Abstract
Infection by the human protozoan parasite Leishmania can lead, depending primarily on the parasite species, to either cutaneous or mucocutaneous lesions, or fatal generalized visceral infection. In the New World, Leishmania (Viannia) species can cause mucocutaneous leishmaniasis (MCL). Clinical MCL involves a strong hyper-inflammatory response and parasitic dissemination (metastasis) from a primary lesion to distant sites, leading to destructive metastatic secondary lesions especially in the nasopharyngal areas. Recently, we reported that metastasizing, but not non-metastatic strains of Leishmania (Viannia) guyanensis, have high burden of a non-segmented dsRNA virus, Leishmania RNA Virus (LRV). Viral dsRNA is sensed by the host Toll-like Receptor 3 (TLR3) thereby inducing a pro-inflammatory response and exacerbating the disease. The presence of LRV in Leishmania opens new perspectives not only in basic understanding of the intimate relation between the parasite and LRV, but also in understanding the importance of the inflammatory response in MCL patients.
Key words: leishmaniasis, Leishmania RNA virus, hyperinflammation, TLR-3, IFNβ
Leishmania are human protozoan parasites endemic in 88 countries, with a disease prevalence of 12 million cases accompanied by 80,000 annual fatalities. These infections induce a large spectrum of clinical pathologies, mainly cutaneous (CL), mucosal (MCL) and visceral leishmaniasis (VL). The differences arise primarily from infection by different Leishmania species, such as L. major, L. braziliensis and L. infantum respectively. Additionally, host factors are thought to play significant roles in determining the clinical course of the disease as well.
Leishmania parasites exist as free-living promastigotes in the sand fly vector. Following differentiation to the infective metacyclic form, parasites are deposited in the skin of vertebrate host by the sand fly bite. There promastigotes encounter several host cell types including neutrophils, dendritic cells and skin macrophages, ultimately transiting and differentiating into amastigotes which go on to replicate within the phagolysosome of macro-phages. Leishmania parasites must change their metabolism and adapt themselves to this new environment, and resist the oxidative and other attacks activated by the innate immune system of the host.
Leishmania species of the L. (Viannia) subgenus, including mainly L. braziliensis, L. guyanensis and L. panamensis, give rise to CL but are also responsible for MCL in up to 5–10% of cases. MCL is clearly distinguishable from other cutaneous leishmaniases by its chronic, latent and metastatic behavior. It is characterized by the dissemination of parasites and secondary distant lesions development (metastasis), especially in the oral and nasopharyngeal areas of the face, and is accompanied by extensive tissue destruction concomitant with high immune cell infiltration, intense activation of inflammatory cells and parasite presence (albeit at low levels).1 MCL can appear concomitantly, several years after the initial infection, or even in patients without any CL history. MCL lesions are not self-healing and are more resistant to antimony treatment than the primary lesions, with frequent relapses. The factors responsible for these relapses are not known; both the emergence of antimony resistance as well as differences among the infecting L. (Viannia) species and its virulence have been suggested.2,3
Reactivation of L. (Viannia) infection can occur following stress or immunosuppression at a site of local inflammation, raising the challenging question of how these factors interact with slow-growing or dormant parasites and the immune system to favor the reemergence of disease pathology. Thus far, little is known about the pathogenesis of MCL, especially factors involved in the immune response of the host, in the parasite dissemination, or in reactivation. It is likely that both L. (Viannia) oxidative stress and antimony resistance as well as genetic background of the host (e.g., particular alleles encoding TNFα, TNFβ, IL-6, CXCR1 and CCL2/MCP1) and particular species and/or isolate specific virulence factors are important parameters in the development of MCL. The definition of such factors and of the immune response of the host could be extremely useful, not only to predict the outcome of the disease and diagnosis tools, but also to understand the metastatic process and the inter-relationships of the parasite with its host. Currently the immunological mechanisms of protection and factors controlling relapse and avoiding reactivation of the infection are not well understood.
