Abstract
Background
Conflicting evidence exists on whether cholinesterase inhibitors and memantine increase the risk of falls, syncope, and related events, defined as fracture and accidental injury.
Objectives
To evaluate the effect of cholinesterase inhibitors and memantine on the risk of falls, syncope, and related events
Design, Setting, Participants, and Intervention
Meta-analysis of 54 placebo-controlled randomized trials and extension studies of cholinesterase inhibitors and memantine that reported falls, syncope, and related events in cognitively impaired older adults. Trials were identified from MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials (no language restriction, through July 2009), and manual search.
Measurements
Falls, syncope, fracture, and accidental injury
Results
Compared to placebo, cholinesterase inhibitor use was associated with an increased risk of syncope (odds ratio [95% confidence interval]: 1.53 [1.02-2.30]), but not with other events (falls: 0.88 [0.74-1.04]; fracture: 1.39 [0.75-2.56]; accidental injury: 1.13 [0.87-1.45]). Memantine use was associated with fewer fractures (0.21 [0.05-0.85]), but not with other events (fall: 0.92 [0.72-1.18]; syncope: 1.04 [0.35-3.04]; accidental injury: 0.80 [0.56-1.12]). There was no differential effect by type and severity of cognitive impairment, residential status, nor length of follow-up. However, due to underreporting and small number of events, a potential benefit or risk cannot be excluded.
Conclusion
Cholinesterase inhibitors may increase the risk of syncope, with no effects on falls, fracture, and accidental injury in cognitively impaired older adults. Memantine may have a favorable effect on fracture, with no effects on other events. More research is needed to confirm the reduction in fractures observed for memantine.
Keywords: Cholinesterase Inhibitors, Memantine, Falls, Syncope, Dementia
INTRODUCTION
Approximately two thirds of cognitively impaired older adults fall annually,1 a rate that is two- to eight-fold greater than generally healthy older adults.2;3 Physical, psychological, and economic consequences of falls in older persons are considerable, including morbidity and mortality from injury, institutionalization, and substantial healthcare costs.2;4;5 Older persons with dementia are particularly prone to these undesirable consequences of falls.2 Impaired gait and balance,6 limited attention,7 psychotropic medications,8 behavioral risk factors,9 and orthostatic hypotension10 may contribute to falls among older adults with dementia. Although multifactorial interventions have proved effective in reducing falls,11 the evidence that such interventions work effectively for persons with dementia or who are institutionalized is very limited.12;13
Cholinesterase inhibitors and the N-methyl-D-aspartate (NMDA) receptor antagonist memantine, are widely prescribed to treat symptoms of Alzheimer's disease (AD) and other dementias.14 Despite their common use, little is known about the impact of these medications on gait, falls, and fall-related adverse events. Safety data from randomized controlled trials have been limited to individual agents or specific dementia types, or not focused on fall-related adverse events.15-18 A few small studies suggest that cholinesterase inhibitors may reduce falls as they improve gait and balance control, possibly through their positive effects on attention and executive function.19;20 By contrast, there have been several case reports of falls, syncope, and accidental injuries related to cholinesterase inhibitors and memantine.21-24 Recently, a large population-based cohort study showed higher rates of syncope, pacemaker insertion, and hip fracture among dementia patients on cholinesterase inhibitors.25
This systematic review and meta-analysis of randomized controlled trials was conducted to evaluate the effect of cholinesterase inhibitors (donepezil, galantamine, rivastigmine, and tacrine) and the NMDA antagonist memantine on the risk of falls and fall-related adverse events (syncope, fracture, and accidental injury) in older adults with mild cognitive impairment and dementia. We also examined whether the risk of falls varied by the type and severity of cognitive impairment, residential status, and length of treatment.
METHODS
Data Sources and Searches
We performed a systematic search of MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials, with no language restriction, from inception through July 2009, to identify all randomized controlled trials of cholinesterase inhibitors (donepezil, galantamine, rivastigmine, and tacrine) and the NMDA antagonist memantine, using the following terms and their variants: donepezil, galantamine, rivastigmine, tacrine, memantine, dementia, Alzheimer, Lewy body, Parkinson, cognitive impairment, and randomized controlled trials (our review protocol is provided in appendix 1). We did not use any specific adverse outcome terms in our search, because a sizable number of reports did not contain text words or indexing terms reflecting adverse events.26;27 In order to identify unpublished safety data of randomized controlled trials, we manually searched the reference lists of the Cochrane Collaboration systematic reviews and selected review articles, pharmaceutical clinical trial registries, and the medical and safety review documents of the Food and Drug Administration (FDA) new drug application available online (http://www.accessdata.fda.gov/Scripts/cder/DrugsatFDA/, accessed July 21, 2009).
Study Selection
At least two investigators independently evaluated all references for their eligibility and any disagreements were resolved by consensus. A report was eligible if it was a randomized placebo-controlled trial or its extension study of any cholinesterase inhibitor or memantine conducted in patients with AD, vascular dementia (VD) or mixed dementia, Parkinson disease with dementia (PDD), dementia with Lewy body (DLB), frontotemporal dementia, or mild cognitive impairment (MCI).
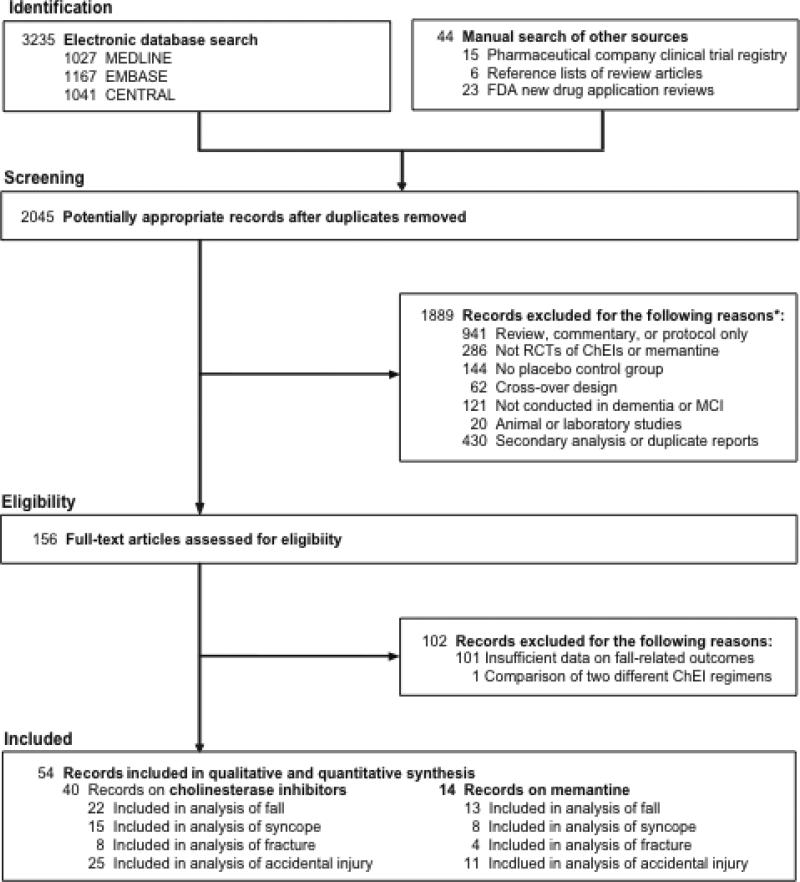
We excluded protocol or design papers, review articles, or commentaries; trials evaluating interventions other than cholinesterase inhibitors or memantine; trials with no placebo group; trials of cross-over design; trials not conducted in patients with dementia or MCI; studies not conducted in humans; and reports of secondary analysis of randomized controlled trials with no additional data on falls, syncope, fracture, and accidental injury. All identified references were manually examined for their report of falls, syncope, and related adverse events and 54 references that contained information on at least one type of events were included (Figure 1).
Figure 1. Study Selection.
Abbreviation: RCT, randomized controlled trial.
* Not mutually exclusive.
Data Extraction and Quality Assessment
The main study outcomes were falls or fall-related adverse events, defined as syncope, fracture, or accidental injury. Because falls and fall-related adverse events were not the primary outcome of the individual studies, few studies described in detail how these events were defined or ascertained. Adverse events that emerged after the initiation of treatment, or “treatment-emergent adverse events”, were extracted when reported. Using a standardized form, at least two investigators independently extracted data on first author, study title, publication year, country, funding source, mean age, gender, and mini-mental state examination (MMSE) score, type and severity of cognitive impairment, residential status (community versus nursing home), regimen and duration of treatment, sample size, length of follow-up, and the number of main outcome events. Unpublished safety data in FDA documents were examined to supplement published data. Any disagreements were resolved by consensus.
The severity of cognitive impairment was defined, using the mean MMSE scores: mild if MMSE score > 20; mild-to-moderate if MMSE score 16-20; moderate-to-severe if MMSE score 11-15; and severe if MMSE score ≤ 10. When the mean MMSE was not reported, qualitative descriptions were used.28-30 Trials were assumed to have been conducted in the community setting, unless specific descriptions of the nursing home or residential care setting were provided. This assumption was justified, because trials31-59 that did not provide detailed information on residential status were conducted in participants with mild-to-moderate cognitive impairment.
