Abstract
Negative surface charge density gradients were prepared on fused silica slides using selective oxidation of a 3-mercaptopropyltrimethoxysilane (MTS) monolayer converting surface thiol groups (-SH) into negatively charged sulfonate (-SO3-) groups. The sulfonate-to-thiol gradient samples were characterized by water contact angle and electron spectroscopy for chemical analysis (ESCA). Gradients were pre-adsorbed with proteins from three different solutions: platelet free plasma (PFP), fibrinogen, or albumin in phosphate buffered saline (PBS). Washed platelets were perfused over gradient samples in a parallel plate flow chamber and platelet adhesion was measured across the gradients using differential interference contrast (DIC) microscopy. Gradients pre-adsorbed with PFP showed adhesion contrast inversely related to the negative surface charge density. The magnitude of the adhesion contrast along the gradient was also dependent on PFP concentration. Gradients pre-adsorbed with fibrinogen showed an adhesion maximum in the center of the gradient region. Albumin coating of the gradients resulted in low overall platelet adhesion with increased adhesion in regions of high negative charge density. The effect of gradient orientation with respect to the flow was also investigated. Gradients pre-adsorbed with 10% PFP showed different adhesion contrast when the platelets were perfused in opposite directions. This suggests that platelet adhesion is, in addition to responding to the local surface properties, also dependent on the upstream conditions.
Keywords: Platelet adhesion, surface gradient, silane, surface charge, fibrinogen, albumin, plasma proteins, blood compatibility
Introduction
The response of blood at biomaterial interfaces has been, and remains a central issue in the biocompatibility of medical devices [1-3]. Material hemocompatibility is determined by complex interactions between the surface, adsorbed plasma proteins, and platelets [1-5]. Platelet surface activation stimulates the activation of plasma coagulation factors resulting in fibrin clot formation therefore extensive effort has been devoted to the development of novel surfaces that minimize both protein adsorption and platelet adhesion [6-8].
Surface chemistry gradients provide a mechanism for high throughput screening over a variety of surface properties as well as insight on potential synergistic phenomena in regions of mixed properties [9]. For example, molecular gradients are a popular tool in studying many biological phenomena and have been used to study both protein adsorption [10-12] and cell and platelet adhesion [13-19]. Specifically, gradients have been used to study the effects of variables such as wettability, charge, and PEO chain length on platelet adhesion [15-19]. The intrinsic value of using surface gradients lies in the ability to identify local effects over a variety of chemistries in a single experiment. However, the degree to which these local effects are reproducible once the homogeneous surface is prepared as well as the effect of gradient orientation have not been well documented.
We present here a versatile method to produce one-dimensional surface gradients based on the selective oxidation of a uniform 3-mercaptopropylsilane (MTS) monolayer by UV irradiation. As a consequence of UV irradiation the surface thiol (-SH) group is oxidized into a sulfonate (-SO3) group [20], thus creating a 1-D variation of surface charge, from fully ionized sulfonate to weakly ionized thiol groups. The dose and duration of UV oxidation control the physical length of the gradient and the extent of thiol-to-sulfonate conversion. Furthermore, the remaining reactive surface thiols on the sulfonate-to-thiol surface gradient can be utilized for a second chemical reaction thus making the MTS + UV oxidation method a versatile basis for several different surface chemistry gradients [V. Hlady, unpublished].
In this study platelet adhesion on negative surface charge density gradients was measured and compared with uniform thiol monolayer control surfaces. Three protein solutions (platelet free plasma (PFP), fibrinogen or albumin in phosphate buffered saline) were used to pre-adsorb proteins onto the gradient and control samples in order to study the role of adsorbed proteins in platelet adhesion. Also the extent to which gradients provide information on local surface effects was studied by comparing the effect of gradient orientation on platelet adhesion patterns.
Materials and Methods
Preparation and Characterization of Gradient Surfaces
Negative surface charge gradient samples were prepared by UV oxidation [20, 21] of an MTS monolayer on 3" fused silica slides (FS, Ted Pella Inc.). FS slides were prepared for surface modification using a four step cleaning procedure. First the FS slides were cleaned in an oxidizing acid bath overnight. This was followed by a 10 min oxygen plasma treatment (Plasmod, Tegal Inc., 50W @ 200 mTorr). The FS slides were then incubated in a 7:3 H2SO4-H2O2 (“piranha”) solution for one hour. This was followed by a final 10 min oxygen plasma treatment to activate the surface prior to the MTS reaction. Clean and dry FS slides were immediately incubated in a 1% (v/v) MTS (United Chemical Technologies) solution in toluene for 4 hours at room temperature. After the reaction, MTS-modified FS slides were rinsed in sequence using solutions of increasing hydrophilicity (toluene, acetone, ethanol, water) and dried with N2 gas. The samples were stored under vacuum until use and were not kept for longer than one week.
Negative surface charge gradients were prepared by the oxidation of the surface thiol groups into negatively charged sulfonate groups using a custom UV patterning chamber (Fig. 1). The sample was placed in a slide holder and selectively exposed to UV light (ELC-4000, Electrolite Corp.) through the linear translation of an edge mask over a 1 cm region in the center of the sample. The translator moved the edge mask at a constant rate of 0.25 cm/min across center of the slide for four minutes, thus creating a 1 cm long linear oxidation gradient in the center of the slide. Such UV exposure changes the wettability of the MTS monolayer which is easily detected using water contact angle measurements. Receding water contact angles were measured by the sessile drop method using a contact angle instrument (CAM 100, KSV Instruments). Equal 5 μl purified water droplets were dispensed along the length of the sample. The extent of the thiol to sulfonate conversion was verified using high resolutions ESCA spectra of S2p peaks. The XPS spectra of MTS-modified silica and UV oxidized MTS-modified silica were recorded with a XPS spectrometer (Axis-Ultra DLD, Kratos) at the University of Utah Nanofab Lab.
