Abstract
This paper describes the development of a new class of N-linked imidazoles as potential pH-sensitive, cleavable linkers for use in cancer drug delivery systems. Kinetic analysis of 8 derivatives of N-ethoxybenzylimidazoles (NEBIs) showed that their rates of hydrolysis are accelerated in mild aqueous acidic solutions compared to in solutions at normal, physiological pH. Incorporation of electron donating or electron withdrawing substituents on the phenyl ring of the NEBI resulted in the ability to tune the rates of hydrolysis under mild acidic conditions with half-lives ranging from minutes to months. A derivative of NEBI carrying doxorubicin, a widely used anticancer agent, also showed an increased rate of hydrolysis under mild acid compared to at normal, physiological pH. The doxorubicin analog resulting from hydrolysis from the NEBI exhibited good cytotoxic activity when exposed to human ovarian cancer cells. These results demonstrate a potentially useful, general strategy for conjugating a wide range of drugs to imidazole-containing delivery vessels via NEBI functionalities for controlled release of therapeutics for drug delivery applications.
Herein, we describe the development of a class of pH-sensitive molecules that hydrolyze with tunable rates under physiological conditions. Organic functional groups that decompose slowly in an aqueous environment have attracted wide interest as potential linkers for the covalent conjugation of drugs to polymers for a range of drug delivery applications (1). Functional groups that decompose in mildly acidic environments, for instance, may be useful for the development of cancer drug delivery systems where an acid-sensitive group could exploit the lower extracellular pH (pH ≈ 6.4 – 6.9) of some tumor cells compared to that of blood or normal tissues (2) to trigger the release of therapeutic agents from suitable delivery vessels (3). Functional groups that cleave under mild acid may also exploit the low pH of endosomes (pH ≈ 5.5 – 6) and lysosomes (pH ≈ 4.5 – 5) to trigger the release of covalently-linked therapeutics from drug delivery carriers after endocytosis by targeted cells (4).
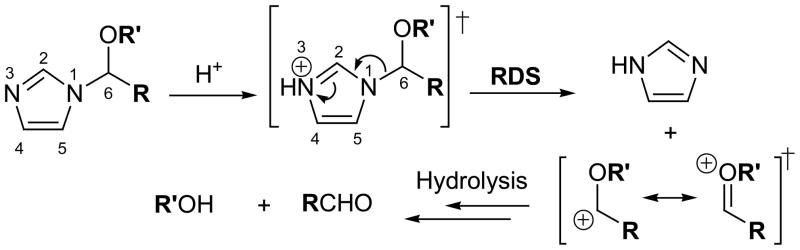
In the course of developing new drug delivery systems, we have explored the possibility of using N-linked imidazoles as functionalities that could undergo hydrolysis in aqueous acidic solutions at physiological temperature. We hypothesized that the expected difference in the protonated state of certain N-linked imidazoles in mild acid (e.g., pH = 5.5) compared to normal, physiological pH (pH = 7.4) (5) might be useful for initiating the rapid release of molecules (e.g., drugs) linked to imidazole-containing delivery vessels when exposed to tumor-like environments. Here, we present a study of the hydrolysis of a new class of N-linked imidazoles that cleave under physiologically relevant conditions upon protonation of the imidazole group. We designed these molecules based on a previously reported (6) ethoxyethyl protecting group for imidazoles that was shown to be hydrolytically unstable in aqueous acidic conditions at elevated temperatures. Mechanistically, the cleavage of ethoxyethyl groups on imidazoles had been proposed to involve the protonation of the imidazole nitrogen (N3 in Figure 1) followed by breaking of the C6-N1 bond as the rate determining step (RDS, Figure 1). To attain a related N-linked imidazole that could undergo acid hydrolysis at physiological temperatures, we explored whether substitution of the ethyl group (i.e., bearing the C6 carbon in Figure 1) with benzyl groups could lower the activation energy required for this hydrolysis reaction and facilitate the cleavage of the C6-N1 bond under physiological conditions.
Figure 1.
