersen TH, Calle EA, Zhao L, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ott HC, Clippinger B, Conrad C, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 25.Skert C, Patriarca F, Sperotto A, et al. Sclerodermatous chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: incidence, predictors and outcome. Haematologica. 2006;91:258–261. [PubMed] [Google Scholar]
- 26.Barausse G, Caramaschi P, Scambi C, et al. Clinical, serologic and instrumental data of ten patients affected by sclerodermatous chronic graft versus host disease: similarities and differences in respect to systemic sclerosis. Int J Immunopathol Pharmacol. 2010;23:373–377. doi: 10.1177/039463201002300139. [DOI] [PubMed] [Google Scholar]
- 27.Martin PJ, Weisdorf D, Przepiorka D, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: VI. Design of Clinical Trials Working Group report. Biol Blood Marrow Transplant. 2006;12:491–505. doi: 10.1016/j.bbmt.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Svegliati S, Olivieri A, Campelli N, et al. Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft-versus-host disease. Blood. 2007;110:237–241. doi: 10.1182/blood-2007-01-071043. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto M, Okano A, Akamatsu S, et al. Rituximab is effective for steroid-refractory sclerodermatous chronic graft-versus-host disease. Leukemia. 2006;20:172–173. doi: 10.1038/sj.leu.2403996. [DOI] [PubMed] [Google Scholar]
- 30.Distler JH, Jungel A, Huber LC, et al. Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis Rheum. 2007;56:311–322. doi: 10.1002/art.22314. [DOI] [PubMed] [Google Scholar]
- 31.Abdollahi A, Li M, Ping G, et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med. 2005;201:925–935. doi: 10.1084/jem.20041393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen MH, Dagher R, Griebel DJ, et al. U.S. Food and Drug Administration drug approval summaries: imatinib mesylate, mesna tablets, and zoledronic acid. Oncologist. 2002;7:393–400. doi: 10.1634/theoncologist.7-5-393. [DOI] [PubMed] [Google Scholar]
- 33.Cohen MH, Johnson JR, Pazdur R. U.S. Food and Drug Administration Drug Approval Summary: conversion of imatinib mesylate (STI571; Gleevec) tablets from accelerated approval to full approval. Clin Cancer Res. 2005;11:12–19. [PubMed] [Google Scholar]
- 34.Cools J, DeAngelo DJ, Gotlib J, et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med. 2003;348:1201–1214. doi: 10.1056/NEJMoa025217. [DOI] [PubMed] [Google Scholar]
- 35.Daniels CE, Wilkes MC, Edens M, et al. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114:1308–1316. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Majhail NS, Schiffer CA, Weisdorf DJ. Improvement of pulmonary function with imatinib mesylate in bronchiolitis obliterans following allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12:789–791. doi: 10.1016/j.bbmt.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 37.DeAngelo DJ, Hochberg EP, Alyea EP, et al. Extended follow-up of patients treated with imatinib mesylate (gleevec) for chronic myelogenous leukemia relapse after allogeneic transplantation: durable cytogenetic remission and conversion to complete donor chimerism without graft-versus-host disease. Clin Cancer Res. 2004;10:5065–5071. doi: 10.1158/1078-0432.CCR-03-0580. [DOI] [PubMed] [Google Scholar]
- 38.Magro L, Mohty M, Catteau B, et al. Imatinib mesylate as salvage therapy for refractory sclerotic chronic graft-versus-host disease. Blood. 2009;114:719–722. doi: 10.1182/blood-2009-02-204750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olivieri A, Locatelli F, Zecca M, et al. Imatinib for refractory chronic graft-versus-host disease with fibrotic features. Blood. 2009;114:709–718. doi: 10.1182/blood-2009-02-204156. [DOI] [PubMed] [Google Scholar]
- 40.Rockey DC. Antifibrotic therapy in chronic liver disease. Clin Gastroenterol Hepatol. 2005;3:95–107. doi: 10.1016/s1542-3565(04)00445-8. [DOI] [PubMed] [Google Scholar]
- 41.Zion O, Genin O, Kawada N, et al. Inhibition of transforming growth factor beta signaling by halofuginone as a modality for pancreas fibrosis prevention. Pancreas. 2009;38:427–435. doi: 10.1097/MPA.0b013e3181967670. [DOI] [PubMed] [Google Scholar]
- 42.Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2005;171:1040–1047. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 43.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 44.Rationale and Design of the Chronic GVHD Cohort Study: Improving Outcomes Assessment in Chronic GVHD. Biol Blood Marrow Transplant. 2011;17:1114–1120. doi: 10.1016/j.bbmt.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pidala J, Kim J, Anasetti C, et al. NIH Consensus chronic graft vs. host disease global severity is associated with overall survival and non-relapse mortality. Haematologica. 2011 doi: 10.3324/haematol.2011.049841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pidala J, Kurland B, Chai X, et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood. 2011;117:4651–4657. doi: 10.1182/blood-2010-11-319509. [DOI] [PMC free article] [PubMed] [Google Scholar]