Abstract
After decades of mouse and human research, we now know that NK cells have unique properties including memory. Although initially described as MHC unrestricted killers, NK cells have several families of receptors that directly recognize MHC including Ly49 receptors in the mouse and killer immunoglobulin-like receptors (KIR) in humans. The strength of this signal is determined by polymorphisms in NK cell inhibitory receptor genes and their MHC ligands inherited on different chromosomes. Inhibitory receptors protect “self” expressing normal tissue from being killed by NK cells and protecting against autoimmunity. Therefore, for NK cells to kill and produce cytokines they must encounter activating receptor ligands in the context of “missing self” that occurs with some viral infections and malignant transformation. The second property of inhibitory receptors is to educate or license NK cells to acquire function. This is best demonstrated in the mouse and in humans by enhanced function on self inhibitory receptor expressing NK cells when in a host expressing cognate ligate. In contrast, NK cells without inhibitory receptors or with non-self inhibitory receptors are relatively hyporesponsive. The basic biology of NK cells in response to cytokines, education, and viruses will translate into strategies to manipulate NK cells for therapeutic purposes.
Mouse NK cell studies establish biologic paradigms for human translation
There has been much consternation regarding the relevance of the mouse for modeling human immune function and disease states. This is particularly highlighted with the study of natural killer (NK) cells. At a superficial level, there appears to be vast differences between the two. As with any species as divergent as mice and humans there will be significant differences that always need to be taken into consideration before extrapolating results. However, there are enough similarities that mouse studies of NK cells have directly informed the clinical use of NK cells. Thus, a more balanced picture regarding the value of the mouse studies emerges as both an invaluable preclinical model and to delineate the highly complex nature of NK cell differentiation, function and regulation.
NK cells have been an enigma since their activity was first described in 1964 by Cudkowicz observing that lethally irradiated mice could spontaneously reject bone marrow allografts(1). Later, these seminal studies were extended and the phenomena of hybrid resistance (the ability of F1 hybrid mice to reject parental bone marrow allografts) was reported by Bennett et al., which was in direct conflict with the “Laws of Transplantation” in which co-dominant expression of transplantation antigens (and therefore tolerance) occurs(2). The observation of spontaneous MHC-unrestricted killing of tumor targets was reported later and the study of NK cells as a discrete immune cell-type was launched(3). While mouse transplantation studies at times seemed peculiar and restricted to mouse rather than what was observed in clinical settings, it was exactly these studies which paved the way for what we now know about NK cell biology and led to increasing interest in their use in clinical transplantation. Indeed, mouse NK cell studies demonstrated that these cells could inhibit graft-versus-host disease (GVHD) and promote graft-versus-tumor effects(4). These studies led to the seminal report by Ruggeri et al. who demonstrated the value of bypassing the inhibitory receptors in allogeneic cell transplantation (HCT) using both mouse and human data(5). This report demonstrated the usefulness of NK cells to improve outcome after HCT. In addition, a recent report that NKp30+ NK cells are comprised of different functional subsets that contribute to outcome in gastrointestinal stromal cell tumors (GIST)(6) suggests that the more we understand the complex biology of NK cells, the more they can be of use clinically. It is of interest to note that, despite the observation that NK cells can detect and reject allogeneic hematopoietic stem cell grafts was made over 40 years ago, there is still tremendous uncertainty regarding the mechanism(s) underlying this process.
