Abstract
Objective
High-mobility group box 1 (HMGB1), an active receptor for advanced glycation endproducts (RAGE), functions as a potent proinflammatory cytokine-like factor that contributes to the pathogenesis of vasculature. Diabetes mellitus (DM) is associated with accelerated development of both microvascular and macrovascular disease and increases the risk of ischemic stroke. Using a model of streptozotocin-induced type-1 diabetes (T1DM) in rats, we investigated the changes in HMGB and RAGE and tested the effects of Niaspan, a slow release form of niacin, on the expression of pro-inflammatory proteins in rats after stroke.
Research design and methods
T1DM rats were subjected to transient middle cerebral artery occlusion (MCAo) and treated without or with Niaspan (40 mg/kg) daily for 14 days after MCAo. Non-streptozotocin rats (WT) were also subjected to MCAo. Immunostaining for inflammatory mediators including HMGB1, RAGE, matrix metalloproteinase-9 (MMP-9) and toll-like receptor 4 (TLR4) immunostaining (n=8/group) and Western blotting (n=4/group) were performed.
Results
Compared to WT-MCAo rats, T1DM-MCAo rats showed an increased expression of HMGB1 (0.82±0.07 vs. 1.81±0.98, P<0.05), RAGE (1.31±0.22 vs. 3.77±0.72, P<0.05), MMP-9 (0.74±0.08 vs. 1.61±0.09, P<0.05) and TLR4 (2.85±0.22 vs. 6.72±0.44, P<0.05) after stroke. Niaspan treatment significantly attenuated the expression of HMGB1 (1.80±0.98 vs. 1.31±0.01, P<0.05), RAGE (3.77±0.71 vs. 1.78±0.45, P<0.05), MMP-9 (1.61±0.09 vs. 0.97±0.07, P<0.05) and TLR4 (6.72±0.44 vs. 2.28±0.43, P<0.05) in the ischemic brain in T1DM-MCAo rats.
Conclusions
T1DM increases HMGB1/RAGE, TLR4 and MMP-9 expression after stroke. Niaspan treatment of stroke in T1DM rats inhibits HMGB1/RAGE, TLR4 and MMP-9 expression which may contribute to the reduced inflammatory response after stroke in T1DM rats.
Keywords: type-1 diabetes rats, stroke, RAGE, Niaspan, HMGB1, MMP-9
Diabetes mellitus (DM) is a common, complex, and chronic disease associated with accelerated development of both microvascular and macrovascular disease (Lloyd-Jones et al., 2010). Consequently, DM not only increases the risk of ischemic stroke but also increases recurrence of stroke and poorer outcomes after stroke (Mast et al., 1995; Lloyd-Jones et al., 2010). Inflammatory activation plays a vital role in the pathophysiological mechanisms of stroke, exerting deleterious effects on the progression of tissue damage and may lead to the vascular damage in diabetes (Williams and Nadler, 2007; Lakhan et al., 2009). Therefore, investigation of inflammatory activation under conditions of diabetic stroke is warranted.
High-mobility group box 1 (HMGB1), a member of alarmin family of mediators, can either be passively released into the extracellular milieu in response to necrotic signals or actively secreted in response to inflammatory signals (Oozawa et al., 2008; Lakhan et al., 2009). HMGB1 functions as a potent proinflammatory cytokine-like factor that contributes to the pathogenesis of vasculature (Yang et al., 2010). Receptor for advanced glycation endproducts (RAGE), as one of the primary receptors for HMGB1, can both initiate and sustain a proinflammatory phenotype when activated by HMGB1 (Fiuza et al., 2003). RAGE has been implicated in the pathogenesis of diabetic complications, neurodegenerative diseases and inflammatory disorders (Barile and Schmidt, 2007; Maillard-Lefebvre et al., 2009). The expression of RAGE is upregulated in human tissues susceptible to the long-term complications of diabetes, such as aorta, kidneys, glomerular epithelial cells, endothelial cells, vascular smooth muscle cells, and inflammatory mononuclear phagocytes (Farmer and Kennedy, 2009). In addition, matrix metalloproteinase-9 (MMP-9), a potentially deleterious neurovascular protease, can be upregulated by HMGB1 via toll-like receptor 4 (TLR4) and lead to acute inflammatory response after cerebral ischemia (Qiu et al., 2010). However, the changes of the inflammatory mediators, HMGB1, RAGE, MMP-9 and TLR4 expression after stroke in T1DM rats have not been investigated.
Niaspan is a slow release form of niacin, and is the most effective medication in clinical use for increasing high-density lipoprotein (HDL) cholesterol (Elam et al., 2000; Chen et al., 2007). Niacin also improves endothelial function, reduces inflammation and increases plaque stability (Rosenson, 2003). Our previous study has found that the Niaspan treatment of stroke reduces cerebral vascular damage and improves functional recovery in wild-type rats (Chen et al., 2007). However, whether Niaspan treatment regulates inflammatory activity and HMGB1/RAGE expression after stroke in diabetic rats has not been investigated.
