Abstract
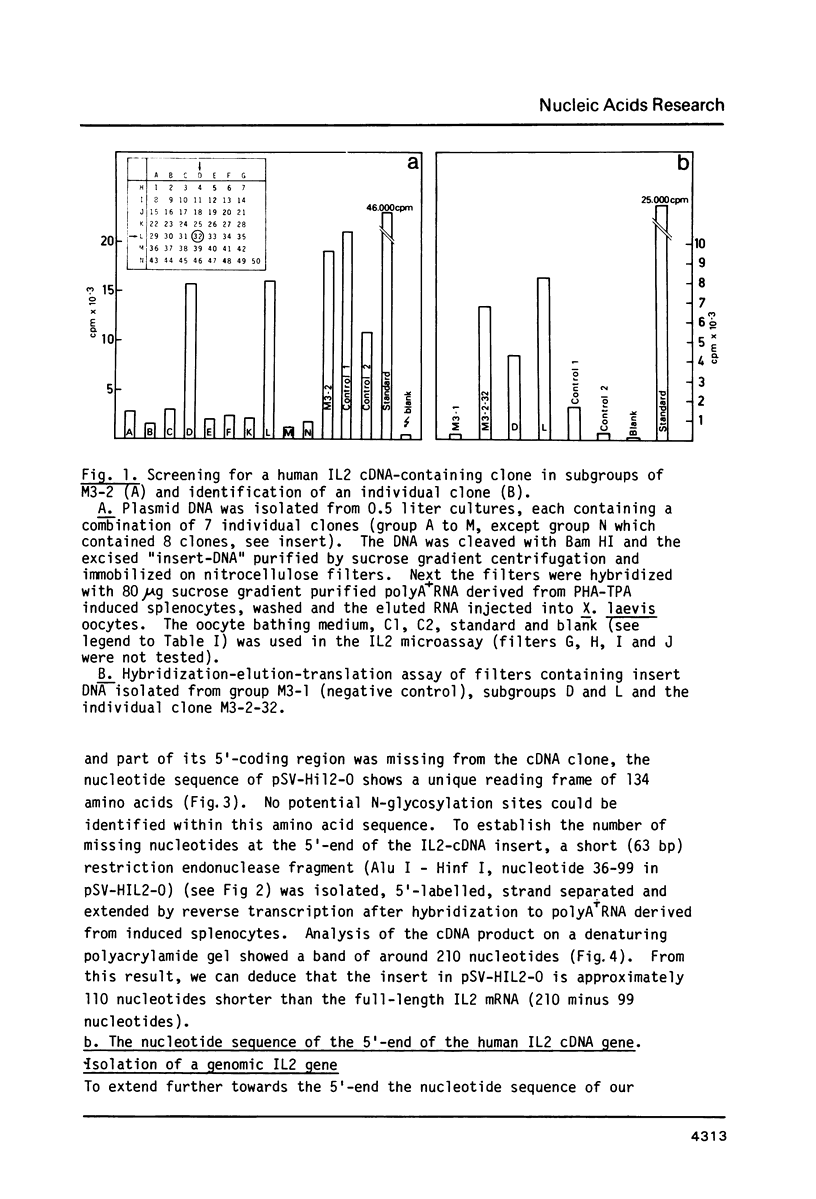
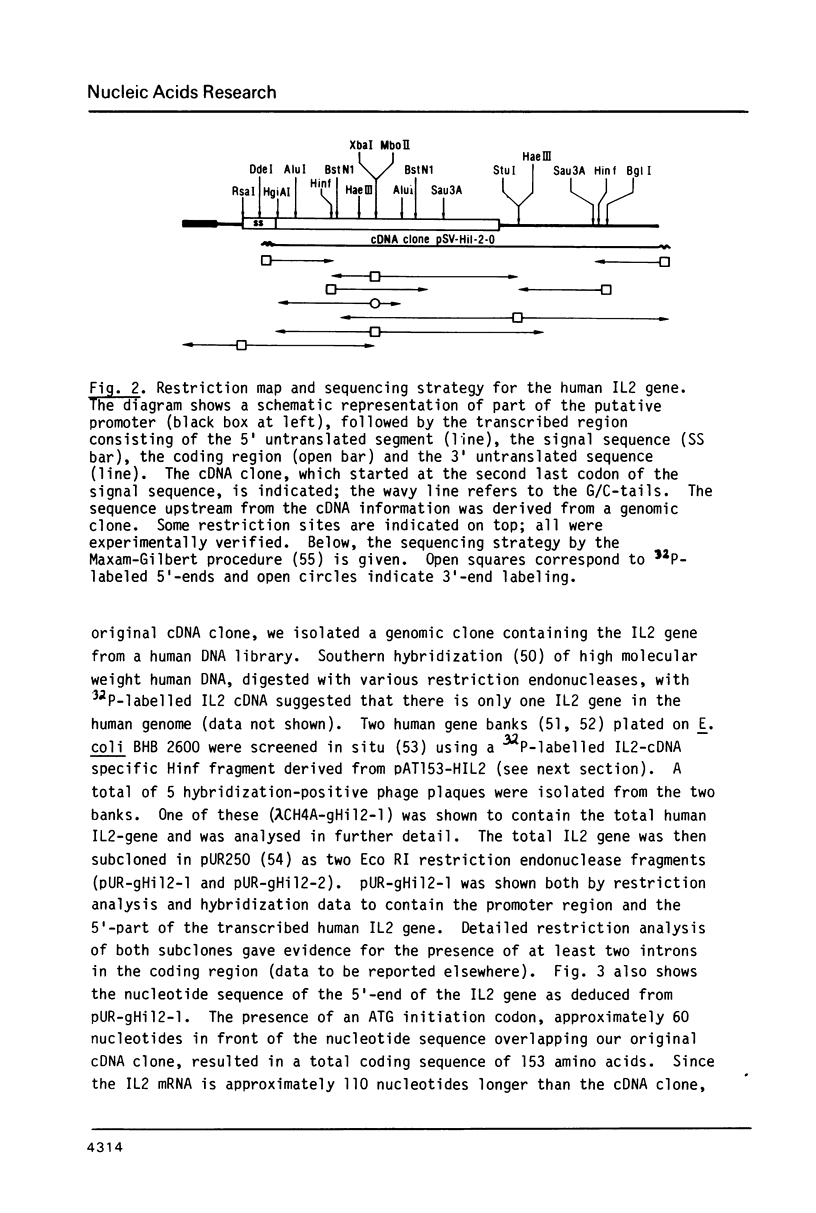
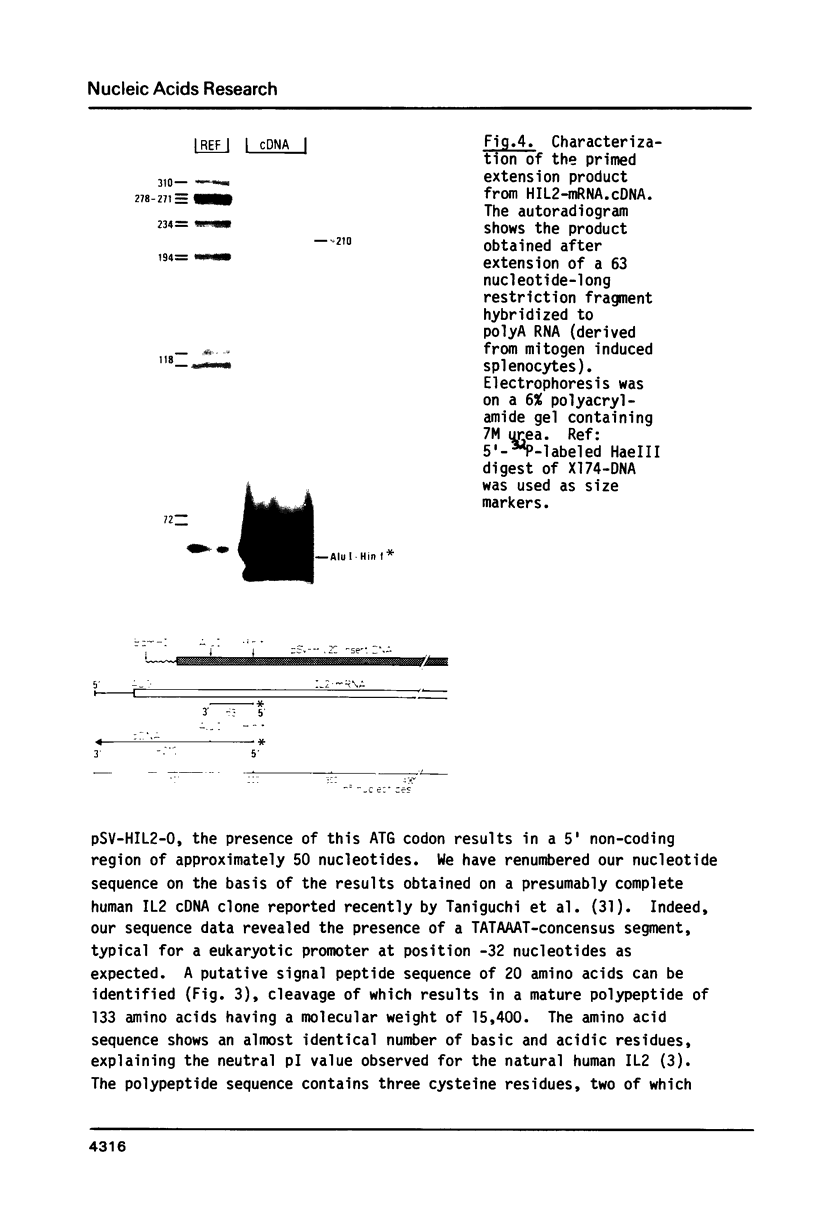
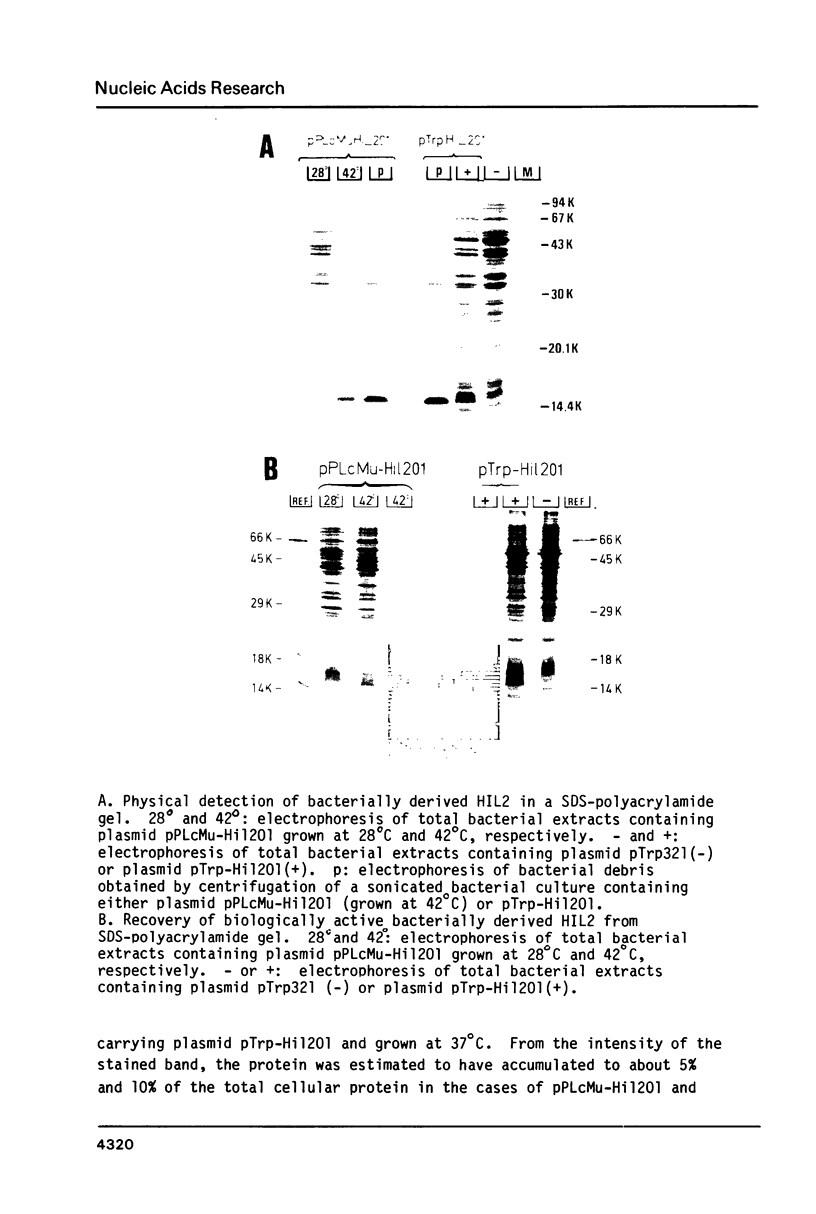
A recombinant plasmid containing human interleukin 2 (IL2) cDNA was identified in a cDNA library constructed from mRNA derived from PHA-TPA induced splenocytes. Using this cDNA as a hybridization probe, a DNA fragment containing the IL2 gene was isolated from a collection of hybrid phages derived from human genomic DNA. A unique reading frame was identified from the nucleotide sequence derived from these plasmids coding for a polypeptide of 153 amino acids and containing a putative signal sequence of 20 amino acids. A mature polypeptide starting with either Met-Ala-Pro or Met-Pro was expressed in E. coli under control of the E. coli trp promoter or using a combination of the phage lambda PL promoter and a ribosome binding site derived from phage Mu. The bacterial IL2 polypeptide had a molecular weight of 15,000 daltons and accounted for more than 10% of the total E. coli proteins in fully induced cells; it was biologically active in the T-cell specific DNA synthesis assay, even after recovery from a SDS-containing polyacrylamide gel.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcocer-Varela J., Alarcón-Segovia D. Decreased production of and response to interleukin-2 by cultured lymphocytes from patients with systemic lupus erythematosus. J Clin Invest. 1982 Jun;69(6):1388–1392. doi: 10.1172/JCI110579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bleackley R. C., Caplan B., Havele C., Ritzel R. G., Mosmann T. R., Farrar J. J., Paetkau V. Translation of lymphocyte mRNA into biologically-active Interleukin 2 in oocytes. J Immunol. 1981 Dec;127(6):2432–2435. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Colman A., Morser J. Export of proteins from oocytes of Xenopus laevis. Cell. 1979 Jul;17(3):517–526. doi: 10.1016/0092-8674(79)90260-5. [DOI] [PubMed] [Google Scholar]
