Abstract
Background
Botulinum toxin A has been used in children to treat spastic disorders and recently for gastrointestinal conditions. Open label studies in adults with gastroparesis have reported an improvement in symptoms and gastric emptying after endoscopic intrapyloric botulinum injections (IPBI) although placebo controlled trials have shown conflicting results. Only a single case report of IPBI is available in children.
Objective
Determine the long term clinical outcomes and predictive factors of IPBI response in children with gastroparesis refractory to medical therapy.
Design
Retrospective review.
Setting
Single tertiary care center.
Patients
Children with refractory gastroparesis symptoms undergoing IPBI.
Interventions
IPBI.
Main outcome measurements
Clinical improvement and predictive factors of response.
Results
A total of 70 injections were given to 47 patients (mean age 9.98 ± 6.5 years and 23 were female) with follow up in 45. Only 15 reported failure and 30 reported success to IPBI. The median duration of response of the first IPBI was 3.0 months (1.2-4.8 months CI). A total of 29 patients received a single IPBI and 18 received multiple IPBI. Older age and vomiting predicted response to initial IPBI and male gender predicted response to repeat IPBI. Only 1 patient reported exacerbation of vomiting after IPBI resolving within a week.
Limitations
Open label and retrospective nature of the study.
Conclusion
IPBI is safe and may be effective in the management of children with symptoms of gastroparesis. Subgroups identified with response to first IPBI include older patients and those presenting with vomiting whereas males responded better to repeat IPBIs.
INTRODUCTION
Botulinum toxin A has been used as a medical therapy for a few decades. In pediatrics it is commonly used to treat spastic disorders associated with cerebral palsy(1). A novel use for Botulinum toxin A has been for gastrointestinal conditions targeting the lower esophageal sphincter and more recently the internal anal sphincter, in order to decrease tone and/or increase relaxation.(2, 3) Gastroparesis is a condition with historical limitations in medical therapy. When medical therapy fails in patients with gastroparesis, they often resort to surgical treatment such as gastrostomy(4, 5) and jejunostomy(6) for nutrition and pyloromyotomy(7), pyloroplasty(7-9) and gastrectomy(10, 11)) to improve gastric emptying. It has recently been suggested that the use of intrapyloric botulinum toxin A injections (IPBI) may be a medical alternative to some surgical procedures.(12) IPBI effects on the pylorus muscle seem to be mediated by decreasing contractility and acetylcholine release from cholinergic nerves at low doses, and directly affecting the muscle tone at higher doses.(13) The effect of Botulinum toxin A may not be limited just the pyloric muscle. It has also been reported to be absorbed from stomach and intestine producing peripheral neuromuscular blockade.(14)
In adult literature there have been contradictory results reported with IPBI. Almost all open label studies report an important clinical benefit and improvement in gastric emptying in patients with idiopathic,(15-18) diabetic(19) and post-surgical gastroparesis.(20) However, two recent small randomized placebo controlled trials comparing IPBI with saline injection showed no advantage on symptom improvement and acceleration of gastric emptying.(21, 22) Among the factors associated with IPBI response included higher dose, idiopathic etiology, female gender and age younger than 50 years old.(23) Given that young age may be associated with a better response, it is possible that children may respond better than adults. There is, however, very little literature on the use of IPBI in children, except for isolated case reports. Therefore the primary aim of our study was to determine the long term clinical outcomes after IPBI in the treatment of children with gastroparesis. The secondary aim was to evaluate potential predicting factors of clinical efficacy of IPBI.
METHODS
Institutional review board approval was obtained for this retrospective review of patients undergoing endoscopic intrapyloric botulinum toxin A injection from January 2004 to October 2010 at Children’s Hospital in Boston.
Population and data collection
We reviewed the medical history of patients with symptoms of gastroparesis (nausea, vomiting, early satiety, abdominal distention) refractory to medical therapy. All subjects either had a gastric emptying study with scintigraphy demonstrating delayed emptying for solids or liquids and/or post-prandial antral hypomotility by antroduodenal manometry. All patients had gastric outlet obstruction, or other anatomic or mucosal abnormalities ruled out by either an upper gastrointestinal endoscopy or a contrast radiological study. Demographic data, presenting symptoms, follow up symptoms, type of gastroparesis and results of gastric emptying study and antroduodenal manometry results were obtained.
Gastric emptying study (GES) was considered abnormal when there was >50% retention of solids and/or liquids at 60 minutes (24, 25). Antroduodenal manometry responses were categorized as follows: normal antroduodenal motility (NAM) if there was a normal motor migrating complex (MMC) during fasting with both normal fed response and normal antral response to EES, post-prandial antral hypomotility (PPAHM) when there was no increase in antral activity after a meal, and normal antral response to erythromycin (ARE) if there were antral contractions in response to erythromycin.
