Abstract
Objective
We aimed to investigate the efficacy of 20 Hz repetitive transcranial magnetic stimulation (rTMS) of either right or left dorsolateral prefrontal cortex (DLPFC) as compared to sham rTMS for the relief of posttraumatic stress disorder (PTSD)–associated symptoms.
Method
In this double-blind, placebo-controlled phase II trial conducted between October 2005 and July 2008, 30 patients with DSM-IV–diagnosed PTSD were randomly assigned to receive 1 of the following treatments: active 20 Hz rTMS of the right DLPFC, active 20 Hz rTMS of the left DLPFC, or sham rTMS. Treatments were administered in 10 daily sessions over 2 weeks. A blinded rater assessed severity of core PTSD symptoms, depression, and anxiety before, during, and after completion of the treatment protocol. In addition, a battery of neuropsychological tests was measured before and after treatment.
Results
Results show that both active conditions—20 Hz rTMS of left and right DLPFC—induced a significant decrease in PTSD symptoms as indexed by the PTSD Checklist and Treatment Outcome PTSD Scale; however, right rTMS induced a larger effect as compared to left rTMS. In addition, there was a significant improvement of mood after left rTMS and a significant reduction of anxiety following right rTMS. Improvements in PTSD symptoms were long lasting; effects were still significant at the 3-month follow-up. Finally, neuropsychological evaluation showed that active 20 Hz rTMS is not associated with cognitive worsening and is safe for use in patients with PTSD.
Conclusions
These results support the notion that modulation of prefrontal cortex can alleviate the core symptoms of PTSD and suggest that high-frequency rTMS of right DLPFC might be the optimal treatment strategy.
Posttraumatic stress disorder (PTSD) is an incapacitating anxiety disorder characterized by intrusive thoughts, hyperarousal, flashbacks, nightmares, sleep disturbances, emotional numbing, and withdrawal, among other clinical symptoms (as classified by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [DSM-IV]).1 Posttraumatic stress disorder has a lifetime prevalence of about 6.8% and may develop in susceptible individuals after exposure to a terrifying ordeal that involved physical harm or the threat of physical harm.2 This severe anxiety disorder affects about 7.7 million people each year and remains challenging to treat, with persistent symptoms leading to considerable social, occupational, and interpersonal dysfunction.3
Although selective serotonin reuptake inhibitors (SSRIs)—among other antidepressants—have resulted in various degrees of improvement in patients with PTSD, there is no definitive pharmacotherapy available to date for the treatment of this debilitating disorder. A review of 37 clinical trials of pharmacotherapies4 found inadequate evidence to determine the value of antidepressants, benzodiazepines, anticonvulsants, α-blockers, and second-generation antipsychotics for the treatment of PTSD. Even so, according to an American Psychiatric Association guideline,5 SSRIs remain the first line of treatment for PTSD. In addition, due to the complex nature of this disorder, individuals with PTSD also seem to benefit from 10 to 12 sessions of cognitive-behavioral therapy, prolonged-exposure therapy, or cognitive-processing therapy. Nevertheless, many individuals respond inadequately to currently available therapies, and research for more effective treatment paradigms is ongoing.
Most recently, repetitive transcranial magnetic stimulation (rTMS)—a method of noninvasive neuromodulation—has been emerging as a potentially effective technique in the treatment of PTSD. Indeed, rTMS has already been shown to be highly effective in the treatment of medically refractory depression6 and is now a clinically available form of treatment in certain settings. Similarly, there is evidence that rTMS can also be effective for the treatment of PTSD. In a prior open-label study,7 a single session of low-frequency (0.3 Hz) transcranial magnetic stimulation (TMS) applied to the left and right motor cortex was found to be transiently effective in lowering the core PTSD symptom of avoidance as well as somatization and symptoms of anxiety and depression. Stimulation with 10 Hz rTMS to the right DLPFC was then shown to generate even greater effects with an especially marked improvement in symptoms of re-experiencing and avoidance; these effects lasted for at least 2 weeks after the end of stimulation.8 Furthermore, 2 case studies suggest that stimulation of the right DLPFC with 1 Hz rTMS can, in fact, normalize the right frontal and paralimbic metabolic hyperactivity that is associated with PTSD as measured with positron emission tomography studies.9 In sum, these previous studies suggest that modulation of prefrontal activity, perhaps particularly on the right frontal cortex, with rTMS holds promise as a form of therapy in the treatment of PTSD.
Therefore, in this study, we aimed to investigate the clinical efficacy of high-frequency rTMS in the relief of core PTSD symptoms (such as hyperarousal, flashbacks, vigilance, intrusive thoughts, emotional numbness, and withdrawal) as well as PTSD-associated symptoms of anxiety and depression. In contrast to previous studies, here we investigate treatment with 20 Hz rTMS (higher frequency than previous studies), as there is evidence that higher-frequency stimulation may result in more substantial effects. In addition, we compare the effects of treatment of either right or left DLPFC (“left rTMS” and “right rTMS”), since rTMS is known to have side-specific effects. For example, in patients with major depression, rTMS can induce antidepressant effects either by enhancing left DLPFC excitability via high-frequency stimulation or by decreasing right DLPFC excitability via low-frequency stimulation.10 Finally, our study here also offers a longer follow-up period of 3 months’ duration and an inclusion of an extensive battery of neuropsychological assessments. The main goal of this study was to evaluate the effects of high-frequency rTMS of right and left DLPFC, as compared to sham stimulation, on the clinical symptoms of PTSD. As secondary aims, we explored whether the clinical effects of stimulation were long lasting and associated with any cognitive changes as indexed by a battery of neuropsychological tests.