According to available guidelines on harms reporting,27;60 we examined two important aspects of the quality assessment of adverse event data: the rigorousness of monitoring and the quality of reporting. The rigorousness of monitoring was assessed using the following criteria: 1) description of the methods for adverse event monitoring; 2) use of standardized and validated definitions of adverse events; and 3) use of active surveillance versus passive monitoring. The quality of reporting was assessed based on whether the study reported all events versus selected events observed above a certain frequency. Because most reports did not provide detailed information on each adverse event, we assessed the quality of overall adverse event reporting rather than specific adverse events. We also examined the adequacy of a given trial according to the following quality standards: generation of random sequences, concealment of randomization, blinding of participants, and blinding of outcome assessors to treatment group. If there was not enough information to assess the quality, it was assumed inadequate.
Data Synthesis and Analysis
Trials of cholinesterase inhibitors were analyzed separately from trials of memantine, due to their differences in pharmacologic properties. For each adverse outcome, the pooled odds ratio (OR) and 95% confidence intervals (CIs) comparing cholinesterase inhibitors and memantine versus placebo were calculated using a random-effects model by DerSimonian and Laird.61 When no events occurred in a treatment arm, we added 0.5 to each cell to calculate the OR and 95% CI. When more than one dose or formulation was used, the doses were combined into a single group and compared with placebo. Between-study heterogeneity was assessed by the Cochran Q test and the I2 statistic.62
Prespecified subgroup meta-analysis and meta-regression analysis were performed to evaluate whether the risk of fall-related adverse events differed by the type (AD, VD or mixed dementia, PDD or DLB, and MCI) and severity of cognitive impairment (mild, mild-to-moderate, moderate-to-severe, and severe), residential status, and length of follow-up (< 6 months, 6-11 months, 12-17 months, and ≥ 18 months). In the subgroup analysis by the length of follow-up, data from both placebo-controlled, blind phase and open-label extension phase were included.
Three sensitivity analyses were performed. First, we repeated main analyses after including studies with complete follow-up (placebo-controlled, blind phase and open-label extension phase). Second, we analyzed studies with total sample size ≥ 500 to minimize the influence of small studies where fall-related adverse events were infrequent. Third, we examined the robustness of the overall estimate and influence of each study on the pooled estimate by excluding one study at a time. In addition, we assessed whether various measures of poor study quality were associated with fall-related adverse events. Publication bias was examined graphically via Begg's funnel plot and rank adjusted correlation test63 as well as Egger's weighted regression test.64 All analyses were performed with Stata SE version 11 (StataCorp, College Station, TX) and two-sided P < 0.05 was considered statistically significant.
RESULTS
The characteristics of the included studies are summarized in Appendix 2. There were 40 studies of cholinesterase inhibitors (16 donepezil,28;37;44;50;51;58;59;65-73 17 galantamine,30;34;38-40;42;43;48;49;52;53;55-57;74;75 6 rivastigmine,33;35;36;41;47;76 and 1 tacrine54) and 14 studies29;31;32;45;46;77-88 of memantine, including 1133;54-56;65;82;84-88 unpublished and 1432;33;39;40;43;45;47;49;52;55;56;79;82;84;88 open-label extension studies. The study participants averaged 69 to 86 years of age, 15 to 67% male, and had mean scores of 6 to 27 on MMSE. Diagnosis was AD in 39 studies28;29;38;40;43-46;48-51;53-57;59;67-76;78-82;84-88 or VD or mixed dementia in 10 studies.33-35;37;39;42;52;58;65;77 Cognitive impairment was mild or mild-to-moderate in 39 studies.30;32-53;55-59;65;66;68;70;71;75-77;80-82 Five studies28;72-74;85 were conducted in nursing home residents. Total sample size was < 500 in 31 studies28;29;32;36;39;40;44-47;49-52;54;59;67-74;78-82;85;86;88 and the duration was shorter than one year in 38 studies.28;29;34-38;41;42;46;48;50;51;53-58;65-78;80;81;85-87 Only 1629;32;38;45;46;48;53;55;56;58;59;71;73;75;82;84;85;87;88 reported 3 or more types of the events of interest.
Meta-Analysis of Cholinesterase Inhibitors
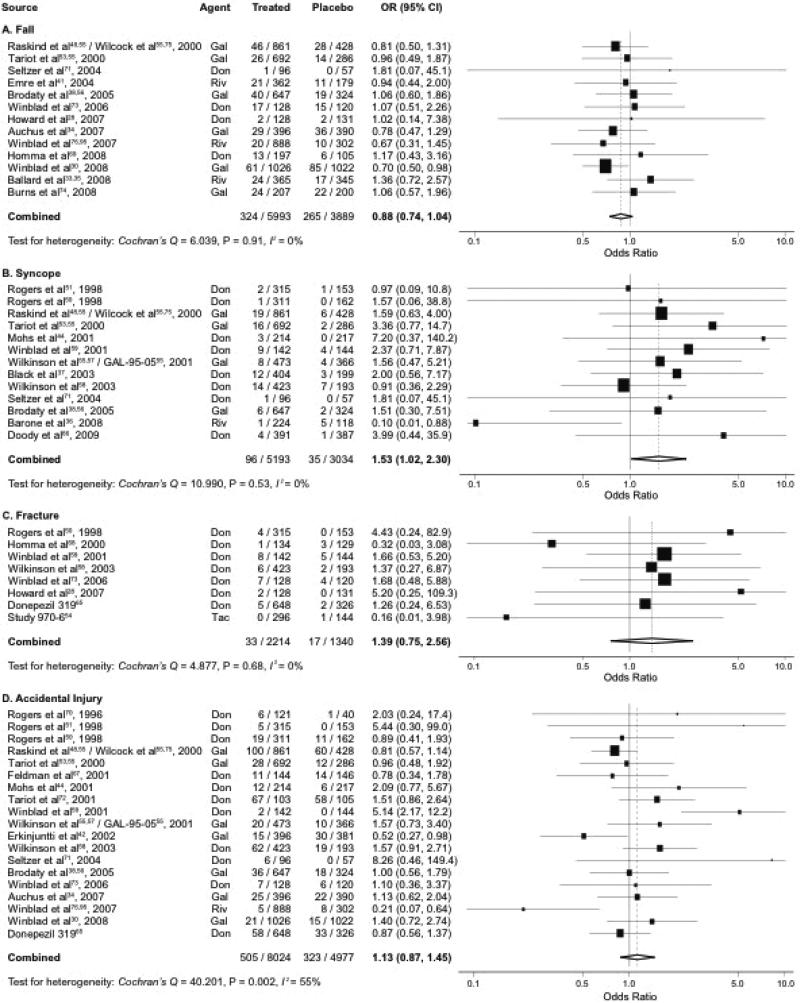
Meta-analysis of cholinesterase inhibitors pooled data from 40 studies that included 9882 participants and 589 events for falls; 8227 participants and 131 events for syncope; 3554 participants and 50 events for fracture; and 13001 participants and 828 events for accidental injury. The pooled ORs (95% CIs) comparing cholinesterase inhibitors and placebo were 0.88 (0.74-1.04; P = 0.14) for fall, 1.53 (1.02-2.30; P = 0.04) for syncope, 1.39 (0.75-2.56; P = 0.29) for fracture, and 1.13 (0.87-1.45; P = 0.37) for accidental injury (Figure 2). There was no evidence of subgroup differences in effects for fall, syncope, and fracture, but there was moderate heterogeneity for accidental injury. Notably, in subgroup analyses, the risk of syncope somewhat differed by type of cognitive impairment (P = 0.05), with higher pooled ORs (95% CI) of 1.90 (1.14-3.15; P = 0.01) for AD and 3.99 (0.44-35.9) for MCI (Table 1). Otherwise, there was little variation by the type and severity of cognitive impairment, residential status, and length of follow-up (P > 0.05 for all other subgroups).
Figure 2. Meta-Analysis of Cholinesterase Inhibitors and Falls, Syncope, and Related Events.*†.
Abbreviations: CI, confidence interval; Don, donepezil; Gal, galantamine; OR, odds ratio; Riv, rivastigmine; Tac, tacrine.
* Pooled odds ratio and 95% confidence interval of each study are represented by a square and a horizontal line, respectively. The size of a square is proportional to the weight of the study.
† I2 measures the proportion of heterogeneity in individual studies that cannot be explained by chance.
Table 1.
