Abstract
Caloric restriction and physical exercise have proven beneficial against age-associated changes in body composition and declining physical performance; however, little is known regarding what benefit these interventions might have when initiated late in life. The study of mimetics of diet and exercise and the combination thereof may provide additional treatments for a vulnerable elderly population; however, how and when to initiate such interventions requires consideration in developing the most safe and efficacious treatment strategies. In this review, we focus on preclinical late-life intervention studies, which assess the relationship between physical function, sarcopenia, and body composition. We provide a conceptual framework for the ever-changing definition of sarcopenia and a rationale for the use of an appropriate rodent model of this condition. We finish by providing our perspective regarding the implications of this body of work and future areas of research that may also contribute to the ultimate goal of extending healthspan.
Keywords: Renin angiotensin system, Enalapril repamycin, Physical function, Body composition
SARCOPENIA is a hallmark of aging, occurs in multiple species, and its progression usually mirrors changes in body composition. Age-related changes in body composition have important clinical implications, given that the loss of muscle mass, gains in fat mass, and the consequent ramifications on muscle strength may be independently associated with declining performance as well as increased risk for disability and mortality in older persons (1–4). In some experimental settings, behavioral interventions, such as moderate caloric restriction and physical exercise, have proven beneficial against age-associated changes in body composition and physical performance in targeted populations [eg, (5,6)]; however, very little is known regarding what benefit these interventions might have when initiated late in life. This is an extremely important empirical question given that these behavioral interventions are not appropriate for all older adults (those not interested or are physically or cognitively incapable). The study of pharmacological mimetics of diet and exercise and the combination thereof may provide additional treatments for this vulnerable population. Funding agencies are conducting late phase clinical trials, which study the impact of diet, exercise, and pharmacological interventions on the functional status of older individuals (7–9). Complementary preclinical studies represent an important translational link insofar as they may inform and guide the direction of and help in the interpretation of the results of these trials in terms of understanding the biological mechanisms of these treatments.
Incredible strides have been made over the last 70 years regarding our understanding of the basic biology of aging, which have provided the basis for the development of preclinical models of late-life intervention strategies for combating physical decline. The bulk of this work has been driven by the finding that a life-long reduction (40%) in caloric intake prolongs life and reverses many cellular, molecular, and physiological changes, which occur with age, including increased inflammation, oxidative damage, and metabolic dysregulation, and reverses or attenuates the impact of sarcopenia (10–29). Although it is true that interventions that prolong life span seem to be correlated with increased physical function (30–32), it is not clear that the application of these interventions late in life will have the same effect. In fact there is very little evidence, clinically or preclinically, to suggest they will be effective. Furthermore, the biological changes that occur in the context of these interventions that relate to improvements in quality in muscle function themselves may or not occur when initiated as late-life interventions.
With the continued “graying” of the worldwide population, the number of individuals at risk for developing physical disability continues to increase along with the social, emotional, and economic cost (33) of caring for such individuals, mandating the need for testing the effectiveness of health-promoting interventions within this cohort. We have focused on the use of a variety of late-life interventions designed to mitigate sarcopenia and changes in body composition but have prioritized functional measures as our primary outcome. This is essential given that changes in physical function/activity are themselves observed across a wide variety of species and the primary outcome of interest. Of course, this is not to the exclusion of the study of biological outcomes as the relationship between changes in behavior and biology is a major advantage and imperative for using preclinical models to further our understanding of human health. Furthermore, once the biological correlate or mechanisms of the behavior change of interest are identified, additional targets for alternative interventions such as pharmaceutical therapeutics may be revealed.
Therefore, in this review, we focus on those late-life intervention studies from our laboratories, which directly assess the relationship between physical function, sarcopenia, and body composition. We begin with providing a conceptual framework for the ever-changing definition of sarcopenia and a rationale for the use of an appropriate rodent model of this condition. We follow with a description of the evolution of these studies then finish by providing our perspective regarding the implications of this body of work and future areas of research that may also contribute to the ultimate goal of maintaining functional independence in older individuals through extending their healthspan.
SARCOPENIA DEFINED AND REFINED
The term “sarcopenia,” first coined by Rosenberg (34), is from the Greek meaning “poverty of flesh.” Sarcopenia has been referred to as a syndrome that includes not only the loss in muscle mass but also concomitant losses in muscle strength and poor physical function, although this has been debated recently (35,36). Nevertheless, the sequela of sarcopenia has been identified as a major cause underlying physical decline with age. Despite its importance, there is still much debate as to a universal set of criteria that defines the condition. In brief, most definitions have derived from epidemiological studies which present the issue in the context of clearly defining levels of muscle mass and/or indices of upper and lower extremity strength that predispose individuals to high risk of poor physical performance and disability (37–39). A major caveat to the approach is that one specific level is not necessarily more predictive of changes in functional status than another. This suggests that to demonstrate a relationship between sarcopenia and functional status, a more nuanced expansion of this definition is needed.
Emerging approaches to defining sarcopenia focus on both the strength and the muscle mass elements of the syndrome. For example, Clark and Manini (35,36) have coined the term “dynapenia” to refer to the age-related loss of strength, which cleverly translates from the Greek to “poverty of strength.” This is based on an analysis of the literature, demonstrating that (a) longitudinal aging studies indicate a dissociation between the loss of muscle mass and strength; (b) interventions such as growth hormone or testosterone supplementation, which clearly result in the hypertrophy of muscle, fail to provide the expected level of gains in strength; and (c) changes in muscle mass and changes in strength resulting from alterations in physical activity levels (ie, exercise training or disuse) do not follow the same time course. Instead, they suggest that rather than loss of mass per se, age-related alterations in biological systems afferent to or localized within the muscle undermine strength. This is not an entirely new perspective, but the conceptualization of dynapenia brings to the forefront the caveats involved in the classic definition of sarcopenia and draws attention away from establishing criteria for its diagnosis and treatment in terms of mass or strength in isolation. Finally, this concept highlights and gives priority to the importance of biological viability of the muscle tissue and upstream factors.
Of particular relevance to this review, is that the study of sarcopenia should also incorporate measures of overall body composition. In humans, there is a gradual increase in adiposity and loss of lean mass in both men and women from 25 to approximately 65 years of age, after which total body weight declines (40). As alluded to in the introduction, loss of muscle and gain of fat mass are independently associated with declining performance as well as increased risk for disability and mortality in older persons (1–4). Adipose tissue is a major endocrine organ, and far from its previously innocuous reputation as simply providing protection for internal organs, increased adiposity with age results in the convergence of several deleterious processes, including increased inflammation, insulin resistance, and oxidative stress. This situation may lead directly to accelerated loss of number and quality of myocytes (41), and each has been shown to correlate with declining physical performance and/or strength in both human and animal models (11,41–51). In addition, with the increased overaccumulation of abdominal fat, which is observed during aging (52,53), fat may also be ectopically deposited in nonadipose tissues, such as muscle, as the oxidative capacity of the adipose depots is exceeded. This results in a lipotoxic environment such that an excess of fatty acids may feed into cytotoxic metabolic pathways such as de novo ceramide production and diacylglycerol deposition (54), both of which may lead to dysregulation of muscle function including cell death. Collectively, these data suggest that increased adiposity with age is an important determinant of both declining function and loss of muscle viability.
