Abstract
Individual differences in vulnerability to neurobehavioral performance impairment during sleep deprivation are considerable and represent a neurobiological trait. Genetic polymorphisms reported to be predictors have suggested the involvement of the homeostatic and circadian processes of sleep regulation in determining this trait. We applied mathematical and statistical modeling of these two processes to psychomotor vigilance performance and sleep physiological data from a laboratory study of repeated exposure to 36 h of total sleep deprivation in 9 healthy young adults. This served to quantify the respective contributions of individual differences in the two processes to the magnitudes of participants’ individual vulnerabilities to sleep deprivation. For the homeostatic process, the standard deviation for individual differences was found to be about 60% as expressed relative to its group-average contribution to neurobehavioral performance impairment. The same was found for the circadian process. Across the span of the total sleep deprivation period, the group-average effect of the homeostatic process was twice as big as that of the circadian process. In absolute terms, therefore, the impact of the individual differences in the homeostatic process was twice as large as the impact of the individual differences in the circadian process in this study. These modeling results indicated that individualized applications of mathematical models predicting performance on the basis of a homeostatic and a circadian process should account for individual differences in both processes.
Keywords: Sleep homeostasis, Circadian rhythm, Inter-individual differences, Psychomotor vigilance test, Polysomnography, Biomathematical modeling
1. Introduction
In recent years it has become widely recognized that there are trait individual differences in vulnerability to performance impairment due to sleep deprivation (Van Dongen et al., 2004). It has been suggested that such trait vulnerability may at least partially be explained by habitual sleep restriction (Rupp et al., 2009), but no correlation has been found between individual differences in vulnerability to sleep deprivation and self-reported habitual sleep duration (Van Dongen et al., 2004). Furthermore, experimental manipulation of prior sleep ration only marginally affects the expression of the trait (Van Dongen et al., 2004). Recent reports of genetic predictors of vulnerability to sleep deprivation cast further doubt on a mere behavioral explanation of the trait (King et al., 2009), suggesting instead that it may be fundamentally neurobiological in nature. Genetic polymorphisms identified as candidate predictors of vulnerability to sleep deprivation (Goel et al., 2010; Rétey et al., 2006; Viola et al., 2007) are believed to be associated with the sleep homeostatic and circadian regulation of sleep. This suggests involvement of the homeostatic and circadian processes in determining trait vulnerability to performance impairment during sleep deprivation, and raises interest in quantifying the respective contributions of individual differences in these two underlying processes (Van Dongen, 2006).
The laboratory study that first established the trait-like nature of vulnerability to sleep loss (Van Dongen et al., 2004) involved repeated exposure to 36 h of total sleep deprivation. A neurobehavioral test battery was administered every 2 h, and impairment was assessed by averaging performance measurements across the test bouts in the final 24 h (i.e., one circadian cycle) of each sleep deprivation period. This yielded multiple assessments of vulnerability to performance impairment during sleep deprivation per subject, which is essential for the disentanglement of systematic between-participants variance from within-participants variance and measurement noise. By averaging the data within each sleep deprivation period, however, the sleep homeostatic and circadian rhythm processes driving performance deficits (Van Dongen and Dinges, 2005) remained intertwined. Therefore, questions about the relative contributions of the two processes to trait vulnerability to sleep loss could not be addressed in this data analysis approach. Here, we revisit this issue in a new laboratory data set of repeated sleep deprivation, using mathematical and statistical modeling to disentangle the circadian rhythm from the sleep homeostat at the level of individual participants.
2. Methods
2.1. Participants and experiment
Nine healthy volunteers (2 men, 7 women; ages 22 - 40 years) completed an 11-day laboratory study. They were physically and psychologically healthy and free of traces of drugs, as assessed by physical examination, blood chemistry, urine analysis, and history. They reported to be good sleepers, habitually sleeping between 7 and 9 h per night and regularly getting up between 06:30 h and 08:30 h. Participants were instructed to maintain their habitual sleep/wake pattern and not to take naps, as verified by means of actigraphy and diary, and to abstain from caffeine, tobacco, alcohol and drugs during the seven days before the experiment.