In this study, we investigated the changes of inflammatory mediators, HMGB1, RAGE, MMP-9 and TLR4 between type-1 diabetic (T1DM) and non-streptozotocin (WT) rats subjected to stroke. We also tested whether Niaspan treatment of stroke in T1DM rats reduces HMGB1, RAGE, MMP-9 and TLR4 expression in a rat model of middle cerebral artery occlusion (MCAo). In the present study, data relating to functional improvement have been previously reported (data not shown).
EXPERIMENTAL PROCEDURES
Diabetes induction
Adult male Wistar rats (225–250 g) purchased from Charles River (Wilmington, MA, USA) were used. Diabetes was induced by a single intraperitoneal injection of streptozotocin (STZ, 60 mg/kg, Sigma Chemical Co., St. Louis, MO, USA) to rat (Like and Rossini, 1976). The fasting blood glucose level was tested by using a glucose analyzer (Accu-Chek Compact System; Roche Diagnostics, Indianapolis, IN, USA). Animals were subjected to MCAo 2 weeks after diabetes induction (fasting blood glucose >300 mg/dl).
MCAo model and experiment groups
Wild-type (WT) and T1DM rats were anesthetized and subjected to transient (2 h) MCAo by using a previously described method of intraluminal vascular occlusion (Chen et al., 2001). Rats were gavaged starting 24 h after MCAo with: (1) saline for T1DM-MCAo-control; (2) saline for WT-MCAo-control; (3) T1DM-Niaspan treatment: 40 mg/kg Niaspan (Kos Pharmaceuticals, Inc., Cranbury, NJ, USA; dissolved in saline) daily for 14 days in T1DM-rats. Rats were sacrificed at 14 days after MCAo for immunostaining (n=8/group). Blood serum also was collected for Western blot assay (n=4/group).
Histological and immunohistochemical assessment
Rats were sacrificed 14 days after MCAo. The brains were fixed by transcardial perfusion with saline, followed by perfusion and immersion in 4% paraformaldehyde before being embedded in paraffin. Seven coronal sections of tissue were processed. For immunostaining, a standard paraffin block was obtained from the center of the lesion (bregma −1 mm to +1 mm). A series of 6-µm-thick sections was cut from the block. Every 10th coronal section for a total of five sections was used for immunohistochemical staining for antibody against RAGE (1:400; Dako, Carpenteria, CA, USA), MMP9 (1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA), TLR4 (1:800; Dako, Carpenteria, CA, USA). Control experiments consisted of staining brain coronal tissue sections as outlined above, but non-immune serum was substituted for the primary antibody. The immunostaining analysis was performed by an investigator blinded to the experimental groups.
Double immunohistochemical staining
To specifically identify RAGE, TLR4 and MMP-reactive cells co-localized with microglia/macrophages (ED1), double immunofluorescence staining of RAGE/ED1, TLR4/ED1 and MMP9/ED1 was performed. FITC (Calbiochem, San Diego, CA, USA) and cyanine-5.18 (CY5, Jackson Immunoresearch, Baltimore Pike POB 9, West Grove, PA, USA) were used for double-label immunoreactivity. Each coronal section was first treated with the primary anti-RAGE, anti-TLR4 or MMP9 antibody with Cy5, and then followed by ED1 with FITC. Control experiments consisted of staining brain coronal tissue sections as outlined above, but using nonimmune serum for the primary antibody.
Western blot assay
Blood serum was isolated. Equal amounts of lysate were subjected to Western blot analysis, as previously described (Chen et al., 2003a). Protein concentration was measured from serum samples using the BCA Protein Assay Kit (Thermo Scientific, Waltham, MA, USA). 40 µg of protein/lane was loaded in a 10% SDS PAGE precast gel (Invitrogen). Gel was transferred to a nitrocellulose membrane (Bio Rad) by running the transfer at 400 mA for 2 h. Nitrocellulose membrane was blocked in 5% milk in 1× TBS-T for 1 h, and then either HMGB1 (Abcam. 1:1000), MMP-9 (Santa Cruz 1:500), or b-actin (Abcam, Cambridge, MA, USA, 1:10000) primary antibodies were added in 5% milk in TBS-T, and incubated on a shaker overnight at 4 °C. The following morning, the membrane was washed three times for 10 min with 1× TBS-T. Secondary antibodies (Jackson ImmunoResearch) was added at 1:1000 dilution in 5% milk in 1× TBS-T on a room temperature shaker for 1 h. After the incubation the membranes were washed three times for 10 min with 1× TBS-T. Luminol reagent (Santa Cruz) was added and allowed to react with the membranes for 1 min. The membranes were then exposed to Kodak BioMax MR film for 5–30 min before developing the film. By using Adobe Photoshop, Western blot data were quantified as previously described (Miller et al., 2009). Briefly, for each lane, the target protein band was normalized to the corresponding B-actin band.