Figure 1.
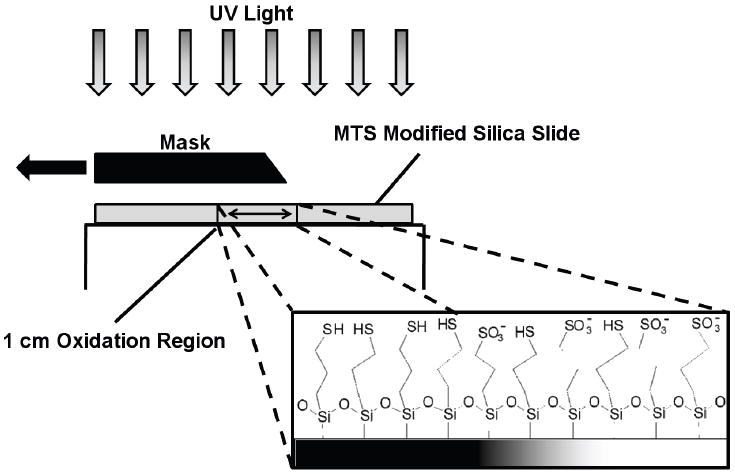
Creation of the surface charge gradient is accomplished by translating the edge mask at a constant rate over a 1 cm region of the uniformly modified MTS silica slide. On the UV exposed region the surface thiol group is oxidized into a negatively charged sulfonate group.
Protein Solution and Washed Platelet Preparation
Three different protein solutions were prepared: platelet free plasma, fibrinogen (plasminogen free, Calbiochem) or albumin (Fraction V, Sigma) in phosphate buffered saline solutions. Platelet free plasma was obtained by filtration of the platelet poor plasma (PPP) collected during the washed platelet preparation using 0.22 μm low protein binding filters (Millipore). For the studies involving testing the effect of PFP concentration on platelet adhesion the PFP concentration was adjusted using PBS (pH 7.4) to 1%, 10% or 50% of normal physiological PFP concentration. Fibrinogen and albumin solutions were prepared in PBS at 10% of each protein’s normal physiological concentration.
Washed platelets were prepared from whole blood from healthy human donors. Blood was collected in 1:7 anticoagulant citrate dextrose (ACD) solution and centrifuged for 15 minutes at 1500 rpm to separate platelet rich plasma (PRP) from the red blood cells. The PRP supernatant was aspirated off using a transfer pipette. Prostaglandin E1 (PGE1, 300 nM) was added to the PRP to inhibit aggregation during preparation [22]. PRP was centrifuged for 15 minutes at 2100 rpm for platelet isolation. The platelet poor plasma (PPP) supernatant was carefully transferred to a clean polypropylene test tube, not disturbing the platelet pellet. The platelet pellet was gently re-suspended in pre-warmed Tyrode’s-HEPES buffer (37 °C, pH 7.4, [23]) and 300 nM PGE1. Platelets were counted using a hemocytometer and the concentration was adjusted to 1.5•107 platelets/ml.
Platelet Perfusion Studies
All protein and washed platelet solutions were maintained at 37°C throughout the experiments. A syringe pump (Kent Scientific) was used to draw solutions through a parallel plate flow chamber (length 7 cm, width 0.5 cm, thickness 0,05 cm) at a constant flow rate (40 ml/hr) thus creating a shear rate of 92 s-1. The flow cell was connected to the solutions using 15 cm long polyethylene tubing (shear rate of 24 s-1) (Fig. 3).
Figure 3.
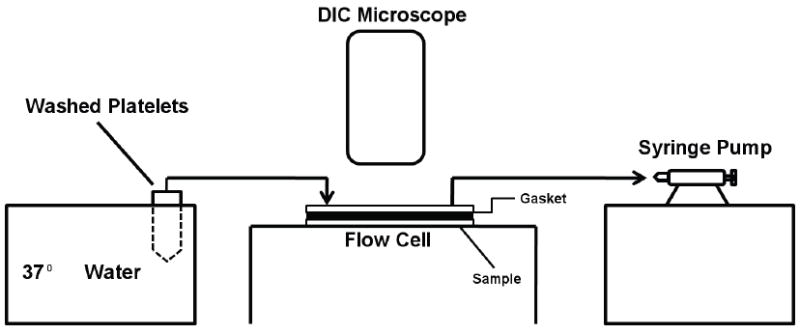
Schematic of the flow system for platelet perfusion studies.
A three step perfusion process was used to study platelet adhesion. First a protein solution was perfused through the flow cell for 15 minutes to allow for plasma protein adsorption to the gradient surface. The protein solution was followed by a 5 minute perfusion of a washed platelet suspension avoiding any surface-air contact. The washed platelet suspension was diluted to 107 platelets/ml and activated by adding Ca2+ (2 mM) and ADP (1 μM) to the solution prior to perfusion. The platelet perfusion time of 5 minutes was selected to minimize the formation of platelet aggregates which may be more indicative of platelet-platelet interactions than of interactions with the protein covered gradient surface. In the final step a 1% glutaraldehyde solution was injected into the chamber for 15 minutes to fix the sample. All unbound platelets were washed from the sample with PBS prior to counting.