- Dennert G. Cloned lines of natural killer cells. Nature. 1980 Sep 4;287(5777):47–49. doi: 10.1038/287047a0. [DOI] [PubMed] [Google Scholar]
- Devos R., Cheroutre H., Taya Y., Degrave W., Van Heuverswyn H., Fiers W. Molecular cloning of human immune interferon cDNA and its expression in eukaryotic cells. Nucleic Acids Res. 1982 Apr 24;10(8):2487–2501. doi: 10.1093/nar/10.8.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devos R., Cheroutre H., Taya Y., Fiers W. Isolation and characterization of IFN-gamma mRNA derived from mitogen-induced human splenocytes. J Interferon Res. 1982;2(3):409–420. doi: 10.1089/jir.1982.2.409. [DOI] [PubMed] [Google Scholar]
- Devos R., van Emmelo J., Contreras R., Fiers W. Construction and characterization of a plasmid containing a nearly full-size DNA copy of bacteriophage MS2 RNA. J Mol Biol. 1979 Mar 15;128(4):595–619. doi: 10.1016/0022-2836(79)90295-x. [DOI] [PubMed] [Google Scholar]
- Eberlein T. J., Rosenstein M., Rosenberg S. A. Regression of a disseminated syngeneic solid tumor by systemic transfer of lymphoid cells expanded in interleukin 2. J Exp Med. 1982 Aug 1;156(2):385–397. doi: 10.1084/jem.156.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efrat S., Pilo S., Kaempfer R. Kinetics of induction and molecular size of mRNAs encoding human interleukin-2 and gamma-interferon. Nature. 1982 May 20;297(5863):236–239. doi: 10.1038/297236a0. [DOI] [PubMed] [Google Scholar]
