HERACLES (Hercules according to the Romans) was probably the earliest and greatest body builder of all times and, without doubt, the greatest hero of Greek mythology. His conception, the result of a brief affair between the supreme deity Zeus and a mortal woman, made Zeus's wife Hera furious. She surreptitiously inserted two deadly snakes into Heracle's crib. According to legend, that evening, a nurse found the delighted baby giggling and joyfully playing with a strangled serpent in each hand. Are stronger people already stronger at birth, as the Heracles legend seems to suggest? Is the destiny of our muscles already encoded in our genes (1)? Perhaps more importantly, what is the role of genetics as opposed to behavioral and environmental factors in the maintenance of muscle mass and strength with aging?
A decline in muscle mass and strength with aging can be found in every living creature endowed with muscle tissue. Worms, flies, fish, frogs, mice, rats, dogs, and primates are among the many species that have been studied that show atrophic and structural degeneration in aging muscles often with the accumulation of abnormal degenerative proteins. Studies conducted in isogenic Caenorhabditis elegans, maintained under constant environmental conditions, show high interindividual variability in the rate of age-associated loss of muscle mass and mobility impairment, which highly predicts survival (2). Interestingly, interventions that preserve muscle integrity and delay muscle aging also tend to delay aging in the whole animal and to extend life span (3). In order to better understand the underlying biological control of body composition with aging in humans, we need better animal models, including evidence from longitudinal studies in mice.
In humans, both muscle mass and muscle strength begin to decline at about the fourth decade. This process is virtually inextricably connected with aging, spares no one, is widely heterogeneous in time and magnitude across individuals, and accelerates with advancing age (4). A substantial literature claims that this process can be delayed with exercise, especially by resistance training (5–7). However, some researchers have questioned whether even the best exercise truly interferes with the underlying process that leads to the decline in muscle mass and strength with aging. In fact, it is difficult to exclude the possibility that exercise simply obscures the phenotypic manifestations of aging muscle by superimposing muscle hypertrophy. Indeed, there is no currently known effective intervention that prevents muscle impairment with aging.
Interestingly, mass and strength track together almost perfectly during development; changes in muscle mass are accompanied by almost perfectly proportional increments in strength (8). This should not be surprising; if the new tissue is perfect and expresses maximal function, then adding new tissue will generate proportionally more strength. With aging, however, changes in muscle mass and strength tend to dissociate. No one has yet tracked muscle mass and strength across the life course from childhood to old age, so currently, we are only able to estimate this process by patching together information from longitudinal studies that evaluated limited portions of the life span. Using data from the Baltimore Longitudinal Study of Aging, Metter and colleagues examined longitudinal changes in muscle quality, operationalized as the strength/mass ratio (9). Interestingly, when muscle mass was estimated from dual-energy X-ray absorptiometry, the strength/mass ratio declined dramatically with aging, or in other words, the decline in strength was much greater than that predicted by the decline in mass. These findings have been confirmed in analyses conducted in the Health ABC study, where muscle mass was estimated by computerized tomography (10) and by further data collected in the Baltimore Longitudinal Study on Aging (Figure 1). However, when Metter and colleagues estimated muscle mass from 24-hour creatinine excretion (a proxy measure of the body's store of contractile proteins), the age-associated decline in muscle quality was much less evident (9). These findings suggest that, in parallel to changes in muscle mass with aging, structural changes in muscle tissue occur that contribute to the global loss of muscle function. Combined, these two effects magnify the differences in strength among individuals (Figure 2). Some age-related changes, such as extracellular and intracellular lipid infiltration, deposition of abnormal proteins, contractile and structural proteins misfolding, and mitochondrial dysfunction, have been identified and are the object of intensive research (11–13). Less studied are the neurological aspects of muscle function. Muscle fibers work together according to predefined patterns of activation that are regulated by the central nervous system. Neuromuscular coupling may be affected by age at both the level of the single fiber and in networks. The gaps in knowledge are great; we need more basic research in isolated fibers or muscle tissue, in animal models, in muscle biopsies, and in acute and moderate-term clinical experiments. Thus, progress toward new interventions depends on better understanding of quantitative and qualitative changes that occur with aging in muscle, the underlying causative mechanisms for these changes, and their consequences on global muscle function. Opportunities for successful translation of new basic knowledge into clinical benefit could be tremendously enhanced by a coordinated effort involving a wide spectrum of expertise from basic biology to clinical science.
