Abstract
The story of prevention and control of infectious diseases remains open and a series of highly virulent pathogens are emerging both in and beyond the hospital setting. Antibiotics were an absolute success story for a previous era. The academic and industrial biomedical communities have now come together to formulate consensus beliefs regarding the pursuit of novel and effective alternative anti-infective countermeasures. Photodynamic therapy was established and remains a successful modality for malignancies but photodynamic inactivation has been transformed recently to an antimicrobial discovery and development platform. The concept of photodynamic inactivation is quite straightforward and requires microbial exposure to light energy, typically wavelengths in the visible region, that causes the excitation of photosensitizer molecules (either exogenous or endogenous), which results in the production of singlet oxygen and other reactive oxygen species that react with intracellular components, and consequently produce cell inactivation. It is an area of increasing interest, as research is advancing (1) to identify the photochemical and photophysical mechanisms involved in inactivation, (2) to develop potent and clinically compatible photosensitizer, (3) to understand how photoinactivation is affected by key microbial phenotypic elements (multidrug resistance and efflux, virulence and pathogenesis determinants, biofilms), (4) to explore novel delivery platforms inspired by current trends in pharmacology and nanotechnology and (5) to identify photoinactivation applications beyond the clinical setting such as environmental disinfectants.
Key words: photodynamic inactivation and therapy, photosensitizer, reactive oxygen species, permeability barrier, multidrug efflux systems, biofilms, drug delivery, nanomedicine, antimicrobial resistance, clinical applications
The Reality of Infection and Antimicrobial Resistance
One of the scientific highlights of the 20th century was, without doubt, the development of successful prevention and control efforts for infectious diseases worldwide. Since the development of penicillin and subsequent development and synthesis of other antibiotics, vaccines and antiseptics, victory against pathogens has been repeatedly declared.1 By the 1980s, pharmaceutical companies were convinced that there were already enough antibiotics. It was time to “close the book on infectious diseases” and the emphasis was shifted to more threatening clinical problems such as cancer, diabetes and heart disease.
Microorganisms, however, had a different opinion. The extensive and inappropriate use of antibiotics gradually led to the development of pervasive antimicrobial resistance. Penicillin was first put into widespread use in the early 1940s and by 1944 half of all clinical Staphylococci spp isolates were resistant to this proclaimed “miracle drug.”2 Today infectious disease is the second most important killer in the world, number three in developed nations and fourth in the USA.3 It is the third leading cause of death in Europe, mostly in elderly and debilitated populations, and despite existing antibiotic therapies and vaccines, infectious diseases remain the leading cause of mortality and morbidity.4 Worldwide, 17 million people die each year from bacterial infections.5
Five classes of antibiotic-resistant pathogens are emerging as major threats to public health: methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus faecalis (VRE), multidrug-resistant mycobacteria, Gram-negative pathogens and fungi.6 In addition to these established threats we are confronting even more challenging clinical scenarios including carbapenem-resistant Klebsiella pneumonia, New Delhi metalloβ-lactamase containing enterobactericae, as well as the German Escherichia coli outbreak caused by a previously unknown strain, all of which are responsible for significant morbidity and mortality.7–9
Photodynamic Therapy (PDT) as an Antimicrobial Approach
As the efficacy of antibiotics decreases and the end of the “antibiotic era” gets closer, major international research efforts to discover new ways to eradicate bacteria are evolving. The emphasis is now on how to comprehend, prevent and if possible eliminate multidrug resistance in concert with exploring new ways to kill microbial pathogens. In addition to target-based conventional discovery there is an array of promising novel approaches currently under investigation. A prominent member of this list is the light-based platform of photodynamic therapy (PDT). PDT was accidentally discovered over 100 years ago by Oskar Raab and Hermann von Tappiener when they noticed that Paramecium spp protozoans stained with acridine orange died upon exposure to bright light.10 In the 1970s, PDT began to be explored for the selective destruction of cancer.11 Since then, PDT has emerged as a tool for the treatment of various malignancies and is the principle tool for the treatment of age-related macular degeneration.12 Recently, it has been transformed to a discovery and treatment alternative for localized infections.13
PDT involves the use of harmless visible light combined with a light-sensitive dye—the photosensitizer (PS)—and oxygen present in and around cells. After illumination with the light of the appropriate visible wavelength, the PS is energized to an excited state that can undergo molecular collisions with oxygen, resulting in the formation of reactive oxygen species (ROS) and singlet oxygen. PDT is a highly selective modality as (1) hyperproliferating cells selectively uptake PS14 and (2) cell death is spatially limited to regions where light of the appropriate wavelength is applied. As microbial cells possess very fast growth rates, much like that of malignant cells, it was suggested that PDT could be used for microbial cell destruction—this became a reality in the mid-1990s.15
Since then, antimicrobial photodynamic-inactivation (PDI) and therapy has been developed as a prolific discovery and development platform, exploring many aspects of the microbial phenotype related to multidrug resistance such as efflux systems, biofilms, bacterial spores and virulence determinants. This trend, in concert with rationalized synthesis and delivery efforts for new PS, has populated the literature with a variety of preclinical and clinical antimicrobial PDT applications.
The Photophysical Processes of PDT
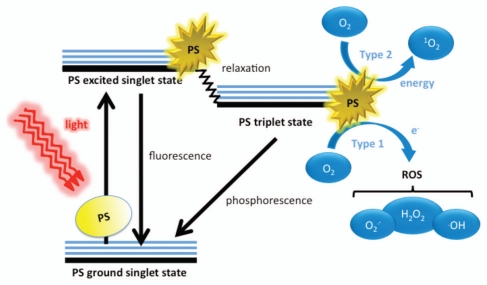
The three principle elements of PDT are the PS, visible light and oxygen. These elements, when combined, yield potent oxidizing species (Fig. 1). In the ground state, a PS is said to be in the singlet state, whereby all of its electrons are spin paired in low energy orbitals. Upon application of light corresponding to the absorption peak of the PS, the electron in the highest occupied molecular orbital (HOMO) of the PS is excited to the lowest unoccupied molecular orbital (LUMO), causing the PS to reach the unstable and short-lived excited singlet state. In this state, several processes may rapidly occur.16 The most critical of these to PDT is the reversal of the excited electron's spin, known as intersystem crossing to the triplet state of the PS. This triplet state is less energetic than the excited singlet state, but has a considerably longer lifetime, as the excited electron, now with a spin parallel to its former paired electron, may not immediately fall back down (as it would then have identical quantum numbers to that of its paired electron, thus violating the Pauli Exclusion Principle). Accordingly, the excited electron in the PS triplet state may first obtain correct spin orientation (a relatively slow process) and then fall to ground levels (phosphorescence) or the PS may interact with molecules abundant in its immediate environment. Because of the Selection Rules that specify that triplet-triplet interactions are spin-allowed while triplet-singlet interactions are spin-forbidden, the PS triplet can react readily with molecular oxygen that is a triplet in its ground state (Fig. 1).
Figure 1.
Schematic illustration of photodynamic therapy including the Jablonski diagram. The PS initially absorbs a photon that excites it to the first excited singlet state and this can relax to the more long-lived triplet state. This triplet PS can interact with molecular oxygen in two pathways, type I and type II, leading to the formation of reactive oxygen species (ROS) and singlet oxygen respectively.
The Photochemical Generation of Oxidizing Species
The discussion of PDT's oxidizing characteristics is centered on molecular oxygen (O2). As mentioned above, the ground state of oxygen is a triplet state, whereby the two outermost orbitals are unpaired but spin parallel. When the PS is in the long-lived triplet state, it may interact with O2 in two distinctly different ways.17,18 The Type I process occurs when the PS directly transfers an electron, sometimes in concert with proton donation, to O2, yielding superoxide anion (O2•-), which can then go on to form other ROS including the hydroxyl radical (•OH), and hydrogen peroxide (H2O2). Alternatively, the Type II process occurs when the energy used to excited the PS to the triplet state is transferred to O2, thus “flipping the spin” of an outermost O2 electron and shifting it into the orbital containing the other electron, which in turn leaves one orbital entirely unoccupied (a violation of Hund's rule). Termed singlet oxygen (1O2), this form of oxygen (not considered a radical as its electrons are spin-paired) is extremely short-lived and reactive, owing to its electron configuration instability.
The formation of ROS through the Type 1 process results in the stripping of electrons from biological macromolecules, including lipids, proteins and nucleic acids.16•OH, arguably the most reactive of the three ROS formed, will abstract electrons to become a hydroxide ion, which then may easily form water via obtaining a proton. O2•- too, may abstract electrons, forming a peroxide ion that immediately abstracts protons to form H2O2; however, in biological systems it is not particularly reactive. Nonetheless, O•- may be converted to HO and O by superoxide dismutase. H2O2 is only considered truly reactive when it reacts with ferrous iron in what is known as the Fenton reaction:
which results in the homolytic fission of the oxygen-oxygen bond in H2O2 to yield a hydroxide ion and •OH via the oxidation of ferrous iron to ferric iron.19 H2O2 is removed through catalase, forming water and oxygen gas. Although •OH is not broken down by enzymatic reactions, it may be quenched by antioxidants, including antioxidant peptides (e.g., glutathione) or by antioxidant sugars (e.g., ascorbic acid).20
Because 1O2 is not a radical, it reacts with biological molecules through quite different mechanisms, making the Type 2 pathway responsible for different macromolecular modifications. 1O2 tends to favor reacting with double bonds and sulfur moieties (both of which have high electron densities) and may interact with aromatic components of macromolecules in Diels-Alder cycloadditions.21,22 Unlike ROS, 1O2 cannot be broken down by enzymes but can be quenched by antioxidants.
