Abstract
In 2008, we published an article arguing that the age-related loss of muscle strength is only partially explained by the reduction in muscle mass and that other physiologic factors explain muscle weakness in older adults (Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63:829–834). Accordingly, we proposed that these events (strength and mass loss) be defined independently, leaving the term “sarcopenia” to be used in its original context to describe the age-related loss of muscle mass. We subsequently coined the term “dynapenia” to describe the age-related loss of muscle strength and power. This article will give an update on both the biological and clinical literature on dynapenia—serving to best synthesize this translational topic. Additionally, we propose a working decision algorithm for defining dynapenia. This algorithm is specific to screening for and defining dynapenia using age, presence or absence of risk factors, a grip strength screening, and if warranted a test for knee extension strength. A definition for a single risk factor such as dynapenia will provide information in building a risk profile for the complex etiology of physical disability. As such, this approach mimics the development of risk profiles for cardiovascular disease that include such factors as hypercholesterolemia, hypertension, hyperglycemia, etc. Because of a lack of data, the working decision algorithm remains to be fully developed and evaluated. However, these efforts are expected to provide a specific understanding of the role that dynapenia plays in the loss of physical function and increased risk for disability among older adults.
Keywords: Strength, Weakness, Atrophy, Function, Disability
IN 2006, there were 37.3 million adults more than the age of 65 years living in the United States (2). Demographers expect the number of older persons to double to 86.7 million—or to 20.6% of the U.S. population—by the year 2050. This surge in growth of the aging population has prompted the Institute on Medicine to develop specific literature focused on retooling medical resources for an aging America (3) to cope with the 42% or 15.6 million who report having one or more limitations performing daily tasks (e.g., walking two to three blocks, transferring from the chair) that are essential for maintaining independence in the community (2). This population growth and associated incidence of physical disability have led to increased scientific interest on the biology of aging. Over the past several decades, the scientific and medical communities have recognized that skeletal muscle dysfunction (e.g., muscle weakness, muscle atrophy, poor muscle coordination, etc) is a debilitating and life threatening condition in older persons. For example, the age-associated loss of muscle strength is highly associated with both mortality and physical disability (4–8), and maintenance of muscle mass with advancing age is critical because it serves as a metabolic reservoir that is needed to effectively withstand disease (9–11).
In recent years, there has been a growing effort to develop criteria for the clinical diagnosis of “sarcopenia.” For example, a European team recently published a consensus statement on the definition and diagnosis of sarcopenia (12), and a team of scientists and practitioners from the United States are currently working to formulate a similar statement (13). Both of these groups criteria for diagnosing sarcopenia involve incorporating aspects of (a) physical function (i.e., gait speed), (b) muscle strength, and (c) muscle mass. Indeed, the term “sarcopenia,” which was initially defined as the age-related loss of muscle mass (14), has also become synonymous with the age-related loss of muscle strength as well as the age-related loss of physical function. In 2008, we published an article arguing that the age-related loss of muscle strength is only partially explained by the reduction in muscle mass and that other physiologic factors explain muscle weakness in older adults (1). Accordingly, we proposed that these events (strength loss and mass loss) need to be defined independently, and in this article we proposed that the term “sarcopenia” be used in its original context to describe the age-related loss of muscle mass and we coined the term “dynapenia” to describe the age-related loss of muscle strength (1). The new terminology is gaining support for use in clinical environments and research settings (15–18); however, despite it's growing popularity, there remains some resistance because such new terminology might confuse efforts for building a consensus decision algorithm for sarcopenia.
We have been asked by the editors of the Journals of Gerontology: Biological and Medical Sciences to provide an update on dynapenia, and herein we will summarize the salient points made in our original article and provide information on recent findings in this field. Additionally, to stimulate discussion along this line we will propose a working decision algorithm to define dynapenia.
SARCOPENIA ≠ DYNAPENIA
What Is the Relationship Between Skeletal Muscle Mass and Muscle Strength?
As stated previously, the term sarcopenia was originally defined as the age-related loss of muscle mass (14). However, one of the first articles on sarcopenia explicitly stated in its abstract (19): “Advancing adult age is associated with profound changes in body composition, the principal component of which is a decrease in skeletal muscle mass. This age-related loss in skeletal muscle has been referred to as sarcopenia. Age-related reduction in muscle is a direct cause of the age-related decrease in muscle strength. Muscle mass (not function) appears to be the major determinant of the age- and sex-related differences in strength… Reduced muscle strength in the elderly is a major cause for their increased prevalence of disability.” Thus began the intimate linking of the age-associated changes in muscle mass, muscle strength, and physical function. The linking of changes in muscle mass and strength (maximal voluntary force) via the same word implies that these are causally linked and that changes in skeletal muscle mass are directly and fully responsible for changes in strength. However, it has been known for more than three decades that muscle strength is not solely dependent upon muscle size (20). In fact, recent longitudinal data from the Health ABC Study indicates that the decline in muscle strength is much more rapid than the concomitant loss of muscle mass and that the change in quadriceps muscle area only explains about 6–8% of the between-subject variability in the change in knee extensor muscle strength (21). This finding is consistent with our experimental models of muscle weakness where we observe that the loss of muscle mass associated with disuse explains less than 10% of the associated loss of muscle strength (22,23). Further, maintaining or gaining muscle mass does not prevent aging-related declines in muscle strength (Figure 1; 21). These findings indicate that the loss of muscle strength in older adults is weakly associated with the loss of lean body mass. Rather they suggest that muscle weakness in older adults is more related to impairments in neural (central) activation and/or reductions in the intrinsic force-generating capacity of skeletal muscle (force/unit tissue; for reviews, see 24–26).
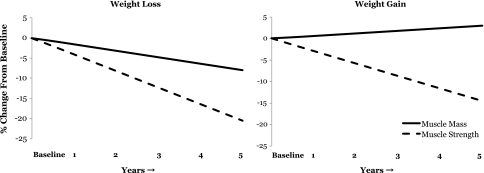
Figure 1.
The age-related loss of muscle strength is weakly associated with the loss of muscle mass. These figures were adapted from published data obtained from the Health ABC Study to examine the relationship between changes in knee extensor strength and quadriceps femoris cross-sectional area muscle (measured via computed tomography) in a 5-y longitudinal study of older adults (21). These data represent the annualized rate of loss more than 5 y in older adults that lost body weight (left panel; n = 309 men) and gained body weight (right panel; n = 143 men). Note that (a) muscle strength is lost at a substantially faster rate than muscle mass and (b) that gaining muscle mass does not prevent the aging-related loss of muscle strength (right panel). Adapted from Delmonico and colleagues (21). Created figure approved by the corresponding author (M. J. Delmonico).
What Is the Relationship Between Health Outcomes and Skeletal Muscle Mass and Muscle Strength?
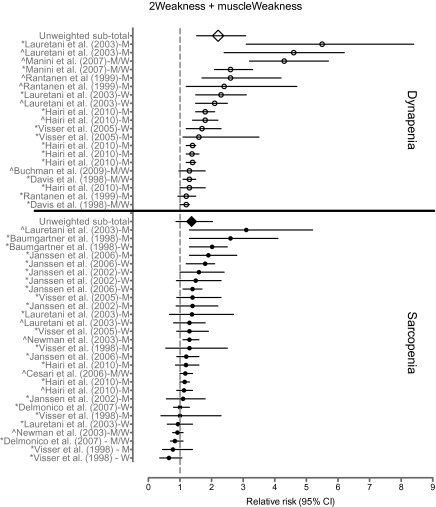
From a clinical perspective, perhaps the more significant question relates to the relative influence of skeletal muscle mass versus muscle strength on physical disability or poor physical performance (e.g., mobility limitation, poor physical performance in activities of daily living). In an attempt to summarize these associations, we conducted a systematic literature search of MEDLINE articles yielding 2,666 hits, and in Figure 2 we present data from seven studies for muscle strength (5,27–32) and nine studies for muscle mass (29,30,32–39) that met our predetermined criteria for evaluation (for complete details of our methodological approach in identifying the selected studies, see Supplementary Table). Unfortunately, a formal meta-analysis between muscle mass and strength and physical disability is not appropriate because of the following issues: (a) the outcomes were not uniform across studies, (b) there were limited prospective cohorts available, (c) there is excessive variability in the measurement of muscle mass and strength, and (d) there are well-known biases in observational studies. Additionally, although we acknowledge the pioneering studies that established the association between muscle strength and size on physical function (40–43), we were unable to include them because they did not meet our predefined criteria for inclusion. However, despite these limitations, this analysis did provide some interesting findings. Specifically, studies examining the association between low muscle strength and poor physical performance or disability were significant 90% of the time (18 out of 20 associations), whereas those examining the same association with low muscle mass were significant 35% of the time (10 out of 28 associations). Furthermore, the unweighted average of the relative risks for low muscle strength was 2.20 (95% CI: 1.5–3.1), whereas low muscle mass exhibited a relative risk of 1.37 (0.87–2.0). We should note that there are limitations in presenting unweighted average relative risks (44); however, the findings suggest that the number and magnitude of associations for low physical performance or disability are greater for low muscle strength than low muscle mass.
