Abstract
Background.
Theoretical definitions of sarcopenia traditionally emphasize age-related loss of muscle strength; however, most analyses of the association between strength and mobility examine strength at a single time point. This study sought to identify sex-specific cutpoints for muscle strength and power (at one time point) and 3-year changes in strength and power that would maximize prediction of 3-year mobility decline.
Methods.
Longitudinal analysis of 934 adults aged ≥65 years enrolled in the Invecchiare in Chianti study was conducted. Grip strength, knee extension strength, and lower extremity power were measured at baseline and 3 years postenrollment. Mobility function (gait speed and self-reported mobility disability) was measured at 3 and 6 years postenrollment. Classification and regression tree analysis was used to predict mobility decline from Years 3 to 6.
Results.
Men with knee extension strength <19.2 kg and grip strength <39.0 kg had clinically meaningful declines in gait speed of .24 m/s. Furthermore, men with power <105 W were nearly nine times more likely to develop incident mobility disability (likelihood ratio = 8.68; 95% confidence interval = 3.91, 19.44). Among women, knee extension strength <18.0 kg was associated with a minimal gait speed decline of 0.06 m/s, and women with leg power <64 W were three times more likely to develop incident mobility disability (likelihood ratio = 3.01; 95% confidence interval = 1.79, 5.08). Three-year changes in strength and power did not predict mobility decline in either sex.
Conclusions.
Findings suggest that strength and power measured at one time point are more predictive of mobility decline than 3-year changes and that low strength and power are particularly powerful risk factors in men.
Keywords: Strength, Sarcopenia, Mobility decline
SARCOPENIA, age-associated loss of muscle mass and strength, is associated with increased risk for functional limitation, mobility decline, and mortality (1–3). As increasing evidence suggests that muscle strength is a better predictor of mobility decline and disability than muscle mass, the emphasis of sarcopenia research has shifted to understanding how muscle strength is related to mobility (4–5), which is the focus of this article. Although theoretically, strength loss occupies a central role in our understanding of sarcopenia (ie, changes in strength), the majority of research has used distribution-based cutpoints to define low strength (6–8). This approach has two important limitations. First, a distribution-based cutpoint may not distinguish groups most at risk for disability. Second, existing cutpoints rely on muscle strength at a single time point, which could be problematic, given that the intended focus of sarcopenia is loss of muscle strength over time.
Muscle strength cutpoints predictive of future mobility decline that can be quickly and accurately measured in the clinical setting would provide clinicians an important tool for identifying persons at risk of mobility loss who might benefit from targeted intervention. However, a focus on muscle strength at a single time point alone may miss older persons who experience significant declines in strength but never cross below a given strength criterion. Therefore, attention to changes in strength as well may identify additional persons who might benefit from early intervention.
The primary objectives of this study were to determine which combination of muscle strength and power, assessed at one time point and as 3-year changes, are most predictive of mobility decline and to determine optimal sex-specific cutpoints in order to identify older adults at greatest risk of mobility decline in the Invecchiare in Chianti (InCHIANTI) study. We also examined whether cutpoints differed by age, weight, or body mass index (9). Because leg power, another measure of muscle function that accounts for force and velocity, has been proposed as a better predictor of mobility than strength alone (10–11), we also explored the aforementioned objectives using lower extremity muscle power in the place of strength.
Methods
Participants
InCHIANTI is a prospective population-based study of the factors that contribute to mobility decline in older Italian adults. The study sample (1,155 participants aged 65–102 years) was randomly selected using a multistage stratified sampling method from two towns in the Chianti geographic area of Italy (Greve in Chianti and Bagno a Ripoli, Tuscany, Italy). The details of the data collection and sampling procedures have been described elsewhere (12). All participants gave written consent for study participation and the Italian National Research Council of Aging Ethical Committee approved the study.
Data Collection
Participants provided data at baseline and at 3 and 6 years postbaseline. Baseline assessments occurred between 1998 and 2000, and 3- and 6-year visits occurred between 2001 and 2003 and between 2004 and 2006, respectively. Participants responded to in-home surveys administered by trained interviewers. Physicians and therapists performed medical examinations and physical function tests, respectively, in the study clinic. Participants were excluded from this analysis for the following reasons: diagnosis of (a) Parkinson's disease (n = 17), (b) a stroke (n = 61), (c) neuropathy (n = 6), or cognitive impairment as defined by a Mini-Mental State Examination score less than 21 (n = 171). These participants were excluded as the neurological impairments associated with these diagnoses can interfere with and compromise the accurate measurement of muscle function and are likely to cause mobility limitations in excess of those predicted by muscle weakness (7). Some participants had multiple exclusionary restrictions resulting in 221 unique individuals excluded. Of the remaining 934 participants, 67 died before the 3-year visit, and an additional 99 participants died before the 6-year visit. Also, 96 and 52 participants were alive but did not return for the 3- and 6-year visits, respectively.