In MCL, the immune response to infection differs from that observed in other types of leishmaniases. After a primary lesion, metastatic lesions can appear at other body sites, accompanied by tissue inflammation. This pathology has been associated with hyperactivity of the specific T cell immune response, with an exuberant, usually progressive, inflammatory response, that is not yet well understood.4 High levels of pro-inflammatory cytokines such as IFNγ and TNFα, and decreased responses to IL-10 and TGFβ, have been described in references 5 and 6. MCL development is associated with persistent immune responses having elevated pro-inflammatory mediator expression (higher levels of TNFα, CXCL10 and CCL4), with a mixed intra-lesional Th1/Th2 phenotype and elevated cytotoxic T cell activity. However, cells from MCL patients display impaired control of the immune response due to a defect in their ability to respond to IL-10.7–10 The production of the different inflammatory cytokines by the host is likely to increase cellular recruitment and contribute to the pathology of the disease. Thus by these and potentially other mechanisms, immunological hyperactivity contributes to MCL pathology. In turn measures diminishing uncontrolled inflammation could be one promising alternative or complement to the conventional drug therapy. Interestingly, treatment with the anti-inflammatory TNFα inhibitor pentoxyphylline in combination with antimony was effective in MCL patients unresponsive to antimonial therapy alone.11
The susceptibility of the golden hamster to infection with species of the L. (Viannia) subgenus has provided a useful experimental model of mucocutaneous leishmaniasis. Hamsters infected with L. (Viannia) guyanensis isolated from human MCL lesions reproduce the metastatic phenotype with primary and metastatic lesion development.12 Different species and individual strains often differ in their propensity to cause hyperinflammatory cutaneous secondary metastatic lesions.13 Diversity was even seen within a single strain, as infective clones from the isolate of L. (Viannia) guyanensis (L.g.) (WHI/BR/78/M5313) were either highly metastatic, moderately metastatic or non-metastatic in the hamster model. Non-metastatic (M−) clones formed lesions only at the site of inoculation and did not disseminate, whereas metastatic (M+) clones gave rise to metastases in 60% to 80% of hamsters. The metastatic phenotype was stable over several passages and exacerbated by non-specific or immunologically induced inflammatory responses.14,15
Molecular approaches have provided some insights into factors potentially playing a role in MCL. One of the most surprising difference between the genomes of L. braziliensis, L. major and L. infantum is the maintenance in L. braziliensis of genes encoding the RNA-mediated interference (RNAi) machinery, telomere-associated transposable elements and splice leader-associated SLACS.16 The RNAi machinery was recently shown to be functional in L. braziliensis and in L. guyanensis.17 A second remarkable feature the presence of Leishmania RNA viruses in many isolates of the L. (Viannia) species. These Leishmaniaviruses have been classified as Totiviridae, which includes RNA viruses detected in other protozoa such as Trichomonas vaginalis and Giardia lamblia and a variety of fungi including Saccharomyces cerevisiae. Totiviruses have a small unsegmented dsRNA genome between 5–7 kb in length, which encodes a capsid protein and a capsid-RNA dependent RNA polymerase (RDRP) fusion protein essential for replication.
The existence of cytosolic dsRNA viruses within Leishmania was first shown in two L. guyanensis strains: MHOM/SR/81/CUMC1A and MHOM/BR/75/M4147.18,19 Currently Leishmania viruses are given arbitrary identifiers at the time of discovery, namely LRV1-1 and LRV1-4 for the viruses of the L. guyanensis CUMC-1 and L. guyanensis M4147 strain respectively. These two viruses share an overall 76% nucleotide sequence identity.20,21 LRV1s have since been identified in many isolates of New World Leishmania (L. braziliensis and L. guyanensis), but in just one isolate of Old World species L. major, which was showed sufficient nucleotide sequence divergence to be termed LRV2-1 (compare taxonomy browser at www.ncbi.nlm.nih.gov). LRV1 are present not only in laboratory strains of L. guyanensis and L. braziliensis but importantly also in biopsies and parasite cultures isolated from clinical cases of leishmaniasis.18,19,22,23 LRV-positive strains of Leishmania originated from both active and healing lesions or scars of patients living in Brazil, Peru, Guyana and Colombia. It was also shown that LRV1 can be occasionally be lost, thus far in just one line of L. guyanensis.24 Such isogenic lines are invaluable tools in evaluating the impact of LRVs specifically on the parasite and on the immune response.