Figure 2.
Relative risk of poor physical performance, functional limitation, or physical disability in older adults with dynapenia (low muscle strength), or sarcopenia (low muscle mass). The counterfactuals are older adults with normal muscle strength or mass. Studies investigating multiple outcomes or expressing findings by sex are repeated. The author of each study is followed with whether the relative risk was estimated in men (M), women (W), or both (M/W). Symbols indicate whether outcome was self-report physical function/disability (*) or observed physical performance (^). Specific information on each study is provided in the Supplementary Table.
Another important question relates to the association between skeletal muscle mass/strength and mortality, and over the past decade, several longitudinal studies have enhanced our understanding of these associations (4,6,32). The most recent data from the InChianti Study demonstrated that muscle cross-sectional area of the calf was not associated with an increased risk of mortality when covariates were considered (4). Additional analyses that defined sarcopenia according to sex-specific categories through regression techniques confirmed no association with mortality. In another large cohort of older adults, Newman and colleagues (6) found that whole leg-muscle mass and thigh cross-sectional area were not associated with risk of mortality. However, both grip and knee extensor muscle strength was highly associated with mortality, despite accounting for muscle mass, suggesting that sarcopenia may be secondary to the effects of dynapenia (6). Collectively, these findings indicate that muscle strength—and not simply muscle mass—is a critical factor for determining both physical disability and mortality in older adults. However, with this stated, it should be noted that losses in muscle strength with age is not the sole determinant of the loss of physical function, as there are numerous conditions that can dramatically impair physical function (e.g., poor cardiopulmonary function, cognitive deficits, etc.; 45,46).
If Sarcopenia Does Not Cause Muscle Weakness in Older Adults Then What Does?
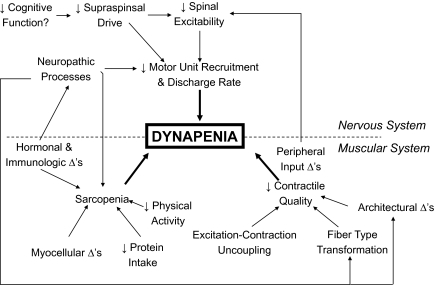
The mechanisms accounting for a decline in muscle strength can be attributed to a combination of “neural” and “muscular” factors. For example, impairments in neural (central) activation, such as that due to a reduction in descending excitatory drive from supraspinal centers and/or suboptimal motor unit recruitment and rate coding, could result in dynapenia. Additionally, a reduction in the intrinsic force-generating capacity of muscle, changes in actomyosin structure and function, and infiltration of adipocytes into muscle fibers could result in dynapenia. Figure 3 depicts a theoretical model of the neurologic and muscular factors potentially leading to dynapenia (this model has been updated since our 2008 article on dynapenia; 1). We will first summarize recent findings on nervous system form and function in the context of muscle force production followed by providing an update on recent findings on muscular factors that are not related to size.
Figure 3.
Theoretical model of the potential neurologic and muscular factors leading to dynapenia. Reprinted with permission from (47).
Neurological mechanisms of muscle weakness.—
There is evidence to suggest that dynapenia is, to some extent, attributable to neurologic mechanisms. For example, function of the cortex, spinal cord, and neuromuscular junction are well-known to influence voluntary activation of muscle fibers (e.g., 48). We will first provide a brief overview of the physiologic processes involved in muscle activation and the assessment of neural activation.
The assessment of muscle strength requires a voluntary effort. Volitional activation comprises the recruitment of motor neurons, and hence muscle fibers, by increased descending drive. With an increased force of contraction, there is increased activation of neurons in the primary motor cortex with increased firing of corticospinal neurons (for review, see 49). The larger this descending drive, the greater the number of motor neurons recruited in the spinal cord and the faster they fire. Accordingly, the two primary ways to increase voluntary force output is to recruit additional motor units within a given alpha-motoneuron pool and/or increase their discharge rate. When these two physiologic properties are optimized, maximal muscle activation results. The most common way to globally investigate whether neural impairments are responsible for a reduction in strength is to deliver a supramaximal electrical stimulus to a peripheral nerve or muscle during a maximal voluntary contraction and evaluate the “added force.” Although this technique is not without limitations (50,51) it does provide insight into the degree of central (voluntary) muscle activation.
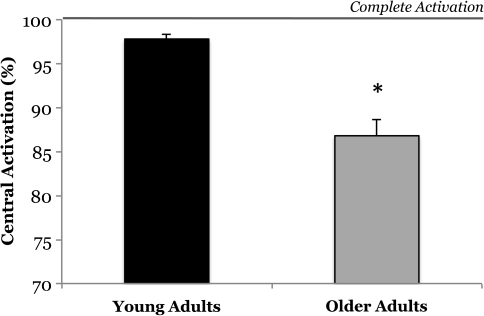
There are equivocal reports in the literature on whether or not advancing age reduces central activation capacity. A synthesis of the literature however does provide some insight into potential explanations of these equivocal reports. Several studies examining the effect of age on central isometric activation of the knee extensors and the elbow flexors suggest that older adults, particularly those greater than 70–75 years of age, exhibit a decrease in central activation, whereas investigations on the age-related changes in central activation of the dorsiflexors yield null findings (for review, see 24,52). Due to the functional differences between these muscles, as well as differences in their physiologic profiles (e.g., motor unit innervations and fiber type characteristics), these muscle-group specific effects are not overly surprising. With respect to the elbow flexors, central activation is consistently reported as 1–5% lower in older adults than in young adults, and this difference is significant in over half the studies (53–58). Of particular interest is a study by Jakobi and Rice (56) that reported a novel and interesting finding: that central activation is less consistent across trials in older men compared with younger men. Specifically, they observed no differences between older and younger adults when central activation was compared based on the single best trial; however, when central activation was calculated based on an average of ten trials a dramatic age difference was observed (79% vs 95% activation). With respect to studies on the knee extensors, a number of reports show no differences between old and young adults (59–62), but a few reports stand out as showing a deficit in central activation with aging (63,64). The first of these studies was conducted by Harridge and colleagues (63). This study examined the oldest cohort of individuals that, to our knowledge, has been examined to date (n = 11, age range: 85–97 years). Here, it was observed that very old adults exhibited significant impairments in central activation (mean: 81%; range: 69–93%). Another study of interest is an article by Stevens and colleagues (64) that combined previously collected data sets on the effect of aging on knee extensor central activation. This study deserves particular attention because it is the largest to date (young adults: n = 46, 18–32 years, older adults: n = 46, 64–84 years). Here, central activation in older adults was significantly less than that of young adults (87% vs 98% activation; Figure 4). The previously mentioned findings are interesting because they demonstrate that clinically meaningful deficits in central activation do exist when a population of older individuals is considered indicating that deficits in neural drive can contribute to some of the muscle weakness observed in older adults—particularly in the very elderly and in certain muscle groups. These findings are particularly meaningful when one considers that older adults, on average, require a relative effort of about 88% to perform a chair rise task (65). Thus, small to modest deficits in neural activation of muscle in older adults may have profound implications on physical function.
Figure 4.
Older adults exhibit impairment in their nervous systems ability to fully activate the knee extensor muscles. This cross-sectional data are the largest study to date examining differences between young and old in central activation and represent data from 46 young (18–32 y) and 46 older (64–84 y) humans. Adapted from Stevens and colleagues (64). Created figure approved by the corresponding author (S. K. Stackhouse).
In recent years, the specific cortical changes associated with aging have begun to be explored. These findings indicate that aging is associated with widespread qualitative and quantitative changes in the motor cortex and spinal cord. For example, there are an overwhelming number of morphometric changes in the motor cortex with advancing age, including a dramatic volumetric reduction in the premotor cortex neuron cell body size (66), significant cortical atrophy of areas near the primary motor cortex (67), and a reduction in the total length of myelinated fibers and integrity of the brain white matter (68,69). Age-related changes have also been observed in the serotonergic (70,71), cholinergic (72), adrenergic (71), dopaminergic (73–76), γ-aminobutyric acidergic (73,75), and glutamatergic systems (73,75), as well as in reductions in neurotrophic factors within the motor cortex (77). In addition to the age-related anatomical and cellular changes as discussed previously, aging also affects motor cortical properties at the systems level. Specifically, aging has been shown to result in cortical hypoexcitability (78–81), a reduced ability to modulate the activity of inappropriate motor networks when required (82–84) and a reduction cortical plasticity (85,86). Collectively, these changes are likely to contribute to age-related reductions in motor performance although the exact relationship to strength loss is yet to be determined.