IPBI Procedure
All patients fasted overnight before undergoing general anesthesia for the endoscopic procedure. The botulinum toxin A was given endoscopically through a submucosal injection using a sclerotherapy needle at a dose of 6 units/kg up to a maximum of 100 units.(2, 3) Botulinum toxin A was diluted in 1 ml of saline and injection was divided in 4 aliquots and applied in 4 quadrants.
Outcome
Overall response to IPBI was classified as follows: a) failure (no response), b) mild response (symptoms improved but still on medications), c) moderate response (symptoms improved and able to stop medications) and d) asymptomatic (complete resolution of the symptoms). We considered responders to the treatment if their overall response was mild to asymptomatic at the time of the last clinic visit after the IPBI
Overall duration of response to the first injection was evaluated as per patient analysis, so overall duration of response after the first IPBI was calculated. Relapse was defined as a return or worsening of any symptom after the first IPBI.
Response to repeat IPBI was evaluated on those receiving multiple injections. Only those with a favorable response to the first IPBI received a repeat IPBI.
Statistical analysis
Statistical analysis was conducted using PASW Statistics 18.0. Continuous variables were expressed as means ± SD, or medians as appropriate. Non-parametric tests Mann-Whitney and independent samples median test were used for continuous variables and Chi square and Fisher’s exact test were used to assess the difference between proportions. Pearson’s correlation was used to evaluate the relationship between continuous variables. Different subgroup analyses were also performed: Patients < 12 or > 12 years old, gastric emptying severity (emptying <20% and >20%). Multivariate logistic regression analysis was used to assess factors responsible for long term response. A Kaplan-Meier curve was generated to demonstrate the duration of response.
RESULTS
A total of 70 injections were given to 47 patients with gastroparesis. Follow up information was available for 45 patients. The mean age was 9.98 ± 6.5 years and 23 (51.1%) were female. Symptoms prompting evaluation and therapy included the following: vomiting in 28 (62.2%), retching post-fundoplication in 7 (15.6%), abdominal distention in 4 (8.9%), nausea in 3 (6.7%), abdominal pain in 2 (4.4%) and feeding intolerance in 1 (2.2%). The etiology of the gastroparesis was idiopathic in 31 (66%), post-fundoplication in 7 (15%), chronic intestinal pseudo-obstruction in 3 (6.4%), diabetes mellitus in 2 (4.2%), related to mitochondrial dysfunction in 2 (4.2%) and post-surgical (pyloromyotomy for pyloric stenosis and esophageal atresia with colonic interposition) in 2 (4.2%). A total of 42/47 reported no response to therapy with prokinetics and 5/47 had very minimal response. GES was performed in 39 subjects and it was abnormal in 37 patients and normal in 2 (those 2 had abnormal antroduodenal manometry). A total of 25 patients underwent and antroduodenal manometry, 18 evidenced PPAHM and 7 were normal.
Overall response
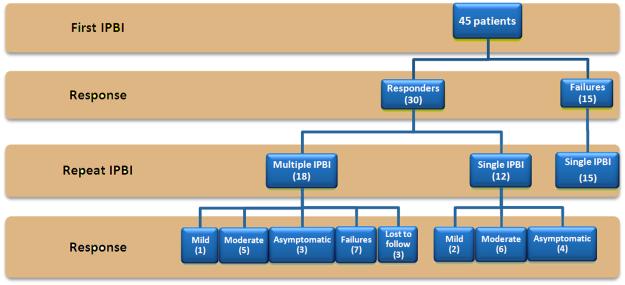
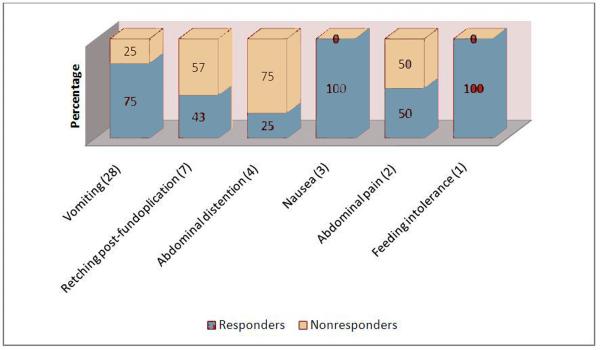
Mean follow up was 17.9 ± 18 months and median was 12 months ranging from 1 to 70 months with all but 1 having follow up of at least 3 months. Of the 45 patients, only 15 (33.3%) reported no response after IPBI and the remainder 30 (66.7%) reported at least mild improvement. The distribution of response of the 30 responders was as follows: 3 (10%) had a mild response, 15 (50%) had a moderate response and 12 (40%) were asymptomatic. The flow diagram with the overall response to IPBI is shown on Figure 1. The rate of response per presenting symptom is shown in Figure 2. We found a significant difference between the median follow up of responders and non-responders to IPBI (20 months range 1-70 months vs. 6 months range 3-69 months respectively, p=0.023).