The study of M+ and M− line is one approach that may shed light on what parameters underlie the metastatic phenotype and the hyperinflammatory response observed in MCL patients. To investigate whether the immune response of the host cell could serve as a readout assay we performed preliminary experiments on the response of host macrophage infected by M+ and M− lines, keeping in mind that L. (Viannia) guyanensis could be infected by a dsRNA virus. Using a 15k cDNA microarray, we concluded that infection of bone marrow derived macrophages (BMMφ) with M+ parasites induced 294 annotated differentially expressed genes when compared with BMMφ infected with non metastatic (M−) parasites that had at least a 1.5-fold change (p ≤ 0.05). Given the importance of the immune response in MCL pathology, we selected for further study genes involved in the immune response and potentially relevant for MCL in the human host. These included ones showing increased expression of surface activation markers, together with the chemokines (CXCL10 and CCL5) and cytokines (IL-6 and TNFα) secretion in BMMφ infected with metastatic (M+) parasites. Furthermore, the increased chemokines and cytokine response to BMM infections by M+ parasites required the TRIF dependent TLR3 signaling pathway. Double stranded RNA is known to bind to TLR3, and induce via TRIF an inflammatory response with production of CCL5, CXCL10, IL-6, TNFα and NO by activating iNOS via NFκB.25,26 This led us to consider the possible involvement parasite dsRNA and specifically the LRV1 found in L. guyanensis. We confirmed that the metastasizing promastigotes (L.g.M+ or h-MCL) contained LRV1 dsRNA, and detected significantly high levels of LRV1 within metastasizing L.g.M+ or h-MCL parasites (LRV1high). In contrast, non-metastasizing L.g.M− or h-CL parasites showed only trace levels of LRV1 RNA (10,000-fold lower; termed LRV1low). Treatment of BMMφ with purified LRV1 dsRNA induced a pro-inflammatory phenotype similar to BMMφ infected with LRV1high metastasizing parasites. In addition, we detected an early upregulation of IFNβ, which is typically a sign of an anti-viral immune response. As with M−/LRV1low parasites, the absence of TLR3 significantly decreased the expression of chemokines and cytokines produced in response to LRV RNA.
While naturally M+ and M− parasites potentially harbor genetic differences other than the presence of LRV, we were able to show definitively that LRV1 was responsible for the cytokine responses by comparing isogenic L. guyanensis bearing or lacking LRV1-4. As before, BMMφ infected with L.g. M4147 LRV1high produce significantly higher cytokine and chemokine than the isogenic virus-free L.g. M4147 (LRV1neg) in a TLR3-dependent manner.17,27 To analyze whether TLR3 and LRV1 play a role in leishmaniasis development in vivo, TLR3-/-, TLR7-/- and C57BL/6 wild-type (WT) mice were infected in the footpad. A significant decrease in footpad swelling peak and diminished parasite load could be observed in mice lacking TLR3 infected with L.g. LRV1high M+ (M5313) or L.g. M4147 (LRV1high) parasites to compared WT mice. No distinguishable difference in disease phenotype was observed in mice infected with L.g. LRV1low M− (Lg17) or L.g. LRV1neg (M4147) or between WT and TLR7-/- infected mice with any parasite isolates.
Our results confirm that metastasizing L.g. (M+) parasites derived from secondary lesions of hamsters or humans activate host BMMφs to secrete TNFα, IL-6, CCL5 and CXCL10, elevated levels of which have been associated with human MCL. These TLR3-dependent responses to infecting L.g. LRV1high/M+ parasites resulted in increased disease severity in mice. Our work provides evidence that LRV1 within metastasizing L.g. parasites is recognized by the host to promote inflammation, and is involved in susceptibility to infection.