Although we have most likely duplicated information that is more extensively, and even more elegantly, reviewed elsewhere in this special issue, we feel that this recapitulation is necessary for several reasons. First, in order to study sarcopenia, a conceptual biological framework, in this case, changes in muscle quality rather than muscle mass, is required to establish a point of intervention. Second, we identify a major contributor to sarcopenia, increased adiposity with age, in terms of its impact on declining function and dysregulation of skeletal muscle. Third, we would argue that because this increase in adiposity is associated with such a broad range of biological factors, one of the most critical outcomes in terms of evaluating the efficacy of interventions designed to ameliorate this rise in adiposity must relate to functional status. Finally, these data provide a rational intersection for translational studies which make use of animal models for establishing new and exciting interventions, for extending our knowledge of current interventions, and most importantly, to provide guidance in the design and interpretation of both approaches when initiated late in life.
THE FISCHER 344 × BROWN NORWAY RAT IS A USEFUL PRECLINICAL MODEL FOR THE TRANSLATIONAL STUDY OF BODY COMPOSITION AND PHYSICAL DECLINE
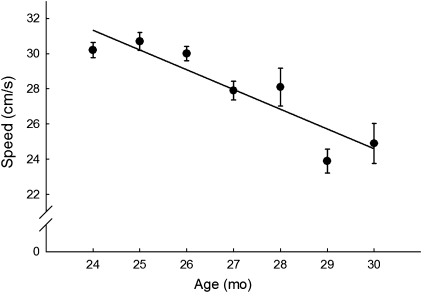
Choosing an animal model should be based solely on the hypothesis one is interested in addressing. Therefore, for several years, we have used the Fischer 344 × Brown Norway (F344BN) rat as our model given the extensive body of published studies reported in this strain, regarding age-related changes in both behavioral function and skeletal muscle biology. Furthermore, there are many conceptual similarities between age-related changes observed in this strain and what is observed in humans. For example, several studies have shown that this strain proceeds from 80% to 50% survival between 24 and 30 months of age (55). In humans, this same pattern of survival mirrors an exponential increase in disability (56). In fact, assessment of functional limitations in vicinity of the 50% survival range is highly predictive of mortality in both rats and humans. For example, we have previously reported that swimming speed performance at 24 months of age predicts longevity in aged F344BN rats (Figure 1). This is particularly relevant in light of a recent paper by Studenski and colleagues (57–59) advocating for the potential use of speed measurements for predicting disability. In the context of changing body composition, a similar association exists. In the F344BN, there is a gradual increase in both lean and fat mass through 24 months of age, a decrease in muscle and further increase in fat through 27 months, and a decrease in both compartments thereafter (16,60,61). These changes in body composition mirror changes in cell signaling pathways in the skeletal muscle itself that are central to the viability and homeostasis of this tissue (inflammation, oxidative stress, insulin resistance, apoptosis, as examples and reviewed in detail elsewhere).
Figure 1.
Least square means of the swimming speed procedures, over a 6-month time period (24–30 months). Longevity was significantly predicted by higher baseline swim speed (hazard ratio 0.88 for 1 cm/s increase; 95% confidence interval 0.81–0.97; p = .009),
Therefore, in many of our experimental designs, we focus the initiation of interventions during this “critical window” during the life span. These studies are longitudinal in nature in that we follow the same animals over time in order to assess responses to treatment within individual subjects. We typically do not use young animals as controls because our hypotheses are designed to address whether functional performance may be improved by our late-life initiated treatments in older animals. The similarity between the F344BN rat strain and humans in terms of timing of late-life, age-related body composition changes, and declining performance makes this strain an excellent model for studying the relationship between age, adiposity, muscle quality, and physical function. What follows is a conceptual rationale and example of our work in which we initiate pharmacological interventions, designed to mitigate age-related increases in adiposity, late in life with an eye toward ameliorating declining function. We also demonstrate a potential dissociation between interventions in terms of life span versus healthspan.
WHAT HAVE WE LEARNED FROM PRECLINICAL STUDIES OF CALORIE RESTRICTION AND EXERCISE
Calorie Restriction
For decades, behavioral interventions, such as short-term moderate caloric restriction (CR) and physical exercise, have proven beneficial against age-associated changes in body composition and physical performance in targeted populations; however, very little is known regarding what benefit these interventions may have when initiated late in life and for extended periods of time. This is especially critical given that NIH is currently sponsoring two large-scale clinical trials to address both of these types of interventions in both younger and older individuals [see (8) for a description of the LIFE study and (7) for CALERIE]. Knowledge garnered from preclinical studies may be especially informative in helping to guide these trials and interpret the resulting data.
From a functional perspective, it is well known that rodents placed on a caloric restrictive diet across the life span show improved survival and a sparing of function in various motor performance tests including increased locomotor activity (62,63). We have contributed to this literature by showing that the F344BN rat, with life-long CR (40%), exhibit improved physical performance and that this effect is mediated by a decrease in adiposity and a lowering of inflammatory status. Certainly, very few people would advocate the use of such severe forms of CR in older individuals, and controversy exists about the circumstances under which older adults should be advised to lose weight (64–67). Furthermore, the second phase of the 2-year CALERIE trial of 25% calorie restriction in nonobese individuals is currently underway; however, the target population is between approximately 20 and 50 years of age, which is well below the age of the large “baby boom” cohort for which interventions are directly needed to curb an impending epidemic of functional decline.
Indeed, there has been no systematic study of the effects of CR when initiated late in life, using functional status as a primary outcome. However, Weindruch and colleagues (68) investigated the effects of varying degrees of CR on health and longevity in mice. They found that CR of all degrees tested, 25%, 55%, and 65% of the ad libitum diet, conferred improved health and longevity over ad libitum–fed control mice. It appears that the greater degree of CR, without inducing malnutrition and starvation, the greater the effects on health and longevity of the mice. Although a lesser degree of CR may not maximize the potential longevity benefits, the potential healthspan benefits have not been extensively described. Restriction to a lesser degree is an intervention that is much more feasible for humans to maintain for the long term. In fact, Holloszy and colleagues (69) have shown that 8% CR increases mean life span without effecting maximal life span. The interpretation of these results was that 8% CR affects health-related outcomes without affecting a basic “aging” process. We have also shown that 8% CR reduces inflammatory status and improves antioxidant production (50). Disease processes as well as the aging process are robustly associated with increased adiposity and inflammatory status in a variety of species. Therefore, regimens such as 8% CR may positively impact declining physical performance by attenuating age-related increases in inflammatory status, oxidative damage, and apoptosis in skeletal muscle (19,20,50,70). These data suggest that, in terms of preclinical studies, a more systematic study of varying “doses” of CR, initiated late in life may provide a translational link to help optimize the design of clinical studies for improving function in older individuals.