Immunostaining quantification
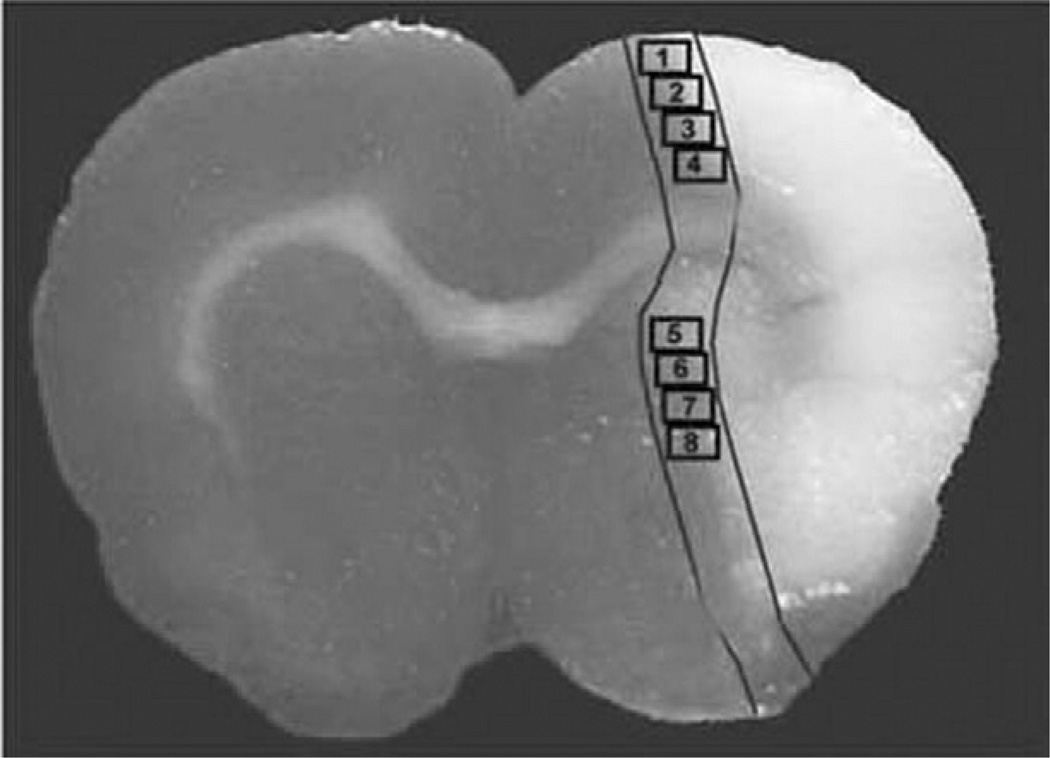
For semiquantitative measurements of RAGE, MMP9 and TLR4, five slides from each brain, with each slide containing eight fields of cortex and stratum from the ischemic border area as shown in Fig. 1 were digitized under a 40× objective (Olympus BX40) using a 3-CCD color video camera (Sony DXC-970MD) interfaced with an MCID image analysis system (Calza et al., 2001; Chen et al., 2003a,b). The ischemic border zone (IBZ) is defined as the area surrounding the lesion, which morphologically differs from the surrounding normal tissue (Nedergaard et al., 1987). RAGE, MMP-9 and TLR4 expression in the IBZ were digitized under a 40× objective (Olympus BX40). The digitalized images were then contrast-enhanced to clearly differentiate positivity from background, and a thresholding procedure was established to determine the proportion of immunoreactive area within each fixed field of view (Calza et al., 2001; Chen et al., 2003a,b, 2005). The quantification was not analyzed stereologically. Data are presented as a percentage of area, in which the RAGE, TLR4 and MMP9 immunopositive areas in each field were divided by the total areas in the field (628×480 mm2) (Calza et al., 2001; Chen et al., 2003a,b, 2005).
Fig. 1.
Schematic representation of quantitative measurements of immunostaining in the ischemic border area (IBZ). Schematic representation of a coronal brain section shows eight fields selected for quantitative measurements of immunostaining in the IBZ.
Statistical analysis
One-way analysis of variance (ANOVA) was used for the evaluation of histology. The analysis started with overall group effect, followed by “Contract/estimate” statement to test the group difference if the overall group effect was detected at 0.05 level.