Subgroup Meta-Analysis of Cholinesterase Inhibitors and Falls, Syncope, and Related Events.
| Study Characteristics (P for interaction*) | No of Studies | Treated (n / N) | Placebo (n / N) | Pooled OR (95% CI) | P for Heterogeneity | I2† |
|---|---|---|---|---|---|---|
| Fall | ||||||
| Type of cognitive impairment (P for interaction = 0.502) | ||||||
| AD | 9 | 189 / 3844 | 116 / 1953 | 0.94 (0.74, 1.20) | 0.98 | 0% |
| VD or mixed dementia | 2 | 53 / 761 | 53 / 735 | 0.99 (0.58, 1.71) | 0.18 | 44% |
| PDD or DLB | 1 | 21 / 362 | 11 / 179 | 0.94 (0.44, 2.00) | - | - |
| MCI | 1 | 61 / 1026 | 85 / 1022 | 0.70 (0.50, 0.98) | - | - |
| Severity of cognitive impairment (P for interaction = 0.263) | ||||||
| Mild | 2 | 62 / 1122 | 85 / 1079 | 0.70 (0.50, 0.99) | 0.56 | 0% |
| Mild to moderate | 7 | 206 / 4211 | 135 / 2254 | 0.91 (0.73, 1.14) | 0.81 | 0% |
| Moderate to severe | 0 | - | - | - | - | - |
| Severe | 4 | 56 / 660 | 45 / 556 | 1.08 (0.71, 1.64) | 1.00 | 0% |
| Residential status (P for interaction = 0.377) | ||||||
| Community | 10 | 281 / 5530 | 226 / 3438 | 0.85 (0.71, 1.02) | 0.81 | 0% |
| Nursing home | 3 | 43 / 463 | 39 / 451 | 1.06 (0.67, 1.69) | 1.00 | 0% |
| Length of follow-up‡ (P for interaction = 0.517) | ||||||
| < 6 months | 2 | 28 / 820 | 16 / 417 | 0.97 (0.51, 1.82) | 0.95 | 0% |
| 6 – 11 months | 11 | 253 / 4651 | 167 / 2669 | 0.98 (0.79, 1.20) | 0.79 | 0% |
| 12 – 17 months | 4 | 138 / 1200 | 98 / 808 | 0.93 (0.70, 1.22) | 0.82 | 0% |
| ≥ 18 months | 3 | 138 / 1678 | 123 / 1308 | 0.76 (0.52, 1.10) | 0.20 | 39% |
| Syncope | ||||||
| Type of cognitive impairment (P for interaction = 0.048) | ||||||
| AD | 9 | 65 / 3751 | 19 / 2137 | 1.90 (1.14, 3.15) | 0.98 | 0% |
| VD or mixed dementia | 2 | 26 / 827 | 10 / 392 | 1.19 (0.56, 2.52) | 0.33 | 0% |
| PDD or DLB | 1 | 1 / 224 | 5 / 118 | 0.10 (0.01, 0.88) | - | - |
| MCI | 1 | 4 / 391 | 1 / 387 | 3.99 (0.44, 35.9) | - | - |
| Severity of cognitive impairment (P for interaction = 0.700) | ||||||
| Mild | 4 | 31 / 1314 | 11 / 836 | 1.37 (0.69, 2.74) | 0.57 | 0% |
| Mild to moderate | 9 | 65 / 3879 | 24 / 2198 | 1.61 (0.94, 2.76) | 0.36 | 9% |
| Moderate to severe | 0 | - | - | - | - | - |
| Severe | 0 | - | - | - | - | - |
| Residential status (P for interaction = N/A) | ||||||
| Community | 13 | 96 / 5193 | 35 / 3034 | 1.53 (1.02, 2.30) | 0.53 | 0% |
| Nursing home | 0 | - | - | - | - | - |
| Length of follow-up‡ (P for interaction = 0.396) | ||||||
| < 6 months | 3 | 26 / 1480 | 7 / 805 | 1.91 (0.80, 4.58) | 0.61 | 0% |
| 6 – 11 months | 8 | 58 / 3357 | 24 / 1868 | 1.26 (0.73, 2.16) | 0.37 | 8% |
| 12 – 17 months | 3 | 13 / 368 | 4 / 367 | 2.64 (0.92, 7.59) | 0.76 | 0% |
| ≥ 18 months | 0 | - | - | - | - | - |
| Fracture | ||||||
| Type of cognitive impairment (P for interaction = 0.916) | ||||||
| AD | 6 | 22 / 1143 | 13 / 821 | 1.42 (0.69, 2.92) | 0.43 | 0% |
| VD or mixed dementia | 2 | 11 / 1071 | 4 / 519 | 1.32 (0.42, 4.16) | 0.94 | 0% |
| PDD or DLB | 0 | - | - | - | - | - |
| MCI | 0 | - | - | - | - | - |
| Severity of cognitive impairment (P for interaction = 0.861) | ||||||
| Mild | 2 | 11 / 1071 | 4 / 519 | 1.32 (0.42, 4.16) | 0.94 | 0% |
| Mild to moderate | 3 | 13 / 591 | 8 / 426 | 1.32 (0.43, 4.09) | 0.31 | 14% |
| Moderate to severe | 0 | - | - | - | - | - |
| Severe | 2 | 9 / 256 | 4 / 251 | 1.98 (0.62, 6.31) | 0.50 | 0% |
| Residential status (P for interaction = 0.482) | ||||||
| Community | 6 | 24 / 1958 | 13 / 1089 | 1.21 (0.59, 2.49) | 0.60 | 0% |
| Nursing home | 2 | 9 / 256 | 4 / 251 | 1.98 (0.62, 6.31) | 0.50 | 0% |
| Length of follow-up‡ (P for interaction = 0.882) | ||||||
| < 6 months | 3 | 6 / 739 | 1 / 428 | 1.66 (0.19, 14.4) | 0.22 | 33% |
| 6 – 11 months | 4 | 19 / 1333 | 11 / 768 | 1.22 (0.55, 2.70) | 0.65 | 0% |
| 12 – 17 months | 1 | 8 / 142 | 5 / 144 | 1.66 (0.53, 5.20) | - | - |
| ≥ 18 months | 0 | - | - | - | - | - |
| Accidental Injury | ||||||
| Type of cognitive impairment (P for interaction = 0.694) | ||||||
| AD | 14 | 324 / 5135 | 204 / 2850 | 1.20 (0.84, 1.71) | 0.002 | 59% |
| VD or mixed dementia | 4 | 160 / 1863 | 104 / 1105 | 0.96 (0.63, 1.47) | 0.07 | 58% |
| PDD or DLB | 0 | - | - | - | - | - |
| MCI | 1 | 21 / 1026 | 15 / 1022 | 1.40 (0.72, 2.74) | - | - |
| Severity of cognitive impairment (P for interaction = 0.990) | ||||||
| Mild | 5 | 162 / 2589 | 97 / 1794 | 1.06 (0.65, 1.72) | 0.04 | 60% |
| Mild to moderate | 11 | 258 / 5060 | 148 / 2812 | 1.18 (0.79, 1.75) | 0.002 | 64% |
| Moderate to severe | 2 | 78 / 247 | 72 / 251 | 1.17 (0.62, 2.19) | 0.20 | 41% |
| Severe | 1 | 7 / 128 | 6 / 120 | 1.10 (0.36, 3.37) | - | - |
| Residential status (P for interaction = 0.441) | ||||||
| Community | 17 | 431 / 7793 | 259 / 4752 | 1.10 (0.83, 1.46) | 0.001 | 58% |
| Nursing home | 2 | 74 / 231 | 64 / 225 | 1.42 (0.86, 2.33) | 0.62 | 0% |
| Length of follow-up‡ (P for interaction = 0.299) | ||||||
| < 6 months | 4 | 59 / 1601 | 23 / 845 | 1.29 (0.79, 2.11) | 0.56 | 0% |
| 6 – 11 months | 13 | 417 / 5545 | 285 / 2968 | 0.90 (0.70, 1.16) | 0.04 | 45% |
| 12 – 17 months | 4 | 104 / 1220 | 74 / 976 | 1.46 (0.79, 2.69) | 0.006 | 73% |
| ≥ 18 months | 2 | 39 / 1189 | 30 / 1098 | 0.85 (0.31, 2.32) | 0.05 | 75% |
Abbreviations: AD, Alzheimer's disease; CI, confidence interval; DLB, dementia with Lewy body; MCI, mild cognitive impairment; N/A, non-applicable; OR, odds ratio; PDD, Parkinson disease with dementia; VD, vascular dementia.
P value for interaction was computed from the meta-regression model that investigated the variations in effect size by a study-level characteristic.
I2 measures the proportion of heterogeneity in individual studies that cannot be explained by chance.
Open-label extension studies of placebo-controlled trials were included so that the number of studies may be greater.