METHOD
Study Population
This study was carried out from October 2005 to July 2008. Participants were recruited by means of newspaper advertisements as well as referred by psychiatrists working with PTSD. We enrolled participants according to the following criteria: participants fulfilling the DSM-IV diagnostic criteria of PTSD, as assessed by the Structured Clinical Interview for DSM-IV (SCID)11 by trained mental health professionals, and aged between 18 and 64 years old. We excluded individuals with head trauma, substance abuse disorder, or any other chronic medical conditions and contraindications to rTMS, such as pregnancy, use of pacemakers, and epilepsy.12 In addition, patients with severe episodes of depression immediately before the traumatic event were excluded. Patients signed an informed consent form that was approved by a local and national research ethics committee (http://portal.saude.gov.br/sisnep). The study was conducted at Mackenzie University (São Paulo, Brazil).
The traumatic events that were associated with PTSD included 6 assaults, 5 cases of sexual abuse, 15 cases of death or severe disease of a relative, and 4 incidences of psychological distress/perceived physical harm (eg, kidnapping, threat of death). The mean age of the subjects was 44.5 ± 4.4 years (mean ± SD). The mean time since occurrence of trauma was 3.9 ± 4.3 years (mean ± SD).
For participants who were taking medication, we used the same strategy used by Grisaru et al7: drug treatment was neither stopped nor changed in the 3 weeks before the study or during the study. In addition, patients continued to receive the same individual and group supportive psychotherapy as before the intervention. Successful use of this procedure has been reported by Cohen et al.8 However, we adopted a strategy of randomization (stratified randomization) to ensure that the groups were balanced regarding medication use.
Experimental Design
In this double-blind, placebo-controlled phase II trial, 30 patients were randomly assigned (1:1:1) to 1 of the 3 stimulation groups: active high-frequency rTMS of the left DLPFC (left rTMS), active high-frequency rTMS of the right DLPFC (right rTMS), and sham rTMS. We used a stratified randomization strategy with random blocks to ensure that the 3 groups had similar use of medications; therefore, we created 3 different groups of patients according to their medication use: antidepressants and/or benzodiazepines, benzodiazepines only, and neither benzodiazepines nor antidepressants.
Rationale for Site of Stimulation
The choice of prefrontal cortex as the site of stimulation is based on the properties of this area and previous research. The prefrontal cortex is involved in many complex cognitive and behavioral functions that are potentially relevant to PTSD, such as working memory,13,14 supervisory attentional control,15 reasoning and decision making,16,17 temporal organization of behavior,18 and emotional processing.19 In fact, structural and functional neuroimaging studies have demonstrated abnormalities in the prefrontal cortex in PTSD patients. For instance, patients with PTSD show a decreased regional cerebral blood flow in the prefrontal cortex (and an increased regional cerebral blood flow in the amygdala) in response to provocation of symptoms by script-driven imagery.20-23 These findings have been documented in both patients with combat-related PTSD and patients in whom PTSD was related to childhood abuse. Overall, functional neuroimaging studies reveal fairly consistent data indicating a hypoactivation of the prefrontal cortex (as well as a hyperresponsive amygdala) in PTSD patients.24-26 Taken together, these findings suggest that the prefrontal cortex (and the amygdala) is intimately involved with PTSD abnormalities and could potentially be the target of stimulation-based treatment strategies. Therefore, the site of stimulation in this study will focus on the right and left dorsolateral prefrontal cortex. We tested treatment with the left DLPFC in addition to the right DLPFC as in the previous study8 because it is not clear whether there is a lateralization of prefrontal dysfunction in PTSD and also because left DLPFC is the site used for the treatment of major depression—a condition commonly comorbid with PTSD—with rTMS.6 Finally, because the DLPFC is involved with cognitive processes, such as working memory and executive function, we performed a cognitive evaluation in these patients before and after the treatment as to assess the safety of this treatment.
Intervention: Transcranial Magnetic Stimulation
Transcranial magnetic stimulation was performed using a commercially available figure-8 coil (outside diameter of each wing = 7 cm) and a Magstim stimulator (1.5 Tesla version; Magstim Company Ltd, Wales, United Kingdom). The current wave form was biphasic, and the orientation of the stimulation coil was 45° from the midline with the handle pointing backward. Patients were randomly assigned to receive active rTMS (left or right DLPFC) and sham rTMS as detailed below.
Active rTMS treatment
There were 2 active rTMS groups (highfrequency rTMS of the left DLPFC [referred to in the text as “left rTMS”] and high-frequency rTMS of the right DLPFC [referred to in the text as “right rTMS”]). Patients received 10 TMS treatments that were administered 5 days per week (weekdays only) for 2 consecutive weeks. The TMS apparatus was equipped with a figure-8–shaped insulated coil. Repetitive transcranial magnetic stimulation was applied by positioning the stimulation coil over the appropriate position (DLPFC) on the subject’s scalp following the guidelines of Pascual-Leone et al27 for the localization of the DLPFC. Transcranial magnetic stimulation at 20 Hz was applied at 80% of the patient’s motor threshold. Each participant received 1,600 pulses per session (40 trains of 2 seconds with an intertrain interval of 28 seconds) over either the right or left DLPFC. These parameters are within the safe parameters according to the safety guidelines published by Wassermann.12 Two trained technicians administered the treatment for patients. These technicians had no contact with patients and were not involved in this study.
