Abstract
Introduction
We recently reported the biochemical characterization of a novel Fasciola hepatica recombinant antigen termed FhSAP2, which has previously shown to elicit protection to F. hepatica infection in rabbits. Further we reported that intramuscular (IM) injections of BALB/c mice with a pFLAG-CMV−2 vector carrying cDNA encoding for FhSAP2 (cDNA-FhSAP2) induce high levels of immune response. The aim of the present study is to ascertain whether the immune response induced by this DNA construct may induce protection in mice against subsequent infection with F. hepatica metacercariae (mc). In addition, protection following subcutaneous (SC) injections with recombinant FhSAP2 was evaluated.
Methods
Mice received three IM injections with 100μg of cDNA-FhSAP2 or three 5C injections with 20μg of FhSAP2. Four weeks after the last vaccination mice were challenged orally with 5 F. hepatica mc and euthanized 45 days after challenge.
Results
Mean worm burdens found in mice vaccinated with cDNA-FhSAP2 was reduced by 83.3% and the mean worm burdens found in mice vaccinated with the recombinant protein was reduced by 60% when compared with controls. All vaccinated animals had less liver damage than challenge controls. Vaccination with cDNA-FhSAP2 seems to favor a mixed Th1/Th2-antibody dependent with higher predominance of Th1-regulated antibody response.
Conclusions
The vaccination with cDNA-FhSAP2 or recombinant FhSAP2 may protect hosts against F. hepatica infections. The vaccination with cDNA form of FhSAP2 appeared to be a little more efficient preventing the infection. The predominance of Th1-dependent antibodies in the vaccinated animals may be responsible for the protection but this should be confirmed by Th1-cytokines determinations.
Keywords: Fasciola hepatica, DNA-vaccination, FhSAP2
Introduction
Fascioliasis is a major parasitic disease of livestock caused by the infection with either Fasciola hepatica or F. gigantica with F. hepatica the most prevalent in the temperate regions of America. Fascioliasis among domestic livestock has important economic implications. Over 700 million production animals are at risk of infection and the losses in productivity have been estimated to cost the agricultural sector in excess of $3.2 billion per year.1 Up until the mid 1990’s, human fascioliasis was considered a coincidental secondary zoonotic disease of humans. However, the recent re-emergence of human fascioliasis in many countries has recently prompted the World Health Organization to recognize fascioliasis as an important human parasitic disease.2 Within the last decade the number of cases for human fascioliasis has been reported to reach as high as 17 million people across 61 countries, with a further 180 million people at risk of infection.3-5 Human fascioliasis is endemic in several regions of Central and South America, Europe, Africa and Asia and the highest rate of infection has been reported in the northern Altiplano of Bolivia with 72–100% of the population infected.6-8
The traditional approaches used to control Fasciola infection include the use of molluscicides and chemotherapy.9 Although molluscicides are cost-effective and have been successfully used to decrease snail population they are toxic to several non-targeted species. The continual risk of contaminating the surrounding environment and nearby animals has seen the use of molluscicides generally abandoned.9 Currently, the most effective approach available for controlling fascioliasis is host treatment with anthelmintics.2,10 Triclabendazol has been the drug of choice against fascioliasis in livestock for over 20 years, and has recently been successful in treating human fascioliasis.10,11 However, the continuing efficacy of this drug is at risk as resistance is emerging.12 Due to the negative impact of molluscicides and development of drug resistance, current research is focusing on developing new approaches, such as effective vaccine therapy, to control fascioliasis.
We recently described a new F. hepatica antigen termed FhSAP2, which is an 11.5-kDa polypeptide belonging to the saposin-like protein family. The saposin signature motif, a compact domain of mainly α-helical character that contains six cysteine residues and 7 hydrophobic conserved residues,13,14 has been implicated in membrane binding, pore formation and subsequent cell lysis in several family members.15-20 FhSAP2 has been shown to induce significant protection levels in rabbits challenged with F. hepatica infection.21
Nucleic acid vaccine technology provides a new approach for prevention of infectious diseases, including those caused by trematode parasites closely related to F. hepatica such as schistosomes.22-24 We previously demonstrated that the pFLAG-CMV−2 vector carrying cDNA encoding for FhSAP2 induces high levels of immune response in mice.25 The aim of the present study is to ascertain whether the immune response induced by this DNA construct may protect mice against subsequent infection with F. hepatica metacercariae. In addition, protection following subcutaneous (SC) injections with recombinant FhSAP2 was evaluated.
Materials and Methods
Mice and Parasites
Inbred female BALB/c (H-2d) mice 10 weeks old were purchased from Harlan, Inc (Indianapolis, IN) and kept under conventional germ-free conditions in the animal care facility of the University of Puerto Rico, School of Medicine and treated according to international regulations for caring for laboratory animals. Metacercariae (mc) 30 days-old used for challenge infection were obtained from Baldwin Aquatic, Inc (Monmouth, Oregon).