In addition to the cortical level changes associated with aging, there are also numerous changes at the spinal level. For example, advancing age has been shown to be associated with a reduction spinal excitability (78,80,87–89), altered motor unit discharge properties (90–93), and reduced motor unit size and numbers (94,95). For example, age-related remodeling of motor units appears to preferentially result in denervation of type II (fast) skeletal muscle fibers with collateral reinnervation allowing for the type I (slow) motor units to gain control of the denervated muscle fibers (25) and that when denervation outpaces reinnervation the motor unit is rendered functionally useless (26). Furthermore, the behavioral properties of motor units are also altered with aging indicating a reduction in the incidence of motor unit doublets (27) and a reduction in maximal motor unit discharge rate (28). Theoretically, alterations in many of the aforementioned neural factors could be mechanistically linked to muscle weakness exhibited with aging, but longitudinal studies are needed to more clearly delineate the effect.
Interestingly, over the past couple of years, there have been several reports suggesting a link between muscle weakness and cognitive decline (96,97). One of the more intriguing of these studies observed that poor physical function and muscle strength coexisted with cognitive impairment and that this relationship was independent of muscle mass and physical-activity level (96). This finding raises the question of the interrelationship between neural activation and cognitive function, and further work is needed to better understand these associations.
Muscular mechanisms of muscle weakness.—
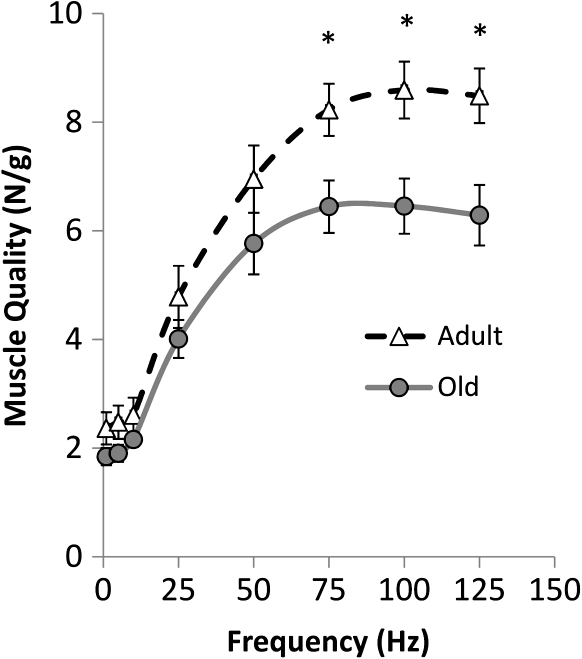
Low levels of skeletal muscle mass are associated with muscle weakness in the elderly, but recent longitudinal studies of aging and disuse indicate that the relative influence of muscle mass on muscle strength is substantially less than originally thought (21–23,98,99). However, there is evidence to suggest that dynapenia is, to some extent, attributable to other muscular mechanisms. The majority of human and animal studies indicate that the intrinsic force-generating capacity of skeletal muscle decreases with age (e.g., force/unit tissue; 100–103). For example, recent animal data from Russ and colleagues (102) provides evidence that older skeletal muscle exhibits a 34% reduction in its intrinsic force-generating capacity (Figure 5). In agreement with this work, human studies also frequently report a reduction in single fiber and whole-muscle contractile quality (101,103,104). Accordingly, these findings illustrate that older skeletal muscle exhibits a reduction in intrinsic force capacity (that would contribute to muscle weakness). The causes of this reduction are yet to determined, but several studies have noted age-related changes in the excitation–contraction coupling processes (105–108) and an association with adipocyte infiltration (109,110).
Figure 5.
Older animal skeletal muscle exhibits a reduction in it's intrinsic force-generating capacity, particularly at high stimulation frequencies. This cross-sectional data were obtained from young (6–8 mos) and older (24 mo) rats by normalizing the electrically stimulated plantarflexor maximal force to the muscle weight. At the higher stimulation frequencies the older—but not yet senescent—muscle exhibited a greater than 30% reduction in its intrinsic force capacity. It should be noted that this reduction occurred in the presence of a very limited amount of atrophy. Modified with permission from Russ and colleagues (102) Modified figure approved by the corresponding author (D. W. Russ).
Excitation–contraction coupling refers to the physiological process of converting the neural signal for muscle activation (the sarcolemmmal action potential) into muscle contraction and force generation (111). There are a number of key events involved in the excitation–contraction coupling process. Namely, electromechanical transduction in skeletal muscle cells requires the dihydropyridine receptor located at the transverse tubule to activate calcium release from the sarcoplasmic reticulum through the ryanodine receptor. The calcium released into the myoplasm binds to troponin C and—through interactions with troponin I and T along with tropomyosin—results in actomyosin interaction (and the associated sliding of these filaments), which continues until calcium is pumped back into the sarcoplasmic reticulum or competitively bound. Disruption or uncoupling at any point along the excitation–contraction coupling pathway could result in reduced intrinsic force capacity and hence dynapenia.
Over the past 15 years, we have begun to understand the effects of aging on the processes involved excitation–contraction coupling. For example, aging has been shown to result in a reduced number of dihydropyridine receptors, and as a result an uncoupling between these receptors and the ryanodine receptor that results in deficits in calcium release in response to muscle excitation, reduced calcium supply to contractile proteins, and eventually reduced contractile force (105–108). In more recent years, several studies have demonstrated that other skeletal muscle proteins are involved in excitation–contraction coupling that have a direct implication for age-associated muscle weakness. Specifically, results from animal studies suggest that aging results in reduced expression of a protein of the sarcoplasmic reticulum junctional face membrane (JP-45; 112–114). This protein alters the levels of expression of the dihydropiridine receptor subunits (i.e., reduced expression of Cav1.1, increased expression of Cavβ1a; 106,112,114–116) and affects protein–protein interactions involved in excitation–contraction coupling (e.g., ryanodine receptor binding to Fk506-binding protein; 102). It has also been suggested that impaired muscle function with aging may result from structural alterations of myosin causing a change in the kinetics of the cross-bridge cycle (117).
In addition to changes in the excitation–contraction coupling process with aging, changes in muscle morphology have also been observed. Over the past decade, numerous studies have reported that aging increases the adipocyte content between muscle groups (intermuscular adipose tissue) and between muscle fascicles (intramuscular adipose tissue; 21,109,110,118,119). The earliest of these studies suggested that greater muscle fat content was associated with reduced muscle strength (109,110) suggesting a potential mechanistic link between increases in fat infiltration in muscle and muscle weakness. Indeed, cytokine production from adipose tissue has been linked to depressed muscle force production (120,121), thus providing a theoretical basis to this assertion. However, more recent longitudinal data has failed to observe a direct relationship between increased levels of intermuscular adipose tissue and strength loss with age (21).
WORKING DECISION ALGORITHM to DEFINE DYNAPENIA
Recent efforts by the European Working Group on Sarcopenia in Older People have yielded a consensus decision algorithm of sarcopenia aimed at practitioners (12). This algorithm uses the following components to define sarcopenia: age, gait speed, grip strength, and muscle mass. According to the algorithm, all adults more than the age of 65 years should be assessed for gait speed. It is then recommended that individuals with a gait speed slower than 0.80 m/s be tested for appendicular or total muscle mass that is used to diagnose sarcopenia. Older adults without gait speed impairments (>0.80 m/s) would perform a grip strength assessment. Individuals with low grip strength are then referred for measurement of appendicular or total muscle mass to diagnose sarcopenia. The algorithm certainly has some strong points—namely feasibility—as gait speed and grip strength testing could easily be conducted at small clinics. However, the cut points proposed for low muscle mass have not resulted in consistently significant associations with health outcomes (29,38), misclassifies obese older adults (38), and has marginal discriminate ability in identifying older adults at risk of disability (area under the receiver operator curve ∼0.70; 30). It should be noted that the addition of fat mass moderately improves the association with health outcomes (35,38). The algorithm also uses several constructs in defining sarcopenia, and as such resembles a syndrome similar to most well-accepted definition of frailty (e.g., weakness, exhaustion, unintentional weight loss, slow gait speed, and low physical activity; 122). As such, the algorithm is not specific to low muscle mass or low muscle strength, but rather seems to encompass a holistic approach to assessing a geriatric patient.