The platelets adhered to the surfaces were visualized using differential interference contrast microscopy (DIC, Diaphot 300, Nikon) (Fig. 3). Separate counts (n = 10) for 20 positions at 1 mm increments including the gradient region were taken and converted to the number of platelets per mm2. In order to account for variable platelet activity over different donors, all data were normalized using a uniform thiol sample (i.e. MTS modified silica with no gradient) pre-adsorbed with 10% PFP as a baseline. Adhesion contrast on gradient samples was determined to be significant by performing an unpaired t-test comparing adhered platelets found in the sulfonate and the thiol regions (n = 20).
Results
Gradient characterization
Receding water contact angles between 50° - 55° were measured in the thiol region of the sample and between 8°-12° in the sulfonate region (Fig. 2A). Contact angles decreased through the region exposed to variable UV irradiation times indicating the presence of a gradient in the center of the sample. The XPS S2p spectra of the MTS-modified silica and 4 minutes UV oxidized MTS-modified silica are shown in Fig. 2B. The elemental XPS analysis showed ~2 atomic % of S, an expected value for MTS monolayer [21]. For MTS-modified silica (dashed line, Fig. 2B) a strong S2p peak at 163.5 eV confirmed the presence of surface thiols. After 4 minutes of UV irradiation (full line, Fig. 2B), the 163.5 eV peak decreased in magnitude while the peak at 168 eV increased. The higher binding energy peak indicated that the sulfur atoms from the –SH groups has combined with oxygen, forming a sulfonate moiety [20]. The conversion of thiol to sulfonate was incomplete: the fraction of sulfur in the oxidized state was around 0.6. Also, a small fraction of oxidized thiols was present on the MTS-modified silica which was not irradiated by UV, possibly due to spontaneous oxidation of thiols in air.
Figure 2.
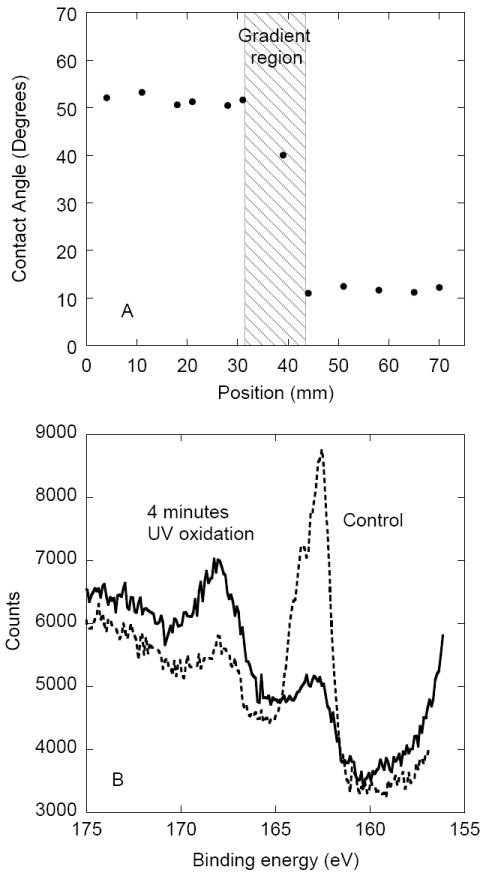
(A) Receding water contact angles plotted as a function of distance from the negatively charged sulfonate end of the gradient sample. (B) High resolution XPS S2p spectra of the MTS-modified silica control (dashed line) and 4 minutes UV oxidized MTS-modified silica (full line).
PFP concentration and Platelet Adhesion
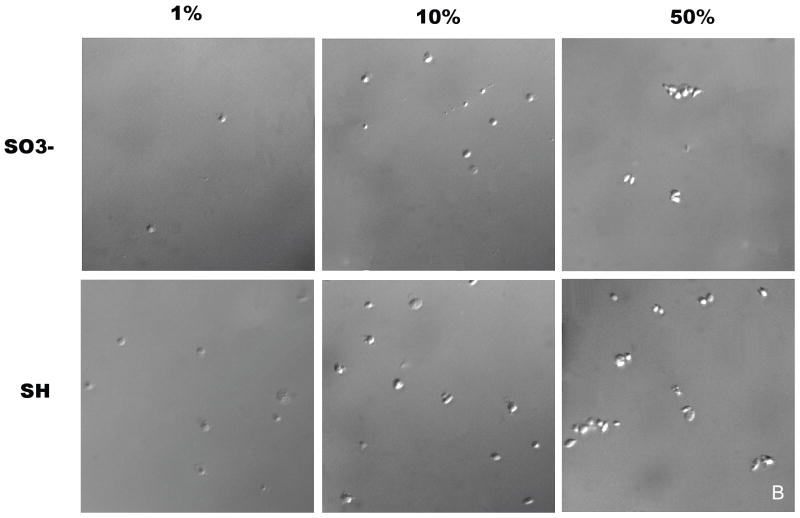
Platelet adhesion on sulfonate-to-thiol gradient surfaces pre-incubated with three different PFP concentrations (1%, 10%, and 50% of physiological PFP concentration) is shown in Figure 4A. Platelet adhesion counts (n = 10) were measured over a 20 mm region of the sample, including the gradient region, at 1 mm intervals with the gradient located between 5 mm and 15 mm. The error bars represent the standard deviation of each sample. An increase in adhesion corresponding to a decrease in negative charge density was observed for all 3 PFP concentrations. An unpaired t-test comparing the sulfonate region with the thiol region was performed for all three concentrations and shown to be significantly different with a confidence interval of p < 0.0001, n = 20. Samples pre-adsorbed with 1% and 50% PFP showed less adhesion contrast across the gradient region compared to samples pre-adsorbed with 10% PFP. A higher number of platelet aggregates was observed on gradient samples pre-adsorbed with 50% PFP compared to 1% and 10% PFP (Fig. 4B).