- Frank M. B., Watson J., Mochizuki D., Gillis S. Biochemical and biologic characterization of lymphocyte regulatory molecules. VIII. Purification of interleukin 2 from a human T cell leukemia. J Immunol. 1981 Dec;127(6):2361–2365. [PubMed] [Google Scholar]
- Friedman S. M., Thompson G., Halper J. P., Knowles D. M. OT-CLL: a human T cell chronic lymphocytic leukemia that produces IL 2 in high titer. J Immunol. 1982 Feb;128(2):935–940. [PubMed] [Google Scholar]
- Fuller-Farrar J., Hilfiker M. L., Farrar W. L., Farrar J. J. Phorbol myristic acetate enhances the production of interleukin 2. Cell Immunol. 1981 Feb;58(1):156–164. doi: 10.1016/0008-8749(81)90157-x. [DOI] [PubMed] [Google Scholar]
- Gheysen D., Fiers W. Expression and excretion of human fibroblast beta 1 interferon in monkey cells after transfection with a recombinant SV40 plasmid vector. J Mol Appl Genet. 1982;1(5):385–394. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gillis S., Gillis A. E., Henney C. S. Monoclonal antibody directed against interleukin 2. I. Inhibition of T lymphocyte mitogenesis and the in vitro differentiation of alloreactive cytolytic T cells. J Exp Med. 1981 Sep 1;154(3):983–988. doi: 10.1084/jem.154.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Mochizuki D. Molecular characterization of interleukin 2 produced from tumor cell lines and T cell hybridomas. Curr Top Microbiol Immunol. 1982;100:211–219. doi: 10.1007/978-3-642-68586-6_24. [DOI] [PubMed] [Google Scholar]
- Gillis S., Smith K. A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977 Jul 14;268(5616):154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- Gramatzki M., Strong D. M., Grove S. B., Bonnard G. D. Cryopreserved human cultured T cells as responder cells for the quantitative measurement of interleukin-2: improvement of the assay. J Immunol Methods. 