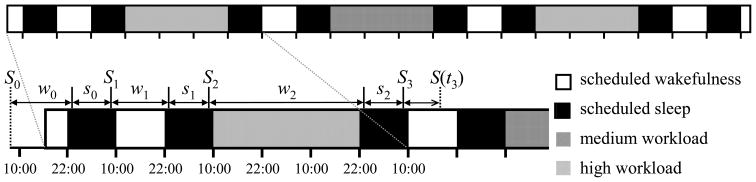
The experiment was conducted in a controlled laboratory environment of the Sleep and Performance Research Center at Washington State University Spokane. Fig. 1 (top) illustrates the study protocol. Upon entering the laboratory, participants practiced the neurobehavioral test battery (described below). They went to bed at 22:00 h and got up 12 h later at 10:00 h the next morning. They performed a low workload (0.2-h) version of the neurobehavioral test battery every 2 h beginning at 10:00 h. They went to bed at 22:00 h and got up 12 h later at 10:00 h the next morning. They were then subjected to 36 h of total sleep deprivation, during which they performed the neurobehavioral test battery every 2 h. The test battery could involve either medium (0.5-h) or high (1.0-h) workload, as further explained below.
Fig. 1.
Schematic of the 11-day laboratory protocol. Top: Tick marks indicate 12-h time intervals (alternating between 22:00 h and 10:00 h). The black sections represent 12-h nocturnal sleep opportunities, which were recorded polysomnographically. The white sections represent periods of wakefulness with low performance testing workload, all of which were 12 h in duration except those at the very beginning and end of the study protocol. The hatched areas reflect the three 36-h periods of total sleep deprivation with medium (single hatched) or high (double hatched) performance testing workload. The high workload condition occurred during the first, second or third sleep deprivation period in randomized, counterbalanced order—for illustration purposes, it is shown here to occur during the second sleep deprivation period. Performance testing occurred at 2-h intervals throughout scheduled wakefulness. Bottom: Subject-specific sleep/wake timeline illustrated for the first few days of the laboratory study. The variables indicated above the timeline are model variables described in the text. Depending on the individual participants, the onsets of sleep and wakefulness, indicated by the tick marks above the time line, typically occurred some time after the beginning and before the end of the 12-h sleep opportunities.
At 22:00 h at the end of the 36-h sleep deprivation period, participants went to bed. After a 12-h recovery sleep opportunity, they got up at 10:00 h, and performed the low workload version of the neurobehavioral test battery every 2 h. They went to bed at 22:00 h and again got up 12 h later at 10:00 h the next morning. This pattern of a 36-h sleep deprivation period with medium or high workload testing, followed by a 12-h recovery sleep period and a 12-h rest day with low workload testing and an additional 12-h sleep period, was repeated two more times. One of the three sleep deprivation periods involved the high workload version of the test battery; the other two sleep deprivation protocols involved the medium workload version. These conditions occurred in randomized, counterbalanced order. At 10:00 h after the last laboratory sleep period, participants performed the low workload test battery one final time, and then went home.
The laboratory environment was strictly controlled in terms of scheduled activities and environmental conditions. Participants were in their own room for performance testing and sleep, and shared a common suite with up to three other participants at other times. Participants were behaviorally monitored continuously, and were allowed only non-vigorous activities between test bouts. Light exposure was less than 50 lux during scheduled wakefulness, and lights were off during scheduled sleep. Ambient temperature was maintained at 21 ± 1 °C. Standardized meals were given at 11:00 h, 15:00 h and 19:00 h each day, and also at 23:00 h, 03:00 h and 07:00 h during sleep deprivation periods. Meals were strictly controlled in terms of calories and nutrients (proteins, fats and carbohydrates); the amount of food participants received during the 36-h sleep deprivation periods matched their normal two-day caloric requirement based on height and weight. No caffeine, alcohol and tobacco were allowed during the experiment.