Sham rTMS treatment
The 10 sham rTMS treatments were administered with the same TMS methodology used for active rTMS treatments, except that no actual magnetic stimulation was released from the coil. We used a specially designed sham TMS coil that has the identical appearance and weight as the real coil. In addition, we installed a small electrical stimulator underneath this coil as to mimic the scalp sensation produced by the active rTMS similarly used by Okabe et al.28 Therefore, this device induced a small electric current (that was near the perception threshold and adjusted for each subject) in order to induce scalp sensation. Although we believe that this method improved the blinding in our study (as compared to standard sham TMS), we did not perform assessment of blinding. With respect to lateralization of sham rTMS, 5 subjects received left hemisphere sham stimulation and 5 subjects received right hemisphere sham stimulation. In this way, a total of 15 subjects had the electrode placed on the right side: 5 subjects received sham and 10 received active stimulation. Similarly, a total of 15 subjects had the electrode placed on the left side: 5 received sham and 10 received active stimulation. This methodology helped to ensure that lateralization would not inherently unblind the subjects. Although it is possible that placing the sham electrode on the left side has a different placebo effect than placing the electrode on the right side, the subjects were not aware that we were interested in comparing left and right rTMS, and so this is unlikely to have made a significant difference.
Methods of Measurement
Before the treatment, we collected data on demographic and clinical information, such as marital status, place of birth, education, place of residence, and the type of trauma that led them to seek help. We then assessed PTSD symptoms using a clinician-administered PTSD scale.29 In order to assess changes before, during, and after treatment, a blinded rater measured PTSD symptoms, anxiety, and depression. These measures were taken at 7 time points: before treatment (baseline), at day 5, at day 10, at day 24 (2 weeks after the end of the intervention), at day 38 (4 weeks after treatment), at day 66 (8 weeks after treatment), and at day 94 (12 weeks after treatment). The participants were assisted in answering the questions, if needed. The interviewer ensured that all participants clearly understood the content of each item and the different aspects of the various component questions. The 4 instruments were used as follows:
PTSD Checklist
The PTSD Checklist30 is a 17-item self-report checklist of PTSD symptoms based closely on the DSM-IV criteria. The respondents rated each item from 1 (“not at all”) to 5 (“extremely”) to indicate the degree to which they have been bothered by that particular symptom over the past month. Thus, the total scores range from 17 to 85.
Treatment Outcome PTSD Scale
The Treatment Outcome PTSD Scale31 is a clinician-rated instrument that measures the presence and severity of PTSD. This 8-item instrument measures symptoms that occur frequently within the PTSD population and is sensitive to the 3 major PTSD symptom dimensions: intrusive thoughts, avoidance behavior, and hyperarousal symptoms. Each symptom is rated on a defined scale (0 to 4). Higher scores reflect greater severity on each measure.
Hamilton Anxiety Rating Scale
The Hamilton Anxiety Rating Scale32 is a clinician-rated instrument that measures the presence and severity of anxiety. This instrument covers 14 symptoms. Each symptom is rated on a defined scale (0 to 4). A higher numeric rating reflects greater symptom severity.
Hamilton Depression Rating Scale
The Hamilton Depression Rating Scale33 is a 28-item instrument that measures the presence and severity of depression. Each symptom is rated on a defined scale (0 to 4), whereby a higher numeric rating reflects greater symptom severity.
In addition to these questionnaires and tasks, a battery of neuropsychological tests using alternate forms for repeated measures was performed to assess whether the treatment was associated with a detrimental effect on cognition and, therefore, to gather preliminary data on the safety of this treatment. This battery consists of the following tests: executive function (Wisconsin Card Sorting Test34 [number of categories and perseverative errors], Controlled Oral Word Association Test35 [phonemic category—letters F, A, S], Victoria version of the Stroop Test36 [colored words and interference card]); reasoning (Raven Colored Progressive Matrices37); and working memory (Digit Span Test38—forward and backward).
Statistical Analysis
The sample size calculation was based on the study of Cohen et al.6 These data show that the scores on the PTSD Checklist after 2 weeks of treatment were 43.5 (± 8.3) for the active group and 55 (± 4.9) for the sham group. For our sample size calculation, we assumed a type I error of 5% (α–2-tailed) and a type 2 error of 10% (β). Therefore, for a 90% power, 8 participants per arm were necessary (total of 24 participants). Conservatively, we increased this number to 30 participants (10 per arm) to account for unexpected factors such as a higher placebo response.
The ratings of psychopathology (PTSD Checklist, Treatment Outcome PTSD Scale, Hamilton Anxiety Rating Scale, and Hamilton Depression Rating Scale) were entered into a mixed 2-way repeated measures analysis of variance (ANOVA). The 2 fixed factors were group (active left rTMS, active right rTMS, and sham) and time (baseline, day 5, day 10) with repeated measures on time. The goal of this model was to detect if there was a significant interaction term between group and time. In addition, we included the random factor subject identification to account for within-subject variability. The main outcome in this study was PTSD symptom changes as assessed with the PTSD Checklist. The other instruments were used as secondary outcomes. If appropriate, paired post hoc tests with Bonferroni correction for multiple comparisons were also undertaken. In addition, we performed similar analyses for the cognitive tests.
We then assessed the long-lasting effects of this treatment, building a model in which we included time of treatment—including all the time points: baseline, day 5, day 10, day 24, day 38, day 66, and day 94—as the main variable and performed this model for the 2 active groups to assess each slope separately.
Finally, we performed exploratory paired correlation tests using Pearson correlation coefficient in which we compared clinical changes in PTSD with demographic characteristics.
There were only 4 dropouts in this study. We used the intentionto- treat analysis to handle missing data using the method of last-observation-carried-forward. This is considered a conservative approach as it leads the results toward the null hypothesis.