Exercise
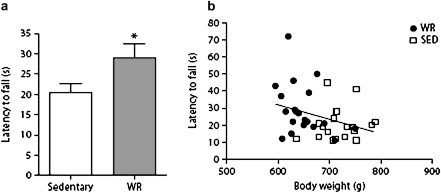
Preclinical studies of life-long exercise interventions from our laboratory have focused on the use of wheel-running regimens pioneered by Holloszy and colleagues (28,71–74) as a model of voluntary exercise in Fischer 344 (F344) rats. This is usually accompanied by a slight reduction in caloric intake (8%) in order to keep animals in a heightened state of motivation to ensure they will maintain wheel running. This situation results in a reduction in adiposity and a preservation of a variety of indices of skeletal muscle function. We have extended this line of research by investigating late-life initiation of voluntary wheel running without accompanied CR in aged F344BN rats (Figure 2). Voluntary wheel running in aged rats significantly reduced adiposity and body weight, although these rats engaged in very low levels of wheel running (as few as nine revolutions per day). The body weight loss was accompanied by an increase in physical performance (75). Although these data show that aged F344BN rats are relatively inactive, a very small amount of increase in physical activity is beneficial to physical performance. The impact on muscle quality is unknown.
Figure 2.
Latency to fall (a) from an incline plane in 24-month-old F344BN male rats that were wheel running for 2 weeks (n = 20) or were sedentary (n = 19). *p = .04 for difference from a wheel-running group by t test. (b) Correlation between latency to fall from an incline plane and body weight, p = .037, r2 = .112.
With forced exercise, our studies demonstrated that relatively high-intensity (25 cm/s, 30 minutes, 5 d/wk) short-term training (4 weeks) in 28-month-old male F344BN rats improves exercise capacity and physical performance (76). In addition, we detected an age-related increase in a variety of markers of apoptosis and apoptotic DNA fragmentation in the extensor digitorum longus muscle of old sedentary rats, which was attenuated/reversed by exercise. We have also collected very preliminary data monitoring changes in body weight and exercise tolerance in aged F344BN male rats after 4 weeks of low-intensity (12 cm/s, 10 min/d, 5 d/wk) treadmill exercise alone or combined with enalapril treatment. Indeed, the combination of enalapril + exercise maintained exercise tolerance and decreased weight gain relative to exercise alone. Therefore, it is quite likely that longer-term treadmill exercise in this strain of rat may also reduce adiposity and, hence, reverse the increased inflammation that leads directly to apoptosis, attenuate the loss of muscle mass, and ultimately protect against declining physical performance. Moreover, exploration of low-intensity exercise in combination with various adjuvant pharmacological therapies may provide a benefit to those aged individuals who may experience difficulty in exercising (see subsequently).
Some controversy does exist regarding the impact of the potentially stressful nature of forced exercise. Indeed, life-long intense treadmill exercise may have adverse consequences (77,78); however, a recent study by Betik and colleagues (79) demonstrated that 5 or 7 months of treadmill exercise training initiated in 29-month-old F344BN rats resulted in greater exercise capacity during an incremental treadmill exercise test and reduced percent body fat. Quite interestingly, this increase in functional performance was observed in the absence of increased muscle aerobic capacity, muscle mass, and other markers of mitochondrial function highlighting the importance of using behavioral outcomes as a measure of the success of a particular intervention. Together, these data suggest that late-life initiation of exercise improves physical function in the context of a reduction in adiposity and preservation of some aspects of muscle quality. Moreover, in the case with voluntary wheel running, very little exercise is necessary to achieve beneficial outcomes.
PHARMACOLOGICAL INTERVENTION: CR MIMETICS
Pharmacological approaches may be particularly relevant late in life because not all older individuals benefit from or are capable of participating in traditional diet and/or exercise programs (eg, those that are obese, frail, or arthritic). This notion has benefited from an evolving area of research focused on the application of CR “mimetics” (80) which target cell signaling pathways that may be involved in the basic biology of aging. The National Institute on Aging is on the forefront of this movement and has spearheaded the development of an interventions testing program to investigate CR mimetics across three different institutes in genetically diverse mice (80). The objective of the program is to develop interventions that produce the same prolongevity effects that CR provides but without reducing caloric intake or inhibiting food intake. However, this does not preclude the notion that such mimetics may still affect differential regulation of adiposity. Independent investigators were encouraged to “sponsor” compounds with adequate rationale. Most importantly, due to experimental caveats, the administration of many of these compounds began at 20 months of age, thereby creating an experimental situation of late-life administration of prolongevity pharmacological interventions.
Our interest was piqued given that we and others have previously shown that physical function is predictive of longevity (albeit over a compressed period of time) and that life-long CR improves function (30). Therefore, a major empirical question is whether or not CR mimetics that purport to increase life span necessarily translate into mimetics of increased functional performance. Here, we highlight results of our tests on physical function/activity and adiposity in the F344BN rat using two compounds used in the interventions testing program and recently reported on (81): one which extended life span (rapamycin) and another which our group proposed (enalapril) that did not extend life span. We show that using both treatments, regardless of the impact on longevity observed in interventions testing program mice, resulted in decreased adiposity/weight loss and improved functional/activity outcomes.
Rapamycin
To be sure, some of the most promising data to emerge from the interventions testing program are related to the use of the compound rapamycin (81). This compound was accidentally discovered in the soil of the island of Rapa Nui (hence its name) and was found to have powerful antifungal properties. Since that time, the compound has been used as a therapy for organ rejection in kidney transplantation and is currently in trials for cancer treatment. Rapamycin inhibits the TOR (target of rapamycin) pathway, which is central to the regulation of nutrient sensing, cell survival/death, protein synthesis, and many other metabolic processes. Although inhibition of the TOR pathway by genetic or pharmacological means has been shown to extend life span in invertebrates, the effect in mammals was not known. As stated above, when rapamycin was fed to male and female mice beginning at 600 days of age, it led to an increase in life span of 14% for females and 9% for males. A subsequent study initiated at 270 days of age has also been show to increase survival in both male and female mice, based on an interim analysis conducted near the median survival point. The real strength of these data is threefold because the life-span enhancing effect of rapamycin is (a) observed in both males and females, (b) observed when initiated very late in life, and (c) highly replicable given that it was observed at three separate institutions.