2.2. Measurements
The neurobehavioral test battery contained a number of cognitive performance tasks described in detail elsewhere (Van Dongen et al., 2004), and included a psychomotor vigilance test (PVT) which was used for the present analyses. The PVT is a sustained attention reaction time task measuring behavioral alertness (Dorrian et al., 2005). The number of PVT lapses (reaction times ≥ 500 ms) was used as the primary outcome variable. As mentioned above, the experiment involved manipulation of the duration of the test battery, which was done for reasons beyond the scope of the present paper. During the sleep deprivation periods there was either a high workload version of the test battery totaling 1 h in duration, which included a 20-minute PVT; or a medium workload version of 0.5 h duration, which included a 10-minute PVT. Only data from the two sleep deprivation periods with medium workload, containing the 10-minute version of the PVT, are considered here. Since the test battery was administered every 2 h, there were 18 test bouts during each sleep deprivation period, and thus each participant contributed 36 PVT data points. The data points acquired immediately after awakening, which were potentially affected by sleep inertia, were discarded, leaving 34 data points per participant.
All eight sleep periods were recorded polysomnographically with digital equipment (Nihon Kohden). The electrode montage included frontal (Fz), central (C3, C4), and occipital (Oz) electroencephalogram (EEG) referenced against the mastoids (A1/A2), bilateral electro-oculogram (EOG), and submental electromyogram (EMG). The records were scored visually in 30-second epochs using conventional criteria (Rechtschaffen and Kales, 1968). Sleep latency, total sleep time, and time of final awakening were assessed from the scored records. Using these data, a sleep/wake timeline across the experiment was constructed for each participant, as illustrated in Fig. 1 (bottom). The sleep homeostatic impact of brief intermittent awakenings during sleep periods was deemed negligible, and as such it was ignored for modeling purposes (In our sample of good sleepers, intermittent awakenings were relatively infrequent and brief. The average number of polysomnographically assessed, intermittent awakenings per sleep period was 15.2, with a between-participants standard deviation of 6.5. The average duration of intermittent awakenings was 2.5 minutes, with a between-participants standard deviation of 1.3 minutes.).
In the seven days before the beginning of the laboratory experiment, participants were required to maintain their habitual sleep/wake pattern and not to take any naps, and to abstain from caffeine, tobacco, alcohol, and drugs. Their sleep times were monitored by actigraphy (Ambulatory Monitoring) and diary. Each participant’s average daily sleep duration in the seven days before the experiment was assessed from the actigraph records. Furthermore, the time of awakening on the day of laboratory admission was determined from each participant’s actigraph record, providing the starting point of the sleep/wake timeline (see Fig. 1, bottom).
2.3. Mathematical modeling
Mathematical model predictions of performance across each participant’s entire sleep/wake timeline were derived using published equations for the homeostatic and circadian processes (Borbély and Achermann, 1999). The equation for the homeostatic process for wakefulness was transformed from its published iterative form to an equivalent closed form. In this form, the homeostatic state S during scheduled waking period j at time awake tj, measured in hours from the time of the most recent awakening, is given by:
| (1) |
where Sj is the homeostatic state at the onset of waking period j (j = 1, ..., 8). Here Sj can be determined by iterating over the following:
| (2) |
where rs and rw are rate constants respectively defined as the inverse of the published time constants for the decline of homeostatic pressure during sleep, rs = 1/4.2, and the build-up of homeostatic pressure during wakefulness, rw = 1/18.2 (Borbély and Achermann, 1999). Wakefulness period number 1 was defined as the first complete waking period spent inside the laboratory, sj–1 as the duration of the preceding sleep period in hours, and wj–1 as the duration of the preceding waking period in hours (see Fig. 1, bottom).
S0 was defined as the equilibrium homeostatic state at the estimated time of awakening on the day the subject entered the laboratory, as given by:
| (3) |
where D is the subject’s average daily sleep duration (in hours) in the seven days before the experiment (as measured with actigraphy).
For the circadian process C, the published equation was used (Borbély and Achermann, 1999). In terms of clock time T expressed in hours from midnight, the equation is given by:
| (4) |
in which ϕ determines the circadian phase position in hours. Here a1 = 0.97, a2 = 0.22, a3 =0.07, a4 = 0.03 and a5 = 0.001 as defined previously (Borbély and Achermann, 1999).
From the two processes S and C, neurobehavioral performance P was predicted using an additive model (Achermann and Borbély, 1994):
| (5) |
where β and γ are scaling factors representing the magnitude of the contributions of the homeostatic and circadian process, respectively, and κ is an intercept modulating the basal level of performance.