Proposed mechanism for the hydrolysis of N- alkoxyalkylimidazoles in aqueous acidic solutions. RDS = rate determining step.
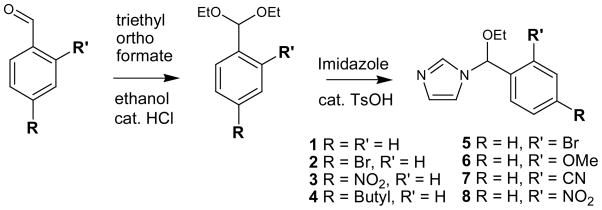
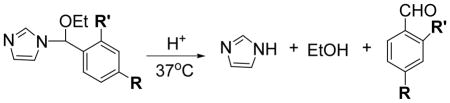
We synthesized N-ethoxybenzylimidazole (NEBI) 1 from benzaldehyde using a simple and mild two step procedure (Scheme 1). The Supporting Information summarizes the details of the synthesis. At 37 °C in 0.5 M HEPES buffer in D2O (pD = 7.4), we measured by 1H-NMR that 1 hydrolyzes with a half-life of 25 hours to give imidazole, ethanol, and benzaldehyde as the only products detected (Figure 1) (7). At 37 °C in 0.5 M MES buffer in D2O (pD = 5.5), 1 hydrolyzed to give the same products as at higher pD (Figure 1) but with a much shorter half-life of 1.7 hours for hydrolysis. Kinetic analysis indicated that the hydrolysis of 1 is first-order dependent on the concentration of 1 and zero-order dependent on the concentration of buffering agent (see Figure S1 in the Supporting Information) (8). As expected from the proposed mechanism shown in Figure 1, the benzyl group (R = Phenyl) appears to stabilize the transition state of the rate determining step better than the previously reported (6) ethyl group (R = Methyl) and, thus, facilitates hydrolysis of 1 at physiological temperatures. Having measured the pKa of the protonated form of 5 (as a representative NEBI) to be 5.4 (9), the observed acceleration of the rate of hydrolysis of 1 in mild acidic solution compared to physiological pH is also consistent with the proposed mechanism (Figure 1).
Scheme 1.
General procedure for the synthesis of N-ethoxybenzylimidazoles (NEBIs) 1–8 from the corresponding benzaldehydes.
To investigate whether electronic effects might influence the rate of hydrolysis of NEBIs, we incorporated electron donating and electron withdrawing groups at the ortho or para positions of the phenyl ring and compared the rates of hydrolysis for 1–8. Incorporation of an electron donating methoxy group at the ortho position in 6, for instance, resulted in a half-life of 0.6 hours for the hydrolysis of 6 at 37 °C in 0.5 M MES buffered D2O (pD = 5.5); we observed a slower rate of hydrolysis for 6 (t1/2 ≈ 5 hours) at 37 °C in 0.5 M HEPES buffered D2O (pD = 7.4) compared to the rates of hydrolysis at pD = 5.5 (Table 1). Incorporation of electron withdrawing substituents at the ortho or para positions of the phenyl ring of 1, on the other hand, resulted in slower rates of hydrolysis in acidic and neutral solutions. Incorporation of a relatively weak electron withdrawing bromo substituent at the ortho position in 5, for example, resulted in a significant decrease in the rate of cleavage of the NEBI moiety, with measured half-lives of 280 and 2300 hours for the hydrolysis of 5 at 37 °C in buffered D2O at pD = 5.5 and pD = 7.4, respectively. Furthermore, incorporation of strong electron withdrawing nitro substituents at the ortho or para position of the phenyl ring resulted in remarkably slow rates of hydrolysis of the NEBI moiety at 37 °C, with measured half-lives of 6900 hours for both 3 and 8 in buffered D2O at pD = 5.5 and no hydrolysis detected after >8 weeks at pD = 7.4.
Table 1.