We have taken a different approach in developing and proposing a working decision algorithm specific for dynapenia. It is well-known that the etiology of poor physical performance (e.g., slow gait speed) is a multifactorial and complex process that manifests itself due to biological (cognitive, musculoskeletal, hormonal, neural etc.), psychological, environmental, and sociological origins (123). As such, multiple conditions and/or risk factors are involved in the loss of physical function, of which dynapenia is only one factor. This philosophy is similar to building a risk profile for cardiovascular disease that is composed of several factors that include: hypercholesterolemia, hypertension, elevated inflammation, and glucose dysregulation. Each risk factor has it's own set of clinical criteria and definition that is used to build the risk profile (i.e., Framingham Risk Score). Muscle weakness is one factor involved in the etiology of a complex health problem of functional limitation or physical disability and should be treated as such. We propose that the decision algorithm needs to screen for the specific condition being ascertained—low muscle strength.
Description of Proposed Algorithm
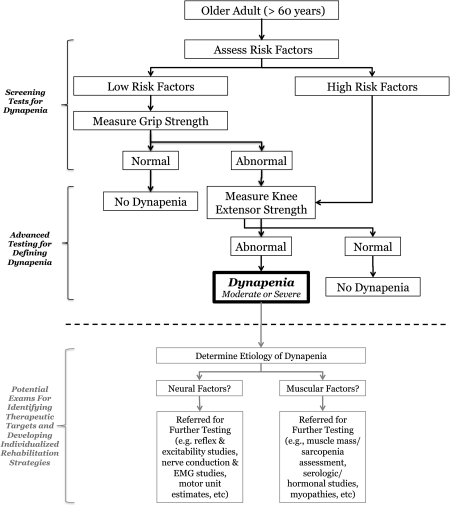
In Figure 6, we propose a decision algorithm for dynapenia. This should be considered a work in progress because there is lack of empirical support for several of its components. The approach is to use a combination of risk factors and screening tests to advise practitioners and scientists about whether muscle weakness is likely a factor in an individual's disability or poor physical performance profile. Specifically, the algorithm begins by screening individuals greater than 60 years for dynapenia—although it could easily be applied to younger individuals at risk. Next, we propose that individuals with sufficiently severe risk factors for the development of dynapenia be referred for a knee extension strength assessment. Conversely, if an individual has no or low risk factors, it is proposed that they undergo an easy to administer grip strength assessment to determine whether a lower extremity strength test is warranted. It should be noted that our proposed algorithm is designed to first determine whether an individual presents with dynapenia and then recommends follow-up testing based on this outcome to determine the etiology of dynapenia (e.g., is the weakness due to neurologic or muscular origins). In an attempt to facilitate discussions on dynapenia, we have established a website blog where we invite comments and input on general and specific components of the algorithm: http://dynapenia.blogspot.com.
Figure 6.
Working decision algorithm to define dynapenia. In an attempt to facilitate discussions on dynapenia, we have established a Web site blog where we invite comments and input on general and specific components of the algorithm: http://dynapenia.blogspot.com.
Frequently Asked Questions About the Proposed Algorithm
Subsequently, we address some anticipated questions and concerns over this algorithm:
Is there sufficient data to define a limited series of relevant risk factors of sufficient predictive value for dynapenia? The simple answer to this question is: no, at present, there is insufficient data identifying specific risk factors for the development of muscle weakness with aging. Accordingly, further research is needed to better define risk factors for dynapenia. Potential risk factors may include certain lifestyle or anthropometric factors (e.g., low levels of physical activity, malnutrition, obesity), diseases or health conditions (e.g., osteoarthritis, vitamin D deficiency, anemia, osteoporosis, cardiorespiratory diseases, active cancer, low cognitive function), medical history (e.g., unexplained weight loss, history of falls), psychological factors (e.g., kinesiophobia), and/or self-reported limitations (e.g., self-reported muscle weakness, mobility limitations, fatigue, or exhaustion). Most of these risk factors can be ascertained in a clinic environment and be used to construct a risk profile.
Knee extension strength is difficult to measure in clinical settings, so, why not just use grip strength? We believe that the assessment of knee extension muscle strength is warranted as lower extremity muscle strength is critically relevant to gait speed and physical function (124). We do believe grip strength is important and that it is useful in the screening phase; however, the use of grip strength alone is likely to misclassify individuals as grip strength only explains about 40% of the variance in lower extremity strength (125–128). Indeed sophisticated and sensitive dynamometers for assessing lower extremity muscle strength are not readily available in most clinics; thus, referral to a separate assessment venue would likely be required in many instances. This approach would be similar to that used in cardiology where a practitioner refers patients for stress testing at specialized clinics. In many developed countries, dynamometers are commonly available in physical therapy and physiotherapy clinics, and these facilities could serve in this capacity.
Is there sufficient data to define the cutoff points for dynapenia? No, one limitation to the proposed algorithm relates to the lack of data to define cutoff points for dynapenia. With this stated, there is already some literature in this area (5,129–133), and epidemiological studies of aging have routinely collected data on upper and/or lower extremity muscle strength. Therefore, it may be feasible to establish cutoff points for defining dynapenia in the near future. Consistent with others (5,126), correction for anthropometric variability is recommended to define dyanpenia (e.g., strength normalized to body mass and/or height).
Why haven’t you incorporated measurements of gait speed or other indexes of physical function? It is clear that physical function (e.g., gait speed, chair rise time) is a critical risk factor of health in older persons. However, the etiology of poor physical function (e.g. slow gait speed) is a multifactorial and complex process that is influenced by biological, psychological, environmental, and sociological factors (123). As such, multiple factors are involved in the loss of physical function, of which muscle weakness is only one factor. To properly treat a patient with low physical function, researchers and practitioners would greatly benefit from understanding the origin of these functional deficits; thus, an evaluation that is specific to muscle weakness would help to either (a) develop targeted treatments or (b) to discount the involvement of muscle weakness on physical function and allow for other systems to be therapeutically targeted. As such, we propose that this decision algorithm should screen for the specific condition being ascertained (low muscle strength), which is in alignment with criteria commonly used to define such conditions as hypercholesteremia, hypertension, and hyperglycemia.
Why define dynapenia based on muscle strength as opposed to muscle power? Muscle power is defined as the time rate of doing work (134). Indeed muscle power is strongly correlated to performance of daily tasks (135–137) and declines at a faster rate with increasing age than muscle strength (138–140). However, muscle power performs similarly to muscle strength when identifying individuals with poor physical function and self-reported disability (30,124,140). Additionally, recent work from Bean and colleagues indicates that older adults with mobility limitations who participated in a 16-week “power-training” exercise program increased leg press power about 10% more than a traditional “strength-training” exercise program, but—despite the greater increase in power—both groups exhibited equivalent improvements in muscle strength and mobility performance (141). Another aspect we considered when faced with the choice of defining dynapenia based on muscle strength or power was the availability of data to evaluate statistically valid cut points. Epidemiological studies of aging have routinely collected data on upper and/or lower extremity muscle strength (isokinetic or isometric), whereas there is considerably less data available on muscle power. Moreover, the equipment to assess muscle power is less readily accessible than that to assess muscle strength. Based on these previously mentioned factors, we felt that the assessment of muscle strength was reasonable and that it was a more clinically viable assessment tool.
Isn’t defining the loss of muscle mass as sarcopenia and loss of strength as dynapenia as separate entities just going to lead to confusion? We recognize and respect this commonly expressed concern. However, recent findings strongly suggest that (a) the loss of muscle strength with aging is largely independent of the loss of muscle mass (Figure 1) and (b) muscle weakness poses a greater relative risk for the development of disability than does low muscle mass (Figure 2). As such, it seems scientifically unsound to define a clinical condition based on factors that are weakly associated with one another. Additionally, we should again note that in other medical disciplines, it is common to define and treat specific conditions based on particular biomarkers that are related to a more global disease or disorder. For example, cholesterol, C-reactive protein, and blood pressure are all independently defined conditions—with different treatment strategies—that are used to predict ones risk for heart disease. Similarly, we believe that predicting ones risk for physical disability needs to consist of a combination of independently defined conditions.