2.4. Statistical modeling
Two-process model fitting was performed using the NLMIXED procedure in SAS 9.1.3 (SAS Institute). The performance model was fitted to the primary neurobehavioral performance outcome variable of the study, that is, the number of PVT lapses observed during the two sleep deprivation periods with medium workload. The time points associated with these data y were defined by the midpoints of each of the 10-minute PVT bouts, relative to the individual participants’ sleep/wake timelines. These time points were expressed as time since awakening tj for the relevant waking period j, and as corresponding clock time T relative to midnight. A regression model was used to estimate the model parameters, as follows:
| (6) |
where ε is the error term, assumed to be normally distributed with mean zero and standard deviation σε.
We assigned β, γ, κ and ϕ to be free parameters in the model. To estimate individual differences, random effects over participants were placed on β, γ and κ. These random effects were assumed to be independently, normally distributed with mean zero and standard deviations σβ, σγ and σκ, respectively, which were also free parameters in the model. To examine the importance of each of the random effects in the full model, reduced models were fitted with the random effects removed, one at a time. The adequacy of the reduced models was tested against the full model using likelihood ratio tests.
3. Results and Discussion
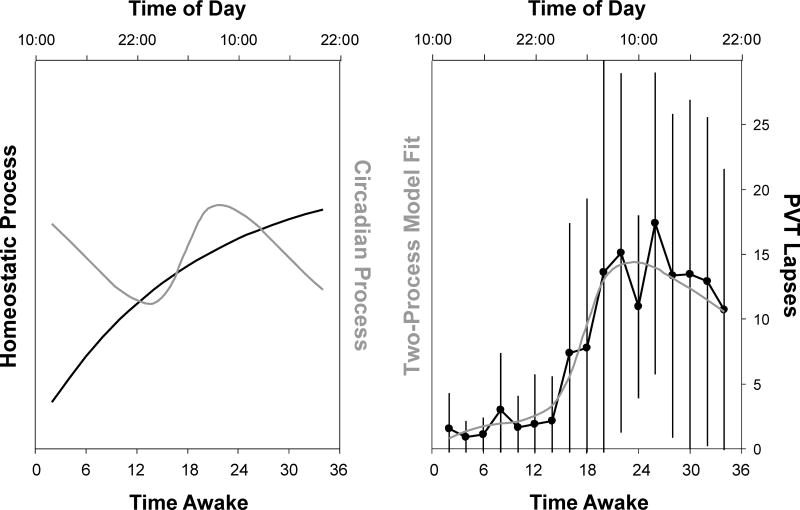
Fig. 2 shows the group-average profiles for the fitted homeostatic and circadian processes during each of the 36-h total sleep deprivation periods (left panel). It also shows the overall two-process model fit (right panel), as well as the group-average data and their standard deviations across time awake (collapsed over the sleep deprivation periods). The figure shows that the model captured the group-average data well. The size of the standard deviations (over participants) was proportional to the magnitude of group-average performance impairment (Pearson’s r = 0.92). This pattern suggests that individual differences were exposed by increased homeostatic and/or circadian influences across the sleep deprivation period (Doran et al., 2001).
Fig. 2.
Two-process model fit for neurobehavioral performance impairment across repeated 36-h periods of total sleep deprivation. Left: Best-fitting curves for the homeostatic (black) and circadian (gray) processes across the repeated sleep deprivation periods. Right: Averages (dots) and standard deviations (whiskers on each side) over participants (n = 9) for the number of PVT lapses at every time point, after collapsing the data within participants over the repeated sleep deprivation periods; and two-process model fit (gray) as a linear combination of the homeostatic and circadian processes. The abscissas show cumulative clock time over the collapsed 36-h sleep deprivation periods, ranging from 10:00 h until 22:00 h the next day.
Table 1 (top) shows the parameter estimates and their standard errors for the full model (i.e., with all three random effects). The error term standard deviation was fairly large, and indicated that at the level of individuals, the model did not capture the data as well as in the collapsed, group-average representation of Fig. 2 (right). This may be due to known order effects (i.e., gradually increasing vulnerability) across the repeated sleep deprivations (Tucker et al., 2007; Van Dongen et al., 2004), which are not accounted for in the present modeling. Nonetheless, the model captured 64.2% of the variance in the data set.
Table 1.