Half-lives (t1/2) and rate constants (k) for the hydrolysis of NEBIs in D2O at pD = 5.5 and pD = 7.4. Rates of hydrolyses were measured by 1H-NMR. See Supporting Information for details.
 | ||||||
|---|---|---|---|---|---|---|
| R | R′ | pH=5.5a | pH=7.4b | |||
| t1/2[h] | k[h−1] | t1/2[h] | k[h−1] | |||
| 1 | H | H | 1.7 | 0.41 | 25 | 0.028 |
| 2 | Br | H | 16.2 | 0.04 | 270 | 0.003 |
| 3 | NO2 | H | 6900 | <0.001 | N/Ac | - |
| 4 | Bu | H | 1.0 | 0.69 | 8.8 | 0.079 |
| 5 | H | Br | 280 | 0.003 | 2310 | < 0.001 |
| 6 | H | OMe | 0.6 | 1.13 | 5.1 | 0.136 |
| 7 | H | CN | 3500 | <0.001 | N/Ac | - |
| 8 | H | NO2 | 6900 | <0.001 | N/Ac | - |
0.5M MES buffer solution
0.5M HEPES buffer
No hydrolysis detected after > 8 weeks
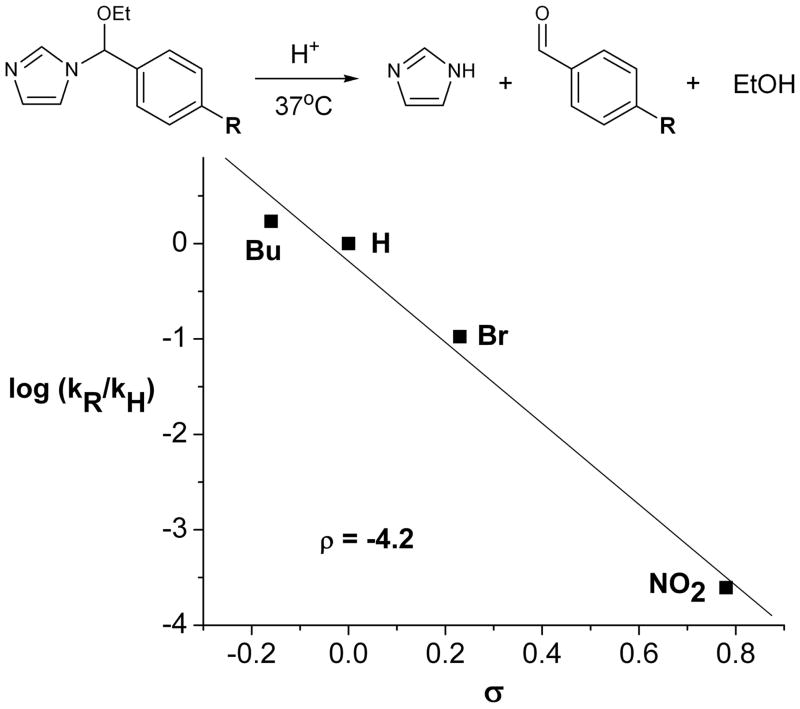
To further probe the mechanism of cleavage of NEBIs in acidic solutions, we plotted the linear free energy relationship (10) for the hydrolysis of 1–4 in D2O at pD = 5.5 (Figure 2). We calculated a ρ-value of −4.2 for the hydrolysis of the NEBIs, which is consistent with an increase in positive charge (e.g., on the benzylic carbon of 1–8) in the rate determining step of the reaction. This observed linear Hammett correlation, combined with the finding that the rate of hydrolysis of 1 is independent of the concentration of buffering agent (see Figure S1 in the Supporting Information), supports a specific acid catalyzed mechanism (10) for the hydrolysis of 1–8 as shown in Figure 1.
Figure 2.
Linear Hammett correlation for the hydrolysis of 1–4 in 0.5 M MES buffered D2O (pD = 5.5) at 37 °C, where R = n-butyl (Bu), H, Br, and NO2.