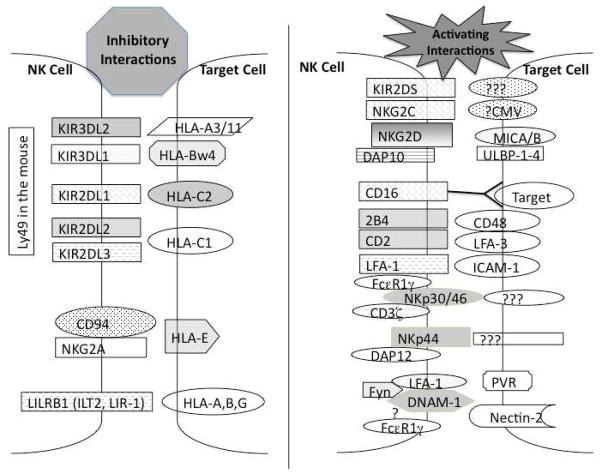
NK cell biology has evolved from what was largely an in vitro activity (MHC-unrestricted killing of tumor targets) or in vivo bone marrow allograft rejection by a homogenous population of distinct “large granular lymphocytes” to a cluster of complex and distinct subpopulations bearing various inhibitory and activating receptors (Figure 1). Mouse studies have led the way regarding the characterization of various inhibitory receptors (Ly49, NKG2A, 2B4). Indeed, the postulation of such inhibitory receptors was initially described by Karre et al. as a means to explain hybrid resistance(7). The characterization of inhibitory receptors (Ly49 in mouse and KIR in humans) has allowed for a mechanism regarding recognition and control of NK cells. These inhibitory receptors, which are capable of binding MHC and MHC-like molecules, are potent due to ITIM motifs and represent at least a partial mechanism to avoid spontaneous “self” attack(8). This has now evolved with the concept of NK cell “licensing” or “arming” in which NK cells bearing inhibitory receptors for “self” MHC are allowed to differentiate and develop into more efficient effector cells(9). The mechanism and functions of licensing is not well characterized and data suggests that NK cells can be reeducated when transferred into new hosts(10). There has been tremendous interest in attempting to ascribe physiologic NK cell functions with licensing. This has been controversial with some reports suggesting that it is the unlicensed NK phenotype subset that exerts greater protection under physiologic conditions such as viral infection(11). With regard to the physiological roles of NK cells (other than tumor cell killing), there have been ample evidence with regard to their role in viral infections (both CMV in humans and MCMV in mice) that suggest they play critical roles by the virus pirating MHC-like domains to evade NK attack and by the existence of activating Ly49 molecules in mice that directly bind to viral proteins(12). At issue with discerning the role of activating versus inhibitory receptors in humans is the extremely close surface homology and lack of reagents able to discern them. In mice, however, with the exception of 2B4, the activating and inhibitory receptors can be easily discerned with antibodies. It is these activating receptors that have been extensively studied with evidence of positive selection occurring during development. Most intriguingly, Sun et al. has reported evidence for NK “memory” after expansion of these subsets during viral infection(13). Thus, the line between the innate and adaptive arms of the immune system has been increasingly blurred with regard to NK cells. These receptors also play a critical role with regard to NK cell expansion. IL15 has emerged as the pivotal cytokine required for NK cell development and maintenance. Whereas mice deficient in IL2 (previously the cytokine of choice to expand and activate NK cells) have normal NK cells, IL15 deficient mice totally lack NK cells(14). The need for IL15 to be “trans-presented” by dendritic cells complicates therapeutic application of this cytokine but also highlights the need for cellular interactions with NK cells and other cell-types for optimal development and function. Interestingly, it was recently reported that common gamma chain null mice, which totally lack signaling via this cytokine family (IL2, IL7, IL15) and are devoid in NK cells, can have NK cells develop via interaction with viral activating receptors (Ly49H in the mouse) and cytokines such as IL12(15) indicating that during pathogeneic states a much broader picture of the needs for NK cell development exists .
Figure 1.
NK cells express a number of inhibitory and activating receptors that determine function. Some of these interactions are definitively established with known signaling pathways, while others are less clear. Most receptors interact with cellular targets but CD16 delivers a potent signal by binding the Fc portion of immunoglobulin-coated targets. The ability of NK cells to kill a target is determined by the net balance of these inhibitory, activating, antibody-dependent and adhesion interactions.