1982 Sep 17;53(2):209–220. doi: 10.1016/0022-1759(82)90143-0. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B., Lane C. D., Woodland H. R., Marbaix G. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature. 1971 Sep 17;233(5316):177–182. doi: 10.1038/233177a0. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Henney C. S., Kuribayashi K., Kern D. E., Gillis S. Interleukin-2 augments natural killer cell activity. Nature. 1981 May 28;291(5813):335–338. doi: 10.1038/291335a0. [DOI] [PubMed] [Google Scholar]
- Hinuma S., Onda H., Naruo K., Ichimori Y., Koyama M., Tsukamoto K. Translation of interleukin 2 mRNA from human peripheral blood leukocytes in Xenopus oocytes. Biochem Biophys Res Commun. 1982 Nov 30;109(2):363–369. doi: 10.1016/0006-291x(82)91729-6. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Lin Y., Stadler B. M., Rabin H. Synthesis of biologically active interleukin 2 by Xenopus oocytes in response to poly(A)-RNA from a gibbon T-cell line. J Biol Chem. 1982 Feb 25;257(4):1587–1590. [PubMed] [Google Scholar]
- Loenen W. A., Brammar W. J. A bacteriophage lambda vector for cloning large DNA fragments made with several restriction enzymes. Gene. 1980 Aug;10(3):249–259. doi: 10.1016/0378-1119(80)90054-2. [DOI] [PubMed] [Google Scholar]
- Lotze M. T., Rosenberg S. A. In vitro growth of cytotoxic human lymphocytes. III. The preparation of lectin-free T cell growth factor (TCGF) and an analysis of its activity. J Immunol. 1981 Jun;126(6):2215–2220. [PubMed] [Google Scholar]