Properties of Photosensitizers
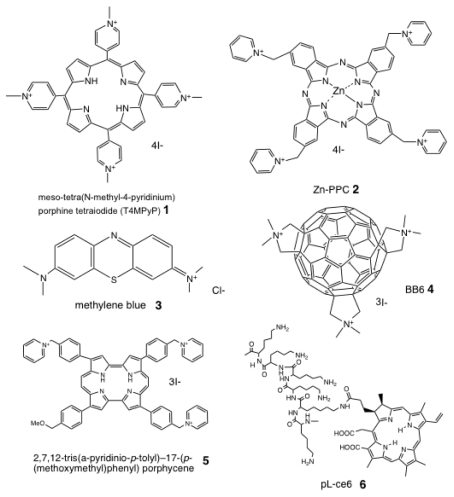
PSs are usually organic delocalized aromatic molecules consisting of a central chromophore with auxiliary branches (auxochromes) that are responsible for further electron delocalization of the PS, thus altering the absorption spectra of the PS.23 Due to extensive electron delocalization, PSs tend to be deeply colored. This means that the energy required to excite the electrons in the HOMO to the LUMO is low compared with less delocalized molecules and therefore the absorption bands are in the longer wavelength (red) spectral region and are large, reflecting the high probability of excitation. Acridine orange was the first photodynamic agent used.24 Most of the PSs that have been employed for the treatment of cancer and other tissue diseases are based on the tetrapyrrole nucleus, with emphasis in the use of porphyrins. Chlorins, bacteriochlorins phthalocyanines as well as a plethora of dyes with different molecular frameworks have been frequently proposed as antimicrobial PSs (Fig. 2).25,26 These include halogenated xanthenes, such as Rose Bengal (RB),27 perylenequinones, such as hypericin28 phenothiazinium salts, such as toluidine blue O (TBO) and methylene blue (MB),29 cationic buckminsterfullerenes (e.g., C60),30,31 psoralens (furanocoumarins).32
Figure 2.
Chemical structures of some representative antimicrobial PS. (1) Cationic porphyrin, meso-tetra(N-methyl-4-pyridinium) porphine tetraiodide (T4MPyP); (2) cationic phthalocyanine, ZnPPC; (3) phenothiazinium salt, methylene blue; (4) cationic functionalized fullerene, BB6; (5) cationic porphycene, 2,7,12-tris(a-pyridinio-p-tolyl)-17-(p-(methoxymethyl)phenyl) porphycene; (6) poly-l-lysine chlorin(e6) conjugate, pL-ce6.
In contrast, fairly recently a genetically encoded PS was developed from the hydrozoan chromoprotein anm2CP, a homolog of green fluorescent protein (GFP). The PS was named KillerRed: It generates ROS upon irradiation with green light and has been proven potent against E. coli and malignant cells in vitro.33 As microorganisms produce and accumulate porphyrins the appealing hypothesis of endogenous photosensitization is also an alternative pathway of photoirradication.34,35
PDI for Microbial Pathogens: The Permeability Barrier
The permeability barrier in Gram-negative bacteria is accountable for the observed susceptibility differences between Gram-positive and Gram-negative species when treated with neutral or anionic PS.36 These observations then prompted the use of molecules such as polymyxin nonapeptide to enhance permeabilization of Gram-negative bacterial outer membranes in combination with PDT.13 Moreover, investigators took cell envelope chemical properties of Gram-negative and Gram-positive bacteria into account (namely that both possess inherently anionic structures) and developed cationic PS effective against both bacterial groups.37 This critical discovery guided the efforts in exploring PDT as a potential modality for the eradication of resistant pathogens.
Yeasts and fungal pathogens are variable in their cell envelopes, possessing outer wall mixtures of glucans, mannan and chitin polymers. This feature makes them inherently more permeable to external substances than Gram-negative bacteria. The hypothesis that cationic PS would more efficient in PDI has been tested in Candida albicans, arguably the most common fungal pathogen. C. albicans has been inactivated by the PSs Photofrin (Porfimer sodium), RB and Al(III)-tetrasulphonated phthalocyanine.38–40
Overcoming the permeability barrier and subsequently enhancing the PDI efficacy has been addressed recently both in bacteria using the polycationic biopolymer chitosan41 and fungi employing saponins.42
Antibiotic-Resistant Pathogens
Methicillin-resistant Staphylococcus aureus (MRSA) is estimated to cause ∼19,000 deaths per year in the United States.43 Apart from their high mortality rate, MRSA infections lead to an estimated $3–4 billion of additional health care costs per year. Furthermore, the rising prevalence of MRSA increases the likelihood that vancomycin-resistant S. aureus (VRSA)44—just as deadly as MRSA but more challenging to treat—will become a new scourge in hospitals. Vancomycin-resistant Enterococcus faecalis (VRE) has been also a common threat in hospital settings for at least 15 years.45 An important feature of antimicrobial PDT is that antimicrobial-resistant isolates are just as susceptible to PDT as their naïve counterparts are, as best demonstrated by a reduction in survival fractions between MRSA and wild-type S. aureus.46 The use of the phenothiazinium dye TBO with a 632.8 nm He laser can completely eradicate MRSA.46 A number of PS, including cationic substituted Zn(II)-phthalocyanines,47 poly-S-lysine-porphyrin conjugates,48 meso-tetrahydroporphyrin, tetrahydroporphyrin-tetratosylat (THPTS),49,50 and cationic water-soluble gallium(III) phthalocyanines (GaPcs),51 can substantially reduce MRSA populations (4–5 log10). Moreover, it has been shown that a PS conjugate of polyethylenimine and chlorin(e6) (pEI-ce6) in concert with red light is capable of reducing MRSA colony viable counts by 2.7 log10 in a murine skin abrasion model.52 MB was active against VRE53 as well as vancomycin-porphyrin conjugates are able to eliminate in vitro vancomycin-sensitive and vancomycin-resistant Enterococci.54
Multidrug-resistant (MDR) and pandrug-resistant (PDR) Gram-negative bacteria are less prevalent than MRSA but pose an equally grave threat of truly untreatable infections.55,56 In a model study, it was demonstrated that 60 MDR Pseudomonas aeruginosa isolates could be killed (up to 6–7 log10 reduction in viable cell counts) using TBO-PDT.57 This study also demonstrates that antibiotic-resistant P. aeruginosa was just as susceptible to PDI as antibiotic-susceptible strains. Another report demonstrated that PDI with cationic phthalocyanines has a substantial phototoxic effect in MDR strains of Aeromonas hydrophila.58 Using the PS Tri-P(4), other investigators have obtained PDI reduction of Yersinia enterolitica viable counts by 5 log10.59
MDR and extensively drug-resistant (XDR) strains of Mycobacterium tuberculosis (MDR-TB and XDR-TB), are a rising threat in the developing world.60 MDR-TB treatment requires a 2 y course of antibiotics with serious side effects; XDR-TB is even more difficult to cure and often fatal.61 PDT for TB studies have been focused on the homologous system M. bovis Bacille de Calmette et Guerin (BCG) both in vitro employing phenothiaziniums and in murine models of localized mycobacterial induced granulomatous infection.62,63 In a similar fashion the effects of PDT have been assessed in infections caused by M. marinum64 and on rapidly growing nontuberculous mycobacteria keratitis.65
Candida spp are the third leading cause of catheter-related infections, and are associated with the highest mortality of all catheter-related infections.66 Although prevention of invasive candidiasis using azole prophylaxis can be effective in selected high-risk patient populations, selection for invasive infection by resistant non-albicans Candida species or molds is a potentially devastating consequence. Despite improvements in antifungal therapy, the high attributable mortality rate due to Candida infections has improved little from two decades ago. Even with appropriate therapy, attributable mortality remains 15–49%.67 Moreover, an episode of candidemia significantly increases length of hospital stay and cost of care. In one analysis, the estimated cost of an episode of care for candidemia was $34,123 per Medicare patient and $44,536 per private insurance patient (1997 US$), with an overall economic impact of $2 billion annually in the US.68
It has also been demonstrated that C. albicans biofilms are sensitive to Photofrin PDT and that C. albicans germ tubes, which are able to survive H2O2 stress, have been eliminated by PDT.69 A recent study employing Photogem® as a PS with a light emitting diode (LED) had a significant effect in PDI against fluconazole-resistant C. albicans and C. glabrata.70
PDI for Fungal Pathogens
Cryptococcus neoformans is an encapsulated yeast that may cause cryptococcosis, a potentially fatal disease affecting immunocompromised patients, which occurs with the inhalation of the infectious inoculum. In an attempt to explore the role of the cell wall integrity pathway in PDI, Fuchs et al. has shown that employing pEI-ce6 in combination with 665 nm red light leads to reduction in C. neoformans KN99α (wild-type serotype with intact cell wall) viability by 2 log10 whereas the PDI-effect in the isogenic mutant rom2 (cell wall defective) was substantially higher (4 log10). PDI employing pEIce6 in concert with the cell wall specific antifungal capsofungin, potentiates cell killing.71
PDT also has promising potential in the treatment of superficial fungal skin infections caused by dermatophytes. Trichophyton rubrum is responsible for Tinea pedis (athelete's foot), fungal folliculitis, onychomycosis and dermatophytosis (ringworm). Employing an ex vivo infection model of human stratum corneum of T. rubrum, Smijs et al. incubated samples with the PS 5,10,15-tris(4-methylpyridinium)-20-phenyl-(21H,23H)-porphine trichloride (Sylsens B) and deuteroporphyrin monomethylester.72 Upon light application, both PS were shown to be active antifungals. Moreover, 5-aminolevulinic acid (5-ALA) and red light has an effect in the treatment of onychomycosis.73
The list of PDT-inactivated fungi includes Trychophyton mentagrophytes, Trychophyton tonsurans, Microsporum cookei, Microscporum gypseum, Microsporum canis, Epidermophyton floccosum, Nannizia cajetani, Metarhizium anisopliae, Aspergillus nidulans, A. fumigatus and Fusarium sp, employing a variety of PS, offering a new avenue for antifungal therapies.74,75
PDI and Multidrug Efflux
The role of multidrug efflux in antimicrobial PDT is a fairly recent trend under investigation. Efflux mechanisms are broadly recognized as major components of resistance to many classes of antimicrobials.76 Efflux occurs due to the activity of membrane transporter proteins widely known as multidrug efflux systems (MES).77,78 MES are implicated in a variety of physiological roles other than efflux and identifying natural substrates and inhibitors is an active and expanding research topic.79 There is an apparent structural similarity between designated efflux substrates and a number of PS especially in their amphilicity. Therefore, it is important to understand how the interaction of PS with efflux systems will affect their PDI potential. This has been explored for phenothiazinium PS and both bacterial and fungal MES.80,81 This interaction seems to be less obvious for different PS chemotypes. Porphyrin uptake and efflux seems to be regulated by the TolC system in E. coli.82 In Streptococcus agalactiae two coregulated efflux transporters modulate intracellular heme and protoporphyrin IX availability.83 The participation of MES in porphyrin-mediated PDI has been implied in ATP Binding Cassette (ABC) mammalian systems.84 In contrast, the PDI pattern of amphipilic protoporphyrin diarginate PPArg(2) in a variety of efflux-related S. aureus strains showed no correlation for the PS with MES.85
The use of small molecules that block MES, known as multi-drug efflux pump inhibitors (EPIs), in combination with conventional antibiotics has been proposed as a plausible antimicrobial alternative. An array of biochemical approaches have yielded a number of promising EPIs in a series of pathogenic systems.86 This synergistic discovery platform has been exploited in PDT for the potentiation of the phototoxic action of PS that are designated substrates of efflux systems.87 It has been shown that near-infrared light can cause selective photodamage of multidrug-resistant pathogens.88 In a recent study, it has been demonstrated that photodamage of multidrug-resistant Gram-positive and Gram-negative bacteria by near-infrared (870 nm/930 nm) light potentiates erythromycin, tetracycline and ciprofloxacin.89 Although the antibiotics used in this study are MES substrates and therefore it is reasonable to assume that near infrared light may play role in efflux inhibition the experimental evidence is rather weak and this possibility requires further exploration. The mechanism is hypothetical at this stage and not clearly distinct from PDI as it potentially involves an optically mediated mechano-transduction of cellular redox pathways, decreasing DeltaPsi and increasing ROS.