RESULTS
Diabetes increases serum HMGB1 and MMP-9 levels compared to WT-MCAo rats. Niaspan treatment decreases serum HMGB1 and MMP-9 levels compared to non-treatment T1DM-MCAo rats
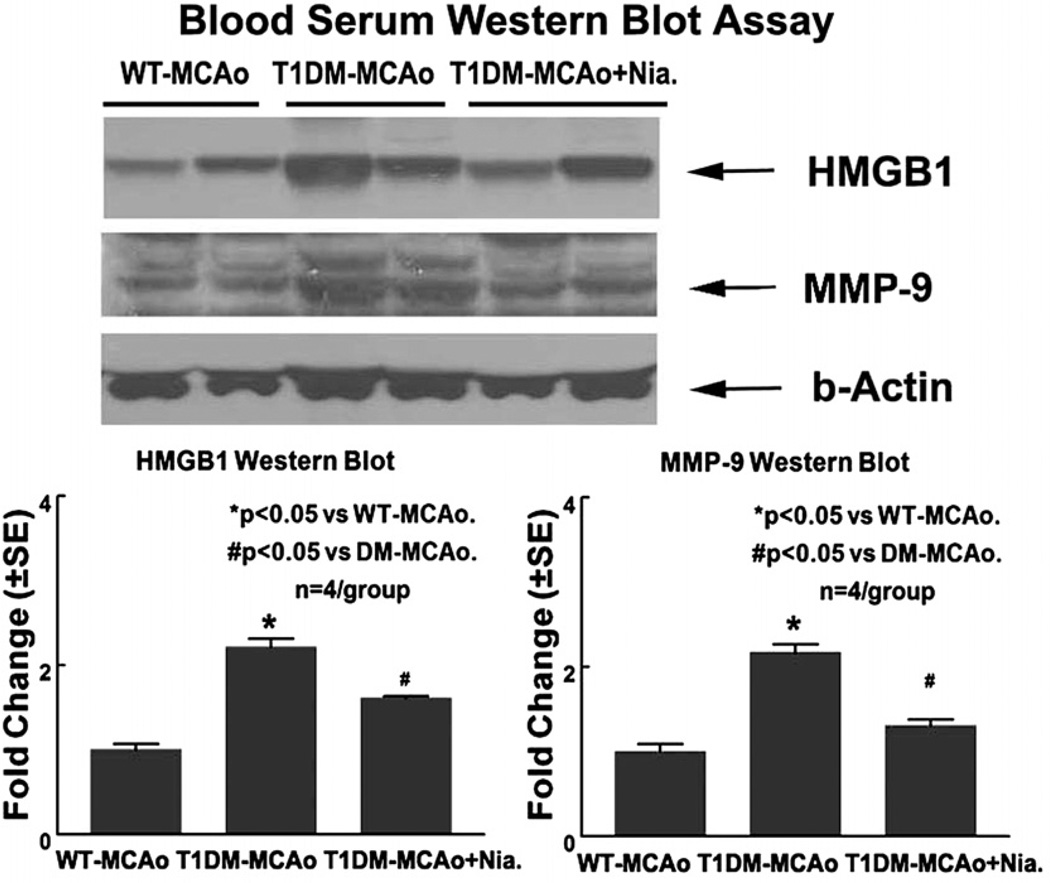
Activated macrophages and monocytes secrete HMGB1 as a cytokine mediator of inflammation (Oozawa et al., 2008). HMGB1 is a potent innate inflammatory mediator when released extracellularly (Han et al., 2008). To test whether T1DM stroke rats increase serum HMGB1 and MMP-9 levels and whether Niaspan treatment regulates HMGB and MMP-9, serum HMGB1 and MMP-9 was measured by Western blot. Fig. 2 shows that T1DM-MCAo rats significantly increased serum HGMB1 and MMP-9 levels compared to WT-MCAo control (P<0.05). Niaspan treatment of T1DM-MCAo rats significantly attenuated serum HMGB-1 and MMP-9 levels compared to non-treatment T1DM-MCAo control rats (P<0.05). These data suggest that HMGB1 and MMP-9 secretion is increased in T1DM-MCAo rats and Niaspan treatment attenuates the secretion of HMGB1 and MMP-9 in T1DM-MCAo rats.
Fig. 2.
T1DM increases serum HMGB and MMP-9 levels after stroke. Niaspan treatment decreases serum HMGB and MMP-9 levels in T1DM-MCAo rats. Using Western blot assay, the data show that T1DM-MCAo rats significantly increases serum HGMB1 and MMP-9 levels compared to WT-MCAo rats (P<0.05). Niaspan treatment significantly decreases serum HGMB1 and MMP-9 levels in T1DM-MCAo rats compared to non-Niaspan treatment T1DM-MCAo rats (P<0.05). n=4/group. * P<0.05 vs. WT-MCAo control. # P<0.05 vs. T1DM-MCAo control.