CONCLUSIONS
In this article, we argue that the age-related loss of muscle strength (dynapenia) is only partially explained by the reduction in muscle mass (sarcopenia), and that these two conditions need to be defined independent of one another. Salient points for this argument are that (a) recent data from longitudinal studies on aging indicate that maintaining or gaining muscle mass does not prevent aging-related declines in muscle strength and (b) muscle weakness is independently associated with physical disability and mortality. The physiologic mechanisms of muscle weakness with aging are multifactorial and arise from deficits in neural activation, reductions in the intrinsic force-generating capacity of muscle, as well as muscle wasting. Accordingly, we propose a working decision algorithm for defining dynapenia and address some of the limitations and future directions for improving the algorithm. Specifically, it is suggested that future research is needed to further develop a clinically and scientifically sound decision algorithm (e.g., identification of risk factors for predicting dynapenia, identification of clinically relevant cut points for muscle strength), and to determine the relative contribution of the various segmental components of the neuromuscular system associated with muscle weakness.
SUPPLEMENTARY MATERIAL
Supplementary table can be found at: http://biomed.gerontologyjournals.org/.
FUNDING
National Institute on Aging Claude D. Pepper Center (P30AG028740) and National Institute on Aging (R21AG031974) to T.M.M; Eunice Kennedy Shriver National Institute of Child Health and Human Development (R15HD065552) to B.C.C.
Supplementary Material
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, or the National Institutes of Health.
References
- 1.Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63:829–834. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- 2.Statistics FIFoA-R. Older Americans 2008: Key Indicators of Well-being. Washington, DC: U.S. Government Printing Office; 2008. [Google Scholar]
- 3.Committee on the Future Health Care Workforce for Older Americans IoM. Retooling for an Aging America: Building the Health Care Workforce. Washington, DC: National Academies Press; 2008. [PubMed] [Google Scholar]
- 4.Cesari M, Pahor M, Lauretani F, Zamboni V, Bandinelli S, Bernabei R, et al. Skeletal muscle and mortality results from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2009;64:377–384. doi: 10.1093/gerona/gln031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manini TM, Visser M, Won-Park S, Patel KV, Strotmeyer ES, Chen H, et al. Knee extension strength cutpoints for maintaining mobility. J Am Geriatr Soc. 2007;55:451–457. doi: 10.1111/j.1532-5415.2007.01087.x. [DOI] [PubMed] [Google Scholar]
- 6.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 7.Visser M, Newman AB, Nevitt MC, Kritchevsky SB, Stamm EB, Goodpaster BH, et al. Reexamining the sarcopenia hypothesis. Muscle mass versus muscle strength. Health, Aging, and Body Composition Study Research Group. Ann N Y Acad Sci. 2000;904:456–461. [PubMed] [Google Scholar]
- 8.Visser M, Simonsick EM, Colbert LH, Brach J, Rubin SM, Kritchevsky SB, et al. Type and intensity of activity and risk of mobility limitation: the mediating role of muscle parameters. J Am Geriatr Soc. 2005;53:762–770. doi: 10.1111/j.1532-5415.2005.53257.x. [DOI] [PubMed] [Google Scholar]
- 9.Lainscak M, Podbregar M, Anker SD. How does cachexia influence survival in cancer, heart failure and other chronic diseases? Curr Opin Support Palliat Care. 2007;1:299–305. doi: 10.1097/SPC.0b013e3282f31667. [DOI] [PubMed] [Google Scholar]
- 10.Muscaritoli M, Bossola M, Bellantone R, Rossi Fanelli F. Therapy of muscle wasting in cancer: what is the future? Curr Opin Clin Nutr Metab Care. 2004;7:459–466. doi: 10.1097/01.mco.0000134366.07148.2e. [DOI] [PubMed] [Google Scholar]
- 11.Tan BH, Fearon KC. Cachexia: prevalence and impact in medicine. Curr Opin Clin Nutr Metab Care. 2008;11:400–407. doi: 10.1097/MCO.0b013e328300ecc1. [DOI] [PubMed] [Google Scholar]
- 12.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Biomarkers Consortium: Sarcopenia Project. Foundation for the National Institutes of Health; 2010. www.thebiomarkersconsortium.org/index.php?option=com_content&;task=view&id=138&Itemid=61. Accessed November 15, 2010. [Google Scholar]
- 14.Rosenberg IH. Summary comments. Am J Clin Nutr. 1989;50:1231–1233. [Google Scholar]
- 15.Janssen I. Evolution of sarcopenia research. Appl Physiol Nutr Metab. 2010;35:707–712. doi: 10.1139/H10-067. [DOI] [PubMed] [Google Scholar]
- 16.Bouchard DR, Janssen I. Dynapenic-obesity and physical function in older adults. J Gerontol A Biol Sci Med Sci. 65:71–77. doi: 10.1093/gerona/glp159. [DOI] [PubMed] [Google Scholar]
- 17.Glover EI, Phillips SM. Resistance exercise and appropriate nutrition to counteract muscle wasting and promote muscle hypertrophy. Curr Opin Clin Nutr Metab Care. 13:630–634. doi: 10.1097/MCO.0b013e32833f1ae5. [DOI] [PubMed] [Google Scholar]
- 18.Barbat-Artigas S, Dupontgand S, Fex A, Karelis AD, Aubertin-Leheudre M. Relationship between dynapenia and cardio-respiratory functions in healthy postmenopausal women: a novel clinical criteria. Menopause. doi: 10.1097/gme.0b013e3181f7a596. In press. [DOI] [PubMed] [Google Scholar]
- 19.Evans WJ. What is sarcopenia? J Gerontol A Biol Sci Med Sci. 1995;50:5–8. doi: 10.1093/gerona/50a.special_issue.5. [DOI] [PubMed] [Google Scholar]
- 20.Moritani T. deVries HA. Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med. 1979;58:115–130. [PubMed] [Google Scholar]
- 21.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark BC, Fernhall B, Ploutz-Snyder LL. Adaptations in human neuromuscular function following prolonged unweighting: I. Skeletal muscle contractile properties and applied ischemia efficacy. J Appl Physiol. 2006;101:256–263. doi: 10.1152/japplphysiol.01402.2005. [DOI] [PubMed] [Google Scholar]
- 23.Clark BC, Manini TM, Bolanowski SJ, Ploutz-Snyder LL. Adaptations in human neuromuscular function following prolonged unweighting: II. Neurological properties and motor imagery efficacy. J Appl Physiol. 2006;101:264–272. doi: 10.1152/japplphysiol.01404.2005. [DOI] [PubMed] [Google Scholar]
- 24.Clark BC, Taylor JL. Age-related changes in motor cortical properties and voluntary activation of skeletal muscle. Curr Aging Sci. doi: 10.2174/1874609811104030192. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delbono O. Expression and regulation of excitation-contraction coupling proteins in aging skeletal muscle. Curr Aging Sci. doi: 10.2174/1874609811104030248. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kostek MC. Delmonico MJ. Age-related changes in adult muscle morphology. Curr Aging Sci. doi: 10.2174/1874609811104030221. In press. [DOI] [PubMed] [Google Scholar]
- 27.Buchman AS, Boyle PA, Leurgans SE, Evans DA, Bennett DA. Pulmonary function, muscle strength, and incident mobility disability in elders. Proc Am Thorac Soc. 2009;6:581–587. doi: 10.1513/pats.200905-030RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis JW, Ross PD, Preston SD, Nevitt MC, Wasnich RD. Strength, physical activity, and body mass index: relationship to performance-based measures and activities of daily living among older Japanese women in Hawaii. J Am Geriatr Soc. 1998;46:274–279. doi: 10.1111/j.1532-5415.1998.tb01037.x. [DOI] [PubMed] [Google Scholar]
- 29.Hairi NN, Cumming RG, Naganathan V, Handelsman DJ, Le Couteur DG, Creasey H, et al. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the concord health and ageing in men project. J Am Geriatr Soc. 2010;58:2055–2062. doi: 10.1111/j.1532-5415.2010.03145.x. [DOI] [PubMed] [Google Scholar]