Biofilm Eradication
Microorganisms in nature thrive through adherence to both living and inanimate surfaces, doing so via forming biofilms.90 Biofilms have been found to be involved in approximately 80% of all infections. The dense and protected environment of the film as well as the significantly different properties from free-floating bacteria of the same species have been implicated to as much as 1,000-fold resistance to detergents, antiseptics and antibiotics.91 There is an expanding body of literature regarding PDT-based biofilm eradication strategies, with emphasis in the use of different PS for biofilm related phenotypes and microbial species.92 By using isogenic pairs of wild-type and transposon mutants deficient in capsular polysaccharide and slime production in S. epidermidis and S. aureus, it has been established that the cat-ionic PSs pL-ce6 and MB can overcome the protective effect of extracellular slime and stationary bacterial growth to PDI susceptibility.93 TBO has a substantial impact on PDI of staphylococcal biofilms decreasing cell numbers (5 log10 after irradiation with red light) disrupting biofilm architecture and suggests damage to bacterial cell membranes.94 PDI with merocyanine 540 has a comparable effect in viability of biofilms from both Gram-positive pathogens when 400 Jcm-2 green light is used.95,96 PDI with the cationic porphyrin, tetra-substituted N-methyl-pyridylporphine (TMP) was effective in both biofilm models when combined with antibiotics or host defense mechanisms.97,98
Tri-meso (N-methyl-pyridyl), meso (N-tetradecyl-pyridyl) porphine (C14) has significantly better PDI effect in the eradication of S. epidermidis biofilms when compared with the parental tetra-substituted N-methyl-pyridyl-porphine (C1).99 TBO-mediated PDI has an impact on Streptococcus mutans biofilms in different maturity stages (4 log10 with red light for mature biofilms),100 as well as mature S. sobrinus and S. sanguinis biofilms.101 Erythrosine was found to inactivate S. mutans biofilms better than MB and protoporphyrin and this effect was enhanced at 2 log10 by light fractionation.102,103 Erythrosine is also more potent than MB against Aggregatibacter actinomycetemcomitans biofilms, a system where the anionic PS RB and TBO have a considerable impact.104,105 PDI mediated by both 5-ALA and TMP at different concentrations can eliminate P. aeruginosa biofilms.106,107
PDI eradication of microbial biofilms would address a variety of challenging clinical conditions. This list includes urinary tract infections, catheter infections, middle-ear infections, sinusitis, formation of dental plaque108 periodontitis,109 gingivitis, endodontics,110 osteomyelitis111 infected contact lenses, endocarditis, infections in cystic fibrosis112 and infections of permanent indwelling devices such as joint prostheses and heart valves and implants.113 In the head and neck area, biofilms are a major etiologic factor in periodontitis, wound infections, oral candidiasis,114 and sinus and ear infections. Peri-implantitis involves the bacterial colonization, typically in the form of biofilms, of implant surfaces and may lead to patient infection and damage to the implant surface. Dörtbudak et al. used TBO PDT to successfully decontaminate implants with bacterial colonization of 15 patients, leading to the reduction in bacterial counts by approximately 2 log10.115
One of the major security and bioterrorism threats of the 21st century is that of Bacillus anthracis, which cannot be inactivated by heat, antibiotics or other antimicrobial agents. Demidova and Hamblin demonstrated that Bacillus spp spores, some of which are more robust than that of B. anthracis, may be destroyed by the phenothiazinium dyes dimethylmethylene blue, MB, new methylene blue, and TBO with the application of red light.116 Oliveira et al. demonstrated that Bacillus cereus endospores could be inactivated by porphyrin PS and light.117 This suggests that PDT may be actively applied in military and national security applications for the decontamination of anthrax spores.
PS Delivery and Nanomedicine
The unique physical and chemical properties of nanoparticles, particularly their small size and high surface-to-volume ratio, allow this technology to surpass barriers and gain access to biologic molecules and systems. Since modern science permits the manipulation of nanosized materials, the size, shape and chemical characteristics may be altered in order to facilitate molecular interactions. As such, they can be engineered as vehicles to carry various therapeutic or diagnostic agents and are potentially useful for medical applications including targeted drug delivery, gene therapy and cell labeling.118
PDT has also attracted the interest of nanotechnology as the effectiveness of the treatment can be greatly enhanced by the use of nanoparticles. In the last decade, different approaches to the combination of nanoparticles and PDT have been investigated in relation to the antimicrobial applications of the technique. One use of the nanoparticles is to improve the delivery of PS to the bacteria; others use the nanoparticles to improve the inactivation kinetics.119 Many of the PSs being studied for PDI of bacteria are based on the tetrapyrrole nucleus, such as porphyrins, chlorins and phthalocyanines, are lipophilic and easily form aggregates in aqueous solution, resulting in the loss of photosensitizing activity.120,121 To overcome this problem, suitable PS carriers were designed to deliver PSs, e.g., liposomes,120,122,123 micelles124 and nanoparticles.125,126 Among these systems, liposomes are most commonly employed to incorporate lipophilic PSs and have been proved to enhance the antimicrobial PDI of various PSs, not only because liposomes increase the solubility and stability of PSs, but also because they can facilitate the penetration of PSs into bacteria by means of fusion processes or disturbing the cell walls.127 However, these reported liposomal formulations mainly aimed to deliver PSs passively, while little research was done to apply liposomes actively targeted to bacteria for PDI of bacteria.