Meta-Analysis of Memantine
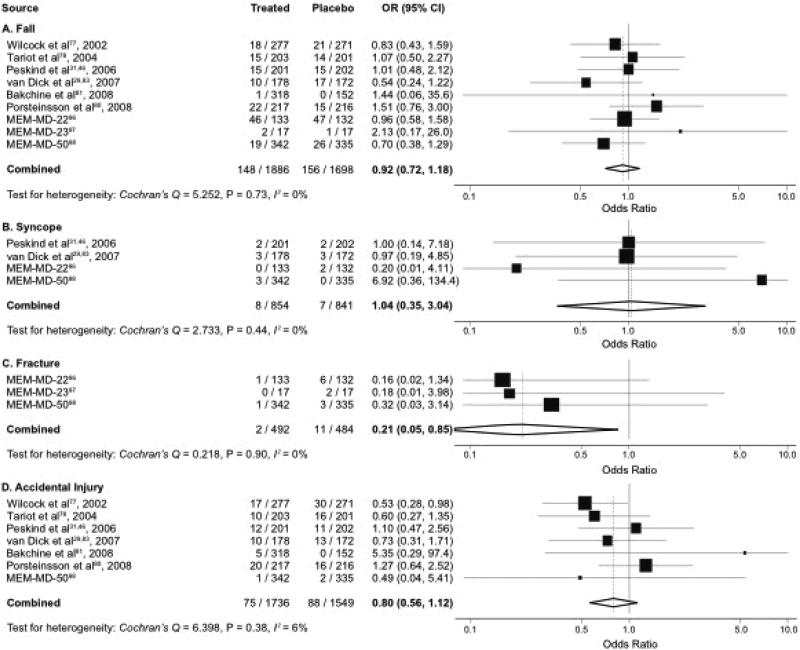
Meta-analysis of memantine pooled data from 14 studies that included 3584 participants and 304 events for fall; 1695 participants and 15 events for syncope; 976 participants and 13 events for fracture; and 3285 participants and 163 events for accidental injury. The pooled ORs (95% CIs) were 0.92 (0.72-1.18; P = 0.51) for fall, 1.04 (0.36-3.04; P = 0.95) for syncope, 0.21 (0.05-0.85; P = 0.03) for fracture, and 0.80 (0.56-1.12; P = 0.19) for accidental injury (Figure 3). There were no significant subgroup differences in the results on any fall-related adverse events. Subgroup analyses were limited by small number of trials (Table 2), but there was no significant variation in the risk of fall-related adverse events by subgroups (P > 0.05 for all subgroups).
Figure 3. Meta-Analysis of Memantine and Falls, Syncope, and Related Events.*†.
Abbreviations: CI, confidence interval; OR, odds ratio.
* Pooled odds ratio and 95% confidence interval of each study are represented by a square and a horizontal line, respectively. The size of a square is proportional to the weight of the study.
† I2 measures the proportion of heterogeneity in individual studies that cannot be explained by chance.
Table 2.
Subgroup Meta-Analysis of Memantine and Falls, Syncope, and Related Events.
| Study Characteristics (P for interaction*) | No of Studies | Treated (n / N) | Placebo (n / N) | Pooled OR (95% CI) | P for Heterogeneity | I2† |
|---|---|---|---|---|---|---|
| Fall | ||||||
| Type of cognitive impairment (P for interaction = 0.728) | ||||||
| AD | 8 | 130 / 1609 | 135 / 1427 | 0.94 (0.72, 1.22) | 0.64 | 0% |
| VD or mixed dementia | 1 | 18 / 277 | 21 / 271 | 0.83 (0.43, 1.59) | - | - |
| PDD or DLB | 0 | - | - | - | - | - |
| MCI | 0 | - | - | - | - | - |
| Severity of cognitive impairment (P for interaction = 0.475) | ||||||
| Mild | 0 | - | - | - | - | - |
| Mild to moderate | 4 | 56 / 1013 | 51 / 841 | 1.08 (0.73, 1.60) | 0.65 | 0% |
| Moderate to severe | 4 | 77 / 670 | 91 / 656 | 0.79 (0.56, 1.12) | 0.55 | 0% |
| Severe | 1 | 15 / 203 | 14 / 201 | 1.07 (0.50, 2.27) | - | - |
| Residential status (P for interaction = 0.868) | ||||||
| Community | 8 | 102 / 1753 | 109 / 1566 | 0.91 (0.69, 1.21) | 0.63 | 0% |
| Nursing home | 1 | 46 / 133 | 47 / 132 | 0.96 (0.58, 1.58) | - | - |
| Length of follow-up‡ (P for interaction = 0.580) | ||||||
| < 6 months | 1 | 2 / 17 | 1 / 17 | 2.13 (0.18, 26.0) | - | - |
| 6 – 11 months | 8 | 146 / 1869 | 155 / 1681 | 0.91 (0.71, 1.17) | 0.68 | 0% |
| 12 – 17 months | 4 | 43 / 865 | 37 / 837 | 1.18 (0.64, 2.15) | 0.20 | 36% |
| ≥ 18 months | 0 | - | - | - | - | - |
| Syncope | ||||||
| Type of cognitive impairment (P for interaction = N/A) | ||||||
| AD | 4 | 8 / 854 | 7 / 841 | 1.04 (0.35, 3.04) | 0.44 | 0% |
| VD or mixed dementia | 0 | - | - | - | - | - |
| PDD or DLB | 0 | - | - | - | - | - |
| MCI | 0 | - | - | - | - | - |
| Severity of cognitive impairment (P for interaction = 0.968) | ||||||
| Mild | 0 | - | - | - | - | - |
| Mild to moderate | 1 | 2 / 201 | 2 / 202 | 1.00 (0.14, 7.19) | - | - |
| Moderate to severe | 3 | 6 / 653 | 5 / 639 | 1.07 (0.21, 5.40) | 0.23 | 27% |
| Severe | 0 | - | - | - | - | - |
| Residential status (P for interaction = 0.251) | ||||||
| Community | 3 | 8 / 721 | 5 / 709 | 1.32 (0.42, 4.16) | 0.49 | 0% |
| Nursing home | 1 | 0 / 133 | 2 / 132 | 0.20 (0.01, 4.11) | - | - |
| Length of follow-up‡ (P for interaction = 0.963) | ||||||
| < 6 months | 0 | - | - | - | - | - |
| 6 – 11 months | 4 | 8 / 854 | 7 / 841 | 1.04 (0.35, 3.04) | 0.44 | 0% |
| 12 – 17 months | 4 | 6 / 865 | 5 / 837 | 1.08 (0.34, 3.38) | 0.75 | 0% |
| ≥ 18 months | 0 | - | - | - | - | - |
| Fracture | ||||||
| Type of cognitive impairment (P for interaction = N/A) | ||||||
| AD | 3 | 2 / 492 | 11 / 484 | 0.21 (0.05, 0.85) | 0.90 | 0% |
| VD or mixed dementia | 0 | - | - | - | - | - |
| PDD or DLB | 0 | - | - | - | - | - |
| MCI | 0 | - | - | - | - | - |
| Severity of cognitive impairment (P for interaction = N/A) | ||||||
| Mild | 0 | - | - | - | - | - |
| Mild to moderate | 0 | - | - | - | - | - |
| Moderate to severe | 3 | 2 / 492 | 11 / 484 | 0.21 (0.05, 0.85) | 0.90 | 0% |
| Severe | 0 | - | - | - | - | - |
| Residential status (P for interaction = 0.726) | ||||||
| Community | 2 | 1 / 359 | 5 / 352 | 0.26 (0.04, 1.65) | 0.76 | 0% |
| Nursing home | 1 | 1 / 133 | 6 / 132 | 0.16 (0.02, 1.34) | - | - |
| Length of follow-up‡ (P for interaction = 0.053) | ||||||
| < 6 months | 1 | 0 / 17 | 2 / 17 | 0.18 (0.01, 3.98) | - | - |
| 6 – 11 months | 2 | 2 / 475 | 9 / 467 | 0.22 (0.05, 1.05) | 0.65 | 0% |
| 12 – 17 months | 1 | 7 / 246 | 3 / 245 | 2.36 (0.60, 9.25) | - | - |
| ≥ 18 months | 0 | - | - | - | - | - |
| Accidental Injury | ||||||
| Type of cognitive impairment (P for interaction = 0.152) | ||||||
| AD | 6 | 58 / 1459 | 58 / 1278 | 0.93 (0.63, 1.37) | 0.54 | 0% |
| VD or mixed dementia | 1 | 17 / 277 | 30 / 271 | 0.53 (0.28, 0.98) | - | - |
| PDD or DLB | 0 | - | - | - | - | - |
| MCI | 0 | - | - | - | - | - |
| Severity of cognitive impairment (P for interaction = 0.784) | ||||||
| Mild | 0 | - | - | - | - | - |
| Mild to moderate | 4 | 54 / 1013 | 57 / 841 | 0.94 (0.52, 1.71) | 0.14 | 46% |
| Moderate to severe | 2 | 11 / 520 | 15 / 507 | 0.70 (0.31, 1.56) | 0.76 | 0% |
| Severe | 1 | 10 / 203 | 16 / 201 | 0.60 (0.27, 1.35) | - | - |
| Residential status (P for interaction = N/A) | ||||||
| Community | 7 | 75 / 1736 | 88 / 1549 | 0.80 (0.56, 1.12) | 0.38 | 6% |
| Nursing home | 0 | - | - | - | - | - |
| Length of follow-up‡ (P for interaction = 0.949) | ||||||
| < 6 months | 0 | - | - | - | - | - |
| 6 – 11 months | 7 | 75 / 1736 | 88 / 1549 | 0.80 (0.56, 1.12) | 0.38 | 6% |
| 12 – 17 months | 4 | 28 / 714 | 34 / 672 | 0.78 (0.46, 1.30) | 0.81 | 0% |
| ≥ 18 months | 0 | - | - | - | - | - |
Abbreviations: AD, Alzheimer's disease; CI, confidence interval; DLB, dementia with Lewy body; MCI, mild cognitive impairment; N/A, non-applicable; OR, odds ratio; PDD, Parkinson disease with dementia; VD, vascular dementia.