Figure 4.
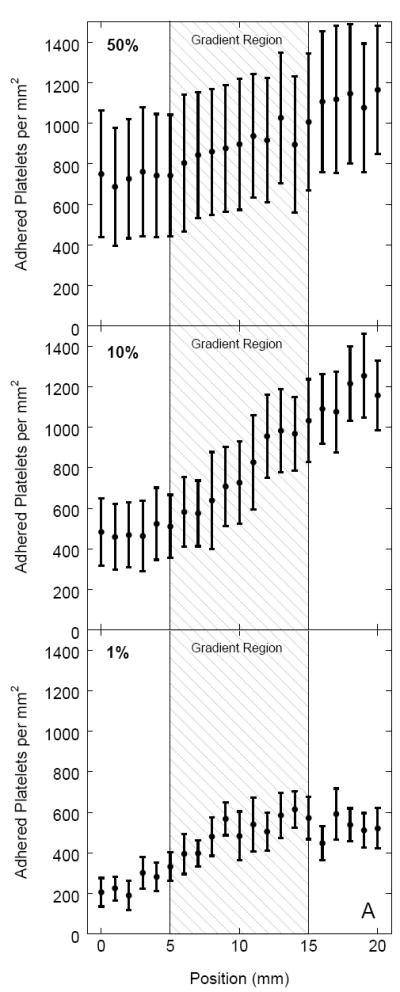
(A) The effect of PFP concentration used to coat the gradient surface on platelet adhesion. For every position the adhesion values represent a mean value where n = 20. Postions 0 – 5 mm represent the sulfonate region and 15 - 20 mm the thiol region with the gradient region between 5 mm – 15 mm. The platelet suspension was perfused in the direction from the sulfonate region, over the gradient, to the thiol region. (B) DIC images of platelet adhesion on sulfonate and thiol regions for 1%, 10%, and 50% pre-adsorbed PFP concentrations.
Platelet Adhesion on Gradient Surfaces
Platelet adhesion on sulfonate-to-thiol gradient surfaces pre-adsorbed with three different 10% protein solutions (PFP, fibrinogen, and albumin) is shown in Figure 5. The platelet suspension was perfused in the sulfonate to thiol direction across the gradient. Adhered platelets were counted over a central 20 mm region covering approximately 5 mm on each side of the gradient at 1 mm intervals (n = 10) with the gradient located between 5 mm – 15 mm. The normalized platelet count for each position is plotted as a function of distance with the error bars representing the standard deviation of each sample. Uniform thiol surface (i.e. with no gradient) served as a control. Control samples pre-adsorbed with 10% PFP displayed increasing adhesion between 0 mm – 5 mm (Fig. 5B). Control samples pre-adsorbed with 10% fibrinogen showed a significant contrast in adhesion as a function of distance from the flow inlet (n = 20, p < 0.001) (Fig. 5D). Control samples pre-adsorbed with 10% human albumin showed a minimal variation of platelet adhesion as a function of distance from the flow inlet (Fig. 5F). Overall, the controls with 10 % PFP and 10 % fibrinogen resulted in similar levels of platelet adhesion while samples pre-incubated with albumin resulted in the lowest adhesion.
Figure 5.
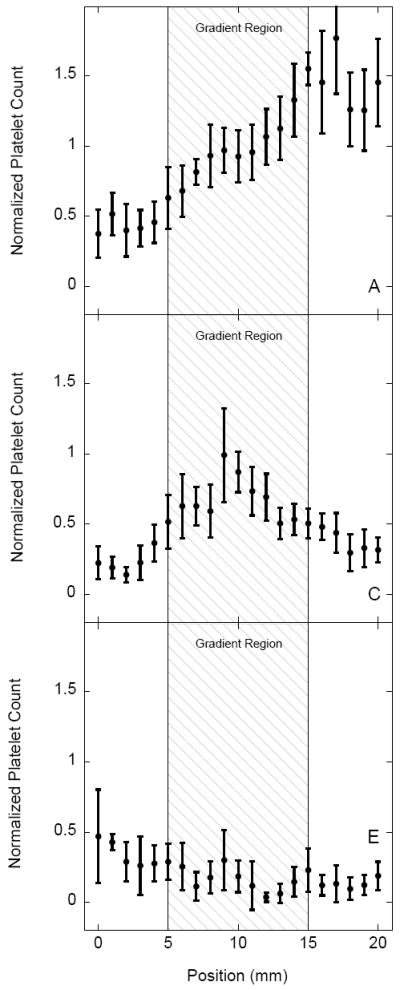
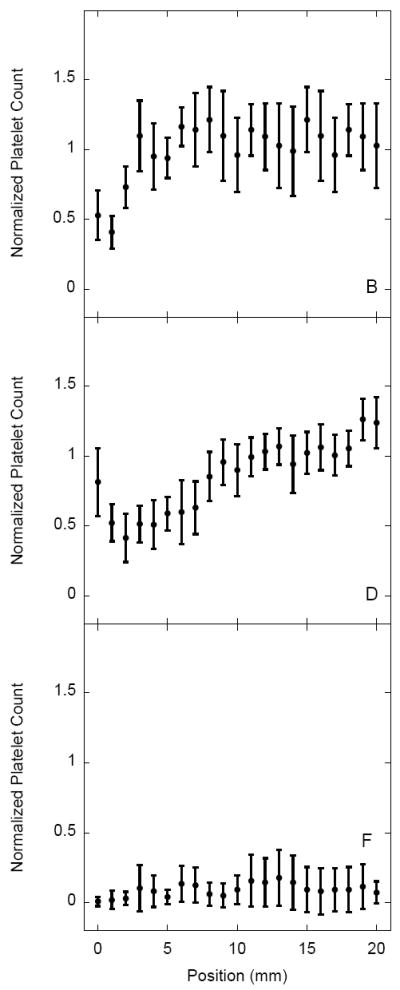
Normalized platelet adhesion on sulfonate-to-thiol gradient samples and on non-gradient MTS controls pre-adsorbed with plasma (panels A,B), fibrinogen (panels C,D) and albumin (panels E,F). Adhesion values represent a mean ± standard deviation; n = 20. Platelet counts were normalized by dividing data from each sample by the average adhesion for a PFP control sample (panel B).