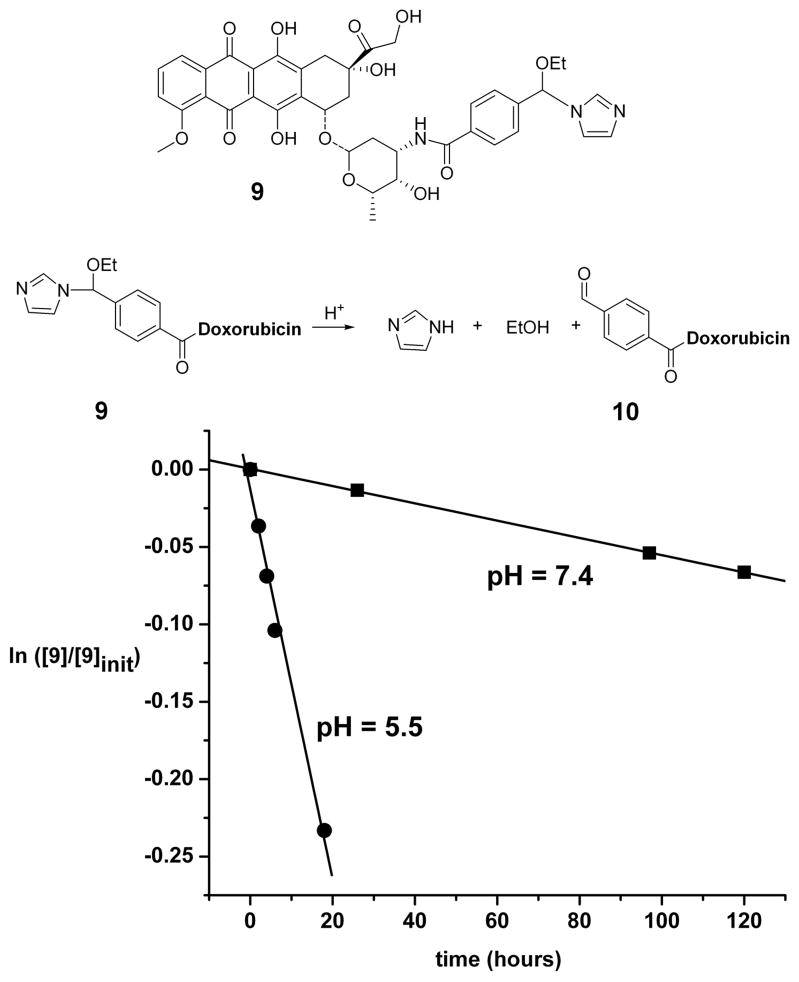
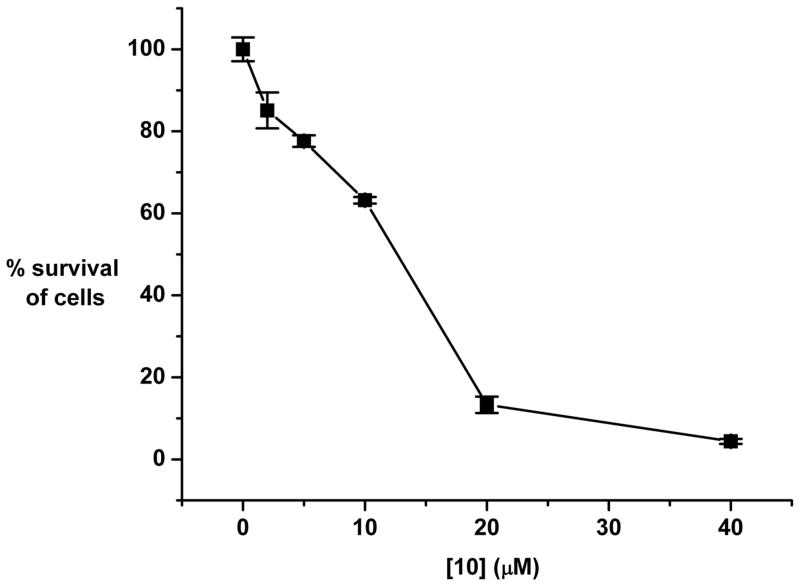
With these results from kinetic and mechanistic studies in hand, we were interested in determining whether a NEBI carrying an anti-tumor agent—here, doxorubicin (11)—would also hydrolyze with accelerated rates under mild acidic solutions compared to at normal, physiological pH. We synthesized 9 by formation of an amide bond between a reactive amine group on doxorubicin and a carboxylate group on the para position of the phenyl ring of the NEBI (Figure 3). The supporting information describes the details of the synthesis and characterization of 9. We measured a half-life by RP-HPLC of 55 hours for the hydrolysis of 9 at 37 °C in MES buffered H2O (pH = 5.5) (12). As expected, we observed a slower rate of hydrolysis of 9 (t1/2 = 1150 hours) at 37 °C in HEPES buffered H2O (pH = 7.4) compared to the rates of hydrolysis at pH = 5.5 (Figure 3). Cytotoxicity studies indicated that 10 (i.e., the analogue of doxorubicin resulting from hydrolysis of 9 in mild acidic solutions) retains anti-cancer activity against human ovarian carcinoma 2008 cells with an IC50 of ~ 12 μM (Figure 4). For comparison, compound 9 had a 2-fold higher IC50 and doxorubicin had a >10-fold lower IC50 than 10 against this cell line. Although less potent than doxorubicin, compound 10 also has similar cytotoxic activity compared to other clinically used anti-cancer agents being developed for controlled release from drug delivery systems via pH-sensitive linkers (4c).
Figure 3.
Hydrolysis of a NEBI conjugated with doxorubicin (9) in MES buffer (pH = 5.5) and HEPES buffer (pH = 7.4). The pH-dependent rate of hydrolysis of 9 to aldehyde 10 was determined by RP-HPLC. See supporting information for details.
Figure 4.
Concentration dependent cytotoxicity of N-(4-formylbenzoyl)doxorubicin 10 on human ovarian carcinoma 2008 cells. Cells were exposed to various concentrations of 10 for 1 hour and survival was determined after a further 7 day period of growth in drug-free medium. See supporting information for details of the cell survival assay.