Figure 1. Flow diagram showing the response to initial and repeat IPBI.
This graph represents the overall response to IPBI of 45 patients with follow up information available. From the 30 responders, 18 underwent a repeat injection. Failures underwent only the initial IPBI.
Figure 2. Response to IPBI by presenting symptom.
Vomiting and retching after a Nissen fundoplication were the most common presenting symptoms. Note the different response to IPBI in patients presenting with vomiting and nausea compared to those presenting with abdominal distention.
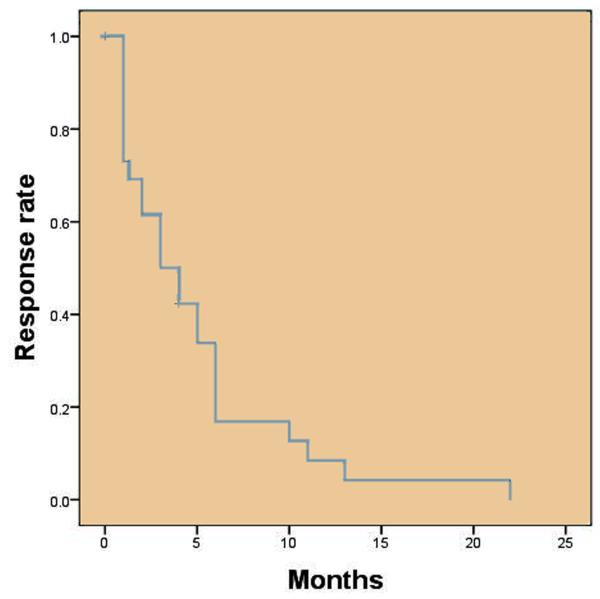
The effect of the first IPBI had a median duration of 3.0 months with a 95% CI of 1.2-4.8 months. (Figure 3). A total of 29 patients received a single injection and 18 patients received more than 1 injection: 14 received 2 injections, 3 received 3 injections and 1 received 4 injections. Of the 18 receiving more than 1 injection 7 failed to respond to subsequent injections, 8 had a sustained response to all injections and 3 were lost to follow after the second IPBI.
Figure 3. Time to symptomatic relapse after first IPBI.
Kaplan-Meier curve showing the time to symptom relapse in each subject after the first IPBI. The median time to relapse was 3.0 months (95% CI 1.2-4.8 months).
Predictors of response
The univariate analysis of factors that may be associated with a response to initial IPBI are shown on Table 1. Twenty nine were < 12 years and 16 > 12 years. The younger group had a lower rate of responders 17/29 (59%) vs. 13/16 (81%) than the older group but was not statistically significant (p=0.19). Response between both genders was also similar: 16/23 (69%) for females and 14/22 (63%) for males (p=0.67). The proportion of patients with vomiting before treatment was higher in responders (21/30; 70%) than in non-responders (7/15; 46%) but that difference was not statistically significant. The responses to IPBI according to etiology were as follows: 18/29 (62%) for idiopathic gastroparesis, 5/9 (55%) for post-surgical gastroparesis (including 3/7 (43%) post-fundoplication patients with retching), 2/3 (66%) for chronic intestinal pseudo-obstruction, 1/2 (50%) for diabetes mellitus and 1/2 (50%) for mitochondrial dysfunction. There was no significant difference in the proportion of responders and non-responders between idiopathic (18/29 or 62%) and non-idiopathic gastroparesis (9/16 or 56%) (p=0.67).
Table 1.