Sulfonate-to-thiol gradients pre-adsorbed with 10% human PFP displayed a gradient of platelet adhesion compared to the non-gradient control with a significant (n = 20, p < 0.001) difference between the negatively charged sulfonate region of the gradient and the thiol region of the sample (Fig. 5A). Platelet adhesion increased with respect to the distance from the flow inlet which was also a direction of decreasing surface charge density. Gradient samples pre-adsorbed with human fibrinogen displayed an adhesion maximum in the center of the gradient region (Fig. 5C) while the gradients pre-adsorbed with 10% human albumin did not show any significant adhesion contrast (Fig. 5E). Overall platelet adhesion, however, on the sulfonate region of the albumin coated gradient sample was somewhat higher than on the thiol region for both the uniform thiol control and sulfonate-to-thiol gradient samples.
Effect of Sample Orientation on Platelet Adhesion
The results of platelet adhesion on sulfonate-to-thiol gradient surfaces pre-adsorbed with 10% human PFP for samples positioned in two different orientations is shown in Figure 6. Platelets were either perfused over the sulfonate region in the upstream position of the flow cell (Fig. 6A) or over the thiol region in the upstream position (Fig. 6B) before reaching the gradient. Platelet adhesion on gradient samples with the upstream sulfonate region displayed similar adhesion contrast seen in previous experiments (Figs. 4A and 5A). When the orientation of the sample was reversed, adhesion contrast was lost and overall adhesion values on the thiol and sulfonate rich regions decreased and increased, respectively.
Figure 6.
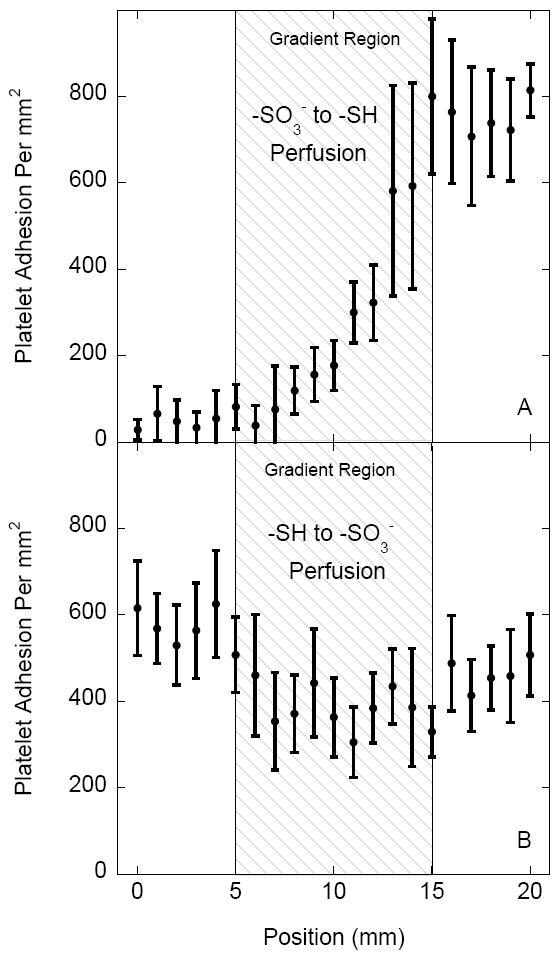
Platelet adhesion comparison on sulfonate-to-thiol gradient surfaces with (A) the sulfonate region in the upstream position and with (B) the thiol region in the upstream position of the flow cell. Samples were pre-adsorbed with 10% human PFP. Adhesion values represent a mean ± standard deviation; n = 20.
Discussion
The events which occur when blood contacts a material interface are complex and not well understood. Here we described a simple method for screening platelet-surface interactions in a single experiment using a surface gradient. Such an approach allows for a correlation between surface properties, adsorbed protein layer, and platelet adhesion to be made.
Blood plasma concentration has been noted to influence the composition of the adsorbed protein layer as well as subsequent platelet-surface interactions [24,25]. In this study, platelet perfusion over gradient surfaces pre-adsorbed with PFP at lower and higher concentrations (1% and 50%) showed less adhesion contrast between sulfonate and thiol regions than 10% PFP pre-adsorbed surfaces. Brash et al. have reported that the amount of adsorbed fibrinogen on glass is dependent on plasma concentration while albumin is not [25]. Thus, one possible explanation for less adhesion contrast at 1% dilutions is that the ratio of adsorbed fibrinogen to adsorbed albumin is not enough to support extensive platelet adhesion. In other words, the overall fibrinogen concentration may not be sufficient to out compete albumin and override its inhibitory effect. At 50% PFP concentration a higher number of platelet aggregates was observed, and the adhesion contrast was low. The increase in aggregate formation may be more representative of platelet-platelet rather than platelet-surface interactions. Gradient surfaces pre-adsorbed with 10% PFP provided the best adhesion contrast and therefore this concentration was used for the remaining platelet adhesion experiments.