We have thus demonstrated that NEBIs hydrolyze in aqueous solutions at physiological temperatures with hydrolysis occurring approximately 10 times faster in mildly acidic solutions compared to solutions at normal, physiological pH. By incorporating electron donating or electron withdrawing substituents, we demonstrated the ability to tune the rates of hydrolysis of these N-linked imidazoles in mild acidic solutions with half-lives ranging from ~30 minutes to > 9 months. A NEBI carrying doxorubicin also exhibited accelerated rates of hydrolysis in acidic solution compared to at neutral pH, with the product of hydrolysis showing potentially useful cytotoxic activity against human cancer cells. Previous studies have shown that drugs conjugated to delivery vessels via pH sensitive linkers with hydrolytic properties similar to 9 can exhibit useful antitumor activity in vivo (1d). These drugs conjugated to delivery vessels using pH sensitive linkers also showed reduced toxicity to normal healthy tissues compared to administration of equivalent concentrations of free drug. The ability to tune the rates of hydrolysis of the NEBIs may make it possible to optimize the rate of release of drugs attached to delivery vessels via these acid sensitive functionalities for maximal efficacy for cancer therapy.
The described N-linked imidazoles possess many desired features (1, 4) for use as acid sensitive linkers in cancer drug delivery. In addition to 1) their tunable rates of hydrolysis, 2) their non-toxic byproducts from hydrolysis (i.e., ethanol), and 3) their versatility for the range of alcohol- and amine-containing therapeutics that can be linked to drug carriers, the known ability to tune the pKa’s of protonated imidazoles (5) may make it possible to optimize operational pH range of NEBIs for specific drug delivery applications. The mild conditions required to synthesize NEBIs carrying anticancer agents also provides a simple route to incorporate this functionality into tumor targeting vessels. It may be possible, for instance, to use NEBI linkers to covalently attach therapeutics to imidazole-containing derivatives of known delivery systems (1, 4) that can target tumors and exploit the endocytotic pathway for internalization in cells. The synthesis and detailed study of a range of potential therapeutic agents conjugated to drug delivery carriers via NEBI linkers is currently under way.