MMP-9 and HMGB1 receptors (RAGE and TLR4) are up-regulated in the ischemic brain in T1DM rats. Niaspan treatment attenuates MMP-9 and HMGB1 receptor expression in T1DM rats
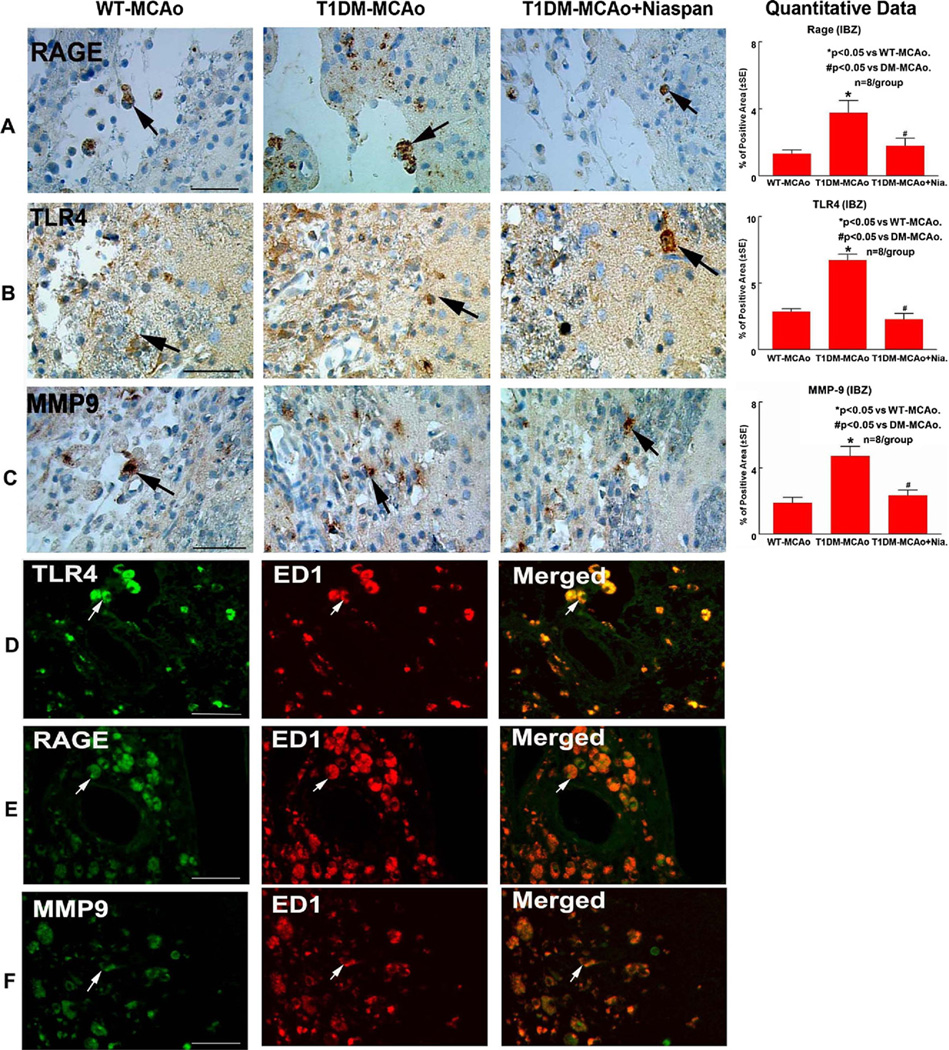
To test whether T1DM induces inflammatory response in the ischemic brain, MMP-9, RAGE and TLR4 immunostaining were performed. Fig. 3 shows that T1DM rats exhibit significantly increased MMP-9, RAGE and TLR4 expression in the ischemic brain compared to WT-MCAo rats (P<0.05, n=8/group). Niaspan treatment significantly decreased MMP-9, RAGE and TLR4 expression in the ischemic brain in T1DM rats compared to non-treated T1DM-MCAo rats (P<0.05).
Fig. 3.
T1DM increases RAGE, TLR4 and MMP-9 expression in the ischemic brain. Niaspan treatment in T1DM-MCAo rats attenuates RAGE, TLR4 and MMP-9 expression in the ischemic brain. RAGE, TLR4 and MMP9 expression are colocalized with ED1 in the ischemic brain in T1DM rats. (A) RAGE immunostaining in the ischemic brain in WT-MCAo, T1DM-MCAo and T1DM-MCAo+Niaspan treatment animals (arrow identifies RAGE positive cell). (B) TLR4 immunostaining in the ischemic brain in WT-MCAo, T1DM-MCAo and T1DM-MCAo+Niaspan treatment animals (arrow identifies TLR4 positive cell). (C) MMP-9 immunostaining in the ischemic brain in WT-MCAo, T1DM-MCAo and T1DM-MCAo+Niasapn treatment animals (arrow identifies MMP9 positive cell). (D–F) Double immunostaining of TLR4 with ED1 (D, red: TLR4 positive cell; green: ED1 positive cell), RAGE with ED1 (E, red: RAGE positive cell; green: ED1 positive cell) and MMP9 with ED1 (F, red: MMP9 positive cell; green: ED1 positive cell) in the ischemic brain. n=8/group. * P<0.05 vs. WT-MCAo control. # P<0.05 vs. T1DM-MCAo control. Scale bar in (A–F)=50 µm.
Previous studies have found that RAGE, TLR4 and MMP9 are expressed in microglia/macrophage (Yang et al., 2005; Bianchi et al., 2007, 2010). TLR4 and RAGE have similar routes leading to inflammation in type-2 diabetic patients (Veloso et al., in press) and both are expressed in astrocytes, as well as in dysplastic neurons (Fan et al., 2009; Zurolo et al., 2011). Consistent with previous finding, Fig. 3 (D–F) shows that RAGE (E), TLR4 (D) and MMP9 (F) are predominantly detected in microglia/macrophage (ED1 positive cell) in the ischemic brain.
DISCUSSION
In this study, we are the first to demonstrate that T1DM-MCAo rats exhibit increased serum HMGB1 and MMP9 levels and increased expression of RAGE, TLR4 and MMP-9 in the ischemic brain compared to WT-MCAo rats. Our data also demonstrate that Niaspan treatment starting 24 h after MCAo in T1DM-MCAo rats significantly decreases serum HMGB and MMP9 level and attenuates the expression of RAGE, TLR4 and MMP-9 in the ischemic brain compared to non-treatment T1DM-MCAo control rats. Niaspan treatment reduces HMGB1, RAGE, TLR4 and MMP-9 expression and thereby may contribute to improved functional outcome after stroke in T1DM-MCAo rats.