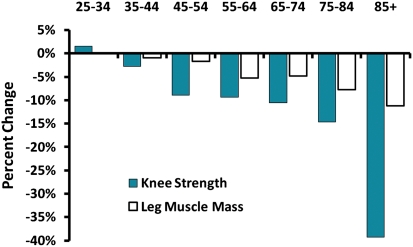
Figure 1.
Decline in knee extension strength and leg muscle mass with aging. This figure uses longitudinal data from the Baltimore Longitudinal Study of Aging to estimate age-related change in muscle strength, measured by isokinetic dynamometry and muscle mass determined by dual-energy X-ray absorptiometry, according to age decade in men. Despite considerable fluctuation in middle age due to measurement error, the rate of decline for both parameters is steeper with older age, but the decline in muscle strength is substantially greater than the decline in muscle mass.
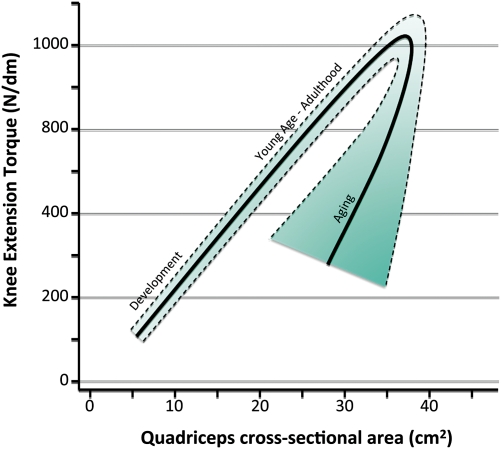
Figure 2.
Theoretical model of the relationship between muscle mass and strength over the life span. Studies have shown that across development from childhood to adulthood, the mass/strength ratio remains constant, probably because newly formed muscle tissue is functioning perfectly (8). However, the decline in strength that occurs with aging is not associated with a proportional decline in mass, and therefore, the strength/mass ratio (a marker of muscle quality) tends to diminish, although with great heterogeneity between individuals.
The effects of aging on muscle and neuromuscular junctions probably involve a plethora of mechanisms and molecular pathways, some influencing others, depending on individual susceptibilities and/or patterns of coexisting diseases. There is emerging evidence that patients with diabetes, peripheral artery disease, chronic pulmonary disease, HIV, CHF, and other chronic conditions experience accelerated age-related declines in muscle mass and strength. Cachexia, the extreme muscle atrophy associated with these conditions, now has proposed diagnostic criteria (14). We do not yet understand the mechanisms that link so many different chronic diseases to the common outcome of muscle atrophy. Probably, the final common pathway may well be the activation of genes that upregulate protein degradation or turnover, but it is likely that diseases can activate this pathway in many different ways (15). It would be important to determine whether the effects of diseases on muscle quality (eg, the strength/mass ratio) are similar to the effects of aging because this information may provide key information to advance our diagnosis capabilities and potentially lead to tailored interventions.
There is currently a lively debate regarding the best way to diagnose age-associated muscle impairment. One widely accepted viewpoint is to define “sarcopenia” as some degree of low muscle mass below a standard threshold, perhaps adjusted for body size (16,17). This condition would be identified based on its association with some measure of current and future limitation in physical performance and/or disability. There are many advantages of using such a definition: Muscle mass could be easily estimated by dual-energy X-ray absorptiometry, a relatively inexpensive and widely accessible technology, as is currently done for osteoporosis. There are also substantial disadvantages to this approach. Several studies have shown that after accounting for the effect of muscle strength on outcomes, such as mobility limitations, disability, and death, muscle mass is no longer independently associated with these outcomes (18–21). These findings are not unexpected. The effects of aging on muscle are more complex than simply atrophy or fiber apoptosis, and the aging effect is greater for strength than for mass. There are practical implications of these findings. It seems illogical to root the definition of age-related muscle impairment on muscle mass alone if this parameter is not independently associated with current and future functional limitation. A valid alternative is to operationalize age-related muscle impairment based on loss of strength.