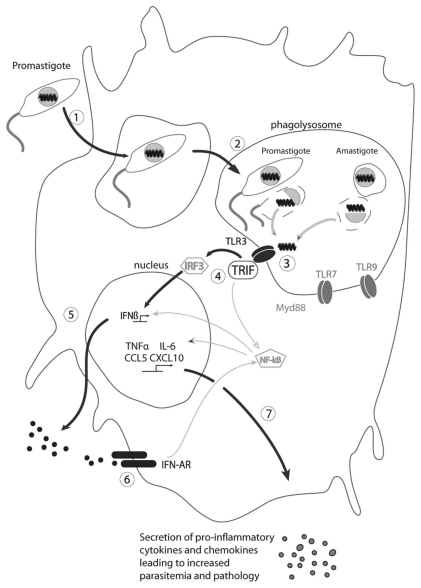
One question is how the dsRNA found normally within the viral particle is able to interact with TLR3. We know from previous studies that 5–10% of the infecting promastigotes are killed during the first hours of infection.28 This killing process takes place in the phagolysosome where endosomal TLRs are present (Fig. 1). As recognition of LRV1 within the metastasizing L.g. parasites arises early after infection, we hypothesize that the viral capsid is destroyed in the acidic milieu prevalent in the phagolysosome, leading to the release of LRV1 dsRNA, recognition by TLR3, and activation of the signaling cascade via TRIF, leading to the secretion of IFNβ (which could act in an autocrine loop on its receptor). In the next hours, inflammatory cytokines and chemokines are produced leading to the attraction of dendritic and T cells. Importantly, both the presence and levels of LRV are factors impacting the host immune response, as parasites bearing only low levels of LRV failed to activate TLR3. Finally, other nucleic acid derived motifs predicted to arise for parasite destruction may contribute to the host's response, as shown by the somewhat diminished cytokine and chemokine production in TLR7-/- BMMφ infected with L.g. (M+) parasites. As these effects were less than seen with TLR3-/- infections, and the TLR7-/- mice did not show any reduction in disease progression or pathology, the TLR3-dependent responses appear to dominate.
Figure 1.
Model of the signaling cascade in response to the release of dsRNA from LRV particles, production of IFNβ and secretion of proinflammatory cytokines and chemokines. The main pathway involved in this process is highlighted in bold. (1) Phagocytosis of LRV infected promastigotes by phagocytes (macrophages); (2) promastigotes differentiate into amatigotes, which reside in phagolysosomes; (3) death of some parasites (promastigotes and amastigotes), release of LRV and of dsRNA, which binds to TLR3; (4) activation of TLR3 via TRIF and signal transmission via the transcription factors IRF3 and NFκB; (5) activation and secretion of IFNβ; (6) binding of IFNβ to its receptor and activation of pro-inflammatory cytokines and chemokines genes (autocrine loop); (7) synthesis and secretion of pro-inflammatory cytokines and chemokines such as TNFα, IL-6, CCL and CXCL10 leading to increased parasitemia and pathology.
Our data show that L. guyanensis LRV induces a specific immune response via dsRNA binding to TLR3 and production of IFNβ early after infection, sufficient to modulate the initial immune response in a way that impairs rather than promotes killing. This is likely mediated through the production of pro-inflammatory chemokines and cytokines, thereby increasing the host's susceptibility to infection and likely parasite dissemination. Thus, Leishmania RNA virus, when present in New World Leishmania guyanensis, plays an important role in subverting the innate immune response. This newly recognized parasite factor could explain some of the differences observed in the different pathologies induced by Old World and New World species. Although the murine model is likely not fully representative of the pathology in humans, it is instrumental for evaluating the role of LRV in MCL. Of course, a role for LRVs in the pathology of MCL does not exclude the likelihood that other parasite or host factors play strong roles as well.