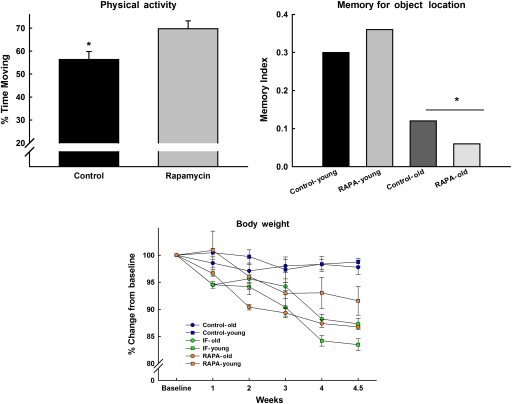
We have conducted a preliminary study (C. Leeuwenburgh, T. C. Foster, C.S. Carter, unpublished data, 2009) (Figure 3) in our laboratory using the F344BN rat to investigate whether rapamycin ameliorates age-related weight loss, cognitive function, and activity levels. In this case, young and old F344BN rats were fed rapamycin for 3 weeks or were given a regimen of intermittent (every other day) calorie restriction (IF). Body weight and food intake were monitored on a daily basis. After 3 weeks, we assessed memory using the object recognition paradigm in which an animal recognizes a novel object when presented along with a variety of other objects, which have been presented 24 hours previously. “Recognition” is measured by the amount of time the animal spends exploring the novel object. All IF- and rapamycin-treated animals lost approximately 15% of their body weight relative to controls. Moreover, the young IF animals lost more weight than their rapamycin-fed counterparts. This was correlated with feeding patterns. Young and old IF as well as old rapamycin-treated animals reduced their food intake by 20–30%, whereas the young rapamycin-treated animals reduced intake by less than 10%. Finally, neither rapamycin nor IF feeding had any impact on cognitive function, although physical activity was increased in both treatment groups, regardless of age. Indeed, we and others have emphasized that tasks which involve exploration as a component of the outcome variable (in this case, time spent exploring objects) may be subject to the confound that movement and activity are influenced by motivational state (in this case, food restriction). Further study is warranted to assess whether longer-term treatment yields similar results or if the nature of weight loss observed is selective to a particular body compartment (fat vs lean) and related to physical function.
Figure 3.
Young and old F344BN rats were fed rapamycin for 3 weeks or were given a regimen of intermittent (every other day) calorie restriction (IF). IF- and rapamycin-treated animals lost approximately 15% of their body weight relative to controls. Moreover, the young IF animals lost more weight than their rapamycin-fed counterparts (lower panel). There was an effect of age on the memory test (upper right); however, rapamycin had no impact on cognitive function, although physical activity was increased with treatment, regardless of age (*all ps < .05).
Elsewhere in the literature, rapamycin’s role in modulating adiposity and skeletal muscle metabolic activity have resulted in paradoxical conclusions. For example, Houde and colleagues (82) have shown that rapamycin administered to young rats for 15 days reduced adiposity and fat cell number and was associated with a coordinated down regulation of genes involved in both lipid uptake and output. However, insulin resistance, severe glucose intolerance, and increased gluconeogenesis were also observed. Similarly, Chang and colleagues (83) have demonstrated that obese KK/HlJ mice treated with rapamycin for 42 days, while on a high-fat diet, lost body weight and adiposity also had higher serum insulin levels and food intake. This resulted in marked decline in glucose tolerance paralleled by increased generation of plasma reactive oxygen species. These findings suggest that rapamycin administration may help to prevent diet-induced obesity, whereas prolonged use of rapamycin may exacerbate glucose intolerance. Furthermore, mTOR (the mammalian target of rapamycin) is known to decrease with age in F344BN rat skeletal muscle and is highly correlated with atrophy (84). However (85), translational signaling of mTOR in young adult and aged plantaris muscle is equally responsive to chronic overload after bilateral synergist ablation. These data suggest that while down regulation of mTOR may be beneficial for overall longevity, aged muscle and the susceptibility to insulin resistance requires adequate levels of mTOR functioning. In addition, aging skeletal muscle may respond adaptively, perhaps via mTOR signaling to interventions such as exercise protocols, which serve to load muscle.
Enalapril
Enalapril is an angiotensin-converting enzyme inhibitor, whose primary use is for the control of hypertension. However, beyond its impact on hemodynamic functioning, enalapril has been shown to modulate an amalgam of biological processes inherent to overall metabolic function, such as insulin sensitivity, inflammation, and oxidative resistance (86). In addition, observational studies in humans and converging evidence from our laboratory suggest that the use of angiotensin-converting enzyme inhibitors attenuate age-related increases in whole-body adiposity and physical performance decline, in the absence of any sizeable muscle hypertrophic effect (60,87–90). We observed no difference in a variety of physiological endpoints which may themselves cause a reduction in body weight (food intake, temperature, glucose and insulin levels, pathology) and which may be secondary to the effects of enalapril. This benefit is not isolated to skeletal muscle in that recently we (91) and others have shown that enalapril treatment mitigates dysfunction in other organ systems, including kidney and heart, which appear to be mediated through reductions in oxidative stress, inflammation, and insulin signaling (92–101). Since then, several other studies have demonstrated similar effects of angiotensin-converting enzyme inhibitors, in particular enalapril, on body composition in various strains of rats and mice, across different ages, and under normal and high-fat feeding scenarios (102,103). Indeed, these data lend credence to the hypothesis that it is not necessary to optimize the quantity of muscle that is preserved with an intervention but that it is the quality of muscle that remains which determines functionality. In order to expand our understanding of how this decrease in adiposity may play a role in preserving skeletal muscle quality, we used gene array technology to identify potential cell signaling pathways that are altered with enalapril and showed that mitochondria-mediated apoptosis is mitigated with this treatment (104). Finally, a very novel finding of this body of work was that the weight loss observed was also not due to some unobservable pathological catabolic state, and in fact, quite the opposite occurred in that enalapril attenuated the age-related increase in spontaneous tumor development relative to placebo-treated animals.
Taken together, these data suggest that while rapamycin is a promising life-span compound, caution should be taken in generalizing this impact in terms extending healthspan due to the negative impact of mTOR blockade on skeletal muscle function and the development of overt insulin resistance. In contrast, with enalapril, no life-span enhancement was observed, although we did observe improvements in whole-body adiposity, muscle quality, and physical performance. These data suggest that there is dissociation between the potential life-span and healthspan effects of treatments, which purport to mimic caloric restriction and that alternative approaches should be considered to maximize both.
CONCLUSIONS
There are key components explicated in this review that we believe that would help to define a new perspective in developing preclinical late-life intervention strategies that may have translational value in combating declining function in older individuals. These include (a) choosing a proper preclinical model in terms of identifying a time frame for a late-life intervention, (b) applying a clear working definition of sarcopenia, (c) assessing a mixture of both functional and biological outcomes, (d) carefully considering the choice of the intervention, and (e) highlighting the translation of findings to clinical studies.
For the most part, the data presented in this review are heavily biased toward the F344BN rat model because it has the qualities necessary to address our hypotheses. Of course, this does not mean that other types of animal models are less important. To be sure, the development and ultimate strength of any scientific set of hypotheses must rely on converging evidence from multiple sources including other strains and species, and genetically altered models. However, in terms of behavior and physiology, the F344BN is an excellent choice for evaluating changes in physical function.