- 30.Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 31.Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, et al. Midlife hand grip strength as a predictor of old age disability. J Am Med Assoc. 1999;281:558–560. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 32.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 33.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 34.Cesari M, Leeuwenburgh C, Lauretani F, Onder G, Bandinelli S, Maraldi C, et al. Frailty syndrome and skeletal muscle: results from the Invecchiare in Chianti study. Am J Clin Nutr. 2006;83:1142–1148. doi: 10.1093/ajcn/83.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delmonico MJ, Harris TB, Lee JS, Visser M, Nevitt M, Kritchevsky SB, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55:769–774. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 36.Janssen I. Influence of sarcopenia on the development of physical disability: the Cardiovascular Health Study. J Am Geriatr Soc. 2006;54:56–62. doi: 10.1111/j.1532-5415.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 37.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 38.Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 39.Visser M, Harris TB, Langlois J, Hannan MT, Roubenoff R, Felson DT, et al. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 1998;53:M214–M221. doi: 10.1093/gerona/53a.3.m214. [DOI] [PubMed] [Google Scholar]
- 40.Bassey EJ, Short AH. A new method for measuring power output in a single leg extension: feasibility, reliability and validity. Eur J Appl Physiol Occup Physiol. 1990;60:385–390. doi: 10.1007/BF00713504. [DOI] [PubMed] [Google Scholar]
- 41.Buchner DM, Larson EB, Wagner EH, Koepsell TD, de Lateur BJ. Evidence for a non-linear relationship between leg strength and gait speed. Age Ageing. 1996;25:386–391. doi: 10.1093/ageing/25.5.386. [DOI] [PubMed] [Google Scholar]
- 42.Ferrucci L, Guralnik JM, Buchner D, Kasper J, Lamb SE, Simonsick EM, et al. Departures from linearity in the relationship between measures of muscular strength and physical performance of the lower extremities: the Women's Health and Aging Study. J Gerontol A Biol Sci Med Sci. 1997;52:M275–M285. doi: 10.1093/gerona/52a.5.m275. [DOI] [PubMed] [Google Scholar]
- 43.Young A. Exercise physiology in geriatric practice. Acta Med Scand Suppl. 1986;711:227–232. doi: 10.1111/j.0954-6820.1986.tb08955.x. [DOI] [PubMed] [Google Scholar]
- 44.Begg CB, Berlin JA. Publication bias and dissemination of clinical research. J Natl Cancer Inst. 1989;81:107–115. doi: 10.1093/jnci/81.2.107. [DOI] [PubMed] [Google Scholar]
- 45.Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology. 2006;20:215–223. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- 46.Paterson DH, Govindasamy D, Vidmar M, Cunningham DA, Koval JJ. Longitudinal study of determinants of dependence in an elderly population. J Am Geriatr Soc. 2004;52:1632–1638. doi: 10.1111/j.1532-5415.2004.52454.x. [DOI] [PubMed] [Google Scholar]
- 47.Clark BC, Manini TM. Functional consequences of sarcopenia and dynapenia in the elderly. Curr Opin Clin Nutr Metab Care. 2010;13:271–276. doi: 10.1097/MCO.0b013e328337819e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 49.Ashe J. Force and the motor cortex. Behav Brain Res. 1997;87:255–269. doi: 10.1016/s0166-4328(97)00752-3. [DOI] [PubMed] [Google Scholar]
- 50.Taylor JL. Point: the interpolated twitch does/does not provide a valid measure of the voluntary activation of muscle. J Appl Physiol. 2009;107:354–355. doi: 10.1152/japplphysiol.91220.2008. [DOI] [PubMed] [Google Scholar]
- 51.de Haan A, Gerrits KH, de Ruiter CJ. Counterpoint: the interpolated twitch does not provide a valid measure of the voluntary activation of muscle. J Appl Physiol. 2009;107:355–357. doi: 10.1152/japplphysiol.91220.2008a. discussion 357–358. [DOI] [PubMed] [Google Scholar]
- 52.Klass M, Baudry S, Duchateau J. Voluntary activation during maximal contraction with advancing age: a brief review. Eur J Appl Physiol. 2007;100:543–551. doi: 10.1007/s00421-006-0205-x. [DOI] [PubMed] [Google Scholar]
- 53.Bilodeau M, Erb MD, Nichols JM, Joiner KL, Weeks JB. Fatigue of elbow flexor muscles in younger and older adults. Muscle Nerve. 2001;24:98–106. doi: 10.1002/1097-4598(200101)24:1<98::aid-mus11>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 54.De Serres SJ, Enoka RM. Older adults can maximally activate the biceps brachii muscle by voluntary command. J Appl Physiol. 1998;84:284–291. doi: 10.1152/jappl.1998.84.1.284. [DOI] [PubMed] [Google Scholar]
- 55.Hunter SK, Todd G, Butler JE, Gandevia SC, Taylor JL. Recovery from supraspinal fatigue is slowed in old adults after fatiguing maximal isometric contractions. J Appl Physiol. 2008;105:1199–1209. doi: 10.1152/japplphysiol.01246.2007. [DOI] [PubMed] [Google Scholar]
- 56.Jakobi JM, Rice CL. Voluntary muscle activation varies with age and muscle group. J Appl Physiol. 2002;93:457–462. doi: 10.1152/japplphysiol.00012.2002. [DOI] [PubMed] [Google Scholar]
- 57.Yoon T, De-Lap BS, Griffith EE, Hunter SK. Age-related muscle fatigue after a low-force fatiguing contraction is explained by central fatigue. Muscle Nerve. 2008;37:457–466. doi: 10.1002/mus.20969. [DOI] [PubMed] [Google Scholar]
- 58.Yue GH, Ranganathan VK, Siemionow V, Liu JZ, Sahgal V. Older adults exhibit a reduced ability to fully activate their biceps brachii muscle. J Gerontol A Biol Sci Med Sci. 1999;54:M249–M253. doi: 10.1093/gerona/54.5.m249. [DOI] [PubMed] [Google Scholar]
- 59.Cannon J, Kay D, Tarpenning KM, Marino FE. Comparative effects of resistance training on peak isometric torque, muscle hypertrophy, voluntary activation and surface EMG between young and elderly women. Clin Physiol Funct Imaging. 2007;27:91–100. doi: 10.1111/j.1475-097X.2007.00719.x. [DOI] [PubMed] [Google Scholar]
- 60.Knight CA, Kamen G. Adaptations in muscular activation of the knee extensor muscles with strength training in young and older adults. J Electromyogr Kinesiol. 2001;11:405–412. doi: 10.1016/s1050-6411(01)00023-2. [DOI] [PubMed] [Google Scholar]
- 61.Roos MR, Rice CL, Connelly DM, Vandervoort AA. Quadriceps muscle strength, contractile properties, and motor unit firing rates in young and old men. Muscle Nerve. 1999;22:1094–1103. doi: 10.1002/(sici)1097-4598(199908)22:8<1094::aid-mus14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 62.Wilder MR, Cannon J. Effect of age on muscle activation and twitch properties during static and dynamic actions. Muscle Nerve. 2009;39:683–691. doi: 10.1002/mus.21233. [DOI] [PubMed] [Google Scholar]
- 63.Harridge SD, Kryger A, Stensgaard A. Knee extensor strength, activation, and size in very elderly people following strength training. Muscle Nerve. 1999;22:831–839. doi: 10.1002/(sici)1097-4598(199907)22:7<831::aid-mus4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 64.Stevens JE, Stackhouse SK, Binder-Macleod SA, Snyder-Mackler L. Are voluntary muscle activation deficits in older adults meaningful? Muscle Nerve. 2003;27:99–101. doi: 10.1002/mus.10279. [DOI] [PubMed] [Google Scholar]
- 65.Hortobagyi T, Mizelle C, Beam S, DeVita P. Old adults perform activities of daily living near their maximal capabilities. J Gerontol A Biol Sci Med Sci. 2003;58:M453–M460. doi: 10.1093/gerona/58.5.m453. [DOI] [PubMed] [Google Scholar]
- 66.Haug H, Eggers R. Morphometry of the human cortex cerebri and corpus striatum during aging. Neurobiol Aging. 1991;12:336–338. doi: 10.1016/0197-4580(91)90013-a. discussion 352–355. [DOI] [PubMed] [Google Scholar]
- 67.Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 68.Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol. 2003;462:144–152. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- 69.Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage. 2004;21:1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 70.Morgan DG, May PC, Finch CE. Dopamine and serotonin systems in human and rodent brain: effects of age and neurodegenerative disease. J Am Geriatr Soc. 1987;35:334–345. doi: 10.1111/j.1532-5415.1987.tb04641.x. [DOI] [PubMed] [Google Scholar]
- 71.Bigham MH, Lidow MS. Adrenergic and serotonergic receptors in aged monkey neocortex. Neurobiol Aging. 1995;16:91–104. doi: 10.1016/0197-4580(95)80012-g. [DOI] [PubMed] [Google Scholar]