In the future, it will be necessary to investigate the mechanism whereby LRVs confer increased susceptibility to infection with L. (Viannia) parasites, and to analyze the critical role of cytokines and chemokines played in the host immune response. Key questions are how LRV1, and the associated hyper-inflammatory immune response conspire together to yield the metastatic phenotype, and whether anti-inflammatory drugs can prevent the development of chronic and secondary metastatic lesions. These new results should help defining the role of LRV1 in MCL pathology and ultimately facilitate the introduction of new clinical strategies to fight MCL. Since most human MCL is caused by infections by L. braziliensis, it will be important to determine whether the LRV-dependent immune subversion observed with L. guyanensis isolates is also a key determinant of L. braziliensis MCL.
Our study on L. guyanensis has possible applications on the prognosis, diagnosis and treatment of MCL. As CL can emerge prior to MCL, or can recede followed by reactivation to MCL, the presence of LRV1 potentially allows some assessment of the degree of MCL risk.29 Similarly, immunization against LRV1, or the identification of anti-LRV chemotherapies, may contribute to amelioration of disease severity. The value of such strategies depends strongly on knowledge of the role of LRV in the etiology of human disease, for which little is known, and the contribution of genetic differences in the parasite nuclear DNA as well. One early study of human biopsy samples did not reveal a strong association between LRV and human disease status, however several key parameters revealed by our recent work were not addressed.30 Future studies are needed with a larger and more diverse panel of MCL isolates, identification of the infecting Leishmania species, quantitation of LRV load, and follows-up to assess the evolution of CL into MCL.30 Since occasionally LRVs can be lost in laboratory culture,27 its absence from laboratory strains must be viewed cautiously, and the role of LRV sequence diversity also remains to be explored.
In conclusion, our study on MCL immune response to a virus infecting a parasite and modulating its virulence will give a better understanding of the host response to infection with New World parasites and will provide clues as to which unique pathways to target in the parasite and in the macrophage so as to diminish and potentially abolish parasite virulence trait. Although genetic polymorphisms in the host or in the parasite could be involved in the outcome of the disease, our study could have a direct impact on MCL diagnosis (presence of a LRV1 in parasite isolates or specific immunodiagnostic direct against LRV1 polypeptides as poor prognosis of developing MCL) and treatment by using or developing new drugs which block LRV1 replication, thereby diminishing the inflammatory response and the non-responsiveness to first line treatment like antimony.
Acknowledgments
The authors are grateful to Annette Ives, Slavica Masina, Haroun Zangger, Florence Prevel, Giulia Ruzzante, Silvia Fuertes-Marraco, Frederic Schutz, Haroun Zangger, Melanie Revaz-Breton, Lon-Fye Lye, Suzanne M. Hickerson, Hans Acha-Orbea and Pascal Launois, for their contribution to the original publication and to Mary-Anne Hartley and Patrik Castiglioni for their participation to the last experiments. This work was funded by the grants FNRS N° 3100A0-116665/1 and IZ70Z0-131421 (N.F.) and NIH grant AI 29,646 (S.M.B.). We thank Adrien Fasel for the drawing of the figure.