Sulfonate-to-thiol gradient surfaces pre-adsorbed with 10% PFP showed a gradient of platelet adhesion compared to non-oxidized control surfaces (Figs 5A vs. 5B). Overall platelet adhesion was related to the surface water contact angles and inversely related to the surface charge density. These results compare well with the previous studies that have shown platelet adhesion to increase with increasing hydrophobicity in the presence of plasma proteins [16,17]. The chemistry difference between thiol and sulfonate mattered as well: sulfonate groups have been incorporated into various biomaterials and shown to improve hemocompatibility [26,27],
The effect of the surface charge gradient on platelet adhesion and its relationship to the nature of the adsorbed protein layer was further investigated by pre-adsorbing a 10% fibrinogen solution prior to platelet perfusion. Interactions between fibrinogen and the GPIIa-IIIb integrin receptors on platelets are central in platelet adhesion and aggregation [28,29]. As noted earlier, sulfonate-to-thiol gradients displayed a platelet adhesion maximum in the middle of gradient region; an effect not observed either on the non-oxidized thiol monolayer control sample (Fig. 5D) or gradient pre-adsorbed with PFP (Fig. 5A). This platelet adhesion maximum on fibrinogen pre-adsorbed gradient sample is different than the additive effect seen on gradient samples pre-adsorbed with PFP, thus confirming the importance of not only the surface properties but the nature of the adsorbed protein layer as well. Autoradiography data on the adsorption of 125I-labeled fibrinogen from 1% solution showed lower amount of adsorbed fibrinogen on the thiol (0.81 μg/cm2) as compared with the sulfonate region (0.35 μg/cm2). The amounts of adsorbed 125I-fibrinogen from 1% PFP were approximately 60% lower than from single protein solution on both regions (V. Hlady, unpublished).
Surprisingly, non-oxidized control samples (uniform thiol monolayers) pre-adsorbed with 10% human fibrinogen and 10% PFP did see a significant increase in adhesion as a function of distance from the flow inlet despite lacking a charge density gradient. Uniform thiol monolayers pre-adsorbed with PFP displayed an adhesion increase in the 0 mm – 5 mm region, while the same surfaces pre-adsorbed with fibrinogen displayed a significant increase in adhesion over the length of the sample (Figs. 5B vs. 5D). We infer that the increased downstream adhesion is most likely due to upstream surface contact activation. Surface fibrinogen-platelet interactions convert platelets from a quiescent to a pro-coagulant state [30]. In addition, once platelets adhere to a substrate they do not always remain there and their contacts can be transient [31]. Taken together, a downstream increase in platelet adhesion may be the result of upstream pre-activation.
Uniform thiol monolayers pre-adsorbed with 10% human albumin did not display adhesion contrast as a function of distance from the flow inlet. The lack of an upstream effect on downstream adhesion was initially interpreted as being the result of albumin inhibition on platelet adhesion [32]. However, contrary to the uniform thiol monolayer data, platelet adhesion in the sulfonate region of the gradient samples was somewhat higher than in the thiol region of the gradient as well as in thiol controls. In a separate study, we found that sulfonate and thiol regions adsorbed similar amounts of albumin, inferring that the lack of inhibition was not due to the lower amount of adsorbed albumin (V. Hlady, unpublished). Significant platelet attachment to albumin coated surfaces has been recently reported, presumably due to exposure of a hidden binding site for platelets on albumin upon surface adsorption [33]. A conjecture can be made that the inhibition of platelet adhesion by adsorbed albumin depends more on its conformation than on the variation of adsorbed amount. The results here may also indicate that low platelet adhesion to surfaces pre-adsorbed with PFP may not be due to increased albumin adsorption but perhaps to protein conformation or to some other variability in the composition of the protein surface layer.
In order to further investigate the effect of upstream conditions on downstream platelet adhesion, the orientation of the gradient sample pre-adsorbed with 10% PFP was reversed and compared. As stated earlier, the adhesion contrast was much lower when gradient surfaces were positioned in the upstream-thiol to downstream-sulfonate orientation. The results support the conclusion that upstream platelet-surface interactions could exhibit enhanced downstream adhesion. This effect can be noticed on both surface gradients (Fig. 6) as well as on homogeneous surfaces (Figs. 5B vs. 5D).
In the surface gradient experiments the relationships between surface composition and platelet adhesion were made by comparing gradients to homogeneous control samples taking into consideration that adhesion at a specific position cannot be directly correlated with the surface properties of that position alone. Thus, it turns out that the history of transient surface-platelet interactions must be considered as well. Discrepancies between results obtained on gradient vs. control samples were also reported by Zelzer et al [13]. It is thus crucial we acknowledge these inconsistencies when using gradients as a tool to study various biological phenomena.
To reduce the complexity of blood-surface interactions and obtain a clearer insight in the cause-effect relationship between surface chemistry, adsorbed protein and platelet adhesion, we used washed platelets instead of whole blood or PRP; however such an approach neglects the effect of red blood cells, which have been shown to increase platelet adhesion through a “mixing” effect [34]. Although the “mixing effect” could have influenced overall adhesion amounts in the experiments described here, it was not expected to significantly affect the adhesion contrast seen in these experiments since red blood cells do not actively interact with the surface.