Muscle Strength and Power
Maximal voluntary isometric knee extension strength was measured in kilograms using handheld dynamometer according to a standardized assessment protocol (13). Participants were asked to perform the task twice with each leg, lying in lateral decubitus (opposite to the examined limb) with the hip and knee in 45° and 60° flexed positions, respectively. Maximal strength obtained from the right leg was used for analysis. Lower extremity muscle power (physical work delivered to the external environment in a unit of time) was assessed using a leg power rig (14). Participants sat in a chair and unilaterally depressed a foot lever in the horizontal plane attached to a flywheel. The task was repeated eight times, and the maximum power output (in Watts) was used. Grip strength was measured with a handheld dynamometer (hydraulic hand “BASELINE”; Smith & Nephew, Milan, Italy) in kilograms using two trials with each hand. The maximum right side grip strength was used for analysis. Strength and power measures were not collected if severe hand or knee pain was present or if significant joint range of motion limitations were present in the hand or knee. In previous studies, the intraclass correlation coefficients for test–retest measures of knee extension isometric strength ranged from .89 to .99 in older adults (13,15) and grip strength ranged from .91 to .97 (16–17). The coefficient of variation for knee extension isometric strength was 4.6 (15), and based on available data from Wang and colleagues (17), we were able to calculate the coefficient of variation for grip strength as 6.64. For lower extremity muscle power, Bassey and Short (14) reported that the coefficient of variation was 9.4.
Mobility Function
Gait speed.—
Using a 4 m course with an optoelectronic system including two photocells connected to a recording chronometer, participants were initially asked to stand with both feet touching the starting line and to begin walking at their usual pace after a verbal command. Use of walking aids was permitted. Walk time was recorded as the time between the activation of the first and the second photocell. Two trials were conducted with the fastest time used for analysis in meters per second. Gait speed was measured at the 3- and 6-year visits. Change in gait speed was calculated as the difference in gait speed from Year 3 to Year 6. Instrumented measures of gait speed have been found to be reliable with an intraclass correlation coefficient of .91 and coefficient of variation of 3.5 (18).
Self-reported mobility disability.—
Participants were asked whether they had any difficulty walking 1 km or climbing a flight of stairs. For each question, responses were coded as no difficulty, able to perform the task with difficulty but without help, able to perform the task with some help from another person, and unable to perform the task. Mobility disability was defined as the inability of a participant to either walk 1 km or climb a flight of stairs at the Year 6 visit when they were able to perform the task at the previous visit.
Other Measures
We also considered age and body mass index (BMI) in kilograms per square meter. Weight was assessed using a high-precision mechanical scale with participants wearing light indoor clothes and no shoes. Height was measured to the nearest 0.1 cm with a wall measure.
Data Analysis
We assessed the relationships of maximum grip strength, knee extension strength, and leg power measured at Year 3 and 3-year changes (Year 3 minus baseline) with incident inability to walk 1 km or climb stairs separately using two-sample t tests. Scatterplots and locally weighted scatterplot smoothing (loess) were used to graph strength and power variables with 3-year changes in gait speed to provide a visual description of the relationships. We used classification and regression tree (CART) analysis (9) to predict (a) 3-year changes in gait speed (Year 6 minus Year 3) and (b) incident inability to walk 1 km or climb stairs separately in men and women. For both outcomes, candidate predictors included maximum grip strength, knee extension strength, and leg power measured at Year 3 and 3-year changes (Year 3 minus baseline). CART identifies the predictors and cutpoints with the strongest relationship with the outcomes based on the criterion of minimum prediction error. To avoid overfitting, the models were evaluated using 40 crossvalidation subsets, and the model was pruned to the most parsimonious model within one standard prediction error from the best-fit model (19). We selected CART over other methods of analysis (eg, stepwise regression or receiver operating characteristic curves), given the goal to optimize prediction and identify cutpoints (20,21). Models for gait speed changes were further evaluated by calculating R2, a measure of model calibration (how well predicted values agree with observed values), from analysis of variance with the CART-identified risk groups as predictors. Models for incident inability to walk 1 km or climb stairs were further evaluated by calculating likelihood ratios and the Brier score (range 0–1, lower is better), which globally measure model calibration and model discrimination (how well the model separates those who do and do not develop incident disability), sensitivity and specificity, and the C-statistic (measure of model discrimination) (21).