The assessment of behavioral outcomes is essential to measuring the efficacy of any late-life intervention in the context of mitigating declining performance. In a recent review, a set of criteria were suggested for assessing the success of any late-life intervention; the primary outcome was stated as slowing healthspan decline, and consisted of the classic set of biomarkers (inflammation, oxidative stress, insulin function, etc.) (105). However, functional/behavioral markers were not mentioned. Studies in humans regarding the nature of the definition of sarcopenia all agree that improved physical functioning is the major determinant of whether an intervention is successful given that measures of muscle strength and mass are often uncorrelated or do not predict this outcome. In addition, interventions may have ubiquitous effects, each of which may be separately or wholly uncorrelated with their functional counterparts. Furthermore, like any good biomarker, behavior may be considered both a predictor and an outcome measure of response to treatment.
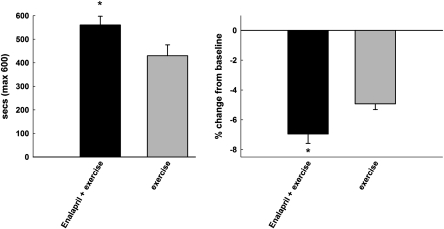
Finally, we should not be dismissive of pharmacological compounds that do not extend life span at the risk of overlooking potential therapeutics for enhancing healthspan. There may in fact be overt dissociations between the two such that life span may be enhanced at the expense of overall function. This is especially relevant to assessing late-life interventions, for which there is a growing need in the context of the world’s rapidly aging population and for which very little clinical data exist. Indeed, healthspan-enhancing therapeutics may create synergy when partnered with behavioral approaches such as mild levels of CR or low levels of exercise/physical activity and may be most effective in those vulnerable aged populations (obese, frail, arthritic, etc.) which are most challenged to engage in exercise/physical activity or for whom excessive weight loss is not recommended. By way of example, we have demonstrated that short-term very low-intensity exercise combined with low-dose enalapril treatment results in greater exercise tolerance compared with exercise alone in aged F344BN rats (C. S. Carter, K. Sweet, A. Posgai, E. Marzetti, unpublished data, 2010) (Figure 4). The converse may also be true, such that exercise or CR or pharmacological interventions may perhaps confer no benefit to function and perhaps may even cause harm. Preclinical models, especially those for which function is a primary outcome, represent a critical translational link for the more rapid translation of treatments to the clinical arena insofar as they may serve as a tool for the relatively rapid systematic assessment of traditional and nontraditional interventions, initiated late in life.
Figure 4.
Four weeks of low-intensity exercise (12 cm/s; 10 combined with low-dose enalapril treatment (20 mg/kg/d) administered to 24-month-old male F344BN rats results in additional weight loss and greater endurance relative to enalapril treatment alone (*p < .05).
FUNDING
This work was supported by National Institute on Aging Grant RO1AG24526, University of Florida Institute on Aging, The Claude D. Pepper Older Americans Independence Center Grant NIH P30 AG028740, and the McKnight Foundation.
Supplementary Material
References
- 1.Newman AB, Haggerty CL, Goodpaster B, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51(3):323–330. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- 2.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol. 2001;90(6):2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 3.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 4.Visser M, Langlois J, Guralnik JM, et al. High body fatness, but not low fat-free mass, predicts disability in older men and women: the Cardiovascular Health Study. Am J Clin Nutr. 1998;68(3):584–590. doi: 10.1093/ajcn/68.3.584. [DOI] [PubMed] [Google Scholar]
- 5.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50(5):1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 6.Miller GD, Rejeski WJ, Williamson JD, et al. The Arthritis, Diet and Activity Promotion Trial (ADAPT): design, rationale, and baseline results. Control Clin Trials. 2003;24(4):462–480. doi: 10.1016/s0197-2456(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 7.Rochon J, Bales CW, Ravussin E, et al. Design and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energy. J Gerontol A Biol Sci Med Sci. 2011;66(1):97–108. doi: 10.1093/gerona/glq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espeland MA, Gill TM, Guralnik J, et al. Designing clinical trials of interventions for mobility disability: results from the lifestyle interventions and independence for elders pilot (LIFE-P) trial. J Gerontol A Biol Sci Med Sci. 2007;62(11):1237–1243. doi: 10.1093/gerona/62.11.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson MR, Reid CM, Ames DA, et al. Feasibility of conducting a primary prevention trial of low-dose aspirin for major adverse cardiovascular events in older people in Australia: results from the ASPirin in Reducing Events in the Elderly (ASPREE) pilot study. Med J Aust. 2008;189(2):105–109. doi: 10.5694/j.1326-5377.2008.tb01932.x. [DOI] [PubMed] [Google Scholar]
- 10.Marzetti E, Privitera G, Simili V, et al. Multiple pathways to the same end: mechanisms of myonuclear apoptosis in sarcopenia of aging. Scientific World Journal. 2010;10:340–349. doi: 10.1100/tsw.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marzetti E, Hwang JC, Lees HA, et al. Mitochondrial death effectors: relevance to sarcopenia and disuse muscle atrophy. Biochim Biophys Acta. 2010;1800(3):235–244. doi: 10.1016/j.bbagen.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hepple RT, Qin M, Nakamoto H, Goto S. Caloric restriction optimizes the proteasome pathway with aging in rat plantaris muscle: implications for sarcopenia. Am J Physiol Regul Integr Comp Physiol. 2008;295(4):R1231–1237. doi: 10.1152/ajpregu.90478.2008. [DOI] [PubMed] [Google Scholar]
- 13.Colman RJ, Beasley TM, Allison DB, Weindruch R. Attenuation of sarcopenia by dietary restriction in rhesus monkeys. J Gerontol A Biol Sci Med Sci. 2008;63(6):556–559. doi: 10.1093/gerona/63.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter CS, Hofer T, Seo AY, Leeuwenburgh C. Molecular mechanisms of life- and health-span extension: role of calorie restriction and exercise intervention. Appl Physiol Nutr Metab. 2007;32(5):954–966. doi: 10.1139/H07-085. [DOI] [PubMed] [Google Scholar]