Mouse versus Human NK cells - similar yet different
Much has been made recently of the differences between mouse and human NK cells. Clearly there are significant differences which can be attributed to species divergence and also how inbred mice are generated and maintained (SPF or specific pathogen free, which may account for the extremely poor lytic ability of resting mouse NK cells). These differences are highlighted by the inhibitory receptors, NK subsets and where NK cell reside. While the NKG2A and D family members are similar, the KIR (immunoglobulin super-family member) and Ly49 (lectin-binding) families of molecules are highly divergent with respect to structure. Importantly, however, their functions are remarkably similar with regard to binding MHC and the mouse studies have paved the way for human NK receptor delineation and clinical use. Another key difference with mouse and human NK cells is the existence of CD56bright and CD56dim subsets in human whereas CD56 is not present on mouse NK. Additionally, the demonstration that CD56bright NK cells reside in the lymph nodes (resting mouse lymph nodes are relatively devoid of NK cells) and are poorly lytic suggests other roles for this subpopulation perhaps in immune regulation. There are other markers and functions that distinguish mouse and human NK cells: CD8 is present on some human NK (mouse NK do not express) and human NK cells can be expanded and cultured in vitro for extended periods of time (mouse NK cells invariably die after several weeks in culture). It is perhaps revealing given the more extensive similarities between mouse and humans on the supposedly more advanced immune system effector cell: the T cell, whereas the “simpler” NK cell appears much more divergent between the species suggesting more recent evolutionary development. Nonetheless, most of the mouse studies with regard to development and function have been retrospectively validated in human studies and the similarities far outweigh the differences. The use of xenogeneic models in which human NK or NK progenitors are transferred into immunodeficient mice is still far from optimal due to MHC, adhesion molecule and cytokine differences between the species. Large animal models simply do not have sophisticated reagents to discern subsets and it is these subsets where more and more appreciation of NK cell biology and function is turning to. This is especially true when considering the now-appreciated complex cellular interactions that occur with NK cells and other immune cell-types, the critical role of MHC in their development as well as organ-specific interactions (NK cells primarily reside in the blood, spleen, and liver) which can have a dramatic impact on clinical extrapolation. Thus, despite the differences between mouse and humans, especially with regard to NK cells, the similarities have (and continue to have) resulted in a clearer picture with regard to their development and function as well as clinical use in cancer and other disease states.
Immunogenetic Modulation of NK Cell Biology
NK cells comprise a heterogeneous subpopulation of lymphocytes that makes important contributions to innate immunity, adaptive immunity and reproduction. Whereas other subpopulations of lymphocytes, the B cells and T cells, use rearranging genes to produce a diversity of antigen receptors, that is not the case for NK cells. Instead, diverse NK cell phenotypes are produced by the differential expression of a wide variety of different cell-surface receptors. Among these are several classes of receptor that recognize determinants of major histocompatibility complex (MHC) class I molecules. In humans these comprise certain members of the leukocyte immunoglobulin-like receptor (LILR) family that recognizes a conserved determinant formed by the a3 and b2-microglobulin domains of HLA class I, the CD94:NKG2 receptors that recognize complexes of conserved HLA-E and peptides derived from the leader sequences of other HLA class I heavy chains, and members of the killer cell immunoglobulin-like receptor (KIR) family that recognize polymorphic determinants of HLA-A, B and C. Whereas the interactions of LILRB1 and CD94:NKG2 with their ligands are conserved within the human population, the interactions of KIR with HLA-A, B and C are highly diversified and provide the major source of immunogenetic modulation of human NK cell biology(16). This modulation occurs both during the NK cell response and during NK cell development, when cognate interaction between KIR and HLA class I ‘educate’ NK cells to respond to perturbations in HLA class I expression(17–20).