References
- 1.Ronet C, Ives A, Bourreau E, Fasel N, Launois P, Masina S. Immune responses to Leishmania guyanensis infection in humans and animal models. In: Jirillo E, Brandonisio O, editors. Immune Response to Parasitic Infections: Protozoa. Bussum: Bentham eBooks; 2010. pp. 165–177. [Google Scholar]
- 2.Arevalo J, Ramirez L, Adaui V, Zimic M, Tulliano G, Miranda-Verastegui C, et al. Influence of Leishmania (Viannia) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis. J Infect Dis. 2007;195:1846–1851. doi: 10.1086/518041. [DOI] [PubMed] [Google Scholar]
- 3.Souza AS, Giudice A, Pereira JM, Guimaraes LH, de Jesus AR, de Moura TR, et al. Resistance of Leishmania (Viannia) braziliensis to nitric oxide: correlation with antimony therapy and TNFalpha production. BMC Infect Dis. 2010;10:209. doi: 10.1186/1471-2334-10-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silveira FT, Lainson R, De Castro Gomes CM, Laurenti MD, Corbett CE. Immunopathogenic competences of Leishmania (V.) braziliensis and L. (L.) amazonensis in American cutaneous leishmaniasis. Parasite Immunol. 2009;31:423–431. doi: 10.1111/j.1365-3024.2009.01116.x. [DOI] [PubMed] [Google Scholar]
- 5.Bacellar O, Lessa H, Schriefer A, Machado P, Ribeiro de Jesus A, Dutra WO, et al. Upregulation of Th1-type responses in mucosal leishmaniasis patients. Infect Immun. 2002;70:6734–6740. doi: 10.1128/IAI.70.12.6734-40.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomes-Silva A, de Cassia Bittar R, Dos Santos Nogueira R, Amato VS, da Silva Mattos M, Oliveira-Neto MP, et al. Can interferon-gamma and interleukin-10 balance be associated with severity of human Leishmania (Viannia) braziliensis infection? Clin Exp Immunol. 2007;149:440–444. doi: 10.1111/j.1365-2249.2007.03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pirmez C, Yamamura M, Uyemura K, Paes-Oliveira M, Conceicao-Silva F, Modlin RL. Cytokine patterns in the pathogenesis of human leishmaniasis. J Clin Invest. 1993;91:1390–1395. doi: 10.1172/JCI116341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faria DR, Gollob KJ, Barbosa J, Jr, Schriefer A, Machado PR, Lessa H, et al. Decreased in situ expression of interleukin-10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis. Infect Immun. 2005;73:7853–7859. doi: 10.1128/IAI.73.12.7853-9.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaze ST, Dutra WO, Lessa M, Lessa H, Guimaraes LH, Jesus AR, et al. Mucosal leishmaniasis patients display an activated inflammatory T-cell phenotype associated with a nonbalanced monocyte population. Scand J Immunol. 2006;63:70–78. doi: 10.1111/j.1365-3083.2005.01707.x. [DOI] [PubMed] [Google Scholar]
- 10.Vargas-Inchaustegui DA, Hogg AE, Tulliano G, Llanos-Cuentas A, Arevalo J, Endsley JJ, et al. CXCL10 production by human monocytes in response to Leishmania braziliensis infection. Infect Immun. 2010;78:301–308. doi: 10.1128/IAI.00959-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lessa HA, Machado P, Lima F, Cruz AA, Bacellar O, Guerreiro J, et al. Successful treatment of refractory mucosal leishmaniasis with pentoxifylline plus antimony. Am J Trop Med Hyg. 2001;65:87–89. doi: 10.4269/ajtmh.2001.65.87. [DOI] [PubMed] [Google Scholar]
- 12.Travi B, Rey-Ladino J, Saravia NG. Behavior of Leishmania braziliensis s.l. in golden hamsters: evolution of the infection under different experimental conditions. J Parasitol. 1988;74:1059–1062. doi: 10.2307/3282237. [DOI] [PubMed] [Google Scholar]
- 13.Martinez JE, Travi BL, Valencia AZ, Saravia NG. Metastatic capability of Leishmania (Viannia) panamensis and Leishmania (Viannia) guyanensis in golden hamsters. J Parasitol. 1991;77:762–768. doi: 10.2307/3282713. [DOI] [PubMed] [Google Scholar]