- 15.Colman RJ, McKiernan SH, Aiken JM, Weindruch R. Muscle mass loss in Rhesus monkeys: age of onset. Exp Gerontol. 2005;40(7):573–581. doi: 10.1016/j.exger.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Rice KM, Linderman JK, Kinnard RS, Blough ER. The Fischer 344/NNiaHSd X Brown Norway/BiNia is a better model of sarcopenia than the Fischer 344/NNiaHSd: a comparative analysis of muscle mass and contractile properties in aging male rat models. Biogerontology. 2005;6(5):335–343. doi: 10.1007/s10522-005-4808-0. [DOI] [PubMed] [Google Scholar]
- 17.Fisher AL. Of worms and women: sarcopenia and its role in disability and mortality. J Am Geriatr Soc. 2004;52(7):1185–1190. doi: 10.1111/j.1532-5415.2004.52320.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim J, Kim D, Choi J, et al. Changes in lipid distribution during aging and its modulation by calorie restriction. Age. 2009;31(2):127–142. doi: 10.1007/s11357-009-9089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marzetti E, Lees HA, Wohlgemuth SE, Leeuwenburgh C. Sarcopenia of aging: underlying cellular mechanisms and protection by calorie restriction. Biofactors. 2009;35(1):28–35. doi: 10.1002/biof.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JH, Kwak HB, Leeuwenburgh C, Lawler JM. Lifelong exercise and mild (8%) caloric restriction attenuate age-induced alterations in plantaris muscle morphology, oxidative stress and IGF-1 in the Fischer-344 rat. Exp Gerontol. 2008;43(4):317–329. doi: 10.1016/j.exger.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marzetti E, Lawler JM, Hiona A, Manini T, Seo AY, Leeuwenburgh C. Modulation of age-induced apoptotic signaling and cellular remodeling by exercise and calorie restriction in skeletal muscle. Free Radic Biol Med. 2008;44(2):160–168. doi: 10.1016/j.freeradbiomed.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 22.rks Naylor AJ, Leeuwenburgh C. Sarcopenia: the role of apoptosis and modulation by caloric restriction. Exerc Sport Sci Rev. 2008;36(1):19–24. doi: 10.1097/jes.0b013e31815ddd9d. [DOI] [PubMed] [Google Scholar]
- 23.Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005;19(6):668–670. doi: 10.1096/fj.04-2870fje. [DOI] [PubMed] [Google Scholar]
- 24.Hepple RT, Baker DJ, Kaczor JJ, Krause DJ. Long-term caloric restriction abrogates the age-related decline in skeletal muscle aerobic function. FASEB J. 2005;19(10):1320–1322. doi: 10.1096/fj.04-3535fje. [DOI] [PubMed] [Google Scholar]
- 25.Drew B, Phaneuf S, Dirks A, et al. Effects of aging and caloric restriction on mitochondrial energy production in gastrocnemius muscle and heart. Am J Physiol Regul Integr Comp Physiol. 2003;284(2):R474–R480. doi: 10.1152/ajpregu.00455.2002. [DOI] [PubMed] [Google Scholar]
- 26.Lee CK, Klopp RG, Weindruch R, Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285(5432):1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- 27.Sprott RL. Diet and calorie restriction. Exp Gerontol. 1997;32(1–2):205–214. doi: 10.1016/s0531-5565(96)00065-4. [DOI] [PubMed] [Google Scholar]
- 28.Holloszy JO. Exercise and food restriction in rats. J Nutr. 1992;122(3 suppl):774–777. doi: 10.1093/jn/122.suppl_3.774. [DOI] [PubMed] [Google Scholar]
- 29.Holloszy JO, Schechtman KB. Interaction between exercise and food restriction: effects on longevity of male rats. J Appl Physiol. 1991;70(4):1529–1535. doi: 10.1152/jappl.1991.70.4.1529. [DOI] [PubMed] [Google Scholar]
- 30.Carter CS, Leeuwenburgh C, Daniels M, Foster TC. Influence of calorie restriction on measures of age-related cognitive decline: role of increased physical activity. J Gerontol A Biol Sci Med Sci. 2009;64(8):850–859. doi: 10.1093/gerona/glp060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duffy PH, Feuers R, Nakamura KD, Leakey J, Hart RW. Effect of chronic caloric restriction on the synchronization of various physiological measures in old female Fischer 344 rats. Chronobiol Int. 1990;7(2):113–124. doi: 10.3109/07420529009056963. [DOI] [PubMed] [Google Scholar]
- 32.Ingram DK, Weindruch R, Spangler EL, Freeman JR, Walford RL. Dietary restriction benefits learning and motor performance of aged mice. J Gerontol. 1987;42(1):78–81. doi: 10.1093/geronj/42.1.78. [DOI] [PubMed] [Google Scholar]
- 33.Olshansky SJ, Goldman DP, Zheng Y, Rowe JW. Aging in America in the twenty-first century: demographic forecasts from the MacArthur Foundation Research Network on an Aging Society. Milbank Q. 2009;87(4):842–862. doi: 10.1111/j.1468-0009.2009.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 suppl):990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 35.Clark BC, Manini TM. Functional consequences of sarcopenia and dynapenia in the elderly. Curr Opin Clin Nutr Metab Care. 2010;13(3):271–276. doi: 10.1097/MCO.0b013e328337819e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63(8):829–834. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- 37.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 38.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Visser M. Towards a definition of sarcopenia—results from epidemiologic studies. J Nutr Health Aging. 2009;13(8):713–716. doi: 10.1007/s12603-009-0202-y. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz RS. Obesity in the elderly. In: Bray GA, editor. Handbook of Obesity. New York: Marcel Dekker, Inc.; 1998. pp. 103–114. [Google Scholar]
- 41.Marzetti E, Hwang JC, Lees HA, et al. Mitochondrial death effectors: relevance to sarcopenia and disuse muscle atrophy. Biochim Biophys Acta. 2009;1800(3):235–244. doi: 10.1016/j.bbagen.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.You T, Sonntag WE, Leng X, Carter CS. Lifelong caloric restriction and interleukin-6 secretion from adipose tissue: effects on physical performance decline in aged rats. J Gerontol A Biol Sci Med Sci. 2007;62(10):1082–1087. doi: 10.1093/gerona/62.10.1082. [DOI] [PubMed] [Google Scholar]
- 43.Cohen HJ, Pieper CF, Harris T, Rao KM, Currie MS. The association of plasma IL-6 levels with functional disability in community-dwelling elderly. J Gerontol Med Sci. 1997;52(4):M201–M208. doi: 10.1093/gerona/52a.4.m201. [DOI] [PubMed] [Google Scholar]
- 44.Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47(6):639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 45.Phillips T, Childs AC, Dreon DM, Phinney S, Leeuwenburgh C. A dietary supplement attenuates IL-6 and CRP after eccentric exercise in untrained males. Med Sci Sports Exerc. 2003;35(12):2032–2037. doi: 10.1249/01.MSS.0000099112.32342.10. [DOI] [PubMed] [Google Scholar]