Crystallographic determination of three-dimensional structures has shown that KIR interact with the upper face of the HLA class I molecule, which comprises the tops of the a helices of the a1 and a2 domains and the peptide bound between them. This is the same face with which HLA class I molecules interact with ab T-cell receptors (TCR). Although overlapping, the two binding sites are not identical: the T cell receptor interacts with all the exposed residues of the bound peptide, but KIR recognition is restricted to peptide residues seven and eight. KIR recognition is also restricted to four epitopes of HLA-A, B, and C that are determined by polymorphisms within residues 79–83 of the a1 helix. The C1 and C2 epitopes, carried by mutually exclusive subsets of HLA-C allotypes, are determined by dimorphism at position 80. For the Bw4 epitope carried by subsets of HLA-A and HLA-B allotypes, arginine 83 is essential, but its function is also modulated by dimorphism at position 80. The A3/11 epitope appears highly peptide-dependant and is less well characterized than the Bw4, C1 and C2 epitopes.
On the basis of amino-acid sequence comparisons, human KIR can be divided into four lineages. Of these, the KIR specific for the C1 and C2 epitopes are of lineage III and the KIR specific for the A3/11 and Bw4 epitopes are of lineage II. KIR2DL4, of lineage I, recognizes HLA-G and the lineage V KIR, KIR3DL3, has no known specificity for HLA class I. The inhibitory KIR specific for C1 (KIR2DL2/3), C2 (KIR2DL1), and Bw4 (KIR3DL1) are all highly polymorphic. The amino-acid differences that distinguish the variants can affect the avidity and/or specificity of the ligand-binding site, the level of cell-surface expression, the frequency of cellular expression, and the capacity for signal transduction(21). Combinations of particular KIR and HLA class I variants are associated with differential resistance and susceptibility to a wide range of diseases. These include infectious and autoimmune diseases, and pregnancy syndromes(22). KIR and HLA class I factors can also influence the success of hematopoietic cell transplantation as therapy for leukemia.
Because of the strong and variable selection imposed on NK cells by pressure from defense and reproduction, MHC class I and KIR are rapidly evolving, and co-evolving, genes. Counterparts to the human KIR are present only in monkeys and apes, and the human KIR system is significantly different from those observed in other species. Distinguishing the human KIR locus has been the evolution of two distinctive groups of haplotypes, the A haplotypes that are enriched for genes encoding KIR that bind to HLA class I and the B haplotypes that enriched for genes encoding KIR that have either reduced or lost their binding to HLA class I. Both A and B haplotypes are present in all populations, though at different relative frequencies. The results of epidemiological studies point to the A haplotypes providing better defense against infection, while the B haplotypes are better for reproduction(23).
Exploiting Basic Biology to Treat Cancer
Therapeutic strategies using allogeneic NK cells are based on our understanding of the signaling pathways that regulate the anti-tumor activity of NK cells. Certain human tumors are more amenable to NK cell based immunotherapy, and the degree of sensitivity to NK mediated killing is often correlated to their expression of ligands for activating NK receptors and not all tumors are targeted through the interactions (24). Most studies have focused on ways to manipulate the NK effectors to decrease the interactions between inhibitory KIR and their MHC ligands. Initially, algorithms were developed to select NK cell or stem cell donors to increase the potential for NK cell alloreactivity. Enthusiasm for this strategy became widespread after the 2002 report from Perugia in which Ruggeri et al. published that KIR ligand mismatch between patients and their donors was associated with improved outcomes in myeloid leukemia after T-cell deplete haploidentical HCT(5). We have made much progress in understanding of NK cell function, informed by mouse studies and by detailed studies of the immunogenetics of NK cell receptors and new algorithms have emerged.