The Question of Resistance Development of Pathogenic Microorganisms to PDI
The studies and reports discussing the potential of microbes to develop resistance to PDT are scattered and quite controversial. The non-selective nature of antimicrobial PDI appears as a competitive advantage in the activation of a specific microbial resistance pathway. In a conventional biological study of routine stress followed by re-growth, 5,10,15-tris(1-methylpyridinium4-yl)-20-(pentafluorophenyl)-porphyrin triiodide [Tri-Py(+)Me-PF] was employed as PS and V. fischeri and E. coli were used as model cells. After ten cycles of partial inactivation followed by re growth, neither of the bacteria developed resistance to the photodynamic process.128
Superoxide dismutase is upregulated following protoporphyrin-mediated PDI in S. aureus and RB-mediated PDI in S. mutans induces the bacterial heat shock protein—responsible for refolding denatured proteins to native conformations and stabilizing lipid membranes during stress—GroEL expression.129 These observations are in accordance with those of St. Denis et al. who demonstrated that sub-lethal PDI stress increased the expression of the two major bacterial heat shock proteins GroEL and Dnak and that exposing E. coli and E. faecalis to heat pretreatment prior to PDI (a positive upregulator) conferred stress tolerance, increasing E. coli cell viability by 2 log10 and E. faecalis cell viability by 4 log10. PDI with RB in the yeast S. cerevisae demonstrated a role of Yap1p and Skn7p in the defense against singlet oxygen.130
From a Conventional Platform to the Alignment with the “Microbial Phenotype”
Advances in microbial physiology shed light on a series of pathways, components and phenotypes that may serve as potential alternative and attractive targets for antimicrobial drug discovery. Recent studies have dissected social (intercellular) interaction at the molecular level through analysis of both synthetic and natural microbial populations.131 These approaches have revealed novel molecular mechanisms that stabilize cooperation among cells and define new roles of population structure for the evolution of cooperative interactions. This knowledge of interaction parameters is changing the view of microbial processes, with emphasis on pathogenesis and antibiotic resistance, and suggests new ways to fight infection by exploiting social interaction.131 Evidently, bacteria have the ability to enter into a dormant (non-dividing) state. The molecular mechanisms that underlie the formation of dormant persister cells are now being unraveled.132 Accumulating evidence suggests that seemingly disparate phenomena as latent bacterial infections, un-culturable microorganisms and biofilm multidrug tolerance are defined by persisters.132 Targeting bacterial virulence factors is also a novel approach under investigation for the development of new antimicrobials that can be used to disarm pathogens in the host.133
The broad-spectrum activity and the non-specific action of antimicrobial PDI should be explored deeper to address these biological phenomena. There is no documented evidence on whether PDT can disrupt these sophisticated microbial defensive lines. We have to take into account that photoinactivation is able to eradicate microorganisms without discriminating resistant isolates, both planktonic and biofilm species. This is in concert with the potential of localized photooxidative stress to inactivate virulence factors134,135 and virulence determinants136–138 in the absence of any documented conventional resistance mechanism. The possibility of active efflux seems to be related with some but not all the molecular classes of PSs although improved delivery methods may overcome this barrier. A single antimicrobial PDT treatment in vitro potently inactivated protease activity and resulted in a 4 log10 reduction in the viability of P. gingivalis.139 Dose and time-of-exposure experiments revealed that protease inactivation occurred at lower concentrations of PS and less time of light exposure. Also, antimicrobial PDT treatment has been shown to be potently and functionally inactivated IL-1β and TNFα.139
Antimicrobial PDT: From Bench Top to Bed Side
With the results from in vitro and animal studies being promising, a number of clinical applications for antimicrobial PDT have been tested and performed in vivo. PDT has been proposed for many dental applications due to the accessibility to the oral cavity. In contrast the complexity of oral microflora makes this microenvironment quite challenging for the deployment of novel antimicrobials. It was demonstrated that phenothiaziniummediated PDT and 660 nm light in root canals infected by the predominant endodontic pathogen E. faecalis leads to 99.9% reduction in viability (TBO) in an approximate 97% reduction in viable enterococci (MB).140,141 Oral biofilms often exacerbate healing of root canals, which led Garcez et al. to demonstrate that P. aeruginosa and Proteus mirabilis biofilms could be reduced in root canals of extracted teeth. Using the pEI-ce6 PS conjugate in concert with 660 nm diode laser, PDT reduced detected cell viability by 95%.142
Helicobacter pylori infections are responsible for stomach ulcers, associated with severe morbidity and contribute to the development of adenocarcinoma of the stomach lining.143 Milson et al. demonstrated that TBO was capable of reducing H. mustelae viability by 90% in infected ferret stomachs.144 A clinical trial with 13 H. pylori-infected patients exposed to oral 5-aminolevulinic acid and 410 nm endoscopic light resulted in the significant viability reduction.145 An important observation is that H. pylori naturally accumulates porphyrins, which may then act as endogenous PS.146 This is actually also true in H. pylori infected patients, whom the application of 405 nm endoscopic light alone was capable of reducing Colony Forming Unit (CFU) counts by about 90%.147
PDT's very nature makes it ideal for the treatment of skin, wound and burn infections, all of which are easily accessible for light therapies.148–150 XF73, a cationic porphyrin PS, is able to reduce MRSA growth by >3 log10 in a porcine skin infection model.151 PDT with polycationic PS conjugates and 665 nm light in murine excisional wounds led to a reduction in infectious organisms, permitting mouse survival to reach 90%, and reduce substantially pathogen viability.152 TBO and light cured mice of otherwise fatal Vibrio vulnificus wound infections in murine models.153 Burns often become infected due to impaired immune responses, destruction of skin vasculature and the cutaneous barrier; accordingly, burn patients typically die of resistant nosocomial infections. PDT was effective in treating Acinetobacter baumannii burn infections in mice, reducing viability of the pathogens at 3 log10.154
Finally, PDT may have prospective applications in the treatment of soft tissue infections. An anti-P. aeruginosa monoclonal antibody-conjugated tin(IV) chlorin(e6) PS and 630 nm light leads to a drop of a >75% in the number of viable P. aeruginosa in a subcutaneous pseudomonad infection.155
Several new PDT clinical applications have been developed in recent years. Ondine Biomedical has a large clinical trial in progress using MB-PDT for nasal decontamination of MRSA before surgery (www.ondinebio.com/wp-content/uploads/2011/04/OBP-NR-041511-Final.pdf). The same company is planning a second clinical trial of photodisinfection for the in situ microbial disinfection of endotracheal tubes as a means to prevent ventilator associated pneumonia (www.ondinebio.com/wp-content/uploads/2011/05/OBP-NR-051011-Final.pdf). A related company called Sinuwave is exploring the use of MB-PDT to combat chronic sinusitis (www.sinuwave.com).
Conclusion
PDT is not a conventional drug discovery platform since three elements (PS, visible light and oxygen) are essential for successful deployment. Though successful methodology to treat infectious disease with PDT will be evolved in due course of time, it is important to realize that photoinactivation of microbes is an exclusively localized process and many other infectious diseases may continue to need systemic therapy unless PDT therapy is developed which can stimulate the host immune system. It is well established in that PDT in anticancer therapy induces host immune responses that have components of innate and adaptive immune systems. In principle the same process should operate when infections are treated with PDT. The effect of PDT on the host immune system is an important implication of PDT that is an open avenue that requires investigation in the area of infection. Researchers have bypassed some of the difficulties associated with new antimicrobial development by developing facile whole-animal screens that utilize the well-studied nematode Caenorhabditis elegans, the great wax moth Galleria mellonella and the fruit fly Drosophila melanogaster as model hosts to identify and develop new classes of antimicrobial agents with anti-virulence or immunomodulatory efficacy and evaluate toxicity or efficacy. The amenability of these non-vertebrate hosts to large screens has made these model hosts useful to identify or develop active compounds against either bacterial or fungal pathogens.156–158 Therefore, the design of host-pathogen studies exploring the ability of PDT to interfere with virulence determinants requires sophisticated tools and approaches. The recent example of a host-parasite model to assess intracellular targeting specificity of novel phthalocyanines159 will inspire similar explorations.
Acknowledgments