Parameter estimates and standard errors (S.E.), and parameter correlation matrix.
| Parameters | Interpretation | Estimate | S.E. |
|---|---|---|---|
| β | Scale of homeostatic process | 21.6 | 4.5 |
| γ | Scale (amplitude) of circadian process | 3.78 | 1.02 |
| κ | Intercept modulating basal performance | −5.12 | 1.27 |
| ϕ | Circadian phase position | 3.62 | 0.59 |
| σβ | Standard deviation over participants for β | 12.1 | 2.9 |
| σγ | Standard deviation over participants for γ | 2.37 | 0.97 |
| σκ | Standard deviation over participants for κ | 0.00 | 1.65 |
| σε | Error term standard deviation | 7.04 | 0.29 |
| Correlations | γ | κ | ϕ | σβ | σγ | σκ | σε |
|---|---|---|---|---|---|---|---|
| β | −0.014 | −0.413 | 0.043 | −0.001 | 0.015 | 0.000 | 0.003 |
| γ | 0.029 | 0.148 | −0.003 | 0.054 | 0.000 | 0.011 | |
| κ | −0.170 | 0.004 | −0.061 | 0.000 | −0.013 | ||
| ϕ | −0.022 | 0.365 | 0.000 | 0.075 | |||
| σβ | −0.013 | 0.000 | −0.007 | ||||
| σγ | 0.000 | 0.090 | |||||
| σκ | 0.000 |
Of primary interest are the random effects for β, γ and κ. Likelihood ratio tests revealed that there was a significant contribution to the model goodness-of-fit from the random effect for β (χ12 = 91.2, P < .001), and a trend for γ (χ12 = 3.7, P = .054). However, there was no significant contribution from the random effect for κ (χ12 < 0.01, P > .99).
The abstract mathematical processes S and C have no physically meaningful absolute scales. The magnitude of the individual differences attributed to each process separately was therefore quantified by expressing the standard deviation of the random effect relative to the corresponding scaling factor. For process S, this yielded σβ/β = 56.0% ± 17.8% (mean ± standard error). For process C, this yielded σγ/γ = 62.7% ± 31.5%. Thus, the relative magnitudes of individual variability were very similar for the two processes S and C, with no significant difference (t6 = 0.19, P = 0.86).
Over the time points considered during each of the sleep deprivation periods (from 2 h until 34 h since scheduled awakening), the range of the modeled effect of the homeostatic process on PVT lapses was 2.02 times as big as that of the circadian process (see Fig. 2, left). Thus, given that the relative magnitudes of the individual differences were found to be similar for the two processes, overall the impact of the individual differences in the homeostatic process was effectively about twice as large as the impact of the individual differences in the circadian process in this study.
Before interpreting these results, it is important to review the parameter correlation matrix shown in Table 1 (bottom). There were no pairwise correlations exceeding 0.5, and most were much smaller. It follows that there were no major linear interdependencies (colinearities) among the parameters. This means that each of the model parameters was well estimable, and as such the processes S and C and their individual variabilities were satisfactorily dissociable in this study.
The absence of individual differences in the intercept κ is noteworthy - in earlier estimates of individual variability in the two-process model used to predict neurobehavioral performance (Van Dongen et al., 2007), individual differences in the intercept were substantial (σκ = 6.02). However, the earlier parameterization of the two-process model was different, and not focused specifically on assessing the individual difference contributions of the dissociated homeostatic and circadian processes. Furthermore, the earlier modeling effort did not include individualization of the initial homeostatic state S0 as in the present work. That said, on a scale theoretically ranging from 0 to 1, the initial homeostatic state S0 varied only modestly among individuals in our data set (between 0.05 and 0.11). In the two-process model, small variations in the value of S0 wash out within one or two sleep/wake cycles, and thus there should have been no noticeable carry-over to the sleep deprivation periods. Indeed, rerunning the model with the value of S0 fixed at the average over participants did not increase the estimate for individual differences in the intercept.