- 72.Bartus RT, Dean RL, III, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 73.Segovia G, Porras A, Del Arco A, Mora F. Glutamatergic neurotransmission in aging: a critical perspective. Mech Ageing Dev. 2001;122:1–29. doi: 10.1016/s0047-6374(00)00225-6. [DOI] [PubMed] [Google Scholar]
- 74.Roth GS. Age changes in signal transduction and gene expression. Mech Ageing Dev. 1997;98:231–238. doi: 10.1016/s0047-6374(97)00110-3. [DOI] [PubMed] [Google Scholar]
- 75.Mora F, Segovia G, Del Arco A. Glutamate-dopamine-GABA interactions in the aging basal ganglia. Brain Res Rev. 2008;58:340–353. doi: 10.1016/j.brainresrev.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 76.Roth GS, Joseph JA. Cellular and molecular mechanisms of impaired dopaminergic function during aging. Ann N Y Acad Sci. 1994;719:129–135. doi: 10.1111/j.1749-6632.1994.tb56824.x. [DOI] [PubMed] [Google Scholar]
- 77.Hayashi M, Yamashita A, Shimizu K. Somatostatin and brain-derived neurotrophic factor mRNA expression in the primate brain: decreased levels of mRNAs during aging. Brain Res. 1997;749:283–289. doi: 10.1016/S0006-8993(96)01317-0. [DOI] [PubMed] [Google Scholar]
- 78.Kossev AR, Schrader C, Dauper J, Dengler R, Rollnik JD. Increased intracortical inhibition in middle-aged humans; a study using paired-pulse transcranial magnetic stimulation. Neurosci Lett. 2002;333:83–86. doi: 10.1016/s0304-3940(02)00986-2. [DOI] [PubMed] [Google Scholar]
- 79.Sale MV, Semmler JG. Age-related differences in corticospinal control during functional isometric contractions in left and right hands. J Appl Physiol. 2005;99:1483–1493. doi: 10.1152/japplphysiol.00371.2005. [DOI] [PubMed] [Google Scholar]
- 80.Smith AE, Ridding MC, Higgins RD, Wittert GA, Pitcher JB. Age-related changes in short-latency motor cortex inhibition. Exp Brain Res. 2009;198(4):489–500. doi: 10.1007/s00221-009-1945-8. [DOI] [PubMed] [Google Scholar]
- 81.McGinley M, Hoffman RL, Russ DW, Thomas JS. Clark BC. Older adults exhibit more intracortical inhibition and less intracortical facilitation than young adults. Exp Gerontol. 2010;45(9):671–678. doi: 10.1016/j.exger.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heuninckx S, Wenderoth N, Debaere F, Peeters R, Swinnen SP. Neural basis of aging: the penetration of cognition into action control. J Neurosci. 2005;25:6787–6796. doi: 10.1523/JNEUROSCI.1263-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Naccarato M, Calautti C, Jones PS, Day DJ, Carpenter TA, Baron JC. Does healthy aging affect the hemispheric activation balance during paced index-to-thumb opposition task? An fMRI study. Neuroimage. 2006;32:1250–1256. doi: 10.1016/j.neuroimage.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 84.Rowe JB, Siebner H, Filipovic SR, Cordivari C, Gerschlager W, Rothwell J, et al. Aging is associated with contrasting changes in local and distant cortical connectivity in the human motor system. Neuroimage. 2006;32:747–760. doi: 10.1016/j.neuroimage.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 85.Fathi D, Ueki Y, Mima T, Koganemaru S, Nagamine T, Tawfik A, et al. Effects of aging on the human motor cortical plasticity studied by paired associative stimulation. Clin Neurophysiol. 2009;121:90–93. doi: 10.1016/j.clinph.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 86.Sawaki L, Yaseen Z, Kopylev L, Cohen LG. Age-dependent changes in the ability to encode a novel elementary motor memory. Ann Neurol. 2003;53:521–524. doi: 10.1002/ana.10529. [DOI] [PubMed] [Google Scholar]
- 87.Clark BC, McGinley MP, Hoffman RL, Thomas JS, Russ DW. Age-related differences in cortical excitability. Med Sci Sports Exerc. 2010;42(5):S10. [Google Scholar]
- 88.Scaglioni G, Ferri A, Minetti AE, Martin A, Van Hoecke J, Capodaglio P, et al. Plantar flexor activation capacity and H reflex in older adults: adaptations to strength training. J Appl Physiol. 2002;92:2292–2302. doi: 10.1152/japplphysiol.00367.2001. [DOI] [PubMed] [Google Scholar]
- 89.Kido A, Tanaka N, Stein RB. Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol. 2004;82:238–248. doi: 10.1139/y04-017. [DOI] [PubMed] [Google Scholar]
- 90.Christie A, Kamen G. Doublet discharges in motoneurons of young and older adults. J Neurophysiol. 2006;95:2787–2795. doi: 10.1152/jn.00685.2005. [DOI] [PubMed] [Google Scholar]
- 91.Kamen G, Sison SV, Du CC, Patten C. Motor unit discharge behavior in older adults during maximal-effort contractions. J Appl Physiol. 1995;79:1908–1913. doi: 10.1152/jappl.1995.79.6.1908. [DOI] [PubMed] [Google Scholar]
- 92.Klass M, Baudry S, Duchateau J. Age-related decline in rate of torque development is accompanied by lower maximal motor unit discharge frequency during fast contractions. J Appl Physiol. 2008;104:739–746. doi: 10.1152/japplphysiol.00550.2007. [DOI] [PubMed] [Google Scholar]
- 93.Nelson RM, Soderberg GL, Urbscheit NL. Comparison of skeletal muscle motor unit discharge characteristics in young and aged humans. Arch Gerontol Geriatr. 1983;2:255–264. doi: 10.1016/0167-4943(83)90029-8. [DOI] [PubMed] [Google Scholar]
- 94.Doherty TJ, Brown WF. The estimated numbers and relative sizes of thenar motor units as selected by multiple point stimulation in young and older adults. Muscle Nerve. 1993;16:355–366. doi: 10.1002/mus.880160404. [DOI] [PubMed] [Google Scholar]
- 95.McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve. 2005;31:461–467. doi: 10.1002/mus.20276. [DOI] [PubMed] [Google Scholar]
- 96.Auyeung TW, Kwok T, Lee J, Leung PC, Leung J, Woo J. Functional decline in cognitive impairment—the relationship between physical and cognitive function. Neuroepidemiology. 2008;31:167–173. doi: 10.1159/000154929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol. 2009;66:1339–1344. doi: 10.1001/archneurol.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 99.Kawakami Y, Akima H, Kubo K, Muraoka Y, Hasegawa H, Kouzaki M, et al. Changes in muscle size, architecture, and neural activation after 20 days of bed rest with and without resistance exercise. Eur J Appl Physiol. 2001;84:7–12. doi: 10.1007/s004210000330. [DOI] [PubMed] [Google Scholar]
- 100.D’Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, et al. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol. 2003;552:499–511. doi: 10.1113/jphysiol.2003.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ochala J, Frontera WR, Dorer DJ, Van Hoecke J, Krivickas LS. Single skeletal muscle fiber elastic and contractile characteristics in young and older men. J Gerontol A Biol Sci Med Sci. 2007;62:375–381. doi: 10.1093/gerona/62.4.375. [DOI] [PubMed] [Google Scholar]
- 102.Russ DW, Grandy JS, Toma K, Ward CW. Aging, but not yet senescent, rats exhibit reduced muscle quality and sarcoplasmic reticulum function. Acta Physiol (Oxf) 2011;201:391–403. doi: 10.1111/j.1748-1716.2010.02191.x. [DOI] [PubMed] [Google Scholar]
- 103.Yu F, Hedstrom M, Cristea A, Dalen N, Larsson L. Effects of ageing and gender on contractile properties in human skeletal muscle and single fibres. Acta Physiol (Oxf) 2007;190:229–241. doi: 10.1111/j.1748-1716.2007.01699.x. [DOI] [PubMed] [Google Scholar]
- 104.D’Antona G, Pellegrino MA, Carlizzi CN, Bottinelli R. Deterioration of contractile properties of muscle fibres in elderly subjects is modulated by the level of physical activity. Eur J Appl Physiol. 2007;100:603–611. doi: 10.1007/s00421-007-0402-2. [DOI] [PubMed] [Google Scholar]
- 105.Delbono O. Regulation of excitation contraction coupling by insulin-like growth factor-1 in aging skeletal muscle. J Nutr Health Aging. 2000;4:162–164. [PubMed] [Google Scholar]
- 106.Renganathan M, Messi ML, Delbono O. Dihydropyridine receptor-ryanodine receptor uncoupling in aged skeletal muscle. J Membr Biol. 1997;157:247–253. doi: 10.1007/s002329900233. [DOI] [PubMed] [Google Scholar]
- 107.Delbono O, O’Rourke KS, Ettinger WH. Excitation-calcium release uncoupling in aged single human skeletal muscle fibers. J Membr Biol. 1995;148:211–222. doi: 10.1007/BF00235039. [DOI] [PubMed] [Google Scholar]