P value for interaction was computed from the meta-regression model that investigated the variations in effect size by a study-level characteristic.
I2 measures the proportion of heterogeneity in individual studies that cannot be explained by chance.
Open-label extension studies of placebo-controlled trials were included so that the number of studies may be greater.
Sensitivity Analyses and Publication Bias
When the main analyses were repeated including studies with complete follow-up or studies with total sample size ≥ 500, the pooled ORs were consistent with the original results (data not shown). In addition, the pooled ORs were not materially affected by the results from exclusion of any single study. When the influence of study quality criteria on the pooled estimates was examined, we found no significant differences by individual study quality criteria (P > 0.05; Appendix 3 and 4). Consistent with the earlier findings of no differences in estimates by sample size, there was also no evidence of publication bias based on graphical examination of funnel plots, Begg's rank adjusted correlation test, nor Egger's weighted regression test.
DISCUSSION
In our meta-analysis of randomized controlled trials, cholinesterase inhibitor use appear to increase the risk of syncope, but have no statistically significant effect on falls, fracture, and accidental injury. We found that memantine use had no statistically significant effect on falls, syncope, and accidental injury, but appeared to reduce the risk of fracture. Subgroup analyses did not suggest that the risk of falls and fall-related adverse events differed by type and severity of cognitive impairment, residential status, or length of follow-up.
Our finding that cholinesterase inhibitors increase the risk of syncope can be explained by the known effect of these drugs on cardioinhibition and bradyarrhythmia through augmentation of parasympathetic activity.89 Neurocardiovascular instability, including bradyarrhythmia and hypotension, is prevalent among older adults with dementia and cognitive impairment and may lead to falls, syncope, and cerebral microvascular pathology.90 These responses can be exacerbated by cholinesterase inhibitors. The pooled estimate from our meta-analysis is consistent with a large population-based Canadian study that found cholinesterase inhibitor use was associated with a 1.76-fold increase in the rate of hospitalization for syncope.25 Although the product label of cholinesterase inhibitors includes a potential risk of bradycardia and syncope, these medications are often continued following hospitalization for syncope or bradycardia. Given that syncope can result in other adverse consequences, such as falls, fracture, accidental injury, and car accidents, clinicians should not overlook a significant increase in the risk of syncope that was suggested in observational studies and confirmed in our meta-analysis.
We did not find a statistically significant effect of cholinesterase inhibitors on falls, fracture, or accidental injury. However, due to small number and possible underreporting of events, we cannot exclude the possibility of small benefits or harms. Previous studies have suggested that cholinesterase inhibitors are associated with improved gait speed and variability in patients with AD.19;20 A double-blind, placebo-controlled, randomized trial (NCT00934531) is underway to evaluate the effect of donepezil on gait velocity, variability, and balance in older adults with MCI. Similarly, we did not find any statistically significant increase in fractures associated with cholinesterase inhibitor use. However, this is consistent with 1.18-fold increase in the rate of hip fracture from a previously reported population-based cohort study.25 Although the direct effects of cholinesterase inhibitors on bone mineral density or falls are not known, it is possible that the risk of fractures may increase as a consequence of syncopal events. In addition, there was moderate between-study heterogeneity on accidental injury for cholinesterase inhibitors, indicating that more variations exist in the study results than would be expected by chance alone. The heterogeneity was not explained by the difference in study population and design characteristics that were considered.
Our finding that memantine is associated with a large reduction in fractures is intriguing. Glutamate is a major excitatory neurotransmitter found in the central nervous system as well as in non-neural peripheral tissues, including bone. The role of glutamate in bone formation and remodeling is not fully understood, but animal and experimental studies have demonstrated that NMDA-receptor agonists promote osteoblast differentiation and osteoclastogenesis, and that NMDA-receptor antagonists inhibit osteoclast formation.91;92 To date, we are not aware of any studies that have examined the effect of memantine on markers of bone turnover, bone mineral density, or fracture. Given that the data were extracted from three small unpublished studies, our finding should be viewed as hypothesis-generating, rather than confirmatory. Future studies should be conducted to replicate our findings. While we did not find any harmful effects of memantine on falls or fall-related adverse events, we cannot eliminate the possibility of any small benefits or harms. Memantine can cause dizziness and elevated blood pressure due to its dopaminergic and antimuscarinic properties. Other cardiovascular effects of memantine have not been studied in humans. Studies in rats have shown that NMDA receptors are present in cardiovascular tissues and produce positive inotropic and negative chronotropic effects.93;94 Adverse cardiac events, including bradycardia, orthostatic hypotension with fall, fainting, heart failure, and sudden death have been reported in a pharmacovigilance study, but a causal relationship could not be established due to its descriptive design, underreporting, and concurrent use of other agents that are well-known to induce bradycardia.23
Strengths and Limitations
Our meta-analysis has several strengths. First, we manually searched various sources to identify unpublished sources and complement published studies. There was no evidence of publication bias. Therefore, our work contributes to the literature by providing a more comprehensive, unbiased view on the safety of cholinesterase inhibitors and memantine in relation to falls and related adverse events that are associated with adverse health outcomes and substantial health care expenditure in older adults. Second, we conducted sensitivity analyses to test the robustness of our findings by repeating our analysis including studies with complete follow-up and studies with larger sample size. We also examined the influence of individual studies on the pooled estimates. Our results remained consistent under different assumptions. Third, since our findings came from randomized controlled trials, residual confounding is unlikely.
Conducting a systematic review that evaluates harms of an intervention is often challenged with difficulty in finding such data.26 First, underreporting of falls, syncope, and related events was common. Our systematic search identified 156 potentially eligible randomized controlled trials, but only 54 reported data on at least one type of fall-related adverse event and 16 reported data on three or more types of adverse events. Despite our best efforts to identify both published and unpublished safety data from various sources, the number of fall-related events reported was much lower than the rates estimated from previous observational studies, even after considering the difference in follow-up duration. This suggests that falls, syncope, and related events may have been systematically underreported or that participants in randomized controlled trials are generally healthier than cognitively impaired adults who did not participate. In addition, if those assigned to cholinesterase inhibitors or memantine were more likely to drop out than those assigned to placebo during the study, the reported number of events could be smaller in the active treatment arm due to shorter follow-up time. Both scenarios would lead to underestimation of the potentially important risks of fall-related adverse events associated with these medications. Nonetheless, this limitation does not reduce the importance of our review. Our findings were consistent with those from observational studies. Furthermore, the absence of publication bias further supports the validity of our findings.
Second, trials did not provide detailed information on how falls, syncope, and related events were defined, monitored, and reported. Although falls can be recorded more accurately by using fall calendars, it is often not practical in the setting of clinical trials in which falls are not the main outcome of interest. As a result, a non-differential misclassification of the events might occur and bias the results toward the null. In addition, we recognize that we evaluated the quality of overall adverse events monitoring and reporting, rather than focusing on specific fall-related adverse events, which might have caused misclassification in assessing study quality. However, in our sensitivity analysis, the results were similar between trials that indicated the use of standardized definitions of adverse events and trials that did not.
Third, there are few quality assessment tools for reporting harms.27 We assessed the rigorousness of monitoring and the quality of reporting by adopting previously suggested criteria,60 in addition to commonly used quality criteria. We were not able to find a set of quality criteria that were uniformly associated with either exaggerated or attenuated effect. Further efforts are needed to develop appropriate tools for reporting harm.
Furthermore, the number of included trials for a certain outcome of interest, in particular, fracture, was small. As a result, we were not able to detect any meaningful variations in the effects of treatment by subgroups and quality criteria. Although we found significant variations by type of cognitive impairment in the effect of cholinesterase inhibitors on syncope, the variation was mostly driven by imprecise estimates from studies of PDD or DLB and MCI. Finally, the majority of participants in randomized controlled trials had mild or mild-to-moderate degree of cognitive impairment and were living in the community, thereby limiting generalizability of our findings to a broader population of severely cognitively impaired, institutionalized older adults. If the true effect of cholinesterase inhibitors on syncope were larger in those with severe cognitive impairment and other multiple comorbidities, our results would underestimate the causal effect at a population level.
Conclusions and Implications
Our meta-analysis of randomized controlled trials provides further support and complements existing evidence from observational studies, by showing that cholinesterase inhibitors may increase the risk of syncope. We observed that cholinesterase inhibitors have little effect on falls, fracture, or accidental injury, and memantine appears to have little effect on fall, syncope, or accidental injury. Nonetheless, our review does not exclude a potentially significant risk due to underreporting and small number of outcome events. In addition, whether memantine reduces the risk of fracture needs to be confirmed in future prospective studies.