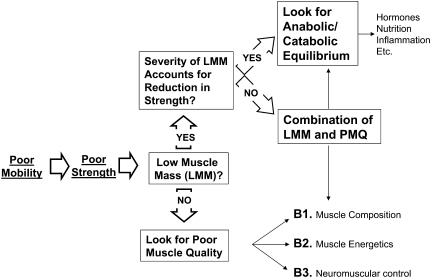
In geriatric medicine, the assessment of muscle function is always the most effective starting point. Based on the outcome of this test, we might consider a diagnostic algorithm similar to the one depicted in Figure 3. The process starts by assessing physical performance, for example, by evaluating walking speed. In those patients who have slow walking speed, the logical next step is to assess the many possible causes of impaired performance, including poor muscle strength. If muscle strength is below a certain “diagnostic threshold,” it would be important to understand to what extent this condition is attributable to reduced muscle mass and/or reduced muscle quality. In the case of poor muscle quality, we may proceed further to identify possible treatable causes of such a condition. On the surface, this is a very simple approach, but … as usual, the devil hides in the details. First, it would be necessary to identify clinically meaningful thresholds. Meaningful thresholds for walking speed have been described, but the determination of critical thresholds for muscle strength is complex. Which muscle groups to test? Should we assess force or power? It would be necessary to identify strength thresholds based on their relationship with relevant outcomes, such as poor lower extremity performance or risk of mobility disability. Attempts in this direction are currently ongoing using pooled data from multiple large longitudinal studies. Unfortunately, poor muscle strength is only one of the many possible causes of mobility disability, and therefore, the relationship between strength and mobility may change based on the presence of other significant impairments. In fact, compensation by other systems may be one key to aging successfully and coping with impairments. For example, muscle strength reserve as well as adaptability and plasticity of the central nervous system are key requirements for compensatory strategies aimed at maintaining the best possible physical function. Thus, posture and gait might compensate for balance problems or joint pain but only if strength reserves are available and an alternative motor program can be implemented. As evidence for this approach, Rantanen and colleagues demonstrated that low muscle strength is a significant but weak predictor of mobility disability over the short term, but the magnitude of the association increased dramatically in people who have balance problems (22). The implication is that the critical threshold for strength to maintain mobility might be different based on the presence or absence of balance problems.
Figure 3.
Proposed clinical approach to the evaluation of age-associated muscle impairment. The first step is always the assessment of physical function, followed by a measure of strength. In patients with poor strength, it is important to distinguish the roles of low muscle mass and poor muscle quality because they may have different causes and, at least theoretically, may respond to different treatments. The major obstacle to trying to implement this model is to determine critical thresholds in strength and mass that could drive the flow of the algorithm.
Even if we were able to determine who has muscle impairment and were able to classify it as due to low muscle mass and/or poor muscle quality, we do not yet have effective treatments other than exercise. Yet, new interventions depend on being able to define who has the condition of interest. It is very possible that a treatment that prevents or reverses decline in muscle mass will not necessarily be the same treatment to address altered muscle quality. In order to develop treatments for loss of muscle quality, further research is needed to determine common causes. Treatment choice and prognosis might well depend on whether the problem is the accumulation of an abnormal protein, reduced oxidative capacity in mitochondria, or dysfunction at the neuromuscular junction. Biomarkers that help differentiate and identify these causal pathways might help determine which patients would respond to certain treatments or interventions. For example, preliminary results from clinical trials presented at scientific meetings suggest that selective androgen receptor modulators increase muscle mass with little or no change in strength. These effects might have differed if the target population were persons in whom reduced muscle mass was a predominant contributor to muscle impairment.
In spite of its complexity, age-related muscle impairment might be the aging phenotype closest to a cure. To move closer to this goal, we need reciprocal communication and teamwork between basic and clinical investigators using a translational perspective. The section presented in this issue of the Journal of Gerontology is a first attempt to foster such communication, to help demolish boundaries between basic and clinical science, and to focus on our ultimate goal, the well-being of the older population. Although inspiring, Heracles’ legend cannot help us here. According to the rest of the story, Eurystheus, the archenemy who became king in Heracles’ place, forced Heracles to carry out his 12 labors. These phenomenal achievements went on to be celebrated by generations of poets and writers who inscribed the name of Heracles eternally in history. Heracles, after accomplishing his incredibly difficult tasks and other mythological adventures, died without ever becoming old and weak. To never age, weaken, and become disabled is the sweet destiny of all heroes and creatures born from our desires and fantasies.