We also performed a sensitivity analysis to determine whether optimal strength or power cutpoints differed according to age, weight, or BMI. This was accomplished by adding age, weight, and BMI as candidate predictors to the CART model.
Results
Table 1 provides participant characteristics. Men and women were aged 73.3 (SD = 6.4) and 74.4 (SD = 6.8) years, respectively. Men and women, on average, declined in knee extension strength from baseline to Year 3 but showed increases in leg power and grip strength. Men and women had mean declines in 4 m gait speed from Year 3 to Year 6. Incident inability to walk 1 km or climb stairs at Year 6 occurred in 11.3% of men and 26.1% of women. Lower Mini-Mental State Examination scores were associated with study dropout and death by Year 3 for women (p < .05). Older age was associated with death by Year 3 for men and women (p < .05).
Table 1.
Participant Characteristics
| Men, N = 419 | SD or % | N* | Women, N = 515 | SD or % | N* | |
| M or Number | M or Number | |||||
| Age, y, M (SD) | 73.3 | 6.4 | 74.4 | 6.8 | ||
| MMSE, M (SD) | 26.3 | 2.2 | 25.6 | 2.5 | ||
| BMI, kg/m2, M (SD) | 27.1 | 3.4 | 382 | 27.9 | 4.5 | 454 |
| Weight, kg, M (SD) | 74.7 | 11.6 | 382 | 65.4 | 11.4 | 455 |
| Height, m, M (SD) | 1.6 | 0.7 | 382 | 1.5 | 0.7 | 454 |
| Knee strength, kg, mean (SD) | ||||||
| Baseline | 20.0 | 6.0 | 374 | 13.5 | 4.2 | 446 |
| Year 3 | 18.8 | 4.3 | 285 | 13.4 | 3.2 | 332 |
| Change (Year 3 − baseline) | −1.9 | 5.2 | 275 | −0.8 | 3.9 | 309 |
| Leg power, W, mean (SD) | ||||||
| Baseline | 156.9 | 61.4 | 369 | 74.0 | 35.0 | 434 |
| Year 3 | 170.9 | 56.7 | 248 | 86.8 | 36.3 | 247 |
| Change (Year 3 − baseline) | 2.4 | 37.1 | 241 | 5.4 | 29.5 | 233 |
| Grip strength, kg, mean (SD) | ||||||
| Baseline | 38.8 | 10.2 | 328 | 22.4 | 7.5 | 410 |
| Year 3 | 40.5 | 9.3 | 303 | 24.0 | 6.1 | 379 |
| Change (Year 3 − baseline) | 0.2 | 7.7 | 255 | 1.1 | 6.4 | 313 |
| 4m gait speed, m/s, mean (SD) | ||||||
| Year 3 | 1.14 | 0.25 | 295 | 0.96 | 0.25 | 361 |
| Year 6 | 1.07 | 0.27 | 261 | 0.91 | 0.27 | 313 |
| Change (Year 6 – Year 3) | −0.09 | 0.19 | 233 | −0.08 | 0.16 | 269 |
| Incident inability to walk 1 km or climb stairs, n (%) | ||||||
| Year 6 | 29 | 11.3 | 257† | 74 | 26.1 | 283† |
Notes: MMSE = Mini-Mental State Examination.
If different from column N.
Among those who were able to walk 1 km and climb stairs at Year 3.
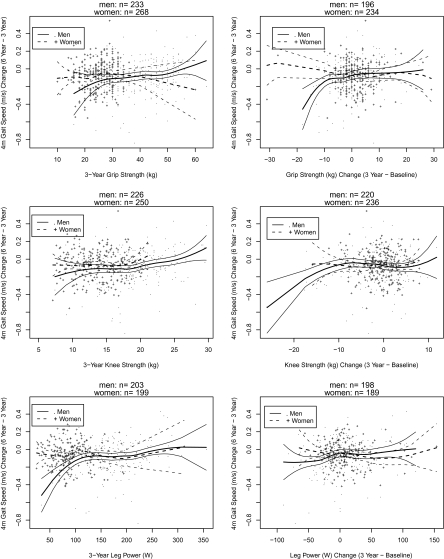
Figure 1 shows sex-specific associations of grip strength, knee extension strength, and leg power (at Year 3 and changes [Year 3 minus baseline]) with changes in gait speed (Year 6 minus Year 3) using loess smoothers. Among men, participants with lower 3-year grip and knee extension strength and lower leg power tended to have greater decreases in gait speed. Also, men with large declines in grip and knee strength (Year 3 minus baseline) tended to have greater declines in gait speed. Among women, only 3-year knee strength appeared to relate to changes in gait speed.