Inflammatory mediators play an important role in the pathophysiology of stroke. HMGB1’s involvement in inflammatory reactions promote progression of ischemic brain injury (Kim et al., 2006, 2008; Muhammad et al., 2008; Qiu et al., 2008). HMGB1 is elevated in serum of stroke patients, is released from ischemic brain tissue and contributes to the initial stages of the inflammatory response post stroke (Muhammad et al., 2008; Qiu et al., 2008). Systemic HMGB1 levels are also elevated in patients with cerebral and myocardial ischemia (Goldstein et al., 2006). HMGB-1 exhibits an inflammatory cytokine-like activity in the extracellular space (Han et al., 2008). Extracellular HMGB1 is a prototypical member of the so-called alarmin family of mediators that mediate cross-talk between injured cells and relative healthy cells around damaged tissues (Qiu et al., 2010). We found that diabetes significantly increased serum HMGB level and induced worse functional outcome after stroke compared to WT-MCAo rats. Thus, HMGB1 may be involved in the progression of neurological disorders in T1DM-MCAo rats.
RAGE, as one of the primary receptors for HMGB1, has been implicated as a sensor of necrotic cell death and contributes to inflammation and ischemic brain damage (Muhammad et al., 2008). A neutralizing anti-HMGB1 antibody and HMGB1 box A, an antagonist of HMGB1 at the receptor RAGE, ameliorated ischemic brain damage (Muhammad et al., 2008). HMGB1 also triggers MMP-9 up-regulation in neurons and astrocytes predominantly via TLR4 after cerebral ischemia (Qiu et al., 2010). TLR4 is linked to HMGB1 and mediates many of the extracellular functions of HMGB1 (Park et al., 2004; Yu et al., 2006; Kruger et al., 2009) and induces the inflammatory response and upregulates the MMP-9 expression, which is a potentially deleterious neurovascular protease (Qiu et al., 2010). MMP-9 protein level and total MMP activity are augmented in isolated cerebrovessels of the Goto-Kakizaki diabetes rats (Li et al., 2010). Type-2 diabetes (T2DM) mice exhibit significantly increased MMP-9 expression and activity in the ischemic brain after stroke (Chen et al., 2011). The levels of HMGB1, RAGE, TLR4 and MMP-9 expression are correlated with inflammatory response after stroke (Yamagishi et al., 2009; Qiu et al., 2010; Yang et al., 2010). HMGB1/RAGE, TLR4 and MMP-9 signaling pathways contribute to the cellular response to neuronal injury via activating the inflammatory response after a cerebral ischemic episode (Qiu et al., 2010; Yang et al., 2010). In this study, we found that T1DM-MCAo rats significantly increased RAGE, TLR4 and MMP-9 expression in the ischemic brain compared to WT-MCAo rats. RAGE, TLR4 and MMP-9 primarily co-localized with microglia/macrophage. Therefore, activation of HMGB1/RAGE, TLR4 and MMP-9 signaling pathways may contribute to T1DM-induced inflammatory response and thereby worsen the functional outcome after stroke.
Targets for inhibiting inflammation are being considered as a therapeutic modality to improve outcome after stroke. Patients with T2DM have increased inflammation and upregulated expression of cell adhesion molecules (CAMs). Niacin improves endothelial function, reduces inflammation and increases plaque stability in patients (Rosenson, 2003). Niaspan treatment in T2DM patients increases HDL cholesterol and significantly reduces monocyte adhesion (Tavintharan et al., 2011). Niaspan treatment of stroke rats also decreased tumor necrosis factor alpha (TNF-a) expression and promotes neuroprotective effects after stroke (Shehadah et al., 2010). In this study, we found that Niaspan treatment significantly decreased RAGE, MMP-9 and TLR4 expression in T1DM-MCAo rats compared to non-treatment T1DM-MCAo animals. We have previously demonstrated that Niaspan treatment significantly improves functional recovery after stroke in T1DM-MCAo rats (data not shown). Decreasing HMGB1, RAGE, TLR4 and MMP-9 in T1DM-MCAo rats by Niaspan treatment thus may contribute to inhibition of inflammation in the ischemic brain, which, at least partially, improves functional outcome after stroke in T1DM-MCAo rats.
CONCLUSION
T1DM increases inflammatory response in the ischemic brain after stroke. HMGB1/RAGE, TLR4 and MMP-9 signaling pathways may play an important role in diabetes-induced inflammatory damage in the ischemic brain. Niaspan treatment decreases HMGB1, RAGE, TLR4 and MMP-9 expression after stroke in T1DM rats. Reduction of the HMGB1/RAGE, TLR4 and MMP-9 signaling pathways by Niaspan treatment provide a beneficial effect after stroke in diabetic rats. Further investigations into the use of Niaspan as a therapeutic agent for the treatment of stroke in diabetics are warranted.
Acknowledgments
The authors wish to thank Qinge Lu and Sutapa Santra for technical assistance. This work was supported by NIA RO1 AG031811 (J.C.), NINDS PO1 NS23393 (M.C.) and 1R41NS064708 (J.C.), and American Heart Association grant 09GRNT2300151 (J.C.).