Immunogenetic Discovery Improves Transplant Outcome
One such approach to exploit the beneficial effects of NK cells after HCT is to consider the KIR genotype of donor/recipient pairs. While recipient KIR genotype had no impact on clinical outcome, in a study of patients undergoing myeloablative HLA matched or mismatched unrelated donor HCT for AML the 3 year overall survival was significantly higher after transplantation from a KIR B/x genotype donor(25). A subsequent analysis of 1409 URD transplant recipients refined a beneficial effect of KIR B genes in AML, but not ALL, where the most benefit from KIR B genotype donors was localized to the centromeric part of the KIR locus(26). Donors homozygous for this region had the most protection from relapse and best disease free survival, in both HLA matched and mismatched transplants. In this large study, donors could be stratified by KIR into those with Best (Cen-B homozygous present in 11% of the population), Better (> 2 B defining domains as seen in 20% of the population) or Neutral donor KIR genotypes. A publically available calculator to determine this stratification is available on-line (www.ebi.ac.uk/ipd/kir/donor_b_content.html) and a prospective trial using KIR genotyping for donor selection has begun. For this trial, KIR genotyping as few as 3 of the best HLA matched donor candidates should substantially increase the frequency of unrelated transplants from donors with favorable KIR gene content from 31% to 79% which is expected to protect against relapse. The question that remains is why these gene patterns are protective.
Understanding How NK cells acquire function
As the first donor-derived lymphocyte subset to reconstitute following hematopoietic cell transplantation (HCT), NK cells may play a pivotal role in the graft versus leukemia (GvL) effect, especially in myeloid leukemia. This has directed attention to how NK cells acquire function, which remains complex. NK cells can acquire function through several mechanisms. Although it is well established that NK cells can be activated by cytokines, there is another layer of complexity that occurs during NK cell development through a process commonly referred to as licensing or NK cell education(9, 27). While the exact timing and location of NK cell education is unknown, it is generally believed that NK cells acquire function following engagement of inhibitory receptors with self-ligand after their differentiation from hematopoietic progenitors. NK cells lacking inhibitory receptors for self do exist, but they remain hyporesponsive and are considered “uneducated”(18). In the early stages of the NK cell development in lymph nodes(28), where NK cells are defined in part by the absence of MHC-specific receptors, these cells lack both cytotoxicity and cytokine production. Upon acquisition of CD94/NKG2A, precursor cells transition to CD56bright NK cells, at which time they acquire the capacity to produce IFNγ. Still, they display low cytotoxic potential. Only upon further development and emigration from the lymph node to the periphery do NK cells acquire CD16 and KIR and become highly cytotoxic.
NK cells early after transplant are partially uneducated
Allo-HCT provides a unique environment in which to study human NK cell education as it recapitulates NK development from hematopoietic stem cells in a short interval. It is unknown whether the acquisition of NK the cytotoxic and cytokine-producing functions occurs in parallel through interactions with inhibitory receptors or if distinct signals are required to generate each effector function. To understand the acquisition of NK cell function early after allo-HCT, use of 9-color flow cytometry to simultaneously measure both degranulation by CD107a expression (as a surrogate marker for cytotoxicity) and IFNγ production in NK cell subsets has been informative. We tested a cohort of 30 patients who received either unmanipulated (T cell replete) or potently T cell depleted (CD34+ selected) grafts from adult unrelated donors(29). Thawed peripheral blood mononuclear cells (PBMC) were rested overnight in cytokine free media and then incubated with K562 cells to trigger cytotoxicity and cytokine production. PBMC were stained with CD107a (a surrogate for cytotoxicity), IFNγ, CD56, CD3, CD45, CD158a, CD158b, CD158e and CD159a simultaneously. Cytotoxicity was intact but modestly suppressed (~35%) at 3 months after both T cell deplete and T cell replete HCT with further recovery of killing at 6 months. By contrast, at 3 months after T cell replete HCT there was potent and sustained suppression of IFNγ production by CD56+ cells (57%±11% suppression, p=0.009). The cohort of patients receiving T cell deplete (CD34-selected) grafts did not receive post transplant immunosuppression and also exhibited significant suppression of IFNγ at 3 and 6 months after HCT, which was partially restored with low concentrations of IL-15. We next compared NK cells that expressed at least one KIR with KIR negative NK cells. KIR expression had no effect on cytotoxicity. In contrast, KIR positive cells produced significantly higher amounts of IFNγ than KIR negative cells. Therefore following HCT, expression of KIR discriminates a population of NK cells that produce IFNγ, but does not correlate with cytotoxicity. This suggests that NKG2A, highly expressed on almost all NK cells early after transplant, selectively mediates education for cytotoxcity but not cytokine production. Because of their critical anti-tumor and infection protection roles, methods to enhance broad in vivo NK cell function, such as the use of post-transplant IL-15 administration are warranted.