- 14.Martínez JE, Valderrama L, Gama V, Leiby DA, Saravia NG. Clonal diversity in the expression and stability of the metastatic capability of Leishmania guyanensis in the golden hamster. J Parasitol. 2000;86:792–799. doi: 10.1645/0022-3395(2000)086[0792:CDITEA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Travi BL, Osorio Y, Saravia NG. The inflammatory response promotes cutaneous metastasis in hamsters infected with Leishmania (Viannia) panamensis. J Parasitol. 1996;82:454–457. doi: 10.2307/3284085. [DOI] [PubMed] [Google Scholar]
- 16.Peacock CS, Seeger K, Harris D, Murphy L, Ruiz JC, Quail MA, et al. Comparative genomic analysis of three Leishmania species that cause diverse human disease. Nat Genet. 2007;39:839–847. doi: 10.1038/ng2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lye LF, Owens K, Shi H, Murta SM, Vieira AC, Turco SJ, et al. Retention and loss of RNA interference pathways in Trypanosomatid protozoans. PLoS Pathog. 2010;6:1001161. doi: 10.1371/journal.ppat.1001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarr PI, Aline RF, Jr, Smiley BL, Scholler J, Keithly J, Stuart K. LR1: a candidate RNA virus of Leishmania. Proc Natl Acad Sci USA. 1988;85:9572–9575. doi: 10.1073/pnas.85.24.9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Widmer G, Comeau AM, Furlong DB, Wirth DF, Patterson JL. Characterization of a RNA virus from the parasite Leishmania. Proc Natl Acad Sci USA. 1989;86:5979–5982. doi: 10.1073/pnas.86.15.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheffter S, Widmer G, Patterson JL. Complete sequence of Leishmania RNA virus 1–4 and identification of conserved sequences. Virology. 1994;199:479–483. doi: 10.1006/viro.1994.1149. [DOI] [PubMed] [Google Scholar]
- 21.Stuart KD, Weeks R, Guilbride L, Myler PJ. Molecular organization of Leishmania RNA virus 1. Proc Natl Acad Sci USA. 1992;89:8596–8600. doi: 10.1073/pnas.89.18.8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cadd TL, Keenan MC, Patterson JL. Detection of Leishmania RNA virus 1 proteins. J Virol. 1993;67:5647–5650. doi: 10.1128/jvi.67.9.5647-5650.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saiz M, Llanos-Cuentas A, Echevarria J, Roncal N, Cruz M, Muniz MT, et al. Short report: detection of Leishmania virus in human biopsy samples of leishmaniasis from Peru. Am J Trop Med Hyg. 1998;58:192–194. doi: 10.4269/ajtmh.1998.58.192. [DOI] [PubMed] [Google Scholar]
- 24.Ro YT, Scheffter SM, Patterson JL. Hygromycin B resistance mediates elimination of Leishmania virus from persistently infected parasites. J Virol. 1997;71:8991–8998. doi: 10.1128/jvi.71.12.8991-8998.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NFkappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 26.Pindado J, Balsinde J, Balboa MA. TLR3-dependent induction of nitric oxide synthase in RAW 264.7 macrophage-like cells via a cytosolic phospholipase A2/cyclooxygenase-2 pathway. J Immunol. 2007;179:4821–4828. doi: 10.4049/jimmunol.179.7.4821. [DOI] [PubMed] [Google Scholar]
- 27.Ro YT, Scheffter SM, Patterson JL. Specific in vitro cleavage of a Leishmania virus capsid-RNA-dependent RNA polymerase polyprotein by a host cysteine-like protease. J Virol. 1997;71:8983–8990. doi: 10.1128/jvi.71.12.8983-8990.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zangger H, Mottram JC, Fasel N. Cell death in Leishmania induced by stress and differentiation: programmed cell death or necrosis? Cell Death Differ. 2002;9:1126–1139. doi: 10.1038/sj.cdd.4401071. [DOI] [PubMed] [Google Scholar]
- 29.Saravia NG, Weigle K, Segura I, Giannini SH, Pacheco R, Labrada LA, et al. Recurrent lesions in human Leishmania braziliensis infection-reactivation or reinfection? Lancet. 1990;336:398–402. doi: 10.1016/0140-6736(90)91945-7. [DOI] [PubMed] [Google Scholar]
- 30.Ogg MM, Carrion R, Jr, Botelho AC, Mayrink W, Correa-Oliveira R, Patterson JL. Short report: quantification of leishmaniavirus RNA in clinical samples and its possible role in pathogenesis. Am J Trop Med Hyg. 2003;69:309–313. [PubMed] [Google Scholar]