Research conducted by Tyler G. St. Denis was supported by the Columbia University I. I. Rabi Fellows Program. George P. Tegos is supported by the NIH (grant 5U54MH084690-02). Research conducted in the Hamblin Laboratory was supported by NIH (RO1 AI050875 to M.R.H.) and US Air Force MFEL Program (FA9550-04-1-0079). T.D. was partially supported by a Bullock-Wellman Fellowship Award and an Airlift Research Foundation Extremity Trauma Research Grant (grant 109421).
References
- 1.Sigerist H. The Great Doctors. New York: Dover Publications; 1971. p. 371. [Google Scholar]
- 2.Livermore DM. Antibiotic resistance in staphylococci. Int J Antimicrob Agents. 2000;16:3–10. doi: 10.1016/S0924-8579(00)00299-5. [DOI] [PubMed] [Google Scholar]
- 3.Kraus CN. Low hanging fruit in infectious disease drug development. Curr Opin Microbiol. 2008;11:434–438. doi: 10.1016/j.mib.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Vicente M, Hodgson J, Massidda O, Tonjum T, Henriques-Normark B, Ron EZ. The fallacies of hope: will we discover new antibiotics to combat pathogenic bacteria in time? FEMS Microbiol Rev. 2006;30:841–852. doi: 10.111/j.1574-6976.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- 5.Butler MS, Buss AD. Natural products-the future scaffolds for novel antibiotics? Biochem Pharmacol. 2006;71:919–929. doi: 10.1016/j.bcp.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Nicolau DP. Current challenges in the management of the infected patient. Curr Opin Infect Dis. 2011;24:1–10. doi: 10.1097/01.qco.0000393483.10270.ff. [DOI] [PubMed] [Google Scholar]
- 7.Norrby SR, Nord CE, Finch R. Lack of development of new antimicrobial drugs: a potential serious threat to public health. Lancet Infect Dis. 2005;5:115–119. doi: 10.1016/S1473-3099(05)01283-1. [DOI] [PubMed] [Google Scholar]
- 8.Cornaglia G, Giamarellou H, Rossolini GM. Metallobeta-lactamases: a last frontier for beta-lactams? Lancet Infect Dis. 2011;11:381–393. doi: 10.1016/S1473-3099(11)70056-1. [DOI] [PubMed] [Google Scholar]
- 9.Turner M. Microbe outbreak panics Europe. Nature. 2011;474:137. doi: 10.1038/474137a. [DOI] [PubMed] [Google Scholar]
- 10.Raab O. Ueber diewirkung fluoreszierender stoffe auf infusori. Z Biol. 1900;39:524–536. (Ger). [Google Scholar]
- 11.Mitton D, Ackroyd R. A brief overview of photodynamic therapy in Europe. Photodiagnosis Photodyn Ther. 2008;5:103–111. doi: 10.1016/j.pdpdt.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Dai T, Huang YY, Hamblin MR. Photodynamic therapy for localized infections—state of the art. Photodiagn Photodyn Ther. 2009;6:170–188. doi: 10.1016/j.pdpdt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–387. doi: 10.1038/nrc1071. [DOI] [PubMed] [Google Scholar]
- 14.Hunt DW. Rostaporfin (Miravant Medical Technologies) IDrugs. 2002;5:180–186. [PubMed] [Google Scholar]
- 15.Nitzan Y, Gutterman M, Malik Z, Ehrenberg B. Inactivation of gram-negative bacteria by photosensitized porphyrins. Photochem Photobiol. 1992;55:89–96. doi: 10.1111/j.1751-097.1992.tb04213.x. [DOI] [PubMed] [Google Scholar]
- 16.Foote CS. Definition of type I and type II photosensitized oxidation. Photochem Photobiol. 1991;54:659. doi: 10.1111/j.1751-097.1991.tb02071.x. [DOI] [PubMed] [Google Scholar]
- 17.Tanielian C, Mechin R, Seghrouchni R, Schweitzer C. Mechanistic and kinetic aspects of photosensitization in the presence of oxygen. Photochem Photobiol. 2000;71:12–19. doi: 10.1562/0031-8655(2000)071<0012:MAKAOp>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 18.Ochsner M. Photophysical and photobiological processes in the photodynamic therapy of tumours. J Photochem Photobiol B. 1997;39:1–18. doi: 10.1016/S1011-1344(96)07428-3. [DOI] [PubMed] [Google Scholar]
- 19.Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 20.Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol and ascorbate. Arch Biochem Biophys. 1993;300:535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 21.Singleton DA, Hang C, Szymanski MJ, Meyer MP, Leach AG, Kuwata KT, et al. Mechanism of ene reactions of singlet oxygen. A two-step no-intermediate mechanism. J Am Chem Soc. 2003;125:1319–1328. doi: 10.1021/ja027225p. [DOI] [PubMed] [Google Scholar]
- 22.Leach AG, Houk KN. Diels-Alder and ene reactions of singlet oxygen, nitroso compounds and triazolinediones: transition states and mechanisms from contemporary theory. Chem Commun (Camb) 2002:1243–1255. doi: 10.1039/b111251c. [DOI] [PubMed] [Google Scholar]
- 23.Wainwright M, Byrne MN, Gattrell MA. Phenothiazinium-based photobactericidal materials. J Photochem Photobiol B. 2006;84:227–230. doi: 10.1016/j.jphotobiol.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi PK, Toups HJ, Greenberg DB, Dimopoullos GT, Rusoff LL. Irradiation of Escherichia coli in the visible spectrum with a tunable organic-dye laser energy source. Appl Microbiol. 1975;29:63–67. doi: 10.1128/am.29.1.63-67.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang L, Huang YY, Mroz P, Tegos GP, Zhiyentayev T, Sharma SK, et al. Stable synthetic cationic bacteriochlorins as selective antimicrobial photosensitizers. Antimicrob Agents Chemother. 2010;54:3834–3841. doi: 10.1128/AAC.00125-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization. Photodiagn Photodyn Ther. 2004;1:279–293. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schäfer M, Schmitz C, Facius R, Horneck G, Milow B, Funken KH, et al. Systematic study of parameters influencing the action of Rose Bengal with visible light on bacterial cells: comparison between the biological effect and singlet-oxygen production. Photochem Photobiol. 2000;71:514–523. doi: 10.1562/0031-8655(2000)071<0514:SSOPIT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Kubin A, Wierrani F, Jindra RH, Loew HG, Grunberger W, Ebermann R, et al. Antagonistic effects of combination photosensitization by hypericin, mesotetrahydroxyphenylchlorin (mTHPC) and photofrin II on Staphylococcus aureus. Drugs Exp Clin Res. 1999;25:13–21. [PubMed] [Google Scholar]
- 29.Harris F, Chatfield LK, Phoenix DA. Phenothiazinium based photosensitisers-photodynamic agents with a multiplicity of cellular targets and clinical applications. Curr Drug Targets. 2005;6:615–627. doi: 10.2174/1389450054545962. [DOI] [PubMed] [Google Scholar]
- 30.Tegos GP, Demidova TN, Arcila-Lopez D, Lee H, Wharton T, Gali H, et al. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem Biol. 2005;12:1127–1135. doi: 10.1016/j.chembiol.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang L, Terakawa M, Zhiyentayev T, Huang YY, Sawayama Y, Jahnke A, et al. Innovative cationic fullerenes as broad-spectrum light-activated antimicrobials. Nanomedicine. 2010;6:442–452. doi: 10.1016/j.nano.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fisher WG, Partridge WP, Jr, Dees C, Wachter EA. Simultaneous two-photon activation of type-I photodynamic therapy agents. Photochem Photobiol. 1997;66:141–155. doi: 10.1111/j.1751-097.1997.tb08636.x. [DOI] [PubMed] [Google Scholar]
- 33.Bulina ME, Chudakov DM, Britanova OV, Yanushevich YG, Staroverov DB, Chepurnykh TV, et al. A genetically encoded photosensitizer. Nat Biotechnol. 2006;24:95–99. doi: 10.1038/nbt1175. [DOI] [PubMed] [Google Scholar]
- 34.Oriel S, Nitzan Y. Photoinactivation of Candida albicans by its own endogenous porphyrins. Curr Microbiol. 2010;60:117–123. doi: 10.1007/s00284-009-9514-8. [DOI] [PubMed] [Google Scholar]
- 35.MacLean M, Macgregor SJ, Anderson JG, Woolsey GA. The role of oxygen in the visible-light inactivation of Staphylococcus aureus. J Photochem Photobiol B. 2008;92:180–184. doi: 10.1016/j.jphotobiol.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Minnock A, Vernon DI, Schofield J, Griffiths J, Parish JH, Brown SB. Mechanism of uptake of a cationic water-soluble pyridinium zinc phthalocyanine across the outer membrane of Escherichia coli. Antimicrob Agents Chemother. 2000;44:522–527. doi: 10.1128/AAC.44.3.522-7.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lazzeri D, Rovera M, Pascual L, Durantini EN. Photodynamic studies and photoinactivation of Escherichia coli using meso-substituted cationic porphyrin derivatives with asymmetric charge distribution. Photochem Photobiol. 2004;80:286–293. doi: 10.1562/2004-03-08-RA-105.1. [DOI] [PubMed] [Google Scholar]
- 38.Bliss JM, Bigelow CE, Foster TH, Haidaris CG. Susceptibility of Candida species to photodynamic effects of photofrin. Antimicrob Agents Chemother. 2004;48:2000–2006. doi: 10.1128/AAC.48.6.2000-6.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lazarova G. Effect of glutathione on rose bengal photosensitized yeast damage. Microbios. 1993;75:39–43. [PubMed] [Google Scholar]
- 40.Bertoloni G, Rossi F, Valduga G, Jori G, Ali H, van Lier JE. Photosensitizing activity of water- and lipid-soluble phthalocyanines on prokaryotic and eukaryotic microbial cells. Microbios. 1992;71:33–46. [PubMed] [Google Scholar]
- 41.Tsai T, Chien HF, Wang TH, Huang CT, Ker YB, Chen CT. Chitosan augments photodynamic inactivation of gram-positive and gram-negative bacteria. Antimicrob Agents Chemother. 2011;55:1883–1890. doi: 10.1128/AAC.00550-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coleman JJ, Okoli I, Tegos GP, Holson EB, Wagner FF, Hamblin MR, et al. Characterization of plant-derived saponin natural products against Candida albicans. ACS Chem Biol. 2010;5:321–332. doi: 10.1021/cb900243b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gould IM. VRSA-doomsday superbug or damp squib? Lancet Infect Dis. 2010;10:816–818. doi: 10.1016/S1473-3099(10)70259-0. [DOI] [PubMed] [Google Scholar]
- 45.Chavers LS, Moser SA, Benjamin WH, Banks SE, Steinhauer JR, Smith AM, et al. Vancomycin-resistant enterococci: 15 years and counting. J Hosp Infect. 2003;53:159–171. doi: 10.1053/jhin.2002.1375. [DOI] [PubMed] [Google Scholar]
- 46.Hajim KI, Salih DS, Rassam YZ. Laser light combined with a photosensitizer may eliminate methicillin-resistant strains of Staphylococcus aureus. Lasers Med Sci. 2010;25:743–748. doi: 10.1007/s10103-010-0803-z. [DOI] [PubMed] [Google Scholar]