NK cells mature and acquire function induced by human CMV infection
One example of a unique mechanism that modulates NK cell function early after transplant is that induced by human cytomegalovirus (hCMV) infection. This work is based on CMV infection, where a population of Ly49H+ NK cells expand and are responsible for disease clearance through the induction of a “memory NK cell response”(13). Whether similar events occur in hCMV infection is unknown. Some studies suggest that the C-type lectin-like receptor NKG2C and killer immunoglobulin receptor (KIR) family may be involved. We studied NK cell function after hCMV reactivation. In cohort 1, samples were triggered by viral reactivation, such that cells were collected at the time of reactivation and at 2, 4, and 8 weeks later (n=10). Most of these patient (9 of 10) received umbilical cord blood transplant grafts, which are CMV naïve and thus considered a CMV negative donor source. NKG2C+ NK cells increased following the detection of virus in the blood of transplant recipients. The peak response (i.e., rise in NKG2C+ NK cells) was at 4 weeks post infection. NK cells that expressed NKG2C produced significantly more IFNγ than NK cells that lacked NKG2C. Four weeks after infection, NK cells that co-expressed NKG2C and KIR produced significantly more IFNγ (11.4±1.66%) than NK cells that expressed only KIR but not NKG2C. In the context of education of KIR+ NK cells through their MHC ligands, only NKG2C+ NK cells with self-KIR (i.e., NK cells that express KIR where the cognate ligand is expressed in the recipient) made IFNγ̃ In contrast, NKG2C+ non-self KIR+ NK cells were hyporesponsive. Remarkably, the expansion and persistence of mature highly functional NKG2C+KIR+ NK cells persisted throughout the first year after transplant. These findings support the emerging concept of memory, viral induced innate cell populations in response to hCMV and show that hCMV infection specifically increases the maturation and functional competence of NK cells that could be important for protecting against relapse or subsequent infections.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cudkowicz G, Stimpfling JH. Hybrid Resistance to Parental Marrow Grafts: Association with the K Region of H-2. Science. 1964 Jun 12;144:1339–40. doi: 10.1126/science.144.3624.1339. [DOI] [PubMed] [Google Scholar]
- 2.Cudkowicz G, Bennett M. Peculiar immunobiology of bone marrow allografts. II. Rejection of parental grafts by resistant F 1 hybrid mice. J Exp Med. 1971 Dec 1;134(6):1513–28. doi: 10.1084/jem.134.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975 Feb;5(2):112–7. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 4.Asai O, Longo DL, Tian ZG, Hornung RL, Taub DD, Ruscetti FW, et al. Suppression of graft-versus-host disease and amplification of graft-versus-tumor effects by activated natural killer cells after allogeneic bone marrow transplantation. J Clin Invest. 1998 May 1;101(9):1835–42. doi: 10.1172/JCI1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002 Mar 15;295(5562):2097–100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 6.Delahaye NF, Rusakiewicz S, Martins I, Menard C, Roux S, Lyonnet L, et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med. Jun;17(6):700–7. doi: 10.1038/nm.2366. [DOI] [PubMed] [Google Scholar]
- 7.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986 Feb 20–26;319(6055):675–8. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 8.Blery M, Olcese L, Vivier E. Early signaling via inhibitory and activating NK receptors. Hum Immunol. 2000 Jan;61(1):51–64. doi: 10.1016/s0198-8859(99)00157-3. [DOI] [PubMed] [Google Scholar]