RESULTS
There were 4 dropouts (2 in the sham group and 2 in the active groups [1 in the left rTMS and 1 in the right rTMS group]). All of the dropouts were associated with difficulties in travel to the rTMS clinic for 10 consecutive days as most of these patients depended on family members to bring them to the rTMS clinic.
The demographic and clinical data are summarized in Table 1. Most of the study subjects (21 of 30) were female. There were no significant differences in demographic and clinical scores at baseline across the 3 groups of treatment. Patients tolerated treatment well. There were no seizures and only mild adverse effects, such as mild headache, neck pain, sleepiness, and dizziness, were reported similarly in the 3 groups of treatment.
Table 1.
Demographic Characteristics of Posttraumatic Stress Disorder Study Patients
| Characteristic | Right rTMS (n=10) | Left rTMS (n=10) | Sham rTMS (n=10) |
|---|---|---|---|
| Sex, n (%), female | 6 (60) | 7 (70) | 8 (80) |
| Age, mean (SD), y | 40.7 (13.65) | 47.1 (12.13) | 45.9 (11.45) |
| Duration of disease, mean (SD), y | 4.12 (4.61) | 4.18 (4.16) | 3.42 (4.48) |
| Type of trauma, n (%) | |||
| Assault | 2 (20) | 2 (20) | 2 (20) |
| Sexual abuse | 2 (20) | 2 (20) | 1 (10) |
| Death of severe disease of relative | 5 (50) | 4 (40) | 6 (60) |
| Psychological distress (kidnapping, death threatening) | 1 (10) | 2 (20) | 1 (10) |
Abbreviation: rTMS=repetitive transcranial magnetic stimulation.
Core PTSD Symptoms
We initially performed a full model to assess whether the interaction term was significant. This analysis showed a significant interaction term group versus time for the PTSD Checklist (F6.54 = 11.2; P < .001) and for the Treatment Outcome PTSD Scale (F6.54 = 12.7; P < .001). We then performed post hoc analysis with Bonferroni correction for multiple comparisons.
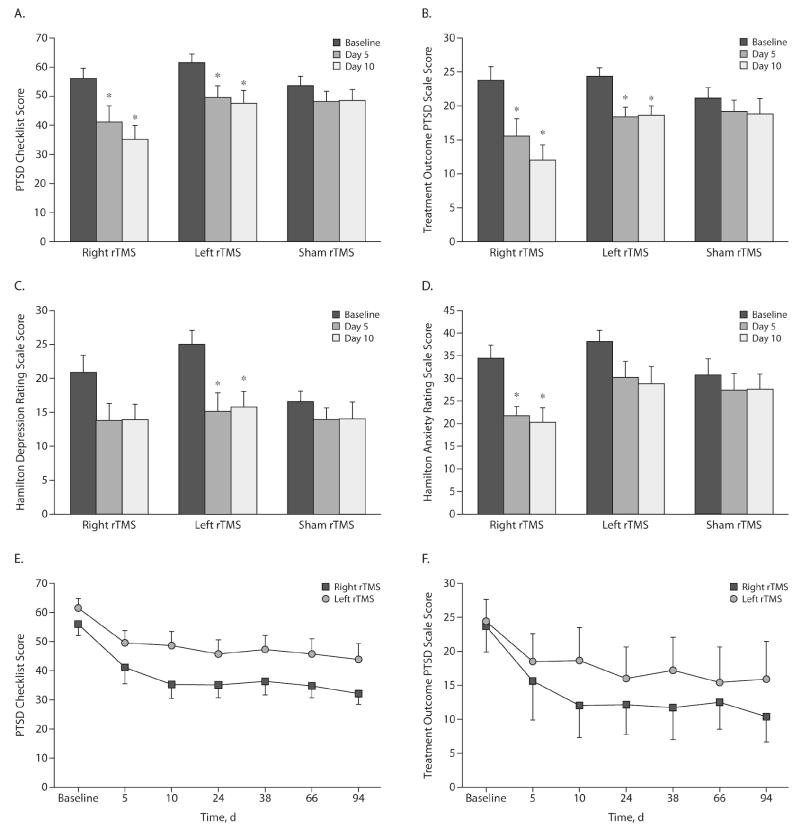
For PTSD Checklist, right rTMS induced a significant decrease in PTSD symptoms after 5 days (P = .018, corrected P value) and 10 days (P = .0042, corrected P value). Similar results were found for left rTMS: significant decrease in core PTSD symptoms after 5 days (P = .012, corrected P value) and 10 days (P = .012, corrected P value). For sham stimulation, there was no significant difference in PTSD Checklist after 5 days (P = .42, corrected P value) and 10 days (P = .32, corrected P value) (Figure 1A).
Figure 1.
Scores on Measures of PTSD Symptoms, Anxiety, and Depression in Study Patients Before, During, and After rTMS Treatment
*P value is significant (P < .05) compared to baseline.
Abbreviations: PTSD = posttraumatic stress disorder, rTMS = repetitive transcranial magnetic stimulation.
Similar results were obtained for the Treatment Outcome PTSD Scale. After 5 and 10 days of stimulation, there was a significant decrease in core PTSD symptoms for right rTMS (P = .02 and P = .008, respectively, corrected P values) and left rTMS treatment (P = .0042 and P = .0039, respectively, corrected P values). For sham rTMS, similarly, there were no changes in PTSD symptoms (P = .18 and P = .36, respectively, corrected P values) (Figure 1B).
Moreover, when comparing left and right rTMS, the results show that improvement after right rTMS is larger as compared to left rTMS, and this difference was marginally significant (for PTSD Checklist: 36.9% [± 20.1] vs 23.1% [± 15.4], right and left rTMS, respectively; P = .03; for Treatment Outcome PTSD Scale: 48.6% [± 27.2] vs 22.8% [± 18.4], P = .051).