F. hepatica ES Antigens and Recombinant FhSAP2
ES antigens were obtained by in vitro maintenance of live F. hepatica adult fluke collected from livers of naturally infected cattle as previously described.26 cDNA encoding FhSAP2 (GenBank AF286903) was cloned into pBAD-HisB, expressed in E. coli as fusion protein with a His-tag and then purified by Ni2+ column affinity chromatography as previously described.
DNA-plasmid Constructs
The construction of plasmid pFLAP-CMV−2 containing the cDNA encoding for FhSAP2 was previously described.25 For immunization, plasmid DNA was prepared using the Endotoxin-Free Maxi Kit according to the manufacturer’s instructions (Qiagen, Hilden, Germany). The DNA was ethanol precipitated and resuspended in sterile endotoxin-free saline (Sigma, St, MO, USA) at a final concentration of 1 mg/mL.
Experimental Design
On day 0 of the experiment the first vaccination was administered to groups of ten mice. Group-1 received three SC injections of 20 μg FhSAP2 each at two-week intervals emulsified in complete Freund’s adjuvant (FA) (first injection) and incomplete FA in the other injections. Group-2 received the same number of injections with PBS in adjuvant and was used as a control. Group-3 received three IM injections of DNA-FhSAP2 over a period of 12 weeks. Group-4 which was also used as a control group, received IM injections with empty pFLAG-CMV−2 vector. Each IM immunization comprised 50 μg of DNA-FhSAP2 or empty vector into each quadriceps muscle (100μg/mouse).
Six weeks after the last injection mice from all groups received an infectious dose of five mc F. hepatica, which were applied with oral gavages. Mice were necropsied 45-days after the infection and their livers examined carefully as described by Harness et al.27 The grade of lesions observed in each liver was summarized semi-quantitatively as previously described.21 Blood samples were collected before vaccination, at challenge and at necropsy.
Antibody Responses
Sera collected during the experiment were examined by ELISA to determine total antibody and antibody isotype levels according to protocol described previously.25 Flat bottomed micro ELISA plates were coated overnight, at 4°C with the recombinant FhSAP2 or ES antigen diluted to 2.5μg/mL with 0.05M carbonate-bicarbonate buffer pH 9.6. After blocking with 3% bovine serum albumin in phosphate buffered saline (PBS) containing 0.05% tween-20 (PBST), each serum sample diluted 1:100 in PBST was incubated in triplicate for 1h at 37°C. For the detection of total antibody response, peroxidase-conjugated sheep anti-mouse immunoglobulin (Bio-Rad, Hercules CA) diluted 1:5,000 in PBST was added and incubation at 37°C was continued for a further hour followed by washing and the addition of substrate solution (O-phenylenediamine). For the detection of antibody isotypes, specific non-conjugated goat anti-mouse IgG1, IgG2a, IgG2b and IgG3 (Sigma) diluted 1:1000 in PBST was added and incubated for 1h at 37°C, followed by washing and the addition of peroxidase-conjugated rabbit anti-goat immunoglobulins (Bio-Rad) diluted 1:2,500 in PBST which was incubated for a further hour. After a last washing step the substrate solution was added and the reaction was stopped by addition of 12.5% sulfuric acid. The results were read at 492nm in an ELISA reader (Bio-Rad).
Statistical Analysis
Each antibody determination was performed in triplicate on two different days and the results expressed as the mean optical density (OD) for each determination. Values yielding an OD492 nm≥.16 were considered positive. When necessary, log-transformation of data was performed for normality test. To assess the significance of differences between groups, two-way ANOVA tests were employed.28 If the assumption of homogeneity of variance was not satisfied, the non-parametric Kruskal-Wallis test was used.29 Kaplan-Meier survival analysis was performed to estimate survival of animals over time and log-rank test was performed to compare survival curves.30 All the statistical analysis was performed with the statistical software STATA, version 10.
Results
Mortality and Fluke Burdens after Challenge Infection
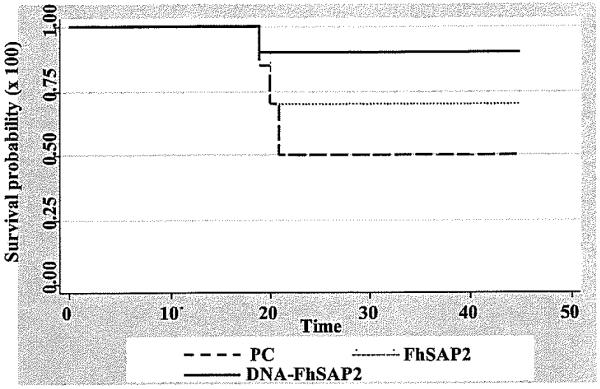
90% of mice vaccinated with plasmid carrying the cDNA encoding for FhSAP2 and 70% of mice vaccinated with the recombinant form survived to the challenge infection. The survival probability was found statistically significant (P<.05) for the group vaccinated with DNA-FhSAP2 (Fig. 1). Overall, the livers from mice vaccinated with PBS or empty vector showed gross-pathological lesions that ranged from moderate to severe with recovery of 1 to 3 flukes. In contrast, the liver from animals vaccinated with recombinant FhSAP2 or DNA presented a normal appearance or exhibited gross-pathological lesions that were scored as mild to moderate. A single fluke was recovered from livers of vaccinated animals that exhibited mild to moderate lesions. The size of flukes recovered from livers of vaccinated mice was slightly smaller than recovered from non-vaccinated animals but these differences were not statistically significant. When a single fluke was recovered its size was larger than those recovered from livers harboring two or three flukes (data not shown).