Abbreviations
- HMGB1
high-mobility group box 1
- MCAo
middle cerebral artery occlusion
- MMP9
matrix metalloproteinase-9
- RAGE
receptor for advanced glycation endproducts
- TLR4
toll-like receptor 4
- T1DM
type-1 diabetes
- WT
wild-type
REFERENCES
- Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976;193:415–417. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- Barile GR, Schmidt AM. RAGE and its ligands in retinal disease. Curr Mol Med. 2007;7:758–765. doi: 10.2174/156652407783220778. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Adami C, Giambanco I, Donato R. S100B binding to RAGE in microglia stimulates COX-2 expression. J Leukoc Biol. 2007;81:108–118. doi: 10.1189/jlb.0306198. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Giambanco I, Donato R. S100B/RAGE-dependent activation of microglia via NF-kappaB and AP-1 co-regulation of COX-2 expression by S100B, IL-1beta and TNF-alpha. Neurobiol Aging. 2010;31:665–677. doi: 10.1016/j.neurobiolaging.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Calza L, Giardino L, Giuliani A, Aloe L, Levi-Montalcini R. Nerve growth factor control of neuronal expression of angiogenetic and vasoactive factors. Proc Natl Acad Sci U S A. 2001;98:4160–4165. doi: 10.1073/pnas.051626998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Cui X, Zacharek A, Cui Y, Roberts C, Chopp M. White matter damage and the effect of matrix metalloproteinases in type 2 diabetic mice after stroke. Stroke. 2011;42:445–452. doi: 10.1161/STROKEAHA.110.596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Cui X, Zacharek A, Jiang H, Roberts C, Zhang C, Lu M, Kapke A, Feldkamp CS, Chopp M. Niaspan increases angiogenesis and improves functional recovery after stroke. Ann Neurol. 2007;62:49–58. doi: 10.1002/ana.21160. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003a;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003b;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- Elam MB, Hunninghake DB, Davis KB, Garg R, Johnson C, Egan D, Kostis JB, Sheps DS, Brinton EA. Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: the ADMIT study: a randomized trial. Arterial Disease Multiple Intervention Trial. JAMA. 2000;284:1263–1270. doi: 10.1001/jama.284.10.1263. [DOI] [PubMed] [Google Scholar]
- Fan HG, Li LT, Zhang XJ, Liu Y, Yang CH, Yang Y, Yin J. Oxymatrine downregulates TLR4, TLR2, MyD88, and NF-kappaB and protects rat brains against focal ischemia. Mediators Inflamm. 2009;2009:704706. doi: 10.1155/2009/704706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer DGS, Kennedy S. RAGE, vascular tone and vascular disease. Pharmacol Ther. 2009;124:185–194. doi: 10.1016/j.pharmthera.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Fiuza C, Bustin M, Talwar S, Tropea M, Gerstenberger E, Shelhamer JH, Suffredini AF. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101:2652–2660. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- Goldstein RS, Gallowitsch-Puerta M, Yang L, Rosas-Ballina M, Huston JM, Czura CJ, Lee DC, Ward MF, Bruchfeld AN, Wang H, Lesser ML, Church AL, Litroff AH, Sama AE, Tracey KJ. Elevated high-mobility group box 1 levels in patients with cerebral and myocardial ischemia. Shock. 2006;25:571–574. doi: 10.1097/01.shk.0000209540.99176.72. [DOI] [PubMed] [Google Scholar]
- Han J, Zhong J, Wei W, Wang Y, Huang Y, Yang P, Purohit S, Dong Z, Wang MH, She JX, Gong F, Stern DM, Wang CY. Extracellular high-mobility group box 1 acts as an innate immune mediator to enhance autoimmune progression and diabetes onset in NOD mice. Diabetes. 2008;57:2118–2127. doi: 10.2337/db07-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB, Lim CM, Yu YM, Lee JK. Induction and subcellular localization of high-mobility group box-1 (HMGB1) in the postischemic rat brain. J Neurosci Res. 2008;86:1125–1131. doi: 10.1002/jnr.21555. [DOI] [PubMed] [Google Scholar]
- Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, Lee MH, Han PL, Park JS, Lee JK. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci. 2006;26:6413–6421. doi: 10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger B, Krick S, Dhillon N, Lerner SM, Ames S, Bromberg JS, Lin M, Walsh L, Vella J, Fischereder M, Kramer BK, Colvin RB, Heeger PS, Murphy BT, Schroppel B. Donor toll-like receptor 4 contributes to ischemia and reperfusion injury following human kidney transplantation. Proc Natl Acad Sci U S A. 2009;106:3390–3395. doi: 10.1073/pnas.0810169106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Prakash R, Kelly-Cobbs AI, Ogbi S, Kozak A, El-Remessy AB, Schreihofer DA, Fagan SC, Ergul A. Adaptive cerebral neovascularization in a model of type 2 diabetes: relevance to focal cerebral ischemia. Diabetes. 2010;59:228–235. doi: 10.2337/db09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Maillard-Lefebvre H, Boulanger E, Daroux M, Gaxatte C, Hudson BI, Lambert M. Soluble receptor for advanced glycation end products: a new biomarker in diagnosis and prognosis of chronic inflammatory diseases. Rheumatology. 2009;48:1190–1196. doi: 10.1093/rheumatology/kep199. [DOI] [PubMed] [Google Scholar]