Finally, because both active treatments resulted in significant improvements in PTSD symptoms, we then analyzed whether one of these strategies is more effective according to the clusters of symptoms (eg, reexperiencing, avoidance, and hyperarousal). Initially, we ran a full model with the factors group (right and left rTMS), cluster, and time. This model revealed that the interaction term group versus time versus clusters was significant (F9.108 = 5.78; P < .0001); suggesting that clusters of symptoms changed differently across the 2 active groups of treatment. In fact, mixed ANOVA models for each cluster showed a significant difference between right and left rTMS for avoidance only (F1.18 = 6.83; P = .018) but not for reexperiencing (F1.18 = 0.18; P = .68). There was a trend for a differential effect for hyperarousal (F1.18 = 2.83; P = .10). In fact, whereas the improvement in avoidance and hyperarousal was larger for right rTMS as compared to left rTMS, for reexperiencing, improvement in both groups was similar.
Anxiety and Depression
We performed an analysis of symptoms of anxiety and depression in a manner similar to that of the PTSD symptoms. We initially executed a full-model analysis to assess whether the interaction term time versus group was significant. This analysis showed a significant interaction term group versus time for both the Hamilton Depression Rating Scale (F6.54 = 17.9; P < .001) and the Hamilton Anxiety Rating Scale (F6.54 = 6.7; P < .001). We then performed post hoc analysis with Bonferroni correction for multiple comparisons.
The post hoc analysis showed an interesting result: while depression scores were significantly improved only after left rTMS treatment (P < .0001 and P = .0006, for after 5 and 10 days, respectively, corrected P values), anxiety scores were significantly decreased only after right rTMS treatment (P = .0066 and P = .0096, after 5 and 10 days, respectively, corrected P values). Sham rTMS induced no significant changes in Hamilton Depression Rating Scale and Hamilton Anxiety Rating Scale scores (Figures 1C and 1D).
Cognitive Function
We then assessed whether rTMS was associated with cognitive worsening. Analysis with a mixed ANOVA model showed that the interaction term time versus group was not significant for the tests— Stroop Test, Digit Span Test (forward and backward), Raven Colored Progressive Matrices, and Wisconsin Card Sorting Test (Table 2, F and P values)—when comparing baseline to day 10 results. Interestingly, in all of these tests, the active groups had an increase in absolute values in performance following rTMS.
Table 2.
Neuropsychological Performance in Study Patients Assessed by a Mixed Analysis of Variance Model at Baseline and After 10 Days
| Measure | Stroop |
Digit Span |
Raven |
COWA |
Wisconsin |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Baseline | ||||||||||
| Right rTMS | 60.70 | 55.73 | 10.30 | 3.43 | 34.80 | 9.85 | 30.60 | 9.88 | 21.30 | 8.08 |
| Left rTMS | 51.60 | 46.36 | 8.80 | 2.82 | 37.40 | 13.28 | 39.90 | 15.06 | 25.90 | 9.01 |
| Sham rTMS | 38.40 | 11.09 | 12.10 | 3.14 | 39.80 | 11.78 | 37.60 | 7.52 | 22.40 | 13.00 |
| Day 10 | ||||||||||
| Right rTMS | 55.60 | 57.40 | 11.40 | 3.89 | 37.00 | 11.71 | 36.30 | 12.07 | 23.09 | 8.10 |
| Left rTMS | 43.30 | 34.64 | 9.50 | 2.22 | 38.40 | 13.75 | 38.60 | 12.50 | 26.50 | 9.35 |
| Sham rTMS | 36.30 | 7.32 | 12.20 | 3.29 | 40.00 | 10.13 | 36.60 | 9.38 | 25.30 | 12.15 |
| F3.27 (P value)a | 2.15 (.11) | 1.51 (.23) | 1.37 (.27) | 3.24 (.04) | 0.75 (.53) | |||||
F and P values indicate the values for the interaction term group versus time.
Abbreviations: COWA = controlled oral word association test, rTMS = repetitive transcranial magnetic stimulation.
However, for the Controlled Oral Word Association Test, the interaction term was statistically significant (F3.27 = 3.24; P = .037). Post hoc comparisons showed that performance was only improved after right rTMS (P = .015, corrected P value). There were no significant changes after left rTMS (P = .99) and sham rTMS (P = .99) (see Table 2 for details).
Effect Durability
We initially performed a full model with all time points (baseline, day 5, day 10, day 24, day 38, day 66, day 94) and treatment groups (right, left, and sham rTMS). This model showed a significant interaction term time versus group for the PTSD Checklist (F18.162 = 6.54; P < .0001) and Treatment Outcome PTSD Scale (F18.162 = 6.85; P < .0001). We then performed separate models for right and left rTMS groups in order to assess whether the slope of time was significantly different from 0. Interestingly, the main effect of time was significant for left and right rTMS for both PTSD instruments: PTSD Checklist and Treatment Outcome PTSD Scale (F6.54 > 5 and P < .0001 for all 4 analyses); showing that the effects were long-lasting (Figures 1E and 1F). In fact, when comparing day 94 against baseline for both PTSD outcomes, this difference remained significant (P < .005 [corrected P value] for all 4 comparisons).
Correlations
Finally, we correlated the effects of rTMS on PTSD symptoms (as indexed by the PTSD Checklist and Treatment Outcome PTSD Scale) with demographic and baseline clinical characteristics. There was a marginally significant correlation between duration disease and PTSD symptom improvement at 10 days (r = −0.45 and P = .045 for PTSD Checklist and r = −0.43 and P = .058 for Treatment Outcome PTSD Scale), such that longer elapsed time since trauma was associated with a larger response.