- 47.Soncin M, Fabris C, Busetti A, Dei D, Nistri D, Roncucci G, et al. Approaches to selectivity in the Zn(II)-phthalocyanine-photosensitized inactivation of wild-type and antibiotic-resistant Staphylococcus aureus. Photochem Photobiol Sci. 2002;1:815–819. doi: 10.1039/b206554a. [DOI] [PubMed] [Google Scholar]
- 48.Tomé JP, Neves MG, Tomé AC, Cavaleiro JA, Soncin M, Magaraggia M, et al. Synthesis and antibacterial activity of new poly-S-lysine-porphyrin conjugates. J Med Chem. 2004;47:6649–6652. doi: 10.1021/jm040802v. [DOI] [PubMed] [Google Scholar]
- 49.Schastak S, Ziganshyna S, Gitter B, Wiedemann P, Claudepierre T. Efficient photodynamic therapy against gram-positive and gram-negative bacteria using THPTS, a cationic photosensitizer excited by infrared wavelength. PLoS ONE. 2010;5:116174. doi: 10.1371/journal.pone.0011674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schastak S, Gitter B, Handzel R, Hermann R, Wiedemann P. Improved photoinactivation of gram-negative and gram-positive methicillin-resistant bacterial strains using a new near-infrared absorbing mesotetrahydroporphyrin: a comparative study with a chlorine e6 photosensitizer photolon. Methods Find Exp Clin Pharmacol. 2008;30:129–133. doi: 10.1358/mf.2008.30.2.1165448. [DOI] [PubMed] [Google Scholar]
- 51.Mantareva V, Kussovski V, Angelov I, W”hrle D, Dimitrov R, Popova E, et al. Non-aggregated Ga(III)-phthalocyanines in the photodynamic inactivation of planktonic and biofilm cultures of pathogenic microorganisms. Photochem Photobiol Sci. 2011;10:91–102. doi: 10.1039/b9pp00154a. [DOI] [PubMed] [Google Scholar]
- 52.Dai T, Tegos GP, Zhiyentayev T, Mylonakis E, Hamblin MR. Photodynamic therapy for methicillin-resistant Staphylococcus aureus infection in a mouse skin abrasion model. Lasers Surg Med. 2010;42:38–44. doi: 10.1002/lsm.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wainwright M, Phoenix DA, Gaskell M, Marshall B. Photobactericidal activity of methylene blue derivatives against vancomycin-resistant Enterococcus spp. J Antimicrob Chemother. 1999;44:823–825. doi: 10.1093/jac/44.6.823. [DOI] [PubMed] [Google Scholar]
- 54.Xing B, Jiang T, Bi W, Yang Y, Li L, Ma M, et al. Multifunctional divalent vancomycin: the fluorescent imaging and photodynamic antimicrobial properties for drug resistant bacteria. Chem Commun (Camb) 2011;47:1601–1603. doi: 10.1039/c0cc04434b. [DOI] [PubMed] [Google Scholar]
- 55.Nordmann P, Naas T, Fortineau N, Poirel L. Superbugs in the coming new decade; multidrug resistance and prospects for treatment of Staphylococcus aureus, Enterococcus spp. and Pseudomonas aeruginosa in 2010. Curr Opin Microbiol. 2007;10:436–440. doi: 10.1016/j.mib.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 56.Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5:939–951. doi: 10.1038/nrmicro1789. [DOI] [PubMed] [Google Scholar]
- 57.Tseng SP, Teng LJ, Chen CT, Lo TH, Hung WC, Chen HJ, et al. Toluidine blue O photodynamic inactivation on multidrug-resistant Pseudomonas aeruginosa. Lasers Surg Med. 2009;41:391–397. doi: 10.1002/lsm.20765. [DOI] [PubMed] [Google Scholar]
- 58.Kussovski V, Mantareva V, Angelov I, Orozova P, W”hrle D, Schnurpfeil G, et al. Photodynamic inactivation of Aeromonas hydrophila by cationic phthalocyanines with different hydrophobicity. FEMS Microbiol Lett. 2009;294:133–140. doi: 10.1111/j.1574-6968.2009.01555.x. [DOI] [PubMed] [Google Scholar]
- 59.Trannoy LL, Terpstra FG, de Korte D, Lagerberg JW, Verhoeven AJ, Brand A, et al. Differential sensitivities of pathogens in red cell concentrates to Tri-P(4)-photoinactivation. Vox Sang. 2006;91:111–118. doi: 10.1111/j.1423-0410.2006.00791.x. [DOI] [PubMed] [Google Scholar]
- 60.Jassal M, Bishai WR. Extensively drug-resistant tuberculosis. Lancet Infect Dis. 2009;9:19–30. doi: 10.1016/S1473-3099(08)70260-3. [DOI] [PubMed] [Google Scholar]
- 61.Keshavjee S, Gelmanova IY, Farmer PE, Mishustin SP, Strelis AK, Andreev YG, et al. Treatment of extensively drug-resistant tuberculosis in Tomsk, Russia: a retrospective cohort study. Lancet. 2008;372:1403–1409. doi: 10.1016/S0140-6736(08)61204-0. [DOI] [PubMed] [Google Scholar]
- 62.O'Riordan K, Sharlin DS, Gross J, Chang S, Errabelli D, Akilov OE, et al. Photoinactivation of Mycobacteria in vitro and in a new murine model of localized Mycobacterium bovis BCG-induced granulomatous infection. Antimicrob Agents Chemother. 2006;50:1828–1834. doi: 10.1128/AAC.50.5.1828-34.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O'Riordan K, Akilov OE, Chang SK, Foley JW, Hasan T. Real-time fluorescence monitoring of phenothiazinium photosensitizers and their anti-mycobacterial photodynamic activity against Mycobacterium bovis BCG in in vitro and in vivo models of localized infection. Photochem Photobiol Sci. 2007;6:1117–1123. doi: 10.1039/b707962a. [DOI] [PubMed] [Google Scholar]
- 64.Wiegell SR, Kongshoj B, Wulf HC. Mycobacterium marinum infection cured by photodynamic therapy. Arch Dermatol. 2006;142:1241–1242. doi: 10.1001/archderm.142.9.1241. [DOI] [PubMed] [Google Scholar]
- 65.Shih MH, Huang FC. Effects of photodynamic therapy on rapidly growing nontuberculous mycobacteria keratitis. Invest Ophthalmol Vis Sci. 2011;52:223–229. doi: 10.1167/iovs.10-5593. [DOI] [PubMed] [Google Scholar]
- 66.Crump JA, Collignon PJ. Intravascular catheter-associated infections. Eur J Clin Microbiol Infect Dis. 2000;19:1–8. doi: 10.1007/s100960050001. [DOI] [PubMed] [Google Scholar]
- 67.Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, et al. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003;37:1172–1177. doi: 10.1086/378745. [DOI] [PubMed] [Google Scholar]
- 68.Rentz AM, Halpern MT, Bowden R. The impact of candidemia on length of hospital stay, outcome and overall cost of illness. Clin Infect Dis. 1998;27:781–788. doi: 10.1086/514955. [DOI] [PubMed] [Google Scholar]
- 69.Chabrier-Roselló Y, Foster TH, Perez-Nazario N, Mitra S, Haidaris CG. Sensitivity of Candida albicans germ tubes and biofilms to photofrin-mediated phototoxicity. Antimicrob Agents Chemother. 2005;49:4288–4295. doi: 10.1128/AAC.49.10.4288-95.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dovigo LN, Pavarina AC, Mima EG, Giampaolo ET, Vergani CE, Bagnato VS. Fungicidal effect of photodynamic therapy against fluconazole-resistant Candida albicans and Candida glabrata. Mycoses. 2011;54:123–130. doi: 10.1111/j.1439-0507.2009.01769.x. [DOI] [PubMed] [Google Scholar]
- 71.Fuchs BB, Tegos GP, Hamblin MR, Mylonakis E. Susceptibility of Cryptococcus neoformans to photodynamic inactivation is associated with cell wall integrity. Antimicrob Agents Chemother. 2007;51:2929–2936. doi: 10.1128/AAC.00121-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smijs TG, Pavel S, Talebi M, Bouwstra JA. Preclinical studies with 5,10,15-Tris(4-methylpyridinium)-20-phenyl-[21H,23H]-porphine trichloride for the photodynamic treatment of superficial mycoses caused by Trichophyton rubrum. Photochem Photobiol. 2009;85:733–739. doi: 10.1111/j.1751-097.2008.00468.x. [DOI] [PubMed] [Google Scholar]
- 73.Donnelly RF, McCarron PA, Lightowler JM, Woolfson AD. Bioadhesive patch-based delivery of 5-aminolevulinic acid to the nail for photodynamic therapy of onychomycosis. J Control Release. 2005;103:381–392. doi: 10.1016/j.jconrel.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 74.Calzavara-Pinton PG, Venturini M, Sala R. A comprehensive overview of photodynamic therapy in the treatment of superficial fungal infections of the skin. J Photochem Photobiol B. 2005;78:1–6. doi: 10.1016/j.jphotobiol.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 75.Gonzales FP, da Silva SH, Roberts DW, Braga GU. Photodynamic inactivation of conidia of the fungi Metarhizium anisopliae and Aspergillus nidulans with methylene blue and toluidine blue. Photochem Photobiol. 2010;86:653–661. doi: 10.1111/j.1751-097.2009.00689.x. [DOI] [PubMed] [Google Scholar]
- 76.Alekshun MN, Levy SB. Molecular mechanisms of antibacterial multidrug resistance. Cell. 2007;128:1037–1050. doi: 10.1016/j.cell.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 77.Piddock LJ. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin Microbiol Rev. 2006;19:382–402. doi: 10.1128/CMR.19.2.382-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cannon RD, Lamping E, Holmes AR, Niimi K, Baret PV, Keniya MV, et al. Efflux-mediated antifungal drug resistance. Clin Microbiol Rev. 2009;22:291–321. doi: 10.1128/CMR.00051-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tegos G. In: Natural substrates and inhibitors of multidrug resistant pumps (MDRs) redefine the plant antimicrobials in naturally occurring bioactive compounds: a new and safe alternative for control of pests and microbial diseases. Carpinella C, Rai M, editors. Cambridge University Press; 2006. [Google Scholar]