Supplementary Material
Acknowledgments
This work was partially supported by the Academic Senate, a Faculty Career Development Award, a Hellman Fellowship Award, and the Department of Chemistry and Biochemistry at UCSD and by Grant #RSG-07-024-01-CDD from the American Cancer Society. S.B.H and G.M. acknowledge support from the National Institutes of Health (CA 95298). The National Science Foundation (CHE-0116662) is gratefully acknowledged for support of the mass spectrometry facilities at UCSD. The authors also thank Professor Charles Perrin for helpful discussions during the preparation of this manuscript.
Footnotes
Supporting Information Available Additional experimental details and characterization of molecules. This material is available free of charge via the Internet at http://pubs.acs.org/BC
References
- 1.a) Gillies ER, Goodwin AP, Frechet JMJ. Acetals as pH-Sensitive Linkages for Drug Delivery. Bioconj Chem. 2004;15:1254–1283. doi: 10.1021/bc049853x. [DOI] [PubMed] [Google Scholar]; b) Heller J, Barr J, Ng SY, Abdellauoi KS, Gurny R. Poly(ortho esters): synthesis, characterization, properties and uses. Adv Drug Deliv Rev. 2002;54:1015–1039. doi: 10.1016/s0169-409x(02)00055-8. [DOI] [PubMed] [Google Scholar]; c) Kruger M, Beyer U, Schumacher P, Unger C, Zahn H, Kratz F. Synthesis and stability of four maleimide derivatives of the anticancer drug doxorubicin for the preparation of chemoimmunoconjugates. Chem Pharm Bull. 1997;45:399–401. [Google Scholar]; d) Patel VF, Hardin JN, Mastro JM, Law KL, Zimmermann JL, Ehlhardt WJ, Woodland JM, Starling JJ. Novel Acid Labile COL1 trityl-linked difluoronucleoside immunoconjugates: Synthesis, characterization, and biological activity. Bioconj Chem. 1996;7:497–510. doi: 10.1021/bc960038u. [DOI] [PubMed] [Google Scholar]; e) Greenfield RS, Kaneko T, Daues A, Edson MA, Fitzgerald KA, Olech LJ, Grattan JA, Spitalny GL, Braslawsky GR. Evaluation in vitro of adriamycin immunoconjugates synthesized using an acid-sensitive hydrazone linker. Cancer Res. 1990;50:6600–6607. [PubMed] [Google Scholar]; f) Shen WC, Ryser HJP. Cis-aconityl spacer between daunomycin and macromolecular carriers: A model of pH-sensitive linkage releasing drug from a lysosomotropic conjugate. Biochem Biophys Res Commun. 1981;102:1048–1054. doi: 10.1016/0006-291x(81)91644-2. [DOI] [PubMed] [Google Scholar]; g) Etrych T, Jelinkova M, Rihova B, Ulbrich K. New HPMA copolymers containing doxorubicin bound via pH-sensitive linkage: synthesis and preliminary in vitro and in vivo biological properties. J Control Release. 2001;73:89–102. doi: 10.1016/s0168-3659(01)00281-4. [DOI] [PubMed] [Google Scholar]; h) Walker GF, Fella C, Pelisek J, Fahrmeir J, Boeckle S, Ogris M, Wagner E. Toward synthetic viruses: Endosomal pH-triggered deshielding of targeted polyplexes greatly enhances gene transfer in vitro and in vivo. Molecular Therapy. 2005;11:418–425. doi: 10.1016/j.ymthe.2004.11.006. [DOI] [PubMed] [Google Scholar]; i) Tomlinson R, Heller J, Brocchini S, Duncan R. Polyacetal-Doxorubicin Conjugates Designed for pH-Dependent Degradation. Bioconj Chem. 2003;14:1096–1106. doi: 10.1021/bc030028a. [DOI] [PubMed] [Google Scholar]; j) Choi jS, MacKay JA, Szoka FC., Jr Low-pH-sensitive PEG-stabilized plasmid-lipid nanoparticles: Preparation and characterization. Bioconj Chem. 2003;14:420–429. doi: 10.1021/bc025625w. [DOI] [PubMed] [Google Scholar]