DISCUSSION
In this study, 30 patients with PTSD were randomly assigned to receive treatment with 10 daily sessions of either 20 Hz rTMS of right DLPFC (“right rTMS”), 20 Hz rTMS of left DLPFC (“left rTMS”), or sham stimulation. Our results demonstrate that active stimulation of the right and left DLPFC were both effective in the relief of core PTSD symptoms (hyperarousal, vigilance, intrusive thoughts, emotional numbness, withdrawal, etc) at days 5 and 10 as determined by scores on the PTSD Checklist and Treatment Outcome PTSD Scale. Interestingly, right rTMS was associated with a greater improvement in core PTSD symptoms (ie, larger reduction in PTSD Checklist and Treatment Outcome PTSD Scale scores) when compared to left rTMS. On the other hand, left rTMS resulted in a significant improvement with respect to symptoms of depression (ie, significant reduction in Hamilton Depression Rating Scale scores) at days 5 and 10 whereas changes in symptoms of depression were not significant for right rTMS. Interestingly, right rTMS generated a significant improvement in the measure of anxiety (Hamilton Anxiety Rating Scale) at days 5 and 10 while left rTMS did not. Finally, with respect to the measures of cognitive performance (Stroop Test, Digit Span Test, Wisconsin Card Sorting Test, and Raven Colored Progressive Matrices), right and left rTMS were both associated with improvements in neuropsychological performance, although these results were not statistically significant (see Table 2). Unexpectedly, there was a significant improvement in verbal fluency—as indexed by Controlled Oral Word Association Test (phonemic category—letters F, A, S)—following right rTMS.
Our findings here support the results of Cohen et al,8 which show that 10 Hz rTMS applied to the right DLPFC results in improvements to both core PTSD symptoms and anxiety. Indeed, our study shows that 20 Hz rTMS of right DLPFC has similar effects as previously described for 10 Hz right TMS, namely improvements in core PTSD and anxiety symptoms; because of our longer follow-up period, we were also able to show that these beneficial effects persist for at least 3 months. Furthermore, here we also tested the efficacy of left DLPFC stimulation in PTSD and showed that, indeed, 20 Hz left rTMS improves symptoms of depression as well as core PTSD symptoms but not symptoms of anxiety. However, because the effect of left rTMS on core PTSD symptoms was significantly smaller in magnitude in comparison to the effects of right rTMS, the improvement seen with 20 Hz rTMS of left DLPF may be secondary to the antidepressant effects of this mode of stimulation.
As demonstrated above, right rTMS has a significant effect in relieving the anxiety dimension of PTSD; this feature may be the primary factor underlying the beneficial effects observed. Longitudinal studies show that anxiety sensitivity and trait anxiety are important components of PTSD onset and vulnerability,39,40 and targeting central nervous system networks involved in the perpetuation of anxiety may be a highly effective approach. In addition, antidepressant SSRIs used in the treatment of PTSD have a common effect in reducing anxiety across the spectrum of anxiety disorders.39 Hence, we propose that high-frequency stimulation of right DLPFC may be an effective approach in the neuromodulatory treatment of PTSD and that it may function by decreasing the anxiety component of this disorder.
A functional magnetic resonance imaging (fMRI) study by Whalley et al41 provides additional evidence for the understanding of a potential mechanism that may explain the demonstrated anxiety-relieving capacity of right rTMS in patients with PTSD. In this imaging study of patients with either PTSD or depression as well as trauma-exposed controls, fMRI results for the contrast between old and new items during an episodic memory retrieval task revealed activation in a predominantly left-sided network of cortical regions, including the left middle temporal, bilateral posterior cingulate, and left prefrontal cortices for all groups. Furthermore, relative to the control and depressed groups, the PTSD group exhibited greater sensitivity to correctly recognized stimuli in the left amygdala/ventral striatum and right occipital cortex, and more specific sensitivity to items encoded in emotional contexts in the right precuneus, left superior frontal gyrus, and bilateral insula. These results are interesting as they demonstrate that, first of all, retrieval of episodic memory is a predominantly left-sided task; thus, it may be speculated that because high-frequency rTMS of right DLPFC is known to inhibit left-sided structures via transcollosal inhibition, excitatory neuromodulation of the right DLPFC with 20 Hz rTMS may, in fact, manifest its PTSD-alleviating effects via inhibition of left-sided memory retrieval networks. In this line, 1 Hz inhibitory rTMS of the left prefrontal cortex might also be an effective approach for the treatment of PTSD. Secondly, the study shows that patients with PTSD may have specific foci of hyperactivity/sensitivity in subcortical nuclei; therefore, prefrontal modulation might inhibit subcortical nuclei that are highly active in PTSD. An alternative suggestion for the mechanism of right rTMS may be based on work by Shin et al,42 which shows hypoactivation of the medial prefrontal cortex in patients with PTSD. In this case, enhancement of activity in right or left prefrontal areas with high-frequency rTMS could be compensating for deficient prefrontal regulation of memory retrieval. These hypotheses are in accordance with proposed neurobiological models for PTSD,43 which hold that the symptoms of flashbacks and intrusive thoughts, among other core PTSD symptoms, may be the result of either hyperactive transmission of fear-relevant information to the amygdala, which is independent of thalamic and hippocampal nuclei, but which relies strongly on visual areas of the inferior temporal cortex, and/or the result of hypoactivation of prefrontal cortex. Indeed, Koenigs et al44 show that ventral medial prefrontal cortex and amygdala are critically involved in the pathogenesis of PTSD.