Univariate analysis of factors associated to response to initial IPBI
| Response to initial IPBI | ||||
|---|---|---|---|---|
| Yes | No | Total | p-value | |
| Demographic factors | ||||
| Age | 0.19 | |||
| < 12 y | 17 | 12 | 29 | |
| > 12 y | 13 | 3 | 16 | |
| Gender | 0.67 | |||
| Male | 14 | 8 | 22 | |
| Female | 16 | 7 | 23 | |
| Symptoms | ||||
| Vomiting | 0.13 | |||
| Yes | 21 | 7 | 28 | |
| No | 9 | 8 | 17 | |
| Retching post-fundoplication | 0.19 | |||
| Yes | 3 | 4 | 7 | |
| No | 27 | 11 | 38 | |
| Diagnosis | ||||
| Idiopathic | 0.67 | |||
| Yes | 18 | 8 | 26 | |
| No | 12 | 7 | 19 | |
| Diagnostic studies | ||||
| GES severity | 0.82 | |||
| Retention <20% | 8 | 4 | 12 | |
| Retention <20% | 19 | 8 | 27 | |
| Antroduodenal Manometry | ||||
| Antral hypomotility | 0.66 | |||
| Yes | 10 | 8 | 18 | |
| No | 5 | 2 | 7 | |
We found no difference in the median % of gastric emptying between responders (30%) and non-responders (37%) (p=0.85). Dividing gastric emptying values in quartiles and comparing extreme values does not alter the results (p=0.67), and classifying GES results by severity using a cut-off of GES <20% as the worse emptying also did not alter results. There was no correlation between GES and duration of response (r=0.03 with p=0.99). A total of 5 patients had a repeat GES and it was significantly improved in all 5 (p=0.04) with 4 of those reporting improvement after the IPBI. All 3 patients that had borderline normal gastric emptying responded to IPBI.
Responders had a lower frequency of antral hypomotility (10/15; 67%) than non-responders (8/10; 80%) but this difference was not statistically significant (p=0.43). Responders and non-responders had similar response to erythromycin during antroduodenal manometry respectively (10/13; 77% and 9/10; 90% for p=0.23).
Multivariate analysis demonstrated that older age and presence of vomiting were the only factors predicting response to IPBI. (Table 2).
Table 2.
Multivariate logistic regression for response to IPBI
| OR 95% CI | ||||
|---|---|---|---|---|
| OR | Lower | Upper | p-value | |
| Age > 12 years | 15.54 | 1.38 | 175.44 | 0.02 |
| Male gender | 0.45 | 0.05 | 4.18 | 0.48 |
| Presence of vomiting | 14.65 | 1.19 | 180.07 | 0.03 |
| GES | 0.99 | 0.94 | 1.04 | 0.70 |
| Idiopathic etiology | 2.52 | 0.29 | 21.60 | 0.40 |
| Absence of PPAH | 26.93 | 0.21 | 345.43 | 0.18 |
| Absent ARE | 0.30 | 0.01 | 13.85 | 0.54 |
Overall duration of response
There was no difference in the duration of response after the first injection according to gender (p=0.58), age (p=0.36), presence of vomiting (p=0.15), presence of retching post-fundoplication (p=0.59), severity of gastric emptying (p=0.35), idiopathic etiology (p=0.50), presence of PPAHM (p=0.90) and ARE (p=0.23). Figure 3 shows a Kaplan-Meier curve for response duration. The median time to relapse was 3.0 months with a 95% CI of 1.2-4.8 months.
Response to repeat IPBIs
Of the 30 responders, 12 required only 1 IPBI and had a sustained response for a median of 3.5 months (range 1-22 months). Of the 18 patients receiving more than 1 injection, follow up information was available in 15 with 7 of those responded favorably to the initial injection and failed to respond to subsequent injections and 8 had a sustained response to all injections. We found male gender to be the only factor associated with response after repeated injections (p<0.01) and a tendency towards an association with older age (p=0.07) and presence of vomiting (p=0.07). (Table 3).
Table 3.
Univariate analysis of factors associated to response to repeat IPBI
| Response to repeat IPBI |
|||||||
|---|---|---|---|---|---|---|---|
| Yes | No | Total | OR | Lower | Upper | p -value | |
| Demographic factors | |||||||
| Age | 0.07 | ||||||
| < 12 years | 6 | 2 | 8 | 0.38 | 0.11 | 1.31 | |
| > 18 years | 2 | 5 | 7 | 2.86 | 0.79 | 10.36 | |
| Gender | <0.01 | ||||||
| Male | 6 | 0 | 6 | 4 | 1.2 | 13.28 | |
| Female | 2 | 7 | 9 | 0.25 | 0.08 | 0.83 | |
| Symptoms | |||||||
| Vomiting | 0.07 | ||||||
| Yes | 4 | 7 | 11 | 0.5 | 0.25 | 1.00 | |
| No | 4 | 0 | 4 | ||||
| Retching post-fundoplication | 0.47 | ||||||
| Yes | 2 | 0 | 2 | ||||
| No | 6 | 7 | 13 | 1.33 | 0.89 | 1.99 | |
| Diagnosis | |||||||
| Idiopathic | 0.31 | ||||||
| Yes | 3 | 5 | 8 | 1.91 | 0.69 | 5.23 | |
| No | 5 | 2 | 7 | 0.46 | 0.13 | 1.66 | |
| Diagnostic studies | |||||||
| GES severity | 1.00 | ||||||
| Emptying <20% | 3 | 3 | 6 | 1.14 | 0.33 | 3.94 | |
| Emptying >20% | 5 | 4 | 9 | 0.91 | 0.39 | 2.11 | |
| Antroduodenal Manometry | |||||||
| Antral hypomotility | 0.14 | ||||||
| Yes | 4 | 1 | 5 | 0.25 | 0.05 | 1.36 | |
| No | 0 | 3 | 3 | ||||
Side effects
Only 1 patient reported short-lived (<1 week) exacerbation of vomiting after injection followed by complete resolution of symptoms.