- 108.Jimenez-Moreno R, Wang ZM, Gerring RC, Delbono O. Sarcoplasmic reticulum Ca2+ release declines in muscle fibers from aging mice. Biophys J. 2008;94:3178–3188. doi: 10.1529/biophysj.107.118786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, et al. Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol. 2001;90:2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 110.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89:104–110. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 111.MacIntosh BR, Gardiner PF, McComas AJ. Skeletal Muscle: Form and Function. 2nd ed. Champaign, IL: Human Kinetics; 2006. [Google Scholar]
- 112.Anderson AA, Altafaj X, Zheng Z, Wang ZM, Delbono O, Ronjat M, et al. The junctional SR protein JP-45 affects the functional expression of the voltage-dependent Ca2+ channel Cav1.1. J Cell Sci. 2006;119:2145–2155. doi: 10.1242/jcs.02935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Anderson AA, Treves S, Biral D, Betto R, Sandona D, Ronjat M, et al. The novel skeletal muscle sarcoplasmic reticulum JP-45 protein. Molecular cloning, tissue distribution, developmental expression, and interaction with alpha 1.1 subunit of the voltage-gated calcium channel. J Biol Chem. 2003;278:39987–39992. doi: 10.1074/jbc.M305016200. [DOI] [PubMed] [Google Scholar]
- 114.Delbono O, Xia J, Treves S, Wang ZM, Jimenez-Moreno R, Payne AM, et al. Loss of skeletal muscle strength by ablation of the sarcoplasmic reticulum protein JP45. Proc Natl Acad Sci U S A. 2007;104:20108–20113. doi: 10.1073/pnas.0707389104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.O’Connell K, Gannon J, Doran P, Ohlendieck K. Reduced expression of sarcalumenin and related Ca2+ -regulatory proteins in aged rat skeletal muscle. Exp Gerontol. 2008;43:958–961. doi: 10.1016/j.exger.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 116.Taylor JR, Zheng Z, Wang ZM, Payne AM, Messi ML, Delbono O. Increased CaVbeta1A expression with aging contributes to skeletal muscle weakness. Aging Cell. 2009;8:584–594. doi: 10.1111/j.1474-9726.2009.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lowe DA, Thomas DD, Thompson LV. Force generation, but not myosin ATPase activity, declines with age in rat muscle fibers. Am J Physiol Cell Physiol. 2002;283:C187–C192. doi: 10.1152/ajpcell.00008.2002. [DOI] [PubMed] [Google Scholar]
- 118.Song MY, Ruts E, Kim J, Janumala I, Heymsfield S, Gallagher D. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr. 2004;79:874–880. doi: 10.1093/ajcn/79.5.874. [DOI] [PubMed] [Google Scholar]
- 119.Zoico E, Rossi A, Di Francesco V, Sepe A, Olioso D, Pizzini F. Adipose tissue infiltration in skeletal muscle of healthy elderly men: relationships with body composition, insulin resistance, and inflammation at the systemic and tissue level. J Gerontol A Biol Sci Med Sci. 65:295–299. doi: 10.1093/gerona/glp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reid MB, Lannergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: involvement of muscle myofilaments. Am J Respir Crit Care Med. 2002;166:479–484. doi: 10.1164/rccm.2202005. [DOI] [PubMed] [Google Scholar]
- 121.Wilcox P, Osborne S, Bressler B. Monocyte inflammatory mediators impair in vitro hamster diaphragm contractility. Am Rev Respir Dis. 1992;146:462–466. doi: 10.1164/ajrccm/146.2.462. [DOI] [PubMed] [Google Scholar]
- 122.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 123.Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38:1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 124.Marsh AP, Miller ME, Saikin AM, Rejeski WJ, Hu N, Lauretani F, et al. Lower extremity strength and power are associated with 400-meter walk time in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2006;61:1186–1193. doi: 10.1093/gerona/61.11.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bohannon RW. Internal consistency of manual muscle testing scores. Percept Mot Skills. 1997;85:736–738. doi: 10.2466/pms.1997.85.2.736. [DOI] [PubMed] [Google Scholar]
- 126.Bohannon RW. Intercorrelations and internal consistency of limb muscle-action strengths of patients with renal disease. Percept Mot Skills. 1998;86:1249–1250. doi: 10.2466/pms.1998.86.3c.1249. [DOI] [PubMed] [Google Scholar]
- 127.Bohannon RW. Is it legitimate to characterize muscle strength using a limited number of measures? J Strength Cond Res. 2008;22:166–173. doi: 10.1519/JSC.0b013e31815f993d. [DOI] [PubMed] [Google Scholar]
- 128.Bohannon RW. Dynamometer measurements of grip and knee extension strength: are they indicative of overall limb and trunk muscle strength? Percept Mot Skills. 2009;108:339–342. doi: 10.2466/PMS.108.2.339-342. [DOI] [PubMed] [Google Scholar]
- 129.Buchner DM, Larson EB, Wagner EH, Koepsell TD, de Lateur BJ. Evidence for a non-linear relationship between leg strength and gait speed. Age Ageing. 1996;25:386–391. doi: 10.1093/ageing/25.5.386. [DOI] [PubMed] [Google Scholar]
- 130.Cress ME, Meyer M. Maximal voluntary and functional performance levels needed for independence in adults aged 65 to 97 years. Phys Ther. 2003;83:37–48. [PubMed] [Google Scholar]
- 131.Ferrucci L, Guralnik J, Buchner D, Kasper J, Lamb S, Simonsick E, et al. Departures from linearity in the relationship between measures of muscular strength and physical performance of the lower extremities: The Women's Health and Aging Study. J Gerentol Med Sci. 1997;52A:M275–M285. doi: 10.1093/gerona/52a.5.m275. [DOI] [PubMed] [Google Scholar]
- 132.Ploutz-Snyder LL, Manini T, Ploutz-Snyder RJ, Wolf DA. Functionally relevant thresholds of quadriceps femoris strength. J Gerontol A Biol Sci Med Sci. 2002;57:B144–B152. doi: 10.1093/gerona/57.4.b144. [DOI] [PubMed] [Google Scholar]
- 133.Sallinen J, Stenholm S, Rantanen T, Heliovaara M, Sainio P, Koskinen S. Hand-grip strength cut points to screen older persons at risk for mobility limitation. J Am Geriatr Soc. 2010;58:1721–1726. doi: 10.1111/j.1532-5415.2010.03035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Harman E. Strength and power: a definition of terms. J Strength Cond Res. 1993;15:18–20. [Google Scholar]
- 135.Cuoco A, Callahan DM, Sayers S, Frontera WR, Bean J, Fielding RA. Impact of muscle power and force on gait speed in disabled older men and women. J Gerontol A Biol Sci Med Sci. 2004;59:1200–1206. doi: 10.1093/gerona/59.11.1200. [DOI] [PubMed] [Google Scholar]
- 136.Herman S, Kiely DK, Leveille S, O’Neill E, Cyberey S, Bean JF. Upper and lower limb muscle power relationships in mobility-limited older adults. J Gerontol A Biol Sci Med Sci. 2005;60:476–480. doi: 10.1093/gerona/60.4.476. [DOI] [PubMed] [Google Scholar]
- 137.Puthoff ML, Nielsen DH. Relationships among impairments in lower-extremity strength and power, functional limitations, and disability in older adults. Phys Ther. 2007;87:1334–1347. doi: 10.2522/ptj.20060176. [DOI] [PubMed] [Google Scholar]
- 138.Foldvari M, Clark M, Laviolette LC, Bernstein MA, Kaliton D, Castaneda C, et al. Association of muscle power with functional status in community-dwelling elderly women. J Gerontol A Biol Sci Med Sci. 2000;55:M192–M199. doi: 10.1093/gerona/55.4.m192. [DOI] [PubMed] [Google Scholar]
- 139.Metter EJ, Conwit R, Tobin J, Fozard JL. Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci. 1997;52:B267–B276. doi: 10.1093/gerona/52a.5.b267. [DOI] [PubMed] [Google Scholar]
- 140.Skelton DA, Greig CA, Davies JM, Young A. Strength, power and related functional ability of healthy people aged 65-89 years. Age Ageing. 1994;23:371–377. doi: 10.1093/ageing/23.5.371. [DOI] [PubMed] [Google Scholar]
- 141.Bean JF, Kiely DK, LaRose S, O’Neill E, Goldstein R, Frontera WR. Increased velocity exercise specific to task training versus the National Institute on Aging's strength training program: changes in limb power and mobility. J Gerontol A Biol Sci Med Sci. 2009;64:983–991. doi: 10.1093/gerona/glp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.