- 80.Tegos GP, Hamblin MR. Phenothiazinium antimicrobial photosensitizers are substrates of bacterial multidrug resistance pumps. Antimicrob Agents Chemother. 2006;50:196–203. doi: 10.1128/AAC.50.1.196-203.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Prates RA, Kato IT, Ribeiro MS, Tegos GP, Hamblin MR. Influence of multidrug efflux systems on methylene blue-mediated photodynamic inactivation of Candida albicans. J Antimicrob Chemother. 2011;66:1525–1532. doi: 10.1093/jac/dkr160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tatsumi R, Wachi M. TolC-dependent exclusion of porphyrins in Escherichia coli. J Bacteriol. 2008;190:6228–6233. doi: 10.1128/JB.00595-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fernandez A, Lechardeur D, Derré-Bobillot A, Couvé E, Gaudu P, Gruss A. Two coregulated efflux transporters modulate intracellular heme and protoporphyrin IX availability in Streptococcus agalactiae. PLoS Pathog. 2010;6:1000860. doi: 10.1371/journal.ppat.1000860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morgan J, Jackson JD, Zheng X, Pandey SK, Pandey RK. Substrate affinity of photosensitizers derived from chlorophyll-a: the ABCG2 transporter affects the phototoxic response of side population stem cell-like cancer cells to photodynamic therapy. Mol Pharm. 2010 doi: 10.1021/mp100154j. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grinholc M, Zawacka-Pankau J, Gwizdek-Wisniewska A, Bielawski KP. Evaluation of the role of the pharmacological inhibition of Staphylococcus aureus multidrug resistance pumps and the variable levels of the uptake of the sensitizer in the strain-dependent response of Staphylococcus aureus to PPArg(2)-based photodynamic inactivation. Photochem Photobiol. 2010;86:1118–1126. doi: 10.1111/j.1751-097.2010.00772.x. [DOI] [PubMed] [Google Scholar]
- 86.Tegos GP, Haynes M, Strouse JJ, Khan MMT, Bologa CG, Oprea TI, et al. Microbial efflux inhibition; tactics & strategies. Curr Pharm Des. 2011 doi: 10.2174/138161211795703726. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tegos GP, Masago K, Aziz F, Higginbotham A, Stermitz FR, Hamblin MR. Inhibitors of bacterial multidrug efflux pumps potentiate antimicrobial photoinactivation. Antimicrob Agents Chemother. 2008;52:3202–3209. doi: 10.1128/AAC.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bornstein E, Hermans W, Gridley S, Manni J. Near-infrared photoinactivation of bacteria and fungi at physiologic temperatures. Photochem Photobiol. 2009;85:1364–1374. doi: 10.1111/j.1751-097.2009.00615.x. [DOI] [PubMed] [Google Scholar]
- 89.Bornstein E, Gridley S, Wengender P, Robbins A. Photodamage to multidrug-resistant gram-positive and gram-negative bacteria by 870 nm/930 nm light potentiates erythromycin, tetracycline and ciprofloxacin. Photochem Photobiol. 2010;86:617–627. doi: 10.1111/j.1751-097.2010.00725.x. [DOI] [PubMed] [Google Scholar]
- 90.Pflumm M. Caught on film. Nat Med. 2011;17:650–653. doi: 10.1038/nm0611-650. [DOI] [PubMed] [Google Scholar]
- 91.Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chemother. 2001;45:999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Biel MA. Photodynamic therapy of bacterial and fungal biofilm infections. Methods Mol Biol. 2010;635:175–194. doi: 10.1007/978-1-60761-697-9_13. [DOI] [PubMed] [Google Scholar]
- 93.Gad F, Zahra T, Hasan T, Hamblin MR. Effects of growth phase and extracellular slime on photodynamic inactivation of gram-positive pathogenic bacteria. Antimicrob Agents Chemother. 2004;48:2173–2178. doi: 10.1128/AAC.48.6.2173-8.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharma M, Visai L, Bragheri F, Cristiani I, Gupta PK, Speziale P. Toluidine blue-mediated photodynamic effects on staphylococcal biofilms. Antimicrob Agents Chemother. 2008;52:299–305. doi: 10.1128/AAC.00988-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lin HY, Chen CT, Huang CT. Use of merocyanine 540 for photodynamic inactivation of Staphylococcus aureus planktonic and biofilm cells. Appl Environ Microbiol. 2004;70:6453–6458. doi: 10.1128/AEM.70.11.6453-8.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sbarra MS, Di Poto A, Arciola CR, Saino E, Sharma M, Bragheri F, et al. Photodynamic action of merocyanine 540 on Staphylococcus epidermidis biofilms. Int J Artif Organs. 2008;31:848–857. doi: 10.1177/039139880803100914. [DOI] [PubMed] [Google Scholar]
- 97.Di Poto A, Sbarra MS, Provenza G, Visai L, Speziale P. The effect of photodynamic treatment combined with antibiotic action or host defence mechanisms on Staphylococcus aureus biofilms. Biomaterials. 2009;30:3158–166. doi: 10.1016/j.biomaterials.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 98.Sbarra MS, Arciola CR, Di Poto A, Saino E, Rohde H, Speziale P, et al. The photodynamic effect of tetra-substituted N-methyl-pyridyl-porphine combined with the action of vancomycin or host defense mechanisms disrupts Staphylococcus epidermidis biofilms. Int J Artif Organs. 2009;32:574–583. doi: 10.1177/039139880903200906. [DOI] [PubMed] [Google Scholar]
- 99.Saino E, Sbarra MS, Arciola CR, Scavone M, Bloise N, Nikolov P, et al. Photodynamic action of Tri-meso (N-methyl-pyridyl), meso (N-tetradecyl-pyridyl) porphine on Staphylococcus epidermidis biofilms grown on Ti6A14V alloy. Int J Artif Organs. 2010;33:636–645. doi: 10.1177/039139881003300909. [DOI] [PubMed] [Google Scholar]
- 100.Zanin IC, Gonçalves RB, Junior AB, Hope CK, Pratten J. Susceptibility of Streptococcus mutans biofilms to photodynamic therapy: an in vitro study. J Antimicrob Chemother. 2005;56:324–330. doi: 10.1093/jac/dki232. [DOI] [PubMed] [Google Scholar]
- 101.Zanin IC, Lobo MM, Rodrigues LK, Pimenta LA, H”fling JF, Gonçalves RB. Photosensitization of in vitro biofilms by toluidine blue O combined with a light-emitting diode. Eur J Oral Sci. 2006;114:64–69. doi: 10.1111/j.1600-0722.2006.00263.x. [DOI] [PubMed] [Google Scholar]
- 102.Wood S, Metcalf D, Devine D, Robinson C. Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms. J Antimicrob Chemother. 2006;57:680–684. doi: 10.1093/jac/dkl021. [DOI] [PubMed] [Google Scholar]
- 103.Metcalf D, Robinson C, Devine D, Wood S. Enhancement of erythrosine-mediated photodynamic therapy of Streptococcus mutans biofilms by light fractionation. J Antimicrob Chemother. 2006;58:190–192. doi: 10.1093/jac/dkl205. [DOI] [PubMed] [Google Scholar]
- 104.Goulart Rde C, Bolean M, Paulino Tde P, Thedei G, Jr, Souza SL, Tedesco AC, et al. Photodynamic therapy in planktonic and biofilm cultures of Aggregatibacter actinomycetemcomitans. Photomed Laser Surg. 2010;28:53–60. doi: 10.1089/pho.2009.2591. [DOI] [PubMed] [Google Scholar]
- 105.Nastri L, Donnarumma G, Porzio C, De Gregorio V, Tufano MA, Caruso F, et al. Effects of toluidine blue-mediated photodynamic therapy on periopathogens and periodontal biofilm: in vitro evaluation. Int J Immunopathol Pharmacol. 2010;23:1125–1132. doi: 10.1177/039463201002300416. [DOI] [PubMed] [Google Scholar]
- 106.Lee CF, Lee CJ, Chen CT, Huang CT. delta-Aminolaevulinic acid mediated photodynamic antimicrobial chemotherapy on Pseudomonas aeruginosa planktonic and biofilm cultures. J Photochem Photobiol B. 2004;75:21–25. doi: 10.1016/j.jphotobiol.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 107.Collins TL, Markus EA, Hassett DJ, Robinson JB. The effect of a cationic porphyrin on Pseudomonas aeruginosa biofilms. Curr Microbiol. 2010;61:411–416. doi: 10.1007/s00284-010-9629-y. [DOI] [PubMed] [Google Scholar]
- 108.Fontana CR, Abernethy AD, Som S, Ruggiero K, Doucette S, Marcantonio RC. The antibacterial effect of photodynamic therapy in dental plaque-derived biofilms. J Periodontal Res. 2009;44:751–759. doi: 10.1111/j.1600-0765.2008.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Raghavendra M, Koregol A, Bhola S. Photodynamic therapy: a targeted therapy in periodontics. Aust Dent J. 2009;54:102–109. doi: 10.1111/j.1834-7819.2009.01148.x. [DOI] [PubMed] [Google Scholar]
- 110.Soukos NS, Chen PS, Morris JT, Ruggiero K, Abernethy AD, Som S. Photodynamic therapy for endodontic disinfection. J Endod. 2006;32:979–984. doi: 10.1016/j.joen.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 111.Bisland SK, Chien C, Wilson BC, Burch S. Preclinical in vitro and in vivo studies to examine the potential use of photodynamic therapy in the treatment of osteomyelitis. Photochem Photobiol Sci. 2006;5:31–38. doi: 10.1039/b507082a. [DOI] [PubMed] [Google Scholar]
- 112.Donnelly RF, McCarron PA, Cassidy CM, Elborn JS, Tunney MM. Delivery of photosensitisers and light through mucus: investigations into the potential use of photodynamic therapy for treatment of Pseudomonas aeruginosa cystic fibrosis pulmonary infection. J Control Release. 2007;117:217–226. doi: 10.1016/j.jconrel.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 113.Schuckert KH, Jopp S, Muller U. De novo grown bone on exposed implant surfaces using photodynamic therapy and recombinant human bone morphogenetic protein-2: case report. Implant Dent. 2006;15:361–365. doi: 10.1097/01.id.0000247856.09740.3f. [DOI] [PubMed] [Google Scholar]
- 114.Donnelly RF, McCarron PA, Tunney MM, Woolfson AD. Potential of photodynamic therapy in treatment of fungal infections of the mouth. Design and characterisation of a mucoadhesive patch containing toluidine blue O. J Photochem Photobiol B. 2007;86:59–69. doi: 10.1016/j.jphotobiol.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 115.D”rtbudak O, Haas R, Bernhart T, Mailath-Pokorny G. Lethal photosensitization for decontamination of implant surfaces in the treatment of periimplantitis. Clin Oral Implants Res. 2001;12:104–108. doi: 10.1034/j.1600-0501.2001.012002104.x. [DOI] [PubMed] [Google Scholar]
- 116.Demidova TN, Hamblin MR. Photodynamic inactivation of Bacillus spores, mediated by phenothiazinium dyes. Appl Environ Microbiol. 2005;71:6918–6925. doi: 10.1128/AEM.71.11.6918-25.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oliveira A, Almeida A, Carvalho CM, Tome JP, Faustino MA, Neves MG, et al. Porphyrin derivatives as photosensitizers for the inactivation of Bacillus cereus endospores. J Appl Microbiol. 2009;106:1986–1995. doi: 10.1111/j.1365-2672.2009.04168.x. [DOI] [PubMed] [Google Scholar]
- 118.Kim BY, Rutka JT, Chan WCW. Nanomedicine. N Engl J Med. 2010;363:2434–2443. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 119.Perni S, Prokopovich P, Pratten J, Parkin IP, Wilson M. Nanoparticles: their potential use in antibacterial photodynamic therapy. Photochem Photobiol Sci. 2011;10:712–720. doi: 10.1039/c0pp00360c. [DOI] [PubMed] [Google Scholar]