Funding
This work was supported in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, Baltimore, Maryland, and received support from NIH grant P30 AG024827.
References
- 1.Sayer AA, Syddall HE, Gilbody HJ, Dennison EM, Cooper C. Does sarcopenia originate in early life? Findings from the Hertfordshire Cohort Study. J Gerontol A Med Sci. 2004;59(9):M930–M934. doi: 10.1093/gerona/59.9.m930. [DOI] [PubMed] [Google Scholar]
- 2.Herndon LA, Schmeissner PJ, Dudaronek JM, et al. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419(6909):808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- 3.Demontis F, Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143(5):813–825. doi: 10.1016/j.cell.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes VA, Frontera WR, Wood M, et al. Longitudinal muscle strength changes in older adults. J Gerontol A Biol Sci. 2001;56(5):B209–B217. doi: 10.1093/gerona/56.5.b209. [DOI] [PubMed] [Google Scholar]
- 5.Nelson ME, Fiatarone MA, Morganti CM, Trice I, Greenberg RA, Evans WJ. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures. JAMA. 1994;272(24):1909–1914. doi: 10.1001/jama.1994.03520240037038. [DOI] [PubMed] [Google Scholar]
- 6.Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. New Engl J Med. 1994;330(25):1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 7.Hanson E, Srivatsan S, Agrawal S, et al. Effects of strength training on physical function: influence of power, strength, and body composition. J Strength Cond Res. 2009;23(9):2627–2637. doi: 10.1519/JSC.0b013e3181b2297b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tonson A, Ratel S, Le Fur Y, Cozzone P, Bendahan D. Effect of maturation on the relationship between muscle size and force production. Med Sci Sports Exerc. 2008;40(5):918–925. doi: 10.1249/MSS.0b013e3181641bed. [DOI] [PubMed] [Google Scholar]
- 9.Metter EJ, Lynch N, Conwit R, Lindle R, Tobin J, Hurley B. Muscle quality and age: cross-sectional and longitudinal comparisons. J Gerontol A Biol Sci. 1999;54(5):B207–B218. doi: 10.1093/gerona/54.5.b207. [DOI] [PubMed] [Google Scholar]
- 10.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 11.Sipilä S, Suominen H. Knee extension strength and walking speed in relation to quadriceps muscle composition and training in elderly women. Clin Physiol. 1994;14(4):433–442. doi: 10.1111/j.1475-097x.1994.tb00402.x. [DOI] [PubMed] [Google Scholar]
- 12.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Phys. 2001;90(6):2157–2165. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 13.Hipkiss AR, Gregory SM. Mitochondrial dysfunction, proteotoxicity, and aging: causes or effects, and the possible impact of NAD+-controlled protein glycation. Adv Clin Chem. 2010;50:123–150. [PubMed] [Google Scholar]
- 14.Evans WJ, Morley JE, Argilés J, et al. Cachexia: a new definition. Clin Nutr. 2008;27(6):793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Tisdale MJ. Is there a common mechanism linking muscle wasting in various disease types? Curr Opin Support Palliat Care. 2007;1(4):287–292. doi: 10.1097/SPC.0b013e3282f35238. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(suppl 5):990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 17.Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159(4):413–421. doi: 10.1093/aje/kwh058. [DOI] [PubMed] [Google Scholar]
- 18.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Phys. 2003;95(5):1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 19.Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the Health, Aging and Body Composition Study Cohort. J Gerontol A Biol Sci Med Sci. 2006;61(1):72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 20.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60(3):324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 21.Visser M, Newman AB, Nevitt MC, et al. Reexamining the sarcopenia hypothesis: muscle mass versus muscle strength. Ann N Y Acad Sci. 2000;904(1):456–461. [PubMed] [Google Scholar]
- 22.Rantanen T, Guralnik JM, Ferrucci L, et al. Coimpairments as predictors of severe walking disability in older women. J Am Geriatr Soc. 2001;49(1):21–27. doi: 10.1046/j.1532-5415.2001.49005.x. [DOI] [PubMed] [Google Scholar]