- 46.Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50(12):1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 47.Penninx BW, Kritchevsky SB, Newman AB, et al. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc. 2004;52(7):1105–1113. doi: 10.1111/j.1532-5415.2004.52308.x. [DOI] [PubMed] [Google Scholar]
- 48.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26(2):372–379. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 49.Chung HY, Cesari M, Anton S, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8(1):18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalani R, Judge S, Carter C, Pahor M, Leeuwenburgh C. Effects of caloric restriction and exercise on age-related, chronic inflammation assessed by C-reactive protein and interleukin-6. J Gerontol A Biol Sci Med Sci. 2006;61(3):211–217. doi: 10.1093/gerona/61.3.211. [DOI] [PubMed] [Google Scholar]
- 51.Marzetti E, Carter CS, Wohlgemuth SE, et al. Changes in IL-15 expression and death-receptor apoptotic signaling in rat gastrocnemius muscle with aging and life-long calorie restriction. Mech Ageing Dev. 2009;130(4):272–280. doi: 10.1016/j.mad.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59(3):242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 53.Cesari M, Pahor M, Bartali B, et al. Antioxidants and physical performance in elderly persons: the Invecchiare in Chianti (InCHIANTI) study. Am J Clin Nutr. 2004;79(2):289–294. doi: 10.1093/ajcn/79.2.289. [DOI] [PubMed] [Google Scholar]
- 54.Kusminski CM, Shetty S, Orci L, Unger RH, Scherer PE. Diabetes and apoptosis: lipotoxicity. Apoptosis. 2009;14(12):1484–1495. doi: 10.1007/s10495-009-0352-8. [DOI] [PubMed] [Google Scholar]
- 55.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol Biol Sci. 1999;54(11):B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 56.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–562. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carter CS, Sonntag WE, Onder G, Pahor M. Physical performance and longevity in aged rats. J Gerontol Biol Sci. 2002;57(5):B193–B197. doi: 10.1093/gerona/57.5.b193. [DOI] [PubMed] [Google Scholar]
- 58.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol Med Sci. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 59.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carter CS, Cesari M, Ambrosius WT, et al. Angiotensin-converting enzyme inhibition, body composition, and physical performance in aged rats. J Gerontol A Biol Sci Med Sci. 2004;59(5):416–423. doi: 10.1093/gerona/59.5.b416. [DOI] [PubMed] [Google Scholar]
- 61.Li H, Matheny M, Nicolson M, Tumer N, Scarpace PJ. Leptin gene expression increases with age independent of increasing adiposity in rats. Diabetes. 1997;46(12):2035–2039. doi: 10.2337/diab.46.12.2035. [DOI] [PubMed] [Google Scholar]
- 62.Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J Gerontol. 1985;40(6):657–670. doi: 10.1093/geronj/40.6.657. [DOI] [PubMed] [Google Scholar]
- 63.Payne AM, Dodd SL, Leeuwenburgh C. Life-long calorie restriction in Fischer 344 rats attenuates age-related loss in skeletal muscle-specific force and reduces extracellular space. J Appl Physiol. 2003;95(6):2554–2562. doi: 10.1152/japplphysiol.00758.2003. [DOI] [PubMed] [Google Scholar]
- 64.Newman AB, Yanez D, Harris T, Duxbury A, Enright PL, Fried LP. Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49(10):1309–1318. doi: 10.1046/j.1532-5415.2001.49258.x. [DOI] [PubMed] [Google Scholar]
- 65.Morley JE. Pathophysiology of weight loss in older persons. Nestle Nutr Workshop Ser Clin Perform Programme. 2005;10:167–172. doi: 10.1159/000083304. [DOI] [PubMed] [Google Scholar]
- 66.Wannamethee SG, Shaper AG, Lennon L. Reasons for intentional weight loss, unintentional weight loss, and mortality in older men. Arch Intern Med. 2005;165(9):1035–1040. doi: 10.1001/archinte.165.9.1035. [DOI] [PubMed] [Google Scholar]
- 67.Dirks AJ, Leeuwenburgh C. Caloric restriction in humans: potential pitfalls and health concerns. Mech Ageing Dev. 2006;127(1):1–7. doi: 10.1016/j.mad.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 68.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116(4):641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- 69.Holloszy JO, Smith EK, Vining M, Adams S. Effect of voluntary exercise on longevity of rats. J Appl Physiol. 1985;59(3):826–831. doi: 10.1152/jappl.1985.59.3.826. [DOI] [PubMed] [Google Scholar]
- 70.Dirks AJ, Leeuwenburgh C. Aging and lifelong calorie restriction result in adaptations of skeletal muscle apoptosis repressor, apoptosis-inducing factor, X-linked inhibitor of apoptosis, caspase-3, and caspase-12. Free Radic Biol Med. 2004;36(1):27–39. doi: 10.1016/j.freeradbiomed.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Holloszy JO, Smith EK. Effects of exercise on longevity of rats. Fed Proc. 1987;46(5):1850–1853. [PubMed] [Google Scholar]
- 72.Holloszy JO. Exercise and longevity: studies on rats. J Gerontol. 1988;43(6):B149–B151. doi: 10.1093/geronj/43.6.b149. [DOI] [PubMed] [Google Scholar]
- 73.Holloszy JO. Exercise increases average longevity of female rats despite increased food intake and no growth retardation. J Gerontol. 1993;48(3):B97–B100. doi: 10.1093/geronj/48.3.b97. [DOI] [PubMed] [Google Scholar]
- 74.Holloszy JO. Mortality rate and longevity of food-restricted exercising male rats: a reevaluation. J Appl Physiol. 1997;82(2):399–403. doi: 10.1152/jappl.1997.82.2.399. [DOI] [PubMed] [Google Scholar]
- 75.Shapiro A, Cheng KY, Gao Y, et al. The act of voluntary wheel running reverses dietary hyperphagia and increases leptin signaling in ventral tegmental area of aged obese rats. Gerontology. doi: 10.1159/000321343. doi:10.1159/000321343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marzetti E, Groban L, Wohlgemuth SE, et al. Effects of short-term GH supplementation and treadmill exercise training on physical performance and skeletal muscle apoptosis in old rats. Am J Physiol Regul Integr Comp Physiol. 2008;294(2):R558–R567. doi: 10.1152/ajpregu.00620.2007. [DOI] [PubMed] [Google Scholar]
- 77.Narath E, Skalicky M, Viidik A. Voluntary and forced exercise influence the survival and body composition of ageing male rats differently. Exp Gerontol. 2001;36(10):1699–1711. doi: 10.1016/s0531-5565(01)00145-0. [DOI] [PubMed] [Google Scholar]
- 78.Judge S, Jang YM, Smith A, et al. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am J Physiol Regul Integr Comp Physiol. 2005;295(3):R773–780. doi: 10.1152/ajpregu.00396.2005. [DOI] [PubMed] [Google Scholar]