Two modeling assumptions we made deserve some discussion. First, we assumed that the random effects were normally distributed over participants, even though there have been no studies assessing whether or not individual differences in the homeostatic and circadian processes are normally distributed. However, it can be demonstrated mathematically (Olofsen et al., 2004) that the results of model fitting do not depend critically on the assumed distributions of the random effects when more than a handful of data points are available per subject (as was the case). Second, we used an additive model to predict neurobehavioral performance based on the processes S and C. This form has been shown previously to describe neurobehavioral function during total sleep deprivation adequately (Achermann and Borbély, 1994; Daan et al., 1984). Nevertheless, a forced desynchrony study has revealed that there is a nonlinear interaction in the contributions of the two processes to neurobehavioral performance (Dijk et al., 1992). This interaction has also been confirmed under conditions of total sleep deprivation (Van Dongen and Dinges, 2003). Yet, in the first 36 h of sleep deprivation the interaction effect was negligible, and it was therefore justifiably ignored here.
Finally, there are some important caveats. The expression of trait individual differences in vulnerability to sleep deprivation depends on the kind of neurobehavioral performance considered (Van Dongen et al., 2004), and possibly on the specific cognitive processes involved (Ratcliff and Van Dongen, 2009). As such, the results of our study could vary upon examining data from other performance tasks in the neurobehavioral test battery. Furthermore, the present results were obtained using data from a (repeated) total sleep deprivation experiment. Whether or not the relative contributions of individual differences in the homeostatic and circadian processes to trait vulnerability to sleep loss are equivalent in other sleep loss and/or circadian disruption paradigms (e.g., Darwent et al., 2010) remains to be determined. Lastly, the present findings may be specific to the population of healthy men and women aged 22 - 40 from which the study sample was drawn.
4. Conclusion
We set out to mathematically dissociate systematic individual differences in sleep homeostatic effects from systematic individual differences in circadian rhythm contributions to neurobehavioral impairment in a study of repeated exposure to 36 h of total sleep deprivation in healthy young adults. We found that for both the homeostatic process and the circadian process, the standard deviation for individual differences was approximately 60% of the group-average magnitude of the contribution of the process to overall neurobehavioral impairment under these conditions. In real-world settings where operational safety and success depend on the performance of individuals or small crews and any impairments they may have need to be anticipated, individualized mathematical models of performance substantially outperform group-average models with regard to their accuracy in predicting impairment (Van Dongen et al., 2007). Our present finding indicates that individualized mathematical models predicting performance on the basis of a homeostatic and a circadian process (Hursh and Van Dongen, 2010) should be specified to account for individual differences in both processes.
Highlights.
Individual differences in performance impairment due to sleep loss represent a trait.
We assessed the homeostatic and circadian process contributions to this trait.
For both, individual differences (SD) were ~60% of the group-average contributions.
Acknowledgments
We thank the staff and the research assistants of the Sleep and Performance Research Center at Washington State University Spokane for their help conducting this study. In addition, we are grateful to Peter Achermann and Thomas Rusterholz for verification of the closed-form equation for process S. This research was supported by NIH grants HL70154 and HL105768, USAMRMC award W81XWH-05-1-0099 and DURIP grant FA9550-06-1-0281, and in part by the National Space Biomedical Research Institute through NASA NCC 9-58.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Hans P.A. Van Dongen, Email: hvd@wsu.edu.
Amy M. Bender, Email: abender@wsu.edu.
David F. Dinges, Email: dinges@mail.med.upenn.edu.