- López-Botet M., Fontán G., Garcia Rodriguez M. C., de Landázuri M. O. Relationship between IL 2 synthesis and the proliferative response to PHA in different primary immunodeficiencies. J Immunol. 1982 Feb;128(2):679–683. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mier J. W., Gallo R. C. Purification and some characteristics of human T-cell growth factor from phytohemagglutinin-stimulated lymphocyte-conditioned media. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6134–6138. doi: 10.1073/pnas.77.10.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mier J. W., Gallo R. C. The purification and properties of human T cell growth factor. J Immunol. 1982 Mar;128(3):1122–1127. [PubMed] [Google Scholar]
- Moretta A., Colombatti M., Chapuis B. Human spleen as a source of T cell growth factor. Clin Exp Immunol. 1981 May;44(2):262–269. [PMC free article] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Okada M., Yoshimura N., Kaieda T., Yamamura Y., Kishimoto T. Establishment and characterization of human T hybrid cells secreting immunoregulatory molecules. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7717–7721. doi: 10.1073/pnas.78.12.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Plasmid vectors for high-efficiency expression controlled by the PL promoter of coliphage lambda. Gene. 1981 Oct;15(1):81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Robb R. J. Human T-cell growth factor: purification and interaction with a cellular receptor. Lymphokine Res. 1982;1(2):37–43. [PubMed] [Google Scholar]
- Robb R. J., Smith K. A. Heterogeneity of human T-cell growth factor(s) due to variable glycosylation. Mol Immunol. 1981 Dec;18(12):1087–1094. doi: 10.1016/0161-5890(81)90024-9. [DOI] [PubMed] [Google Scholar]
- Ruscetti F. W., Gallo R. C. Human T-lymphocyte growth factor: regulation of growth and function of T lymphocytes. Blood. 1981 Mar;57(3):379–394. [PubMed] [Google Scholar]