DISCUSSION
This is the first clinical investigation on the use of IPBI in children. The previous pediatric literature on the subject was a single case report.(26) We have shown for the first time in this non-controlled study that intrapyloric injection of botulinum toxin A in intractable pediatric gastroparesis is safe and effective. This is an important observation because these patients are very symptomatic, and there is a very limited pharmacologic treatment. In fact our patients were refractory to commonly used medical therapy. Therefore the application of Botox is a relatively non-invasive treatment that may provide long term symptomatic improvement in this difficult to treat population.
We found that adolescent patients (> 12 years old) and those with vomiting as the indication for the IPBI had a better response. Our results go along with the adult case series reporting improvement on symptoms(17, 27) as well as gastric emptying of solids(27). The duration of response was also similar to previously reported adult case series.((17)) Large series of adults with gastroparesis have reported a better response in younger patients (<50 years), females and those with idiopathic gastroparesis.(23) In our study we saw an increase response in males, which is similar to other adult literature.(17) The duration of response was similar to previously reported adult case series. Our results go along with the adult case series reporting improvement on symptoms as well as gastric emptying despite the small controlled studies showing no benefit.(23)
The result of the initial gastric emptying study does not seem to predict response to IPBI, possibly because all patients show delayed emptying to start but even dividing by quartiles and comparing extreme values did not make a difference. We found that patients with abnormal antral response to a meal had a lower response rate, although it did not reach statistical significance probably due to the small sample size. Interestingly the antral response to erythromycin during manometry also did not predict response to IPBI.
We also report for the first time on the natural history after IPBI injections. We showed that from the 30 responders, 12 required only one injection. For those that had a relapse, and required repeated injections 50% continued to respond to repeated injections. There is only one previous adult study that showed that repeated injections were also very effective(23). We also did not administer repeated injections in those patients that did not respond to the first injection. In the adult study they showed that some patients responded after a second injection if higher doses were given(23).
This study has some limitations. The most important is its retrospective and open label nature. This limits the ability to capture clinical data in a validated objective format (28) before and after treatment, or to accurately judge the placebo effect. However we think our findings are still important as all patients had been intractable to our usual management, lessening the impact that our physician patient relationship could have had on the outcome. All had had previous endoscopies, which lessens the impact of the endoscopy on the results, and all had failed other medical therapies, so the placebo response to oral medications was already excluded. Of course the main confounding is the possible placebo effect that the actual administration of the IPBI could have had. A placebo controlled study with the intrapyloric injection of saline or Botox would have made the design stronger. However to undertake such a study without having any preliminary data, and which involves an invasive procedure under anesthesia in a pediatric population is very complex. Given the limited medical armamentarium to treat these children, we do not consider that the lack of a placebo arm makes our findings not useful, as they suggest that in intractable patients that have failed all other available therapies, the use of IPBI may be beneficial, before more invasive treatments like surgery are contemplated.
Similar positive results have been reported in two large open label studies of the use of IPBI in adults (17, 23), whereas 2 small placebo-controlled trials failed to show a difference, raising the possibility that the uncontrolled response seen with IPBI may be a placebo effect (21, 22). The reason for the discrepancy in the results of the uncontrolled trials both in adults and children and those of the placebo controlled trials in adults is not clear. One of the placebo controlled trials (23 patients) was a crossover study, making it very difficult to judge the true effect of IPBI as the action after the initial injection may last for many months, as can also be seen in our patients. Also the placebo group had significantly higher symptoms scores at baseline compared to the botox group, raising another important concern that the placebo group was sicker (more room to improve or more functional complains amenable to a higher placebo effect) and also the time between both injections was only 4 weeks(21). The other placebo controlled study (32 patients) demonstrated significant acceleration on gastric emptying in the IPBI group, although there was no difference in symptoms(22), suggesting there are other factors that contribute to symptoms in these patients. Another big consideration is that both controlled studies cluster a very heterogeneous group of patients, and were probably underpowered to achieve a significant difference(23). It is unclear the influence that the placebo response had in our patients, but they were all intractable, and had failed medical therapy under own care and supervision. Our study shows similar response rates than the larger adult studies, and given that we are dealing with younger patients it adds important new information as our findings suggest that children and adolescents may respond better than older populations. Large, multicenter prospective and randomized placebo controlled trials are needed to assess the safety and efficacy of IPBI for gastroparesis symptoms in children, and our findings may serve as the basis to design those studies.