References
- Achermann P, Borbély AA. Simulation of daytime vigilance by the additive interaction of a homeostatic and a circadian process. Biological Cybernetics. 1994;71:115–121. doi: 10.1007/BF00197314. [DOI] [PubMed] [Google Scholar]
- Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. Journal of Biological Rhythms. 1999;14:557–568. doi: 10.1177/074873099129000894. [DOI] [PubMed] [Google Scholar]
- Daan S, Beersma DGM, Borbély AA. Timing of human sleep: Recovery process gated by a circadian pacemaker. American Journal of Physiology. 1984;246:R161–R178. doi: 10.1152/ajpregu.1984.246.2.R161. [DOI] [PubMed] [Google Scholar]
- Darwent D, Ferguson SA, Sargent C, Paech GM, Williams L, Zhou X, Matthews RW, Dawson D, Kennaway DJ, Roach GD. Contribution of core body temperature, prior wake time, and sleep stages to cognitive throughput performance during forced desynchrony. Chronobiology International. 2010;27:898–910. doi: 10.3109/07420528.2010.488621. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. Journal of Sleep Research. 1992;1:112–117. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Doran SM, Van Dongen HPA, Dinges DF. Sustained attention performance during sleep deprivation: Evidence of state instability. Archives of Italian Biology. 2001;139:253–267. [PubMed] [Google Scholar]
- Dorrian J, Rogers NL, Dinges DF. Psychomotor vigilance performance: Neurocognitive assay sensitive to sleep loss. In: Kushida CA, editor. Sleep Deprivation. Clinical Issues, Pharmacology, and Sleep Loss Effects. Marcel Dekker; New York: 2005. pp. 39–70. [Google Scholar]
- Goel N, Banks S, Mignot E, Dinges DF. DQB1*0602 predicts interindividual differences in physiologic sleep, sleepiness, and fatigue. Neurology. 2010;26:1509–1519. doi: 10.1212/WNL.0b013e3181f9615d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Van Dongen HPA. Fatigue and performance modeling. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 5. Elsevier Saunders; St. Louis: 2010. pp. 745–752. [Google Scholar]
- King AC, Belenky G, Van Dongen HPA. Performance impairment consequent to sleep loss: Determinants of resistance and susceptibility. Current Opinion in Pulmonary Medicine. 2009;15:559–564. doi: 10.1097/MCP.0b013e3283319aad. [DOI] [PubMed] [Google Scholar]
- Olofsen E, Dinges DF, Van Dongen HPA. Nonlinear mixed-effects modeling: Individualization and prediction. Aviation, Space and Environmental Medicine. 2004;75:A134–A140. [PubMed] [Google Scholar]
- Ratcliff R, Van Dongen HPA. Sleep deprivation affects multiple distinct cognitive processes. Psychonomic Bulletin and Review. 2009;16:742–751. doi: 10.3758/PBR.16.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human participants. Publication Number 204, Neurological Information Network. National Institutes of Health; Bethesda: 1968. [DOI] [PubMed] [Google Scholar]
- Rétey JV, Adam M, Gottselig JM, Khatami R, Dürr R, Achermann P, Landolt HP. Adenosinergic mechanisms contribute to individual differences in sleep deprivation-induced changes in neurobehavioral function and brain rhythmic activity. Journal of Neuroscience. 2006;26:10472–10479. doi: 10.1523/JNEUROSCI.1538-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp TL, Wesensten NJ, Bliese PD, Balkin TJ. Banking sleep: Realization of benefits during subsequent sleep restriction and recovery. Sleep. 2009;32:311–321. doi: 10.1093/sleep/32.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker AM, Dinges DF, Van Dongen HPA. Trait interindividual differences in the sleep physiology of healthy young adults. Journal of Sleep Research. 2007;16:170–180. doi: 10.1111/j.1365-2869.2007.00594.x. [DOI] [PubMed] [Google Scholar]
- Van Dongen HPA. Shift work and inter-individual differences in sleep and sleepiness. Chronobiology International. 2006;23:1139–1147. doi: 10.1080/07420520601100971. [DOI] [PubMed] [Google Scholar]
- Van Dongen HPA, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: Evidence of trait-like differential vulnerability. Sleep. 2004;27:423–433. [PubMed] [Google Scholar]
- Van Dongen HPA, Dinges DF. Investigating the interaction between the homeostatic and circadian processes of sleep-wake regulation for the prediction of waking neurobehavioural performance. Journal of Sleep Research. 2003;12:181–187. doi: 10.1046/j.1365-2869.2003.00357.x. [DOI] [PubMed] [Google Scholar]
- Van Dongen HPA, Dinges DF. Sleep, circadian rhythms, and psychomotor vigilance. Clinics in Sports Medicine. 2005;24:237–249. doi: 10.1016/j.csm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Van Dongen HPA, Mott CG, Huang JK, Mollicone DJ, McKenzie FD, Dinges DF. Optimization of biomathematical model predictions for cognitive performance impairment in individuals: Accounting for unknown traits and uncertain states in homeostatic and circadian processes. Sleep. 2007;30:1129–1143. doi: 10.1093/sleep/30.9.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viola AU, Archer SN, James LM, Groeger JA, Lo JCY, Skene DJ, Von Schantz M, Dijk DJ. PER3 polymorphism predicts sleep structure and waking performance. Current Biology. 2007;17:613–618. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]