Figure 1.
Sex-specific associations of grip strength, knee extension strength, and leg power with changes in gait speed using loess smoothers.
Table 2 shows sex-specific associations of grip strength, knee extension strength, and leg power (at Year 3 and changes [Year 3 minus baseline]) with incident inability to walk 1 km or climb stairs. For both men and women, mean Year 3 knee strength, grip strength, and leg power were lower for those who developed mobility disability at Year 6 than those who remained free of mobility disability (p < .05). Among men, those with incident mobility disability at Year 6 showed declines in leg power and grip strength between baseline and Year 3, whereas those without incident mobility disability improved leg power and grip strength on average. However, the differences in grip strength and power changes between men with and without mobility disability were not statistically significant (p > .05). Mean knee extension strength declined by approximately the same amount in men irrespective of incident mobility disability. Women had declines in knee extension strength irrespective of incident mobility status, but the decline was larger (1 vs 0.6 kg), although not statistically significant, in those with incident mobility disability. Mean leg power increased for women but to a lesser degree in those with incident mobility disability. Unexpectedly, mean grip strength improved more in women with incident mobility disability. It is important to note that there were no statistically significant differences in the baseline to Year 3 changes for any of the muscle parameters between the women with and without mobility disability at Year 6 (p > .05).
Table 2.
Strength by Sex-Specific Incident Mobility Disability
| Men: Incident Mobility Disability |
Women: Incident Mobility Disability |
|||||||||
| No |
Yes |
No |
Yes |
|||||||
| M (SD) | N | P Value | M (SD) | N | M (SD) | N | M (SD) | N | P Value | |
| Knee strength, kg | ||||||||||
| Year 3 | 20.0 (3.8) | 209 | 16.7 (4.2) | 23 | <.001 | 14.1 (3.0) | 188 | 12.7 (3.1) | 61 | .002 |
| Change (Year 3 − baseline) | −1.9 (5.4) | 205 | −1.8 (4.1) | 23 | .95 | −0.6 (4.0) | 181 | −1.0 (3.4) | 55 | .50 |
| Leg power, W | ||||||||||
| Year 3 | 182.6 (51.4) | 192 | 125.3 (52.5) | 19 | <.001 | 92.9 (35.8) | 157 | 75.2 (32.6) | 44 | .004 |
| Change (Year 3 − baseline) | 4.5 (37.2) | 186 | −6.3 (41.0) | 19 | .24 | 6.4 (31.4) | 151 | 2.3 (22.1) | 41 | .34 |
| Grip strength, kg | ||||||||||
| Year 3 | 43.1 (8.0) | 211 | 35.1 (9.9) | 27 | <.001 | 26.0 (4.9) | 191 | 23.8 (7.0) | 66 | .02 |
| Change (Year 3 − baseline) | 0.6 (7.4) | 172 | −1.8 (8.1) | 26 | .13 | 0.9 (6.2) | 163 | 1.6 (8.3) | 56 | .60 |
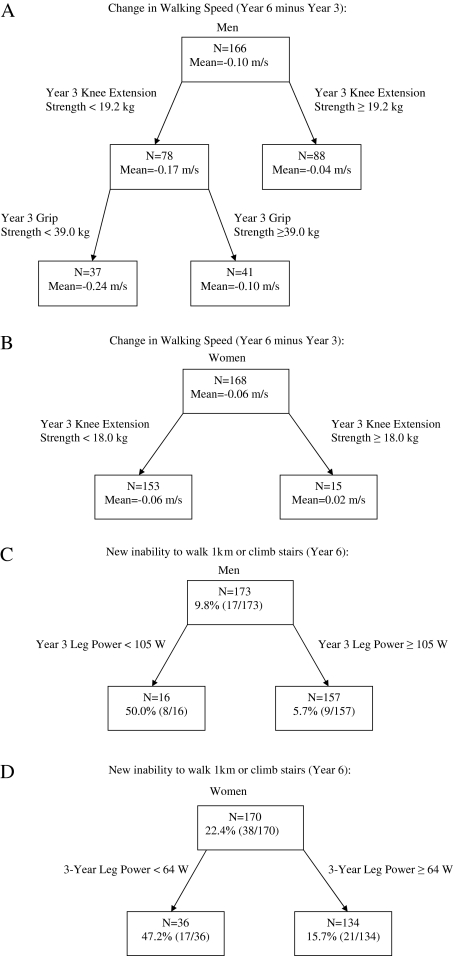