An important limitation when using a device, especially rTMS, in clinical trials is the sham method. Although we tried to improve the sham method with the electrical stimulator attached to the sham coil and divided the sham group in right and left DLPFC stimulation, it is still possible that our blinding method was not effective, and this is an inherent limitation to rTMS studies.
Therefore, in summary, 10 daily sessions of high-frequency 20 Hz rTMS of right and left DLPFC resulted in improvements in core PTSD symptoms (right > left). Right rTMS also improves anxiety, while left rTMS improves depression. Our results show a long-lasting effect of rTMS—consistent with other applications of rTMS as in neuropathic pain45 and major depression46—and indicate that both right and left rTMS are safe as they are not associated with cognitive worsening and show only mild adverse effects in patients with PTSD. This study supports the continuation of clinical investigation of brain stimulation for the treatment of PTSD: our results confirm that high-frequency rTMS over the right DLPFC may be the best approach in most patients, yet we suggest that patients with high levels of depression may show greater benefit from high-frequency rTMS applied over left DLPFC.
Acknowledgments
The authors are thankful to John Scandone, BSc, from Magstim Company LTD for assisting us with equipment needs for this study; to Barbara Bonnetti, BA, from the Instituto Scala in São Paulo, Brazil, for administrative support; and to Carolina Mello Santos, MD, from the University of São Paulo in São Paulo, Brazil, for assistance with patient referrals.
Funding/support: This work was supported by a research grant from Northstar Neuroscience. In addition, Ms Rocha was supported by a research grant (Programa Institucional de Iniciação Cientifica [PIBIC]-Mackenzie).
Footnotes
Potential conflicts of interest: None reported.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 2.Kessler RC, Wang PS. The descriptive epidemiology of commonly occurring mental disorders in the United States. Annu Rev Public Health. 2008;29(1):115–129. doi: 10.1146/annurev.publhealth.29.020907.090847. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell AM, Sakraida TJ, Kameg K. Overview of post-traumatic stress. Disaster Manag Response. 2002 Sep;:10–14. [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Practice Guideline for the Treatment of Patients with Acute Stress Disorder and Posttraumatic Stress Disorder. Arlington, VA: American Psychiatric Association; 2004. p. 57. [PubMed] [Google Scholar]
- 5.Institute of Medicine Committee on Treatment of Posttraumatic Stress Disorder. Treatment of Posttraumatic Stress Disorder: An Assessment of the Evidence. Washington, DC: National Academies Press; 2007. [Google Scholar]
- 6.George MS, Nahas Z, Borckardt JJ, et al. Brain stimulation for the treatment of psychiatric disorders. Curr Opin Psychiatry. 2007;20(3):250–254. doi: 10.1097/YCO.0b013e3280ad4698. discussion 247–249. [DOI] [PubMed] [Google Scholar]
- 7.Grisaru N, Amir M, Cohen H, et al. Effect of transcranial magnetic stimulation in posttraumatic stress disorder: a preliminary study. Biol Psychiatry. 1998;44(1):52–55. doi: 10.1016/S0006-3223(98)00016-X. [DOI] [PubMed] [Google Scholar]
- 8.Cohen H, Kaplan Z, Kotler M, et al. Repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in posttraumatic stress disorder: a double-blind, placebo-controlled study. Am J Psychiatry. 2004;161(3):515–524. doi: 10.1176/appi.ajp.161.3.515. [DOI] [PubMed] [Google Scholar]
- 9.McCann UD, Kimbrell TA, Morgan CM, et al. Repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Arch Gen Psychiatry. 1998;55(3):276–279. doi: 10.1001/archpsyc.55.3.276. [DOI] [PubMed] [Google Scholar]
- 10.Gross M, Nakamura L, Pascual-Leone A, et al. Has repetitive transcranial magnetic stimulation (rTMS) treatment for depression improved? a systematic review and meta-analysis comparing the recent vs the earlier rTMS studies. Acta Psychiatr Scand. 2007;116(3):165–173. doi: 10.1111/j.1600-0447.2007.01049.x. [DOI] [PubMed] [Google Scholar]
- 11.First MB, Spitzer RL, Williams JBW, et al. Structured Clinical Interview for DSM-IV (SCID) Washington, DC: American Psychiatric Association; 1997. [Google Scholar]
- 12.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108(1):1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 13.Levy R, Goldman-Rakic PS. Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res. 2000;133(1):23–32. doi: 10.1007/s002210000397. [DOI] [PubMed] [Google Scholar]
- 14.Postle BR, Berger JS, Taich AM, et al. Activity in human frontal cortex associated with spatial working memory and saccadic behavior. J Cogn Neurosci. 2000;12(suppl 2):2–14. doi: 10.1162/089892900564028. [DOI] [PubMed] [Google Scholar]
- 15.Andrés P. Supervisory attentional system in patients with focal frontal lesions. J Clin Exp Neuropsychol. 2001;23(2):225–239. doi: 10.1076/jcen.23.2.225.1212. [DOI] [PubMed] [Google Scholar]
- 16.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- 17.van ’t Wout M, Kahn RS, Sanfey AG, et al. Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex affects strategic decision-making. Neuroreport. 2005;16(16):1849–1852. doi: 10.1097/01.wnr.0000183907.08149.14. [DOI] [PubMed] [Google Scholar]
- 18.Fuster JM. Memory networks in the prefrontal cortex. Prog Brain Res. 2000;122:309–316. doi: 10.1016/S0079-6123(08)62147-0. [DOI] [PubMed] [Google Scholar]