- Mast H, Thompson JL, Lee SH, Mohr JP, Sacco RL. Hypertension and diabetes mellitus as determinants of multiple lacunar infarcts. Stroke. 1995;26:30–33. doi: 10.1161/01.str.26.1.30. [DOI] [PubMed] [Google Scholar]
- Miller RK, Qadota H, Stark TJ, Mercer KB, Wortham TS, Anyanful A, Benian GM. CSN-5, a component of the COP9 signalosome complex, regulates the levels of UNC-96 and UNC-98, two components of M-lines in Caenorhabditis elegans muscle. Mol Biol Cell. 2009;20:3608–3616. doi: 10.1091/mbc.E09-03-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad S, Barakat W, Stoyanov S, Murikinati S, Yang H, Tracey KJ, Bendszus M, Rossetti G, Nawroth PP, Bierhaus A, Schwaninger M. The HMGB1 receptor RAGE mediates ischemic brain damage. J Neurosci. 2008;28:12023–12031. doi: 10.1523/JNEUROSCI.2435-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M, Gjedde A, Diemer NH. Hyperglycaemia protects against neuronal injury around experimental brain infarcts. Neurol Res. 1987;9:241–244. doi: 10.1080/01616412.1987.11739802. [DOI] [PubMed] [Google Scholar]
- Oozawa S, Mori S, Kanke T, Takahashi H, Liu KY, Tomono Y, Asanuma M, Miyazaki I, Nishibori M, Sano S. Effects of HMGB1 on ischemia-reperfusion injury in the rat heart. Circ J. 2008;72:1178–1184. doi: 10.1253/circj.72.1178. [DOI] [PubMed] [Google Scholar]
- Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- Qiu J, Nishimura M, Wang Y, Sims JR, Qiu S, Savitz SI, Salomone S, Moskowitz MA. Early release of HMGB-1 from neurons after the onset of brain ischemia. J Cereb Blood Flow Metab. 2008;28:927–938. doi: 10.1038/sj.jcbfm.9600582. [DOI] [PubMed] [Google Scholar]
- Qiu JH, Xu JA, Zheng Y, Wei Y, Zhu XX, Lo EH, Moskowitz MA, Sims JR. High-mobility group box 1 promotes metalloproteinase-9 upregulation through toll-like receptor 4 after cerebral ischemia. Stroke. 2010;41:2077–2082. doi: 10.1161/STROKEAHA.110.590463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenson RS. Antiatherothrombotic effects of nicotinic acid. Atherosclerosis. 2003;171:87–96. doi: 10.1016/j.atherosclerosis.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Shehadah A, Chen J, Zacharek A, Cui Y, Ion M, Roberts C, Kapke A, Chopp M. Niaspan treatment induces neuroprotection after stroke. Neurobiol Dis. 2010;40:277–283. doi: 10.1016/j.nbd.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavintharan S, Woon K, Pek LT, Jauhar N, Dong X, Lim SC, Sum CF. Niacin results in reduced monocyte adhesion in patients with type 2 diabetes mellitus. Atherosclerosis. 2011;215:176–179. doi: 10.1016/j.atherosclerosis.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Veloso CA, Fernandes JS, Volpe CM, Fagundes-Netto FS, Reis JS, Chaves MM. Nogueira-Machado JA (in press) TLR4 and RAGE: similar routes leading to inflammation in type 2 diabetic patients. Diabetes Metab. doi: 10.1016/j.diabet.2010.12.005. in press. [DOI] [PubMed] [Google Scholar]
- Williams MD, Nadler JL. Inflammatory mechanisms of diabetic complications. Curr Diab Rep. 2007;7:242–248. doi: 10.1007/s11892-007-0038-y. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Nakamura K, Matsui T. Regulation of advanced glycation end product (AGE)-receptor (RAGE) system by PPAR-gamma agonists and its implication in cardiovascular disease. Pharmacol Res. 2009;60:174–178. doi: 10.1016/j.phrs.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang H, Czura CJ, Tracey KJ. The cytokine activity of HMGB1. J Leukoc Biol. 2005;78:1–8. doi: 10.1189/jlb.1104648. [DOI] [PubMed] [Google Scholar]
- Yang QW, Wang JZ, Li JC, Zhou Y, Zhong Q, Lu FL, Xiang J. High-mobility group protein box-1 and its relevance to cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:243–254. doi: 10.1038/jcbfm.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. doi: 10.1097/01.shk.0000225404.51320.82. [DOI] [PubMed] [Google Scholar]
- Zurolo E, Iyer A, Maroso M, Carbonell C, Anink JJ, Ravizza T, Fluiter K, Spliet WG, van Rijen PC, Vezzani A, Aronica E. Activation of toll-like receptor, RAGE and HMGB1 signalling in malformations of cortical development. Brain. 2011;134:1015–1032. doi: 10.1093/brain/awr032. [DOI] [PubMed] [Google Scholar]