Another concern might be due to the presence of albumin in the platelet wash buffer used to prevent premature platelet activation during handling. One may question its effect on the nature of the adsorbed protein layer during adhesion experiments. The presence of albumin in the wash buffer (3 mg/ml) is expected to have little effect on surfaces pre-coated with albumin since any exchange reactions would not change the nature of the protein layer. Surfaces pre-adsorbed with 10% fibrinogen may be affected by the presence of albumin in the platelet wash buffer due to the Vroman effect; however this may also be unlikely due to the fact that pre-adsorbed fibrinogen has a higher affinity for surfaces than albumin making this exchange energetically unfavorable [35].
Conclusions
In this work platelet adhesion on surfaces with varying negative charge densities was studied. Platelet adhesion was also related to the nature of the adsorbed protein layer (specifically: albumin and fibrinogen). Platelet adhesion onto gradients was affected by both the type and concentration of pre-adsorbed protein as well as related to the negative charge density. The high-throughput potential of surface gradients is a useful concept for testing platelet adhesion and material hemocompatibility in general, however, it is important to consider that local surface effect may be affected by the upstream surface-platelet interactions. The orientation of gradient samples was shown to affect platelet adhesion patterns suggesting that the history of transient surface contacts must be considered.
Acknowledgments
This work is supported by the National Institute of Health (5RO1 HL84586). We would like to thank Dr. Andy Weyrich and his lab for human blood used in our studies and Mr. Nathan Gooch for XPS analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vroman L. Blood. Natural History Press, Doubleday and Co; New York: 1967. [Google Scholar]
- 2.Andrade JD, Nagaoka S, Cooper S, Okano T, Kim SW. Surface and blood compatibility: Current hypotheses. ASAIO J. 1987;10:75–84. [Google Scholar]
- 3.Tanzi MC. Bioactive technologies for hemocompatibility. Expert Rev Med Devices. 2005;2:473–92. doi: 10.1586/17434440.2.4.473. [DOI] [PubMed] [Google Scholar]
- 4.Andrade JD, Hlady V. Protein adsorption and materials biocompatibility: a tutorial review and suggested hypotheses. Adv Polymer Sci. 1986;79:1–63. [Google Scholar]
- 5.Wang YX, Robertson JL, Spillman WB, Jr, Claus RO. Effects of the chemical structure and the surface properties of polymeric biomaterials on their biocompatibility. Pharm Res. 2004;21:1362–73. doi: 10.1023/b:pham.0000036909.41843.18. [DOI] [PubMed] [Google Scholar]
- 6.Zhang M, Horbett TA. Tetraglyme coatings reduce fibrinogen and von Willebrand factor adsorption and platelet adhesion under both static and flow conditions. J Biomed Mat Res A. 2009;89:791–803. doi: 10.1002/jbm.a.32085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li D, Chen H, McClung WG, Brash JL. Lysine-PEG-modified polyurethane as a fibrinolytic surface: Effect of PEG chain length on protein interactions, platelet interactions and clot lysis. Acta Biomater. 2009;5:1864–71. doi: 10.1016/j.actbio.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Yim EKF, Sefton MV. Amidine surface modification of poly(acrylonitrile-co-vinyl chloride) reduces platelet adhesion. J Biomed Mater Res A. 2009;89:780–90. doi: 10.1002/jbm.a.32022. [DOI] [PubMed] [Google Scholar]
- 9.Kim MS, Khang G, Lee HB. Gradient polymer surfaces for biomedical applications. Prog Polym Sci. 2008;33:138–64. [Google Scholar]
- 10.Elwing H, Askendal A, Lundstrom I. Protein exchange reactions on solid surfaces studied with a wettability gradient method. Prog Colloid Polym Sci. 1987;74:103–7. doi: 10.1163/156856292x00042. [DOI] [PubMed] [Google Scholar]
- 11.Golander CG, Lin YS, Hlady V, Andrade JD. Wetting and plasma-protein adsorption studies on surfaces with a hydrophobicity gradient. Colloids Surf. 1990;49:289–302. [Google Scholar]
- 12.Zhijun B, Filiaggi MJ, Sanderson RJ, Lohstreter LB, Mc Arthur MA, Dahn JR. Surface characteristics and protein adsorption on combinatorial binary Ti-M(Cr, Al, Ni) and Al-M (Ta, Zr) library films. J Biomed Mat Res Part A. 2010;92A:3521–32. doi: 10.1002/jbm.a.32398. [DOI] [PubMed] [Google Scholar]
- 13.Zelzer M, Majani R, Bradley JW, Rose FRAJ, Davies MC. Investigation of cell-surface interactions using chemical gradients formed from plasma polymers. Biomaterials. 2008;29:172–84. doi: 10.1016/j.biomaterials.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 14.Ruardy TG, Schakenraad JM, Van Der Mei HC, Busscher HJ. Preparation and characterization of chemical gradient surfaces and their application for the study of cellular interaction phenomena. Surf Sci Rep. 1997;27:3–30. [Google Scholar]