- 2.a) Ojugo ASE, McSheehy PMJ, Stubbs M, Alder G, Bashford CL, Maxwell RJ, Leach MO, Judson IR, Griffiths JR. Influence of pH on the uptake of 5-fluorouracil into isolated tumour cells. Brit J Cancer. 1998;77:873–879. doi: 10.1038/bjc.1998.144. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ojugo ASE, McSheehy PMJ, McIntyre DJO, McCoy C, Stubbs M, Leach MO, Judson IR, Griffiths JR. Measurement of the extracellular pH of solid tumours in mice by magnetic resonance spectroscopy: a comparison of exogenous 19F and 31P probes. NMR Biomed. 1999;12:495–504. doi: 10.1002/(sici)1099-1492(199912)12:8<495::aid-nbm594>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]; c) van Sluis R, Bhujwalla ZM, Raghunand N, Ballesteros P, Alvarez J, Cerdan S, Galons JP, Gilles RJ. In vivo imaging of extracellular pH using 1H MRSI. Magn Reson Med. 1999;41:743–750. doi: 10.1002/(sici)1522-2594(199904)41:4<743::aid-mrm13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Brown DM. Drug Delivery Systems in Cancer Therapy. Humana Press; Totowa: 2004. [Google Scholar]
- 4.a) Gillies ER, Frechet JMJ. pH-responsive copolymer assemblies for controlled release of doxorubicin. Bioconj Chem. 2005;16:361–368. doi: 10.1021/bc049851c. [DOI] [PubMed] [Google Scholar]; b) Rice JR, Howell SB. Polymer-delivered platinum complex. Drugs of the Future. 2004;29:561–565. [Google Scholar]; c) Lin X, Zhang Q, Rice JR, Stewart DR, Nowotnik DP, Howell SB. Improved targeting of platinum chemotherapeutics: the antitumour activity of HPMA copolymer platinum agent AP5280 in murine tumour models. Eur J Cancer. 2004;40:291–297. doi: 10.1016/j.ejca.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 5.The pKa of imidazolium ions have been reported to be between 5 and 7: Bruice TC, Schmir GL. Imidazole Catalysis. 11. The Reaction of Substituted Imidazoles with Phenyl Acetates in Aqueous Solution. J Am Chem Soc. 1958;80:148–156.
- 6.Manoharan TS, Brown RS. I-(1-Ethoxyethyl): An Effective Protecting Group for Imidazole Nitrogen. J Org Chem. 1988;53:1107–1110. [Google Scholar]
- 7.For all hydrolysis studies in D2O, we added 20–40% d6-DMSO (v/v) to improve the solubility of the molecules. The addition of 20–40% DMSO did not affect the pH of the solutions or the rate of hydrolysis of 1.
- 8.Connors KA. Chemical Kinetics: The Study of Reaction Rates in Solution. Wiley-VCH; New York: 1990. [Google Scholar]
- 9.We measured the pKa of 5 in 50:50 H2O:DMSO by titration of 5 with a concentrated solution of HCl. We estimated the pKa of 5 by measuring the pH of the solution when half an equivalent of HCl was added
- 10.Carroll FA. Perspectives on Structure and Mechanism in Organic Chemistry. Brooks/Cole Publishing Company; Pacific Grove: 1998. [Google Scholar]
- 11.Perry MC. The Chemotherapy Source Book. 3. Lipincott, Williams & Wilkins; Philadelphia: 2001. [Google Scholar]; Arcamone F. Doxorubicin Anticancer Antibiotics. Academic Press; New York: 1981. [Google Scholar]
- 12.The observed rate of hydrolysis of 9 at pH = 5.5 is consistent with the expected value for a NEBI group containing an amide functionality attached to the para position of the phenyl ring in 1 as predicted from the linear free energy relationship shown in Figure 2 (R = CONHR’, σ = 0.36, log (kR/kH) = −1.32).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.