Figure 2 shows sex-specific CART results. Figure 2A shows that the optimal prediction model for change in gait speed (Year 6 minus Year 3) in men includes Year 3 knee extension strength and grip strength. Men with knee extension strength <19.2 kg and grip strength < 39.0 kg (the “high-risk” group) had declines in gait speed of 0.24 m/s, which was 0.20 m/s (SE = 0.03) worse than those with knee extension strength ≥19.2 kg (R2 = 16.1%, p < .001). Women with Year 3 knee extension strength <18.0 kg had declines in gait speed of 0.06 m/s (Figure 2B), which was 0.08 m/s (SE = 0.04) worse than those with knee extension strength ≥18.0 kg (R2 = 2.6%, p = .04). Figure 2C shows that 50.0% of men with leg power <105 W had incident mobility disability compared with 5.7% of men with leg power ≥105 W (Brier score = 0.072, likelihood ratio = 8.68, 95% confidence interval = 3.91, 19.44, C-statistic = 71.0%, p < .001). Among women, 47.2% with leg power <64 W had incident mobility disability compared with 15.7% with leg power ≥64 W (Brier score = 0.157, likelihood ratio = 3.01, 95% confidence interval = 1.79, 5.08, C-statistic = 65.1%, p < .001).
Figure 2.
Sex-specific classification and regression tree results.
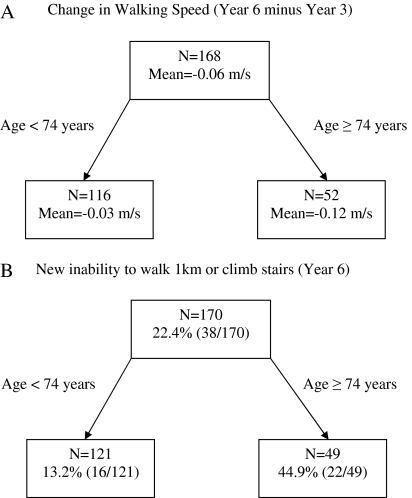
Sensitivity analysis using age and BMI as candidate predictors resulted in the same CART results for men but different results for women. Figure 3A and B shows that the optimal model for predicting both change in gait speed and incident mobility disability in women included only age. Women aged ≥74 years had declines in gait speed of 0.12 m/s (Figure 3A), which was 0.09 m/s (SE = 0.02) worse than those aged <74 years (R2 = 7.5%, p < .001). Figure 3B shows that 44.9% of women aged ≥74 years had incident mobility disability compared with 13.2% aged <74 years (Brier score = 0.153, likelihood ratio = 3.44, 95% confidence interval = 1.96, 5.90, C-statistic = 68.7%, p value < .001).
Figure 3.
Sensitivity analysis: classification and regression tree results for women.
Discussion
The primary objectives of this study were to determine which combination of muscle strength and power, assessed at one time point and as 3-year changes, are most predictive of mobility decline and to determine optimal sex-specific cutpoints in order to identify older adults at greatest risk of mobility decline. Our data indicate that men with knee extension strength <19.2 kg and grip strength <39 kg had clinically meaningful declines in gait speed of .24 m/s over a 3-year period (22–23). Furthermore, men with leg power <105 W were nearly nine times more likely to develop incident mobility disability over time. Among women, knee extension strength <18 kg was associated with a minimal decline in gait speed (0.06 m/s), and women with leg power <64 W were three times more likely to develop incident mobility disability. These cutpoints are similar to those previously reported for InCHIANTI baseline data (7).
To our knowledge, this is the first study of older adults to explore changes in muscle strength and power over time as predictors of decline in mobility. CART analysis did not select change in strength or power values to predict future mobility status. Our findings suggest that absolute strength and power values are better predictors of future mobility decline than changes in strength or power. From a clinical perspective, it is important to know that evaluation of muscle function at a single time point provides the best estimate of risk, independent of the changes that have occurred over the past 3 years.