- 15.Lee JH, Khang G, Lee JW, Lee HB. Platelet adhesion onto chargeable functional group gradient surfaces. J Biomed Mater Res. 1998;40:180–6. doi: 10.1002/(sici)1097-4636(199805)40:2<180::aid-jbm2>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 16.Spijker HT, Bos R, Busscher HJ, Van Kooten TG, Van Oeveren W. Platelet adhesion and activation on a shielded plasma gradient prepared on polyethylene. Biomaterials. 2002;23:757–66. doi: 10.1016/s0142-9612(01)00181-8. [DOI] [PubMed] [Google Scholar]
- 17.Lee JH, Lee HB. Platelet adhesion onto wettability gradient surfaces in the absence and presence of plasma proteins. J Biomed Mater Res. 1998;41:304–11. doi: 10.1002/(sici)1097-4636(199808)41:2<304::aid-jbm16>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Jeong BJ, Lee HB. Plasma protein adsorption and platelet adhesion onto comb-like PEO gradient surfaces. J Biomed Mat Res Part A. 1998;34:105–14. doi: 10.1002/(sici)1097-4636(199701)34:1<105::aid-jbm14>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 19.Iwasaki Y, Ishihara K, Nakabayashi N, Khang G, Jeon JH, Lee JW, et al. Platelet adhesion on the gradient surfaces grafted with phospholipid polymer. J Biomat Sci Polym Ed. 1998;9:801–16. doi: 10.1163/156856298x00163. [DOI] [PubMed] [Google Scholar]
- 20.Bhatia SK, Teixeira JL, Anderson M, Shriver-Lake LC, Calvert JM, Georger JH, et al. Fabrication of surfaces resistant to protein adsorption and application to two-dimensional protein patterning. Anal Biochem. 1993;208:197–205. doi: 10.1006/abio.1993.1027. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Hlady V. Chemical pattern on silica surface prepared by UV irradiation of 3-mercaptopropyltriehtoxy silane layer: surface characterization and fibrinogen adsorption. Colloids Surf B: Biointerfaces. 1996;8:25–37. doi: 10.1016/S0927-7765(96)01298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mustard JF, Packham MA. Factors influencing platelet function: adhesion, release, and aggregation. Pharm Revs. 1970;22:97–187. [PubMed] [Google Scholar]
- 23.McNicol A. Platelet preparation and estimation of functional responses. In: Watson SP, Authi KS, editors. Platelets: a practical approach. New York: Oxford University Press; 2002. p. 5. [Google Scholar]
- 24.Chinn JA, Horbett TA, Ratner BD. Baboon fibrinogen adsorption and platelet adhesion to polymeric materials. Thromb Haemost. 1991;65:608–17. [PubMed] [Google Scholar]
- 25.Brash JL, Ten Hove P. Effect of plasma dilution on adsorption of fibrinogen to solid surfaces. Thromb Haemost. 1984;51:326–30. [PubMed] [Google Scholar]
- 26.Yeh H, Lin J. Surface characterization and in vitro platelet compatibility study of surface sulfonated chitosan membrane with amino group protection-deprotection strategy. J Biomater Sci Polym Ed. 2008;19:291–310. doi: 10.1163/156856208783720985. [DOI] [PubMed] [Google Scholar]
- 27.Han DK, Park K, Park KD, Ahn KD, Kim YH. In vivo biocompatibility of sulfonated PEO-grafted polyurethanes for polymer heart valve and vascular graft. Artif Organs. 2006;30:955–59. doi: 10.1111/j.1525-1594.2006.00327.x. [DOI] [PubMed] [Google Scholar]
- 28.Phillips DR, Charo IF, Parise LV, Fitzgerald LA. The platelet membrane glycoprotein IIb-IIIa complex. Blood. 1988;71:831–43. [PubMed] [Google Scholar]
- 29.Savage B, Shattil SJ, Ruggeri ZM. Modulation of platelet function through adhesion receptors. A dual role for glycoprotein IIb-IIIa (integrin alpha IIb beta 3) mediated by fibrinogen and glycoprotein Ib-von Willebrand factor. J Biol Chem. 1992;267:11300–6. [PubMed] [Google Scholar]
- 30.Grunkemeier JM, Tsai WB, McFarland CD, Horbett TA. The effect of adsorbed fibrinogen, von Willebrand factor and vitronectin on the procoagulant state of adherent platelets. Biomaterials. 2000;21:2243–52. doi: 10.1016/s0142-9612(00)00150-2. [DOI] [PubMed] [Google Scholar]
- 31.Godo MN, Sefton MV. Characterization of transient platelet contacts on a polyvinyl alcohol hydrogel by video microscopy. Biomaterials. 1999;20:1117–26. doi: 10.1016/s0142-9612(99)00012-5. [DOI] [PubMed] [Google Scholar]
- 32.Kottke-Marchant K, Anderson JM, Umemura Y, Marchant RE. Effect of albumin coating on the in vitro blood compatibility of Dacron arterial prostheses. Biomaterials. 1989 Apr;10:147–55. doi: 10.1016/0142-9612(89)90017-3. [DOI] [PubMed] [Google Scholar]
- 33.Sivaraman B, Latour RA. The adherence of platelets to adsorbed albumin by receptor-mediated recognition of binding sites exposed by adsorption-induced unfolding. Biomaterials. 2010;31:1036–44. doi: 10.1016/j.biomaterials.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turitto VT, Weiss HJ. Red blood cells: their dual role in thrombus formation. Science. 1980;207:541–3. doi: 10.1126/science.7352265. [DOI] [PubMed] [Google Scholar]
- 35.Slack SM, Horbett TA. The Vroman effect: a critical review. In: Horbett TA, Brash J, editors. Proteins at interfaces II: fundamentals and applications. Washington DC: American Chemical Society; 1995. pp. 112–28. [Google Scholar]