Another limitation of our study is that we did not repeat the gastric emptying study or the antroduodenal manometry in all patients after the IPBI, so we cannot show if the improvement was correlated to changes in antroduodenal function. This is always an issue in pediatric studies given the radiation exposure, or the invasiveness of the procedures. It is possible that in the future with the advent of newer techniques to assess gastric function (29, 30) prospective studies in which measurements are repeated before and after treatment will be feasible.
We did not observe any significant side effects, showing that the IPBI is safe in this population.
An important concern is the recent reports of botulism in children with cerebral palsy undergoing botulinum toxin A injections to treat spasticity(31, 32) and also an adult death from anaphylaxis.(33) We did not find any side effects, and the maximum doses we are using are much smaller than those that have been associated with botulism (6units/kg up to 100units vs. 23-40units/kg up to 1200units). Furthermore botulinum toxin A has been safely and successfully used in other GI applications in children, particularly lower esophageal sphincter and internal anal sphincter (2, 3, 34-37).
In conclusion we have shown for the first time in a large pediatric cohort that use of IPBI may be an effective medical therapy for children with intractable gastroparesis.
Acknowledgments
This work was supported by NIH grants K23DK073713 (Dr. Rosen) and K24DK082792A (Dr. Nurko).
ACRONYMS
- IPBI
intrapyloric botulinum injection
- GES
gastric emptying study
- NAM
normal antroduodenal manometry
- MMC
motor migrating complex
- PPAHM
post-prandial antral hypomotility
- ARE
antral response to erythromycin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORS CONTRIBUTIONS Conception and design of the study: Drs Rodriguez, Rosen and Nurko
Analysis and interpretation of the data: Drs Rodriguez, Rosen and Nurko
Drafting of the article: Drs Rodriguez, Rosen, Manfredi and Nurko
Critical revision of the article for important intellectual content: Drs Rodriguez, Rosen, Manfredi and Nurko
Final approval of the article: Drs Rodriguez, Rosen, Manfredi and Nurko
REFERENCES
- 1.Koman LA, Mooney JF, 3rd, Smith B, et al. Management of cerebral palsy with botulinum-Atoxin: preliminary investigation. J Pediatr Orthop. 1993;13:489–95. doi: 10.1097/01241398-199307000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Ciamarra P, Nurko S, Barksdale E, et al. Internal anal sphincter achalasia in children: clinical characteristics and treatment with Clostridium botulinum toxin. J Pediatr Gastroenterol Nutr. 2003;37:315–9. doi: 10.1097/00005176-200309000-00020. [DOI] [PubMed] [Google Scholar]
- 3.Chumpitazi BP, Fishman SJ, Nurko S. Long-term clinical outcome after botulinum toxin injection in children with nonrelaxing internal anal sphincter. Am J Gastroenterol. 2009;104:976–83. doi: 10.1038/ajg.2008.110. [DOI] [PubMed] [Google Scholar]
- 4.Kim CH, Nelson DK. Venting percutaneous gastrostomy in the treatment of refractory idiopathic gastroparesis. Gastrointest Endosc. 1998;47:67–70. doi: 10.1016/s0016-5107(98)70301-3. [DOI] [PubMed] [Google Scholar]
- 5.Michaud L, Guimber D, Carpentier B, et al. Gastrostomy as a decompression technique in children with chronic gastrointestinal obstruction. J Pediatr Gastroenterol Nutr. 2001;32:82–5. doi: 10.1097/00005176-200101000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Devendra D, Millward BA, Travis SP. Diabetic gastroparesis improved by percutaneous endoscopic jejunostomy. Diabetes Care. 2000;23:426–7. doi: 10.2337/diacare.23.3.426. [DOI] [PubMed] [Google Scholar]