- Rüther U. pUR 250 allows rapid chemical sequencing of both DNA strands of its inserts. Nucleic Acids Res. 1982 Oct 11;10(19):5765–5772. doi: 10.1093/nar/10.19.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier M. H., Iscove N. N., Tees R., Aarden L., von Boehmer H. Clones of killer and helper T cells: growth requirements, specificity and retention of function in long-term culture. Immunol Rev. 1980;51:315–336. doi: 10.1111/j.1600-065x.1980.tb00326.x. [DOI] [PubMed] [Google Scholar]
- Smith K. A. T-cell growth factor. Immunol Rev. 1980;51:337–357. doi: 10.1111/j.1600-065x.1980.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stadler B. M., Berenstein E. H., Siraganian R. P., Oppenheim J. J. Monoclonal antibody against human interleukin 2 (IL 2). I. Purification of IL 2 for the production of monoclonal antibodies. J Immunol. 1982 Apr;128(4):1620–1624. [PubMed] [Google Scholar]
- Taniguchi T., Matsui H., Fujita T., Takaoka C., Kashima N., Yoshimoto R., Hamuro J. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983 Mar 24;302(5906):305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]
- Tavernier J., Derynck R., Fiers W. Evidence for a unique human fibroblast interferon (IFN-beta 1) chromosomal gene, devoid of intervening sequences. Nucleic Acids Res. 1981 Feb 11;9(3):461–471. doi: 10.1093/nar/9.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Welte K., Wang C. Y., Mertelsmann R., Venuta S., Feldman S. P., Moore M. A. Purification of human interleukin 2 to apparent homogeneity and its molecular heterogeneity. J Exp Med. 1982 Aug 1;156(2):454–464. doi: 10.1084/jem.156.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]