Considering the public health impact of fall-related adverse events and the potential causal role of medications in such events among older adults with cognitive impairment, these events should be routinely included in trial reports. Furthermore, more high-quality observational research is warranted to evaluate the impact of these agents on fall-related adverse events in a more representative population.
ACKNOWLEDGMENTS
The authors thank Yuxia Jia, M.D., Ph.D. (Division of Gerontology, Beth Israel Deaconess Medical Center, Boston, MA) for her assistance in interpreting the Chinese literature. DHK has support from the John A. Hartford Center of Excellence Research Fellowship Award at Harvard Medical School.
Funding Sources: This research was supported by grant P01AG004390 from the National Institute on Aging Research Nursing Home Program Project.
SPONSOR'S ROLE
The funder did not have any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Presentaion: The abstract was presented at the Presidential Poster Session of the 2010 American Geriatrics Society Annual Scientific Meeting, Orlando, FL, on May 13, 2010.
AUTHOR CONTRIBUTIONS
DHK contributed to the study conception and design, data collection, statistical analysis and interpretation of the data, drafting of the manuscript and revising it for important intellectual content. RTB contributed to data collection, interpretation of the data, and revising the manuscript for important intellectual content. ELD contributed to statistical analysis and interpretation of the data and revising the manuscript for important intellectual content. DPK contributed to the study design, interpretation of the data, and revising the manuscript for important intellectual content. SDB contributed to the study conception and design, data collection, interpretation of the data, and revising the manuscript for important intellectual content. All authors have reviewed and approved the final version of the manuscript.
CONFLICT OF INTEREST
DPK has grant support from Merck, Wyeth, Novartis, Pfizer, and Amgen, and has served as a consultant to Merck, Wyeth, Lilly, Novartis, GSK, Amgen, and Procter & Gamble. SDB has grant support from Merck, Pfizer, Amgen, and GSK. The other authors declare no interests relevant to the submitted work.
REFERENCES
- 1.van Dijk PT, Meulenberg OG, van de Sande HJ, et al. Falls in dementia patients. Gerontologist. 1993;33:200–204. doi: 10.1093/geront/33.2.200. [DOI] [PubMed] [Google Scholar]
- 2.Morris JC, Rubin EH, Morris EJ, et al. Senile dementia of the Alzheimer's type: An important risk factor for serious falls. J Gerontol. 1987;42:412–417. doi: 10.1093/geronj/42.4.412. [DOI] [PubMed] [Google Scholar]
- 3.Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319:1701–1707. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- 4.Stevens JA, Corso PS, Finkelstein EA, et al. The costs of fatal and non-fatal falls among older adults. Inj Prev. 2006;12:290–295. doi: 10.1136/ip.2005.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med. 1997;337:1279–1284. doi: 10.1056/NEJM199710303371806. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka A, Okuzumi H, Kobayashi I, et al. Gait disturbance of patients with vascular and Alzheimer-type dementias. Percept Mot Skills. 1995;80:735–738. doi: 10.2466/pms.1995.80.3.735. [DOI] [PubMed] [Google Scholar]
- 7.Sheridan PL, Hausdorff JM. The role of higher-level cognitive function in gait: Executive dysfunction contributes to fall risk in Alzheimer's disease. Dement Geriatr Cogn Disord. 2007;24:125–137. doi: 10.1159/000105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sterke CS, Verhagen AP, van Beeck EF, et al. The influence of drug use on fall incidents among nursing home residents: A systematic review. Int Psychogeriatr. 2008;20:890–910. doi: 10.1017/S104161020800714X. [DOI] [PubMed] [Google Scholar]
- 9.van Doorn C, Gruber-Baldini AL, Zimmerman S, et al. Dementia as a risk factor for falls and fall injuries among nursing home residents. J Am Geriatr Soc. 2003;51:1213–1218. doi: 10.1046/j.1532-5415.2003.51404.x. [DOI] [PubMed] [Google Scholar]
- 10.Passant U, Warkentin S, Gustafson L. Orthostatic hypotension and low blood pressure in organic dementia: A study of prevalence and related clinical characteristics. Int J Geriatr Psychiatry. 1997;12:395–403. [PubMed] [Google Scholar]
- 11.Gillespie LD, Gillespie WJ, Robertson MC, et al. Interventions for preventing falls in elderly people. Cochrane Database Syst Rev. 2003:CD000340. doi: 10.1002/14651858.CD000340. [DOI] [PubMed] [Google Scholar]
- 12.Shaw FE, Bond J, Richardson DA, et al. Multifactorial intervention after a fall in older people with cognitive impairment and dementia presenting to the accident and emergency department: Randomised controlled trial. BMJ. 2003;326:73. doi: 10.1136/bmj.326.7380.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coussement J, De PL, Schwendimann R, et al. Interventions for preventing falls in acute- and chronic-care hospitals: A systematic review and meta-analysis. J Am Geriatr Soc. 2008;56:29–36. doi: 10.1111/j.1532-5415.2007.01508.x. [DOI] [PubMed] [Google Scholar]
- 14.Morden NE, Zerzan JT, Larson EB. Alzheimer's disease medication: Use and cost projections for Medicare Part D. J Am Geriatr Soc. 2007;55:622–624. doi: 10.1111/j.1532-5415.2007.01106.x. [DOI] [PubMed] [Google Scholar]
- 15.Birks J, Harvey RJ. Donepezil for dementia due to Alzheimer's disease. Cochrane Database Syst Rev. 2006;(1):CD001190. doi: 10.1002/14651858.CD001190.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Birks J, Grimley EJ, Iakovidou V, et al. Rivastigmine for Alzheimer's disease. Cochrane Database Syst Rev. 2009;(2):CD001191. doi: 10.1002/14651858.CD001191. [DOI] [PubMed] [Google Scholar]
- 17.Craig D, Birks J. Galantamine for vascular cognitive impairment. Cochrane Database Syst Rev. 2006;(1):CD004746. doi: 10.1002/14651858.CD004746.pub2. [DOI] [PubMed] [Google Scholar]
- 18.Farlow MR, Graham SM, Alva G. Memantine for the treatment of Alzheimer's disease: Tolerability and safety data from clinical trials. Drug Saf. 2008;31:577–585. doi: 10.2165/00002018-200831070-00003. [DOI] [PubMed] [Google Scholar]
- 19.Montero-Odasso M, Wells J, Borrie M. Can cognitive enhancers reduce the risk of falls in people with dementia? An open-label study with controls. J Am Geriatr Soc. 2009;57:359–360. doi: 10.1111/j.1532-5415.2009.02085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Assal F, Allali G, Kressig RW, et al. Galantamine improves gait performance in patients with Alzheimer's disease. J Am Geriatr Soc. 2008;56:946–947. doi: 10.1111/j.1532-5415.2008.01657.x. [DOI] [PubMed] [Google Scholar]
- 21.Bordier P, Lanusse S, Garrigue S, et al. Causes of syncope in patients with Alzheimer's disease treated with donepezil. Drugs Aging. 2005;22:687–694. doi: 10.2165/00002512-200522080-00005. [DOI] [PubMed] [Google Scholar]
- 22.Fisher AA, Davis MW. Prolonged QT interval, syncope, and delirium with galantamine. Ann Pharmacother. 2008;42:278–283. doi: 10.1345/aph.1K514. [DOI] [PubMed] [Google Scholar]
- 23.Gallini A, Sommet A, Montastruc JL. Does memantine induce bradycardia? A study in the French PharmacoVigilance Database. Pharmacoepidemiol Drug Saf. 2008;17:877–881. doi: 10.1002/pds.1620. [DOI] [PubMed] [Google Scholar]
- 24.Kayrak M, Yazici M, Ayhan SS, et al. Complete atrioventricular block associated with rivastigmine therapy. Am J Health Syst Pharm. 2008;65:1051–1053. doi: 10.2146/ajhp070230. [DOI] [PubMed] [Google Scholar]
- 25.Gill SS, Anderson GM, Fischer HD, et al. Syncope and its consequences in patients with dementia receiving cholinesterase inhibitors: A population-based cohort study. Arch Intern Med. 2009;169:867–873. doi: 10.1001/archinternmed.2009.43. [DOI] [PubMed] [Google Scholar]
- 26.Derry S, Kong LY, Aronson JK. Incomplete evidence: The inadequacy of databases in tracing published adverse drug reactions in clinical trials. BMC Med Res Methodol. 2001;1:7. doi: 10.1186/1471-2288-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ioannidis JP, Evans SJ, Gotzsche PC, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004;141:781–788. doi: 10.7326/0003-4819-141-10-200411160-00009. [DOI] [PubMed] [Google Scholar]
- 28.Howard RJ, Juszczak E, Ballard CG, et al. Donepezil for the treatment of agitation in Alzheimer's disease. N Engl J Med. 2007;357:1382–1392. doi: 10.1056/NEJMoa066583. [DOI] [PubMed] [Google Scholar]
- 29.van Dyck CH, Tariot PN, Meyers B, et al. A 24-week randomized, controlled trial of memantine in patients with moderate-to-severe Alzheimer disease. Alzheimer Dis Assoc Disord. 2007;21:136–143. doi: 10.1097/WAD.0b013e318065c495. [DOI] [PubMed] [Google Scholar]
- 30.Winblad B, Gauthier S, Scinto L, et al. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology. 2008;70:2024–2035. doi: 10.1212/01.wnl.0000303815.69777.26. [DOI] [PubMed] [Google Scholar]
- 31. [June 11, 2009];A randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of memantine in patients with mild to moderate dementia of the Alzheimer's type. Available at: http://www.forestclinicaltrials.com/CTR/CTRController/CTRCompletedListStudies.