- 7.Okuyama H, Urao M, Starr GA, et al. A comparison of the efficacy of pyloromyotomy and pyloroplasty in patients with gastroesophageal reflux and delayed gastric emptying. J Pediatr Surg. 1997;32:316–9. doi: 10.1016/s0022-3468(97)90201-3. discussion 319-20. [DOI] [PubMed] [Google Scholar]
- 8.Maxson RT, Harp S, Jackson RJ, et al. Delayed gastric emptying in neurologically impaired children with gastroesophageal reflux: the role of pyloroplasty. J Pediatr Surg. 1994;29:726–9. doi: 10.1016/0022-3468(94)90355-7. [DOI] [PubMed] [Google Scholar]
- 9.Jawaid W, Abdalwahab A, Blair G, et al. Outcomes of pyloroplasty and pyloric dilatation in children diagnosed with nonobstructive delayed gastric emptying. J Pediatr Surg. 2006;41:2059–61. doi: 10.1016/j.jpedsurg.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Watkins PJ, Buxton-Thomas MS, Howard ER. Long-term outcome after gastrectomy for intractable diabetic gastroparesis. Diabet Med. 2003;20:58–63. doi: 10.1046/j.1464-5491.2003.00872.x. [DOI] [PubMed] [Google Scholar]
- 11.Speicher JE, Thirlby RC, Burggraaf J, et al. Results of completion gastrectomies in 44 patients with postsurgical gastric atony. J Gastrointest Surg. 2009;13:874–80. doi: 10.1007/s11605-009-0821-y. [DOI] [PubMed] [Google Scholar]
- 12.Cerfolio RJ, Bryant AS, Canon CL, et al. Is botulinum toxin injection of the pylorus during Ivor Lewis [corrected] esophagogastrectomy the optimal drainage strategy? J Thorac Cardiovasc Surg. 2009;137:565–72. doi: 10.1016/j.jtcvs.2008.08.049. [DOI] [PubMed] [Google Scholar]
- 13.James AN, Ryan JP, Parkman HP. Inhibitory effects of botulinum toxin on pyloric and antral smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2003;285:G291–7. doi: 10.1152/ajpgi.00296.2002. [DOI] [PubMed] [Google Scholar]
- 14.Maksymowych AB, Reinhard M, Malizio CJ, et al. Pure botulinum neurotoxin is absorbed from the stomach and small intestine and produces peripheral neuromuscular blockade. Infect Immun. 1999;67:4708–12. doi: 10.1128/iai.67.9.4708-4712.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ezzeddine D, Jit R, Katz N, et al. Pyloric injection of botulinum toxin for treatment of diabetic gastroparesis. Gastrointest Endosc. 2002;55:920–3. doi: 10.1067/mge.2002.124739. [DOI] [PubMed] [Google Scholar]
- 16.Miller LS, Szych GA, Kantor SB, et al. Treatment of idiopathic gastroparesis with injection of botulinum toxin into the pyloric sphincter muscle. Am J Gastroenterol. 2002;97:1653–60. doi: 10.1111/j.1572-0241.2002.05823.x. [DOI] [PubMed] [Google Scholar]
- 17.Bromer MQ, Friedenberg F, Miller LS, et al. Endoscopic pyloric injection of botulinum toxin A for the treatment of refractory gastroparesis. Gastrointest Endosc. 2005;61:833–9. doi: 10.1016/s0016-5107(05)00328-7. [DOI] [PubMed] [Google Scholar]
- 18.DeSantis ER, Huang S. Botulinum toxin type A for treatment of refractory gastroparesis. Am J Health Syst Pharm. 2007;64:2237–40. doi: 10.2146/ajhp060394. [DOI] [PubMed] [Google Scholar]
- 19.Lacy BE, Crowell MD, Schettler-Duncan A, et al. The treatment of diabetic gastroparesis with botulinum toxin injection of the pylorus. Diabetes Care. 2004;27:2341–7. doi: 10.2337/diacare.27.10.2341. [DOI] [PubMed] [Google Scholar]
- 20.Reddymasu SC, Singh S, Sankula R, et al. Endoscopic pyloric injection of botulinum toxin-A for the treatment of postvagotomy gastroparesis. Am J Med Sci. 2009;337:161–4. doi: 10.1097/MAJ.0b013e318182ee33. [DOI] [PubMed] [Google Scholar]
- 21.Arts J, Holvoet L, Caenepeel P, et al. Clinical trial: a randomized-controlled crossover study of intrapyloric injection of botulinum toxin in gastroparesis. Aliment Pharmacol Ther. 2007;26:1251–8. doi: 10.1111/j.1365-2036.2007.03467.x. [DOI] [PubMed] [Google Scholar]