- 19.LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- 20.Shin LM, Kosslyn SM, McNally RJ, et al. Visual imagery and perception in posttraumatic stress disorder: a positron emission tomographic investigation. Arch Gen Psychiatry. 1997;54(3):233–241. doi: 10.1001/archpsyc.1997.01830150057010. [DOI] [PubMed] [Google Scholar]
- 21.Rauch SL, van der Kolk BA, Fisler RE, et al. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry. 1996;53(5):380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- 22.Bremner JD. Alterations in brain structure and function associated with post-traumatic stress disorder. Semin Clin Neuropsychiatry. 1999;4(4):249–255. doi: 10.153/SCNP00400249. [DOI] [PubMed] [Google Scholar]
- 23.Bremner JD, Staib LH, Kaloupek D, et al. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry. 1999;45(7):806–816. doi: 10.1016/S0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bremner JD, Krystal JH, Charney DS, et al. Neural mechanisms in dissociative amnesia for childhood abuse: relevance to the current controversy surrounding the “false memory syndrome”. Am J Psychiatry. 1996;153(suppl):71–82. doi: 10.1176/ajp.153.7.71. [DOI] [PubMed] [Google Scholar]
- 25.Freeman TW, Cardwell D, Karson CN, et al. In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of subjects with combat-related posttraumatic stress disorder. Magn Reson Med. 1998;40(1):66–71. doi: 10.1002/mrm.1910400110. [DOI] [PubMed] [Google Scholar]
- 26.De Bellis MD, Keshavan MS, Spencer S, et al. N-Acetylaspartate concentration in the anterior cingulate of maltreated children and adolescents with PTSD. Am J Psychiatry. 2000;157(7):1175–1177. doi: 10.1176/appi.ajp.157.7.1175. [DOI] [PubMed] [Google Scholar]
- 27.Pascual-Leone A, Rubio B, Pallardó F, et al. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348(9022):233–237. doi: 10.1016/S0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- 28.Okabe S, Ugawa Y, Kanazawa I. Effectiveness of rTMS on Parkinson’s Disease Study Group. 0.2-Hz repetitive transcranial magnetic stimulation has no add-on effects as compared to a realistic sham stimulation in Parkinson’s disease. Mov Disord. 2003;18(4):382–388. doi: 10.1002/mds.10370. [DOI] [PubMed] [Google Scholar]
- 29.Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8(1):75–90. doi: 10.1002/jts.2490080106. [DOI] [PubMed] [Google Scholar]
- 30.Blanchard EB, Hickling EJ, Buckley TC, et al. Psychophysiology of posttraumatic stress disorder related to motor vehicle accidents: replication and extension. J Consult Clin Psychol. 1996;64(4):742–751. doi: 10.1037/0022-006X.64.4.742. [DOI] [PubMed] [Google Scholar]
- 31.Davidson JR, Colket JT. The eight-item treatment-outcome post-traumatic stress disorder scale: a brief measure to assess treatment outcome in post-traumatic stress disorder. Int Clin Psychopharmacol. 1997;12(1):41–45. doi: 10.1097/00004850-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 34.Grant DA, Berg EA. A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. J Exp Psychol. 1948;38:404–411. doi: 10.1037/h0059831. [DOI] [PubMed] [Google Scholar]
- 35.Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. 3. Iowa City, IA: AJA Associates; 1994. [Google Scholar]
- 36.Regard M. Unpublished doctoral dissertation, University of Victoria, British Columbia. 1981. Cognitive rigidity and flexibility: a neuropsychological study. [Google Scholar]
- 37.Raven JC, Court JH, Raven J. Colored progressive matrices. Oxford: Oxford Psychologists Press; 1995. [Google Scholar]
- 38.Blackburn HL, Benton AL. Revised administration and scoring of the digit span test. J Consult Psychol. 1957;21:139–143. doi: 10.1037/h0047235. [DOI] [PubMed] [Google Scholar]
- 39.Hensley L, Varela RE. PTSD symptoms and somatic complaints following Hurricane Katrina: the roles of trait anxiety and anxiety sensitivity. J Clin Child Adolesc Psychol. 2008;37(3):542–552. doi: 10.1080/15374410802148186. [DOI] [PubMed] [Google Scholar]
- 40.Kiliç EZ, Kiliç C, Yilmaz S. Is anxiety sensitivity a predictor of PTSD in children and adolescents? J Psychosom Res. 2008;65(1):81–86. doi: 10.1016/j.jpsychores.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Whalley MG, Rugg MD, Smith AP, et al. Incidental retrieval of emotional contexts in post-traumatic stress disorder and depression: an fMRI study. Brain Cogn. 2009;69(1):98–107. doi: 10.1016/j.bandc.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071(1):67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- 43.Brewin CR. What is it that a neurobiological model of PTSD must explain? Prog Brain Res. 2008;167:217–228. doi: 10.1016/S0079-6123(07)67015-0. [DOI] [PubMed] [Google Scholar]
- 44.Koenigs M, Huey ED, Raymont V, et al. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat Neurosci. 2008;11(2):232–237. doi: 10.1038/nn2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khedr EM, Kotb H, Kamel NF, et al. Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. J Neurol Neurosurg Psychiatry. 2005;76(6):833–838. doi: 10.1136/jnnp.2004.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bortolomasi M, Minelli A, Fuggetta G, et al. Long-lasting effects of high frequency repetitive transcranial magnetic stimulation in major depressed patients. Psychiatry Res. 2007;150(2):181–186. doi: 10.1016/j.psychres.2006.04.010. [DOI] [PubMed] [Google Scholar]