There are several explanations for our finding that absolute strength and power values are better predictors of future mobility decline than changes in strength or power, and we cannot rule out that changes in strength and power predict changes in mobility. First, strength and mobility decline may occur essentially concurrently such that strength measurement is more closely associated with current mobility than with future mobility decline. Second, participants with greater declines in strength from baseline to Year 3 may have dropped out of the study or died prior to the reassessment at Year 6, limiting our ability to examine the predictive power of changes in strength. A post hoc analysis demonstrated that strength changes from baseline to Year 3 were not related to subsequent dropout or death (p > .05) among participants who survived to Year 3. However, participants with lower strength at Year 3 were more likely to die before the Year 6 visit (p < .05).We also found that participants with lower strength and power at baseline were more likely to die or dropout by the Year 3 visit (p < .05), which could explain the small, but unexpected, net increases in grip strength and power from baseline to Year 3 seen in this sample. Third, the finding that absolute measures were more predictive than changes in strength in our study may be influenced by limited variability in strength change over 3 years in the InCHIANTI data. In persons with strength data at baseline and at Year 3, strength and power values remained relatively stable over time considering the coefficients of variation for these measures. In fact, relatively few participants had true declines in strength. For example, previous work has established that the minimum detectable change for grip strength is 6 kg (24), which means that changes in grip strength less than 6 kg may be due to chance or normal fluctuation. As illustrated in Figure 1, relatively few participants had grip strength declines greater than 6 kg; but, in men, those with grip strength declines greater than 6 kg had greater declines in gait speed. In a sample with greater variability in muscle parameter changes, we might find change in muscle strength and power to have greater predictive ability. Finally, it is possible that the 3-year time period may have been too short to truly capture the trajectory of muscle strength and/or power loss that would be predictive of future changes in mobility function, especially given the variability in most strength measures. As evidence of this point, Lauretani and colleagues demonstrated that InCHIANTI participants do have a net decrease in strength measures over a 6-year period of time (25), which is consistent with the findings of strength loss over time in other epidemiologic studies of older adults.
These finding suggest that the evaluation of muscle strength may have greater clinical utility in predicting mobility declines in older men than in older women as the muscle parameters studied were stronger predictors of future mobility decline in men than in women. Not only were the associations between strength and mobility decline stronger in men than in women but also strength accounted for greater variability in gait speed in men than in women as indicated by higher R2. Furthermore, sensitivity analyses demonstrated that age was the strongest predictor of mobility decline in women, whereas strength remained the strongest predictor of mobility decline in men, even after including age and BMI in the mobility prediction model. In fact, the addition of age, weight, and BMI into the model for the CART sensitivity analysis resulted in the elimination of strength as a significant predictor of mobility decline or incident mobility disability in women. The longitudinal results are similar to previous cross-sectional work that has found that muscle strength is more strongly associated with mobility function in older men than in older women (7,26–27). Although women have, on average, lower muscle mass than men throughout adulthood (28), the rate of decline in muscle mass and strength with advancing age is faster in men (7,28). Thus, factors other than strength decline may explain age-associated disability in older women. For example, nonfatal disabling conditions such as depression, osteoarthritis, and osteoporosis are more prevalent in women than in men and are believed to contribute to their higher disability rates (29–30).
The approach used here represents an extension of an approach used previously in cross-sectional research to define cutpoints for strength based on mobility outcomes (7,31). However, mobility decline is multifactorial and may be affected by many factors other than strength, including balance, cognition, fatigue, and pain. Previous cross-sectional studies in both older men and women have demonstrated that decreased muscle strength may be associated with functional limitations other than the mobility measures considered here (32) and that the association between muscle strength and disability may depend highly on the muscle group tested (33). In an earlier cross-sectional evaluation of older women, lower extremity strength was associated with the ability to achieve a target maximal walking speed (>1.22 m/s) but not with the ability to walk at slower speeds (34). All these studies serve to highlight the challenges of identifying clinically meaningful strength cutpoints in older adults. Although the use of mobility decline as an outcome has been a logical choice for identifying strength cutpoints, future studies should consider defining cutpoints for strength and loss of strength based on other important outcomes, that is, mortality or falls.