- 22.Friedenberg FK, Palit A, Parkman HP, et al. Botulinum toxin A for the treatment of delayed gastric emptying. Am J Gastroenterol. 2008;103:416–23. doi: 10.1111/j.1572-0241.2007.01676.x. [DOI] [PubMed] [Google Scholar]
- 23.Coleski R, Anderson MA, Hasler WL. Factors Associated with Symptom Response to Pyloric Injection of Botulinum Toxin in a Large Series of Gastroparesis Patients. Dig Dis Sci. 2009 doi: 10.1007/s10620-008-0660-9. [DOI] [PubMed] [Google Scholar]
- 24.Montgomery M, Escobar-Billing R, Hellstrom PM, et al. Impaired gastric emptying in children with repaired esophageal atresia: a controlled study. J Pediatr Surg. 1998;33:476–80. doi: 10.1016/s0022-3468(98)90091-4. [DOI] [PubMed] [Google Scholar]
- 25.Rosen PR, Treves S. The relationship of gastroesophageal reflux and gastric emptying in infants and children: concise communication. J Nucl Med. 1984;25:571–4. [PubMed] [Google Scholar]
- 26.Woodward MN, Spicer RD. Intrapyloric botulinum toxin injection improves gastric emptying. J Pediatr Gastroenterol Nutr. 2003;37:201–2. doi: 10.1097/00005176-200308000-00023. [DOI] [PubMed] [Google Scholar]
- 27.Arts J, van Gool S, Caenepeel P, et al. Influence of intrapyloric botulinum toxin injection on gastric emptying and meal-related symptoms in gastroparesis patients. Aliment Pharmacol Ther. 2006;24:661–7. doi: 10.1111/j.1365-2036.2006.03019.x. [DOI] [PubMed] [Google Scholar]
- 28.Revicki DA, Rentz AM, Dubois D, et al. Development and validation of a patient-assessed gastroparesis symptom severity measure: the Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther. 2003;18:141–50. doi: 10.1046/j.1365-2036.2003.01612.x. [DOI] [PubMed] [Google Scholar]
- 29.Gentilcore D, Hausken T, Horowitz M, et al. Measurements of gastric emptying of low- and high-nutrient liquids using 3D ultrasonography and scintigraphy in healthy subjects. Neurogastroenterol Motil. 2006;18:1062–8. doi: 10.1111/j.1365-2982.2006.00830.x. [DOI] [PubMed] [Google Scholar]
- 30.Kuo B, McCallum RW, Koch KL, et al. Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther. 2008;27:186–96. doi: 10.1111/j.1365-2036.2007.03564.x. [DOI] [PubMed] [Google Scholar]
- 31.Crowner BE, Brunstrom JE, Racette BA. Iatrogenic botulism due to therapeutic botulinum toxin a injection in a pediatric patient. Clin Neuropharmacol. 2007;30:310–3. doi: 10.1097/WNF.0b013e31804b1a0d. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein EM. Safety of high-dose botulinum toxin type A therapy for the treatment of pediatric spasticity. J Child Neurol. 2006;21:189–92. doi: 10.2310/7010.2006.00041. [DOI] [PubMed] [Google Scholar]
- 33.Li M, Goldberger BA, Hopkins C. Fatal case of BOTOX-related anaphylaxis? J Forensic Sci. 2005;50:169–72. [PubMed] [Google Scholar]
- 34.Khoshoo V, LaGarde DC, Udall JN., Jr. Intrasphincteric injection of Botulinum toxin for treating achalasia in children. J Pediatr Gastroenterol Nutr. 1997;24:439–41. doi: 10.1097/00005176-199704000-00015. [DOI] [PubMed] [Google Scholar]
- 35.Hurwitz M, Bahar RJ, Ament ME, et al. Evaluation of the use of botulinum toxin in children with achalasia. J Pediatr Gastroenterol Nutr. 2000;30:509–14. doi: 10.1097/00005176-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Langer JC, Birnbaum E. Preliminary experience with intrasphincteric botulinum toxin for persistent constipation after pull-through for Hirschsprung’s disease. J Pediatr Surg. 1997;32:1059–61. doi: 10.1016/s0022-3468(97)90399-7. discussion 1061-2. [DOI] [PubMed] [Google Scholar]
- 37.Irani K, Rodriguez L, Doody DP, et al. Botulinum toxin for the treatment of chronic constipation in children with internal anal sphincter dysfunction. Pediatr Surg Int. 2008;24:779–83. doi: 10.1007/s00383-008-2171-3. [DOI] [PubMed] [Google Scholar]