- 32. [July 21, 2009];A long-term extension study evaluating the safety and tolerability of BID and QD administration of memantine in patients with mild to moderate dementia of the Alzheimer's type. Available at: http://www.forestclinicaltrials.com/CTR/CTRController/CTRCompletedListStudies.
- 33. [July 11, 2009];A 52-week, open-label treatment extension to a 24-week prospective, randomized, multicenter, double-blind, placebo-controlled, parallel-group comparison of the efficacy, tolerability, and safety of rivastigmine capsules 3-12 mg/day in patients with probable vascular dementia. http://www.novartisclinicaltrials.com/webapp/clinicaltrialrepository/public/productjsp?productID=90&diseaseAreaID=3&divisionId=2.
- 34.Auchus AP, Brashear HR, Salloway S, et al. Galantamine treatment of vascular dementia: A randomized trial. Neurology. 2007;69:448–458. doi: 10.1212/01.wnl.0000266625.31615.f6. [DOI] [PubMed] [Google Scholar]
- 35.Ballard C, Sauter M, Scheltens P, et al. Efficacy, safety and tolerability of rivastigmine capsules in patients with probable vascular dementia: The VantagE study. Curr Med Res Opin. 2008;24:2561–2574. doi: 10.1185/03007990802328142. [DOI] [PubMed] [Google Scholar]
- 36.Barone P, Burn DJ, van LT, et al. Rivastigmine versus placebo in hyperhomocysteinemic Parkinson's disease dementia patients. Mov Disord. 2008;23:1532–1540. doi: 10.1002/mds.21997. [DOI] [PubMed] [Google Scholar]
- 37.Black S, Roman GC, Geldmacher DS, et al. Efficacy and tolerability of donepezil in vascular dementia: Positive results of a 24-week, multicenter, international, randomized, placebo-controlled clinical trial. Stroke. 2003;34:2323–2330. doi: 10.1161/01.STR.0000091396.95360.E1. [DOI] [PubMed] [Google Scholar]
- 38.Brodaty H, Corey-Bloom J, Potocnik FC, et al. Galantamine prolonged-release formulation in the treatment of mild to moderate Alzheimer's disease. Dement Geriatr Cogn Disord. 2005;20:120–132. doi: 10.1159/000086613. [DOI] [PubMed] [Google Scholar]
- 39.Bullock R, Erkinjuntti T, Lilienfeld S. Management of patients with Alzheimer's disease plus cerebrovascular disease: 12-month treatment with galantamine. Dement Geriatr Cogn Disord. 2004;17:29–34. doi: 10.1159/000074140. [DOI] [PubMed] [Google Scholar]
- 40.Darreh ST, Kadir A, Almkvist O, et al. Inhibition of acetylcholinesterase in CSF versus brain assessed by 11C-PMP PET in AD patients treated with galantamine. Neurobiol Aging. 2008;29:168–184. doi: 10.1016/j.neurobiolaging.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 41.Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson's disease. N Engl J Med. 2004;351:2509–2518. doi: 10.1056/NEJMoa041470. [DOI] [PubMed] [Google Scholar]
- 42.Erkinjuntti T, Kurz A, Gauthier S, et al. Efficacy of galantamine in probable vascular dementia and Alzheimer's disease combined with cerebrovascular disease: A randomised trial. Lancet. 2002;359:1283–1290. doi: 10.1016/S0140-6736(02)08267-3. [DOI] [PubMed] [Google Scholar]
- 43.Lyketsos CG, Reichman WE, Kershaw P, et al. Long-term outcomes of galantamine treatment in patients with Alzheimer disease. Am J Geriatr Psychiatry. 2004;12:473–482. doi: 10.1176/appi.ajgp.12.5.473. [DOI] [PubMed] [Google Scholar]
- 44.Mohs RC, Doody RS, Morris JC, et al. A 1-year, placebo-controlled preservation of function survival study of donepezil in AD patients. Neurology. 2001;57:481–488. doi: 10.1212/wnl.57.3.481. [DOI] [PubMed] [Google Scholar]
- 45.Ott BR, Blake LM, Kagan E, et al. Open label, multicenter, 28-week extension study of the safety and tolerability of memantine in patients with mild to moderate Alzheimer's disease. J Neurol. 2007;254:351–358. doi: 10.1007/s00415-006-0374-x. [DOI] [PubMed] [Google Scholar]
- 46.Peskind ER, Potkin SG, Pomara N, et al. Memantine treatment in mild to moderate Alzheimer disease: A 24-week randomized, controlled trial. Am J Geriatr Psychiatry. 2006;14:704–715. doi: 10.1097/01.JGP.0000224350.82719.83. [DOI] [PubMed] [Google Scholar]
- 47.Poewe W, Wolters E, Emre M, et al. Long-term benefits of rivastigmine in dementia associated with Parkinson's disease: An active treatment extension study. Mov Disord. 2006;21:456–461. doi: 10.1002/mds.20700. [DOI] [PubMed] [Google Scholar]
- 48.Raskind MA, Peskind ER, Wessel T, et al. Galantamine in AD: A 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology. 2000;54:2261–2268. doi: 10.1212/wnl.54.12.2261. [DOI] [PubMed] [Google Scholar]
- 49.Rockwood K, Dai D, Mitnitski A. Patterns of decline and evidence of subgroups in patients with Alzheimer's disease taking galantamine for up to 48 months. Int J Geriatr Psychiatry. 2008;23:207–214. doi: 10.1002/gps.1864. [DOI] [PubMed] [Google Scholar]
- 50.Rogers SL, Farlow MR, Doody RS, et al. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease. Donepezil Study Group. Neurology. 1998;50:136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- 51.Rogers SL, Doody RS, Mohs RC, et al. Donepezil improves cognition and global function in Alzheimer disease: A 15-week, double-blind, placebo-controlled study. Donepezil Study Group. Arch Intern Med. 1998;158:1021–1031. doi: 10.1001/archinte.158.9.1021. [DOI] [PubMed] [Google Scholar]
- 52.Small G, Erkinjuntti T, Kurz A, et al. Galantamine in the treatment of cognitive decline in patients with vascular dementia or Alzheimer's disease with cerebrovascular disease. CNS Drugs. 2003;17:905–914. doi: 10.2165/00023210-200317120-00004. [DOI] [PubMed] [Google Scholar]
- 53.Tariot PN, Solomon PR, Morris JC, et al. A 5-month, randomized, placebo-controlled trial of galantamine in AD. The Galantamine USA-10 Study Group. Neurology. 2000;54:2269–2276. doi: 10.1212/wnl.54.12.2269. [DOI] [PubMed] [Google Scholar]
- 54.U.S.Food and Drug Administration [2009 July 21];NDA 020070. Available at: http://www.accessdata.fda.gov/Scripts/cder/DrugsatFDA/indexcfm?fuseaction=SearchLabel_ApprovalHistory#apphist.
- 55.U.S.Food and Drug Administration [July 21, 2009];NDA 21-169. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2001/21-169_Reminylcfm.
- 56.U.S.Food and Drug Administration [July 21, 2009];NDA 021615. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/021615s000_RazadyneTOCcfm.
- 57.Wilkinson D, Murray J. Galantamine: A randomized, double-blind, dose comparison in patients with Alzheimer's disease. Int J Geriatr Psychiatry. 2001;16:852–857. doi: 10.1002/gps.409. [DOI] [PubMed] [Google Scholar]
- 58.Wilkinson D, Doody R, Helme R, et al. Donepezil in vascular dementia: A randomized, placebo-controlled study. Neurology. 2003;61(4):479–486. doi: 10.1212/01.wnl.0000078943.50032.fc. [DOI] [PubMed] [Google Scholar]
- 59.Winblad B, Engedal K, Soininen H, et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001;57:489–495. doi: 10.1212/wnl.57.3.489. [DOI] [PubMed] [Google Scholar]
- 60.Loke Y, Price D, Herxheimer A. Including adverse effects in your review. [August 4, 2009];Cochrane Adverse Effects Subgroup. Available at: www.dsru.org/wwwboard/latestdraft.pdf.
- 61.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 62.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 63.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 64.Egger M, Davey SG, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. [July 21, 2009];A 24-week, multi-center, randomized, double-blind, placebo-controlled evaluation of the efficacy, safety, and tolerability of donepezil hydrochloride (E2020) in patients with dementia associated with cerebrovascular disease. Available at: http://www.clinicalstudyresults.org/documents/company-study_1638_0.pdf.