- 79.Betik AC, Thomas MM, Wright KJ, Riel CD, Hepple RT. Exercise training from late middle age until senescence does not attenuate the declines in skeletal muscle aerobic function. Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R744–R755. doi: 10.1152/ajpregu.90959.2008. [DOI] [PubMed] [Google Scholar]
- 80.Ingram DK, Anson RM, de CR, et al. Development of calorie restriction mimetics as a prolongevity strategy. Ann N Y Acad Sci. 2004;1019:412–423. doi: 10.1196/annals.1297.074. [DOI] [PubMed] [Google Scholar]
- 81.Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Houde VP, Brule S, Festuccia WT, et al. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes. 2010;59(6):1338–1348. doi: 10.2337/db09-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chang GR, Wu YY, Chiu YS, et al. Long-term administration of rapamycin reduces adiposity, but impairs glucose tolerance in high-fat diet-fed KK/HlJ mice. Basic Clin Pharmacol Toxicol. 2009;105(3):188–198. doi: 10.1111/j.1742-7843.2009.00427.x. [DOI] [PubMed] [Google Scholar]
- 84.Paturi S, Gutta AK, Katta A, et al. Effects of aging and gender on muscle mass and regulation of Akt-mTOR-p70s6k related signaling in the F344BN rat model. Mech Ageing Dev. 2010;131(3):202–209. doi: 10.1016/j.mad.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 85.Chale-Rush A, Morris EP, Kendall TL, Brooks NE, Fielding RA. Effects of chronic overload on muscle hypertrophy and mTOR signaling in young adult and aged rats. J Gerontol A Biol Sci Med Sci. 2009;64(12):1232–1239. doi: 10.1093/gerona/glp146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Henriksen EJ, Jacob S. Modulation of metabolic control by angiotensin converting enzyme (ACE) inhibition. J Cell Physiol. 2003;196(1):171–179. doi: 10.1002/jcp.10294. [DOI] [PubMed] [Google Scholar]
- 87.Carter CS, Onder G, Kritchevsky SB, Pahor M. Angiotensin-converting enzyme inhibition intervention in elderly persons: effects on body composition and physical performance. J Gerontol A Biol Sci Med Sci. 2005;60(11):1437–1446. doi: 10.1093/gerona/60.11.1437. [DOI] [PubMed] [Google Scholar]
- 88.Onder G, Penninx BW, Balkrishnan R, et al. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet. 2002;359(9310):926–930. doi: 10.1016/s0140-6736(02)08024-8. [DOI] [PubMed] [Google Scholar]
- 89.Bahi L, Koulmann N, Sanchez H, et al. Does ACE inhibition enhance endurance performance and muscle energy metabolism in rats? J Appl Physiol. 2004;96(1):59–64. doi: 10.1152/japplphysiol.00323.2003. [DOI] [PubMed] [Google Scholar]
- 90.Foianini KR, Steen MS, Kinnick TR, Schmit MB, Youngblood EB, Henriksen EJ. Effects of exercise training and ACE inhibition on insulin action in rat skeletal muscle. J Appl Physiol. 2000;89(2):687–694. doi: 10.1152/jappl.2000.89.2.687. [DOI] [PubMed] [Google Scholar]
- 91.Moningka NC, Sasser JM, Croker B, Carter C, Baylis C. Protection against age-dependent renal injury in the F344xBrown Norway male rat is associated with maintained nitric oxide synthase. Mech Ageing Dev. 2010;132(1–2):1–7. doi: 10.1016/j.mad.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Cavanagh EM, Inserra F, Ferder M, Ferder L. From mitochondria to disease: role of the renin-angiotensin system. Am J Nephrol. 2007;27(6):545–553. doi: 10.1159/000107757. [DOI] [PubMed] [Google Scholar]
- 93.Basso N, Cini R, Pietrelli A, Ferder L, Terragno NA, Inserra F. Protective effect of long-term angiotensin II inhibition. Am J Physiol Heart Circ Physiol. 2007;293(3):H1351–H1358. doi: 10.1152/ajpheart.00393.2007. [DOI] [PubMed] [Google Scholar]
- 94.de Cavanagh EM, Piotrkowski B, Basso N, et al. Enalapril and losartan attenuate mitochondrial dysfunction in aged rats. FASEB J. 2003;17(9):1096–1098. doi: 10.1096/fj.02-0063fje. [DOI] [PubMed] [Google Scholar]
- 95.de Cavanagh EM, Inserra F, Toblli J, Stella I, Fraga CG, Ferder L. Enalapril attenuates oxidative stress in diabetic rats. Hypertension. 2001;38(5):1130–1136. doi: 10.1161/hy1101.092845. [DOI] [PubMed] [Google Scholar]
- 96.de Cavanagh EM, Inserra F, Ferder L, Fraga CG. Enalapril and captopril enhance glutathione-dependent antioxidant defenses in mouse tissues. Am J Physiol Regul Integr Comp Physiol. 2000;278(3):R572–R577. doi: 10.1152/ajpregu.2000.278.3.R572. [DOI] [PubMed] [Google Scholar]
- 97.de Cavanagh EM, Ferder L, Carrasquedo F, et al. Higher levels of antioxidant defenses in enalapril-treated versus non-enalapril-treated hemodialysis patients. Am J Kidney Dis. 1999;34(3):445–455. doi: 10.1016/s0272-6386(99)70071-5. [DOI] [PubMed] [Google Scholar]
- 98.de Cavanagh EM, Fraga CG, Ferder L, Inserra F. Enalapril and captopril enhance antioxidant defenses in mouse tissues. Am J Physiol. 1997;272(2 Pt 2):R514–R518. doi: 10.1152/ajpregu.1997.272.2.R514. [DOI] [PubMed] [Google Scholar]
- 99.Inserra F, Romano LA, de Cavanagh EM, Ercole L, Ferder LF, Gomez RA. Renal interstitial sclerosis in aging: effects of enalapril and nifedipine. J Am Soc Nephrol. 1996;7(5):676–680. doi: 10.1681/ASN.V75676. [DOI] [PubMed] [Google Scholar]
- 100.de Cavanagh EM, Inserra F, Ferder L, Romano L, Ercole L, Fraga CG. Superoxide dismutase and glutathione peroxidase activities are increased by enalapril and captopril in mouse liver. FEBS Lett. 1995;361(1):22–24. doi: 10.1016/0014-5793(95)00137-x. [DOI] [PubMed] [Google Scholar]
- 101.Ferder L, Inserra F, Romano L, Ercole L, Pszenny V. Decreased glomerulosclerosis in aging by angiotensin-converting enzyme inhibitors. J Am Soc Nephrol. 1994;5(4):1147–1152. doi: 10.1681/ASN.V541147. [DOI] [PubMed] [Google Scholar]
- 102.Santos EL, de Picoli Souza K, da Silva ED, et al. Long term treatment with ACE inhibitor enalapril decreases body weight gain and increases life span in rats. Biochem Pharmacol. 2009;78(8):951–958. doi: 10.1016/j.bcp.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 103.Weisinger RS, Stanley TK, Begg DP, Weisinger HS, Spark KJ, Jois M. Angiotensin converting enzyme inhibition lowers body weight and improves glucose tolerance in C57BL/6J mice maintained on a high fat diet. Physiol Behav. 2009;98(1–2):192–197. doi: 10.1016/j.physbeh.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 104.Carter CS, Giovannini S, Seo DO, et al. Differential effects of enalapril and losartan on body composition and indices of muscle quality in aged male Fisher 344 Brown Norway rats. Age. doi: 10.1007/s11357-010-9196-y. doi:10.1007/s11357-010-9196-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rae MJ, Butler RN, Campisi J, et al. The demographic and biomedical case for late-life interventions in aging. Sci Transl Med. 2010;2(40):40cm21. doi: 10.1126/scitranslmed.3000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.