- 9.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005 Aug 4;436(7051):709–13. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 10.Joncker NT, Shifrin N, Delebecque F, Raulet DH. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J Exp Med. Sep 27;207(10):2065–72. doi: 10.1084/jem.20100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orr MT, Murphy WJ, Lanier LL. 'Unlicensed' natural killer cells dominate the response to cytomegalovirus infection. Nat Immunol. Apr;11(4):321–7. doi: 10.1038/ni.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kielczewska A, Kim HS, Lanier LL, Dimasi N, Vidal SM. Critical residues at the Ly49 natural killer receptor's homodimer interface determine functional recognition of m157, a mouse cytomegalovirus MHC class I-like protein. J Immunol. 2007 Jan 1;178(1):369–77. doi: 10.4049/jimmunol.178.1.369. [DOI] [PubMed] [Google Scholar]
- 13.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009 Jan 29;457(7229):557–61. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, et al. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002 Nov 15;100(10):3633–8. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 15.Sun JC, Ma A, Lanier LL. Cutting edge: IL-15-independent NK cell response to mouse cytomegalovirus infection. J Immunol. 2009 Sep 1;183(5):2911–4. doi: 10.4049/jimmunol.0901872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005 Mar;5(3):201–14. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- 17.Andersson S, Fauriat C, Malmberg JA, Ljunggren HG, Malmberg KJ. KIR acquisition probabilities are independent of self-HLA class I ligands and increase with cellular KIR expression. Blood. 2009 Jul 2;114(1):95–104. doi: 10.1182/blood-2008-10-184549. [DOI] [PubMed] [Google Scholar]
- 18.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006 Aug;25(2):331–42. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Yawata M, Yawata N, Draghi M, Little AM, Partheniou F, Parham P. Roles for HLA and KIR polymorphisms in natural killer cell repertoire selection and modulation of effector function. J Exp Med. 2006 Mar 20;203(3):633–45. doi: 10.1084/jem.20051884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yawata M, Yawata N, Draghi M, Partheniou F, Little AM, Parham P. MHC class I-specific inhibitory receptors and their ligands structure diverse human NK-cell repertoires toward a balance of missing self-response. Blood. 2008 Sep 15;112(6):2369–80. doi: 10.1182/blood-2008-03-143727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parham P, Norman PJ, Abi-Rached L, Guethlein LA. Variable NK cell receptors exemplified by human KIR3DL1/S1. J Immunol. Jul 1;187(1):11–9. doi: 10.4049/jimmunol.0902332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moffett A, Loke C. Immunology of placentation in eutherian mammals. Nat Rev Immunol. 2006 Aug;6(8):584–94. doi: 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- 23.Parham P. The genetic and evolutionary balances in human NK cell receptor diversity. Semin Immunol. 2008 Dec;20(6):311–6. doi: 10.1016/j.smim.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moretta L, Bottino C, Pende D, Castriconi R, Mingari MC, Moretta A. Surface NK receptors and their ligands on tumor cells. Semin Immunol. 2006 Jun;18(3):151–8. doi: 10.1016/j.smim.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Cooley S, Trachtenberg E, Bergemann TL, Saeteurn K, Klein J, Le CT, et al. Donors with group B KIR haplotypes improve relapse-free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009 Jan 15;113(3):726–32. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Le CT, et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010 Jun 25; doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raulet DH. Missing self recognition and self tolerance of natural killer (NK) cells. Semin Immunol. 2006 Jun;18(3):145–50. doi: 10.1016/j.smim.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Freud AG, Yokohama A, Becknell B, Lee MT, Mao HC, Ferketich AK, et al. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med. 2006 Apr 17;203(4):1033–43. doi: 10.1084/jem.20052507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, et al. NK cell education after allogeneic transplantation: dissociation between recovery of cytokine-producing and cytotoxic functions. Blood. Sep 8;118(10):2784–92. doi: 10.1182/blood-2011-04-347070. [DOI] [PMC free article] [PubMed] [Google Scholar]