- 66.Doody RS, Ferris SH, Salloway S, et al. Donepezil treatment of patients with MCI. A 48-week randomized, placebo-controlled trial. Neurology. 2009;72:1555–1561. doi: 10.1212/01.wnl.0000344650.95823.03. [DOI] [PubMed] [Google Scholar]
- 67.Feldman H, Gauthier S, Hecker J, et al. A 24-week, randomized, double-blind study of donepezil in moderate to severe Alzheimer's disease. Neurology. 2001;57:613–620. doi: 10.1212/wnl.57.4.613. [DOI] [PubMed] [Google Scholar]
- 68.Homma A, Takeda M, Imai Y, et al. Clinical efficacy and safety of donepezil on cognitive and global function in patients with Alzheimer's disease. A 24-week, multicenter, double-blind, placebo-controlled study in Japan. E2020 Study Group. Dement Geriatr Cogn Disord. 2000;11:299–313. doi: 10.1159/000017259. [DOI] [PubMed] [Google Scholar]
- 69.Homma A, Imai Y, Tago H, et al. Donepezil treatment of patients with severe Alzheimer's disease in a Japanese population: Results from a 24-week, double-blind, placebo-controlled, randomized trial. Dement Geriatr Cogn Disord. 2008;25:399–407. doi: 10.1159/000122961. [DOI] [PubMed] [Google Scholar]
- 70.Rogers SL, Friedhoff LT. The efficacy and safety of donepezil in patients with Alzheimer's disease: Results of a US Multicentre, Randomized, Double-Blind, Placebo-Controlled Trial. The Donepezil Study Group. Dementia. 1996;7:293–303. doi: 10.1159/000106895. [DOI] [PubMed] [Google Scholar]
- 71.Seltzer B, Zolnouni P, Nunez M, et al. Efficacy of donepezil in early-stage Alzheimer disease: A randomized placebo-controlled trial. Arch Neurol. 2004;61:1852–1856. doi: 10.1001/archneur.61.12.1852. [DOI] [PubMed] [Google Scholar]
- 72.Tariot PN, Cummings JL, Katz IR, et al. A randomized, double-blind, placebo-controlled study of the efficacy and safety of donepezil in patients with Alzheimer's disease in the nursing home setting. J Am Geriatr Soc. 2001;49:1590–1599. [PubMed] [Google Scholar]
- 73.Winblad B, Kilander L, Eriksson S, et al. Donepezil in patients with severe Alzheimer's disease: Double-blind, parallel-group, placebo-controlled study. Lancet. 2006;367:1057–1065. doi: 10.1016/S0140-6736(06)68350-5. [DOI] [PubMed] [Google Scholar]
- 74.Burns A, Bernabei R, Bullock R, et al. Safety and efficacy of galantamine (Reminyl) in severe Alzheimer's disease (the SERAD study): A randomised, placebo-controlled, double-blind trial. Lancet Neurol. 2009;8:39–47. doi: 10.1016/S1474-4422(08)70261-8. [DOI] [PubMed] [Google Scholar]
- 75.Wilcock GK, Lilienfeld S, Gaens E. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer's disease: Multicentre randomised controlled trial. Galantamine International-1 Study Group. BMJ. 2000;321:1445–1449. doi: 10.1136/bmj.321.7274.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Winblad B, Cummings J, Andreasen N, et al. A six-month double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer's disease--rivastigmine patch versus capsule. Int J Geriatr Psychiatry. 2007;22:456–467. doi: 10.1002/gps.1788. [DOI] [PubMed] [Google Scholar]
- 77.Wilcock G, Mobius HJ, Stoffler A. A double-blind, placebo-controlled multicentre study of memantine in mild to moderate vascular dementia (MMM500). Int Clin Psychopharmacol. 2002;17:297–305. doi: 10.1097/00004850-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 78.Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: A randomized controlled trial. JAMA. 2004;291:317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 79.Reisberg B, Doody R, Stoffler A, et al. A 24-week open-label extension study of memantine in moderate to severe Alzheimer disease. Arch Neurol. 2006;63:49–54. doi: 10.1001/archneur.63.1.49. [DOI] [PubMed] [Google Scholar]
- 80.Porsteinsson AP, Grossberg GT, Mintzer J, et al. Memantine treatment in patients with mild to moderate Alzheimer's disease already receiving a cholinesterase inhibitor: A randomized, double-blind, placebo-controlled trial. Curr Alzheimer Res. 2008;5:83–89. doi: 10.2174/156720508783884576. [DOI] [PubMed] [Google Scholar]
- 81.Bakchine S, Loft H. Memantine treatment in patients with mild to moderate Alzheimer's disease: Results of a randomised, double-blind, placebo-controlled 6-month study. J Alzheimers Dis. 2008;13:97–107. doi: 10.3233/jad-2008-13110. [DOI] [PubMed] [Google Scholar]
- 82. [June 6, 2009];A randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of memantine in patients with mild to moderate dementia of the Alzheimer's type, with a long-term, open-label extension study. Available at: http://www.forestclinicaltrials.com/CTR/CTRController/CTRCompletedListStudies.
- 83. [June 6, 2009];A randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of memantine in patients with moderate-to-severe dementia of the Alzheimer's type. Available at: http://www.forestclinicaltrials.com/CTR/CTRController/CTRCompletedListStudies.
- 84. [June 6, 2009];A long-term extension study evaluating the safety and tolerability of four memantine dosing regimens in patients with moderate to severe dementia of the Alzheimer's type. Available at: http://www.forestclinicaltrials.com/CTR/CTRController/CTRCompletedListStudies.
- 85. [June 6, 2009];A randomized, double-blind, placebo-controlled evaluation of the effectiveness and safety of memantine in nursing home residents with moderate to severe Alzheimer's disease. Available at: http://www.forestclinicaltrials.com/CTR/CTRController/CTRCompletedListStudies.
- 86. [June 6, 2009];A randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of memantine in noninstitutionalized agitated patients with moderate to severe Alzheimer's disease. Available at: http://www.forestclinicaltrials.com/CTR/CTRController/CTRCompletedListStudies.
- 87. [June 6, 2009];A randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of memantine in patients with moderate-to-severe dementia of the Alzheimer's type. Available at: http://www.forestclinicaltrials.com/CTR/CTRController/CTRCompletedListStudies.
- 88. [July 21, 2009];An open-label extension study evaluating the safety and tolerability of memantine in patients with moderate to severe dementia of the Alzheimer's type. Available at: http://www.forestclinicaltrials.com/CTR/CTRController/CTRCompletedListStudies.
- 89.Masuda Y. Cardiac effect of cholinesterase inhibitors used in Alzheimer's disease--from basic research to bedside. Curr Alzheimer Res. 2004;1:315–321. doi: 10.2174/1567205043332009. [DOI] [PubMed] [Google Scholar]
- 90.Kenny RA, Kalaria R, Ballard C. Neurocardiovascular instability in cognitive impairment and dementia. Ann N Y Acad Sci. 2002;977:183–195. doi: 10.1111/j.1749-6632.2002.tb04816.x. [DOI] [PubMed] [Google Scholar]
- 91.Hinoi E, Fujimori S, Yoneda Y. Modulation of cellular differentiation by N-methyl-D-aspartate receptors in osteoblasts. FASEB J. 2003;17:1532–1534. doi: 10.1096/fj.02-0820fje. [DOI] [PubMed] [Google Scholar]
- 92.Merle B, Itzstein C, Delmas PD, et al. NMDA glutamate receptors are expressed by osteoclast precursors and involved in the regulation of osteoclastogenesis. J Cell Biochem. 2003;90:424–436. doi: 10.1002/jcb.10625. [DOI] [PubMed] [Google Scholar]
- 93.Huang CF, Su MJ. Positive inotropic action of NMDA receptor antagonist (+)-MK801 in rat heart. J Biomed Sci. 1999;6:387–398. doi: 10.1007/BF02253670. [DOI] [PubMed] [Google Scholar]
- 94.Leung JC, Travis BR, Verlander JW, et al. Expression and developmental regulation of the NMDA receptor subunits in the kidney and cardiovascular system. Am J Physiol Regul Integr Comp Physiol. 2002;283:R964–R971. doi: 10.1152/ajpregu.00629.2001. [DOI] [PubMed] [Google Scholar]
- 95.U.S.Food and Drug Administration [July 21, 2009];NDA 022083. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022083_exelon_toccfm.
- 96. [June 6, 2009];A randomized, double-blind, placebo-controlled evaluation of the effectiveness and safety of memantine in nursing home residents with moderate to severe Alzheimer's disease. Available at: http://www.forestclinicaltrials.com/CTR/CTRController/CTRCompletedListStudies.
- 97. [June 6, 2009];A randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of memantine in noninstitutionalized agitated patients with moderate to severe Alzheimer's disease. Available at: http://www.forestclinicaltrials.com/CTR/CTRController/CTRCompletedListStudies.
- 98. [June 6, 2009];A randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of memantine in patients with moderate-to-severe dementia of the Alzheimer's type. Available at: http://www.forestclinicaltrials.com/CTR/CTRController/CTRCompletedListStudies.