The many strengths of this study include use of a large, longitudinal population-based cohort with availability of strength and power measures as well as validated measures of mobility. This study extends prior analyses from the InCHIANTI study (7) by using a prospective study design to highlight possible intervention points on the pathway to disability through the identification of muscle strength and power cutpoints that predict mobility decline. Furthermore, we used a novel theoretically driven approach including both absolute strength and/or power levels and changes in strength and power. Despite these strengths, some limitations must be considered. First, individuals in this cohort were relatively healthy, and thus, the small number of participants with very low strength or large declines in strength may have limited the ability to detect cutpoints at the lower levels of the strength distribution. Furthermore, the participants with data at all time points (ie, those who attended all in-clinic visits) may be the most robust members of the study and potentially at lower risk for strength declines over time. This may account for mean increases observed between baseline and Year 3 in grip strength and leg power. Finally, a more complete analysis of sarcopenia would focus on both mass and strength. Unfortunately, whole-body lean mass measures are not available in InCHIANTI, and we are not able to examine the relative predictive power of changes in lean mass to changes in strength.
In conclusion, we determined sex-specific cutpoints in absolute strength and power values predictive of gait speed decline and incident mobility disability. The findings suggest that absolute strength and power are more predictive of mobility decline than changes in strength and power and that evaluation of muscle strength has greater clinical utility in identifying older men at risk for mobility decline as compared with older women. Early identification of individuals at high risk for mobility decline may create opportunities for developing and implementing prevention strategies (ie, resistance training) to delay the development of mobility disability.
Funding
The InCHIANTI study was supported by the Italian Ministry of Health and in part by the U.S. National Institute on Aging (contracts 263 MD 916413 and 263 MD 821336). Dr. G.E.H. was supported by grant R21HD057274, Dr. D.E.A. was supported by grant K12 HD043489, Dr. M.S. was supported by grant R01 AG029315, and Dr. R.M.M. was supported by grant K23 AG019161 from the National Institutes of Health.
References
- 1.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137:231–243. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 2.Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol A Med Sci. 2000;55:M716–M724. doi: 10.1093/gerona/55.12.m716. [DOI] [PubMed] [Google Scholar]
- 3.Fried LP, Guralnik JM. Disability in older adults: evidence regarding significance, etiology, and risk. J Am Geriatr Soc. 1997;45:92–100. doi: 10.1111/j.1532-5415.1997.tb00986.x. [DOI] [PubMed] [Google Scholar]
- 4.Visser M, Newman AB, Nevitt MC, et al. Reexamining the sarcopenia hypothesis. Muscle mass versus muscle strength. Health, Aging, and Body Composition Study Research Group. Ann N Y Acad Sci. 2000;904:456–461. [PubMed] [Google Scholar]
- 5.Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008;63:829–834. doi: 10.1093/gerona/63.8.829. [DOI] [PubMed] [Google Scholar]
- 6.Hairi NN, Cumming RG, Naganathan V, et al. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the Concord Health and Ageing in Men Project. J Am Geriatr Soc. 2010;58:2055–2062. doi: 10.1111/j.1532-5415.2010.03145.x. [DOI] [PubMed] [Google Scholar]
- 7.Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 8.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 9.Breiman L. Classification and Regression Trees. Belmont, CA: Wadsworth; 1984. [Google Scholar]
- 10.Bassey EJ, Fiatarone MA, O’Neill EF, Kelly M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci (Lond) 1992;82:321–327. doi: 10.1042/cs0820321. [DOI] [PubMed] [Google Scholar]
- 11.Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci L. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? J Gerontol A Biol Sci Med Sci. 2003;58:728–733. doi: 10.1093/gerona/58.8.m728. [DOI] [PubMed] [Google Scholar]
- 12.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 13.Bandinelli S, Benvenuti E, Del Lungo I, et al. Measuring muscular strength of the lower limbs by hand-held dynamometer: a standard protocol. Aging (Milano) 1999;11:287–293. doi: 10.1007/BF03339802. [DOI] [PubMed] [Google Scholar]