- 120.Engelhardt V, Krammer B, Plaetzer K. Antibacterial photodynamic therapy using water-soluble formulations of hypericin or mTHPC is effective in inactivation of Staphylococcus aureus. Photochem Photobiol Sci. 2010;9:365–369. doi: 10.1039/b9pp00144a. [DOI] [PubMed] [Google Scholar]
- 121.Sibani SA, McCarron PA, Woolfson AD, Donnelly RF. Photosensitiser delivery for photodynamic therapy. Part 2: systemic carrier platforms. Expert Opin Drug Deliv. 2008;5:1241–254. doi: 10.1517/17425240802444673. [DOI] [PubMed] [Google Scholar]
- 122.Bombelli C, Bordi F, Ferro S, Giansanti L, Jori G, Mancini G, et al. New cationic liposomes as vehicles of mtetrahydroxyphenylchlorin in photodynamic therapy of infectious diseases. Mol Pharm. 2008;5:672–679. doi: 10.1021/mp800037d. [DOI] [PubMed] [Google Scholar]
- 123.Ferro S, Ricchelli F, Mancini G, Tognon G, Jori G. Inactivation of methicillin resistant Staphylococcus aureus (MRSA) by liposome-delivered photosensitising agents. J Photochem Photobiol B. 2006;83:98–104. doi: 10.1016/j.jphotobiol.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 124.Tsai T, Yang YT, Wang TH, Chien HF, Chen CT. Improved photodynamic inactivation of Gram-positive bacteria using hematoporphyrin encapsulated in liposomes and micelles. Lasers Surg Med. 2009;41:316–322. doi: 10.1002/lsm.20754. [DOI] [PubMed] [Google Scholar]
- 125.Schwiertz J, Wiehe A, Grafe S, Gitter B, Epple M. Calcium phosphate nanoparticles as efficient carriers for photodynamic therapy against cells and bacteria. Biomaterials. 2009;30:3324–3331. doi: 10.1016/j.biomaterials.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 126.Guo Y, Rogelj S, Zhang P. Rose Bengal-decorated silica nanoparticles as photosensitizers for inactivation of gram-positive bacteria. Nanotechnology. 2010;21:065102. doi: 10.1088/0957-4484/21/6/065102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jia Y, Joly H, Omri A. Characterization of the interaction between liposomal formulations and Pseudomonas aeruginosa. J Liposome Res. 2010;20:134–146. doi: 10.3109/08982100903218892. [DOI] [PubMed] [Google Scholar]
- 128.Tavares A, Carvalho CM, Faustino MA, Neves MG, Tomé JP, Tomé AC, et al. Antimicrobial photodynamic therapy: study of bacterial recovery viability and potential development of resistance after treatment. Mar Drugs. 2010;8:91–105. doi: 10.3390/md8010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nakonieczna J, Michta E, Rybicka M, Grinholc M, Gwizdek-Wisniewska A, Bielawski KP. Superoxide dismutase is upregulated in Staphylococcus aureus following protoporphyrin-mediated photodynamic inactivation and does not directly influence the response to photodynamic treatment. BMC Microbiol. 2010;10:323. doi: 10.1186/1471-2180-10-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brombacher K, Fischer BB, Rüfenacht K, Eggen RI. The role of Yap1p and Skn7p-mediated oxidative stress response in the defence of Saccharomyces cerevisiae against singlet oxygen. Yeast. 2006;23:741–750. doi: 10.1002/yea.1392. [DOI] [PubMed] [Google Scholar]
- 131.Xavier JB. Social interaction in synthetic and natural microbial communities. Mol Syst Biol. 2011;7:483. doi: 10.1038/msb.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol. 2007;5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 133.Lee YM, Almqvist F, Hultgren SJ. Targeting virulence for antimicrobial chemotherapy. Curr Opin Pharmacol. 2003;3:513–519. doi: 10.1016/j.coph.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 134.Tubby S, Wilson M, Nair SP. Inactivation of staphylococcal virulence factors using a light-activated antimicrobial agent. BMC Microbiol. 2009;9:211. doi: 10.1186/1471-2180-9-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kömerik N, Wilson M, Poole S. The effect of photodynamic action on two virulence factors of gram-negative bacteria. Photochem Photobiol. 2000;72:676–680. doi: 10.1562/0031-8655(2000)072<0676:TEOPAO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 136.Sharma SK, Dai T, Kharkwal GB, Huang YY, Huang L, De Arce VJ, et al. Drug Discovery of Antimicrobial Photosensitizers using Animal Models. Curr Pharm Des. 2011 doi: 10.2174/138161211795703735. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.St. Denis TG, Huang L, Dai T, Hamblin MR. Analysis of the bacterial heat shock response to photodynamic therapy-mediated oxidative stress. Photochem Photobiol. 2011;87:707–713. doi: 10.1111/j.1751-097.2011.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zolfaghari PS, Packer S, Singer M, Nair SP, Bennett J, Street C, et al. In vivo killing of Staphylococcus aureus using a light-activated antimicrobial agent. BMC Microbiol. 2009;9:27. doi: 10.1186/1471-2180-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Braham P, Herron C, Street C, Darveau R. Antimicrobial photodynamic therapy may promote periodontal healing through multiple mechanisms. J Periodontol. 2009;80:1790–1798. doi: 10.1902/jop.2009.090214. [DOI] [PubMed] [Google Scholar]
- 140.Fonseca MB, Junior PO, Pallota RC, Filho HF, Denardin OV, Rapoport A, et al. Photodynamic therapy for root canals infected with Enterococcus faecalis. Photomed Laser Surg. 2008;26:209–213. doi: 10.1089/pho.2007.2124. [DOI] [PubMed] [Google Scholar]
- 141.Soukos NS, Chen PS, Morris JT, Ruggiero K, Abernethy AD, Som S, et al. Photodynamic therapy for endodontic disinfection. J Endod. 2006;32:979–984. doi: 10.1016/j.joen.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 142.Garcez AS, Ribeiro MS, Tegos GP, Nunez SC, Jorge AO, Hamblin MR. Antimicrobial photodynamic therapy combined with conventional endodontic treatment to eliminate root canal biofilm infection. Lasers Surg Med. 2007;39:59–66. doi: 10.1002/lsm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schneider S, Carra G, Sahin U, Hoy B, Rieder G, Wessler S. Complex cellular responses of Helicobacter pylori-colonized gastric adenocarcinoma cells. Infect Immun. 2011;79:2362–2371. doi: 10.1128/IAI.01350-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Millson CE, Wilson M, MacRobert AJ, Bown SG. Ex-vivo treatment of gastric Helicobacter infection by photodynamic therapy. J Photochem Photobiol B. 1996;32:59–65. doi: 10.1016/1011-1344(95)07190-3. [DOI] [PubMed] [Google Scholar]
- 145.Wilder-Smith CH, Wilder-Smith P, Grosjean P, van den Bergh H, Woodtli A, Monnier P, et al. Photoeradication of Helicobacter pylori using 5-aminolevulinic acid: preliminary human studies. Lasers Surg Med. 2002;31:18–22. doi: 10.1002/lsm.10066. [DOI] [PubMed] [Google Scholar]
- 146.Hamblin MR, Viveiros J, Yang C, Ahmadi A, Ganz RA, Tolkoff MJ. Helicobacter pylori accumulates photoactive porphyrins and is killed by visible light. Antimicrob Agents Chemother. 2005;49:2822–2827. doi: 10.1128/AAC.49.7.2822-7.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ganz RA, Viveiros J, Ahmad A, Ahmadi A, Khalil A, Tolkoff MJ, et al. Helicobacter pylori in patients can be killed by visible light. Lasers Surg Med. 2005;36:260–265. doi: 10.1002/lsm.20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mihu MR, Martinez LR. Novel therapies for treatment of multi-drug resistant Acinetobacter baumannii skin infections. Virulence. 2011;2:97–102. doi: 10.4161/viru.2.2.15061. [DOI] [PubMed] [Google Scholar]
- 149.Manolakaki D, Velmahos G, Kourkoumpetis T, Chang Y, Alam HB, De Moya MM, et al. Candida infection and colonization among trauma patients. Virulence. 2010;1:367–375. doi: 10.4161/viru.1.5.12796. [DOI] [PubMed] [Google Scholar]
- 150.Dai T, Kharkwal GB, Tanaka M, Huang YY, Bil de Arce VJ, Hamblin MR. Animal models of external traumatic wound infections. Virulence. 2011:2. doi: 10.4161/viru.2.4.16840. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Maisch T, Bosl C, Szeimies RM, Love B, Abels C. Determination of the antibacterial efficacy of a new porphyrin-based photosensitizer against MRSA ex vivo. Photochem Photobiol Sci. 2007;6:545–551. doi: 10.1039/b614770d. [DOI] [PubMed] [Google Scholar]
- 152.Hamblin MR, Zahra T, Contag CH, McManus AT, Hasan T. Optical monitoring and treatment of potentially lethal wound infections in vivo. J Infect Dis. 2003;187:1717–26. doi: 10.1086/375244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wong TW, Wang YY, Sheu HM, Chuang YC. Bactericidal effects of toluidine blue-mediated photodynamic action on Vibrio vulnificus. Antimicrob Agents Chemothe. 2005;49:895–902. doi: 10.1128/AAC.49.3.895-902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dai T, Tegos GP, Lu Z, Huang L, Zhiyentayev T, Franklin MJ, et al. Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob Agents Chemother. 2009;53:3929–3934. doi: 10.1128/AAC.00027-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Berthiaume F, Reiken SR, Toner M, Tompkins RG, Yarmush ML. Antibody-targeted photolysis of bacteria in vivo. Biotechnology (NY) 1994;12:703–706. doi: 10.1038/nbt0794-703. [DOI] [PubMed] [Google Scholar]
- 156.Vilcinskas A. Coevolution between pathogen-derived proteinases and proteinase inhibitors of host insects. Virulence. 2010;1:206–14. doi: 10.4161/viru.1.3.12072. [DOI] [PubMed] [Google Scholar]
- 157.Fuchs BB, O'Brien E, Khoury JB, Mylonakis E. Methods for using Galleria mellonella as a model host to study fungal pathogenesis. Virulence. 2010;1:475–482. doi: 10.4161/viru.1.6.12985. [DOI] [PubMed] [Google Scholar]
- 158.Fuchs BB, Mylonakis E. Using non-mammalian hosts to study fungal virulence and host defense. Curr Opin Microbiol. 2006;9:346–51. doi: 10.1016/j.mib.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 159.Dutta S, Ongarora BG, Li H, Vicente Mda G, Kolli BK, Chang KP. Intracellular targeting specificity of novel phthalocyanines assessed in a host-parasite model for developing potential photodynamic medicine. PLoS ONE. 2011;6:20786. doi: 10.1371/journal.pone.0020786. [DOI] [PMC free article] [PubMed] [Google Scholar]