- 14.Bassey EJ, Short AH. A new method for measuring power output in a single leg extension: feasibility, reliability and validity. Eur J Appl Physiol Occup Physiol. 1990;60:385–390. doi: 10.1007/BF00713504. [DOI] [PubMed] [Google Scholar]
- 15.Wang CY, Olson SL, Protas EJ. Test-retest strength reliability: hand-held dynamometry in community-dwelling elderly fallers. Arch Phys Med Rehabil. 2002;83:811–815. doi: 10.1053/apmr.2002.32743. [DOI] [PubMed] [Google Scholar]
- 16.Bohannon RW, Schaubert KL. Test-retest reliability of grip-strength measures obtained over a 12-week interval from community-dwelling elders. J Hand Ther. 2005;18:426–427. doi: 10.1197/j.jht.2005.07.003. quiz 428. [DOI] [PubMed] [Google Scholar]
- 17.Wang CY, Chen LY. Grip strength in older adults: test-retest reliability and cutoff for subjective weakness of using the hands in heavy tasks. Arch Phys Med Rehabil. 2010;91:1747–1751. doi: 10.1016/j.apmr.2010.07.225. [DOI] [PubMed] [Google Scholar]
- 18.Menz HB, Latt MD, Tiedemann A, Mun San Kwan M, Lord SR. Reliability of the GAITRite walkway system for the quantification of temporo-spatial parameters of gait in young and older people. Gait Posture. 2004;20:20–25. doi: 10.1016/S0966-6362(03)00068-7. [DOI] [PubMed] [Google Scholar]
- 19.Maindonald JH, Braun J. Data analysis and graphics using R: an example-based approach. 2nd ed. Cambridge, NY: Cambridge University Press; 2007. [Google Scholar]
- 20.Greenland S. The need for reorientation toward cost-effective prediction: comments on ‘Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond’ by M.J. Pencina et al., Statistics in Medicine (DOI: 10.1002/sim.2929) Stat Med. 2008;27:199–206. doi: 10.1002/sim.2995. [DOI] [PubMed] [Google Scholar]
- 21.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 22.Kwon S, Perera S, Pahor M, et al. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study) J Nutr Health Aging. 2009;13:538–544. doi: 10.1007/s12603-009-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 24.Nitschke JE, McMeeken JM, Burry HC, Matyas TA. When is a change a genuine change? A clinically meaningful interpretation of grip strength measurements in healthy and disabled women. J Hand Ther. 1999;12:25–30. [PubMed] [Google Scholar]
- 25.Lauretani F, Semba RD, Bandinelli S, et al. Low plasma carotenoids and skeletal muscle strength decline over 6 years. J Gerontol A Biol Sci Med Sci. 2008;63:376–383. doi: 10.1093/gerona/63.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Visser M, Deeg DJ, Lips P, Harris TB, Bouter LM. Skeletal muscle mass and muscle strength in relation to lower-extremity performance in older men and women. J Am Geriatr Soc. 2000;48:381–386. doi: 10.1111/j.1532-5415.2000.tb04694.x. [DOI] [PubMed] [Google Scholar]
- 27.Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159:413–421. doi: 10.1093/aje/kwh058. [DOI] [PubMed] [Google Scholar]
- 28.Melton LJ, 3rd, Khosla S, Crowson CS, O’Connor MK, O’Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000;48:625–630. [PubMed] [Google Scholar]
- 29.Murtagh KN, Hubert HB. Gender differences in physical disability among an elderly cohort. Am J Public Health. 2004;94:1406–1411. doi: 10.2105/ajph.94.8.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kempen GI, Verbrugge LM, Merrill SS, Ormel J. The impact of multiple impairments on disability in community-dwelling older people. Age Ageing. 1998;27:595–604. doi: 10.1093/ageing/27.5.595. [DOI] [PubMed] [Google Scholar]
- 31.Manini TM, Visser M, Won-Park S, et al. Knee extension strength cutpoints for maintaining mobility. J Am Geriatr Soc. 2007;55:451–457. doi: 10.1111/j.1532-5415.2007.01087.x. [DOI] [PubMed] [Google Scholar]
- 32.Hernandez ME, Goldberg A, Alexander NB. Decreased muscle strength relates to self-reported stooping, crouching, or kneeling difficulty in older adults. Phys Ther. 2010;90:67–74. doi: 10.2522/ptj.20090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasegawa R, Islam MM, Lee SC, Koizumi D, Rogers ME, Takeshima N. Threshold of lower body muscular strength necessary to perform ADL independently in community-dwelling older adults. Clin Rehabil. 2008;22:902–910. doi: 10.1177/0269215508094713. [DOI] [PubMed] [Google Scholar]
- 34.Rantanen T, Guralnik JM, Izmirlian G, et al. Association of muscle strength with maximum walking speed in disabled older women. Am J Phys Med Rehabil. 1998;77:299–305. doi: 10.1097/00002060-199807000-00008. [DOI] [PubMed] [Google Scholar]