Fig 1.
Kapplan-Meier analysis to estimate the survival probability over the time after challenge in mice vaccinated with FhSAP2 or cDNA-FhSAP2 compared to positive controls. PC includes mice vaccinated with PBS in FA and vaccinated with the empty pFLAG-CMV−2 plasmid. Log-rank test determined that there are statistical differences (P<.05) between survival probability of DNA-FhSAP2-vaccinated group compared to the PC group
When we compared the vaccinated groups with the corresponding control group, a significant reduction in the fluke burden was found (Table 1). The odds of finding parasites in the positive control was 18.7-fold (95%CI: 4.35, 80.46) the odds of finding parasites in the group vaccinated with cDNA-FhSAP2 or FhSAP2; this was statistically different (P<.05). In contrast, the odds of finding parasites in the FhSAP2-vaccinated group was only 0.34-fold (95% CI: 0.06, 2.07) the odds of finding parasites in the cDNA-FhSAP2 vaccinated group and this difference was not statistically different.
Table 1.
Fluke burden and gross-pathological lesions in BALB/c mice vaccinated with FhSAP2 protein or DNA construct compared to a control groups. (−) No visible lesions; (+) Mild lesions; (++) Moderate lesions; (+++) Severe lesions
| Vaccinated with FhSAP2 (n=10). Three mice died on days 19 to 20 after infection |
Vaccinated with cDNA-FhSAP2 (n=10). One mouse died on day 18 after infection |
||||
|---|---|---|---|---|---|
| Mouse | # Fluke recovered | Liver lesions | Mouse | # Fluke recovered | Liver lesions |
| 1 | 0 | − | 1 | 0 | − |
| 2 | 0 | − | 2 | 1 | + |
| 3 | 0 | − | 3 | 0 | − |
| 4 | 1 | + | 4 | 0 | − |
| 5 | 1 | ++ | 5 | 0 | − |
| 6 | 1 | + | 6 | 0 | − |
| 7 | 1 | + | 7 | 0 | − |
| Total | 4 | 8 | 0 | − | |
| 9 | 1 | ++ | |||
| Total | 2 | ||||
| Vaccinated with PBS-FA (n=10). Six mice died on days 19-21 after infection |
Vaccinated with empty pFLAG-CMV−2 vector (n=10). Five mice died on days 18 to 22 after infection |
||||
|---|---|---|---|---|---|
| Mouse | # Fluke recovered | Liver lesions | Mouse | # Fluke recovered | Liver lesions |
| 1 | 3 | +++ | 1 | 3 | +++ |
| 2 | 3 | +++ | 2 | 2 | ++ |
| 3 | 1 | ++ | 3 | 3 | +++ |
| 4 | 3 | +++ | 4 | 1 | +++ |
| Total | 10 | 5 | 3 | +++ | |
| Total | 12 | ||||
| Protection: 60% | Protection: 83.3% | ||||
Protection was calculated by the formula (C-E)/C×100, where C is the average number of flukes in the control group and E is the average number of flukes in the vaccinated group of animals.
Anti-FhSAP2 and Anti-FhES Antibody Response
Mice that received SC immunizations with FhSAP2 developed high antibody levels that ranged from 0.8 to 2.66 (mean OD = 1.45 ± 0.56). These animals developed high antibody levels to the FhES antigen that ranged from 0.78 to 2.1 (mean 1.35 ± 0.49). Animals that received IM injections with cDNA-FhSAP2 also developed specific antibody to FhSAP2 and FhES antigens that ranged from 0.75 to 1.4 (mean 1.09 ± 0.23) and 0.55 to 1.43 (mean 0.97 ± 0.25) respectively. Sera of animals immunized with PBS or with the empty pFLAP-CMV−2 vector did not react with FhSAP2 nor FhES antigen. These antibody levels were not substantially modified after the infection. At necropsy, CMV−2 and PBS-vaccinated mice were found to be highly positive to FhSAP2 with OD values ranging from 0.38 to 1.2 (mean 0.59 ± 0.31) and 0.6 to 1.1 (mean 0.81 ± 0.26) respectively. Statistically significant differences (P<.05) were found between mean specific antibody levels stimulated by FhSAP2- or cDNA-FhSAP2 when compared to the controls.
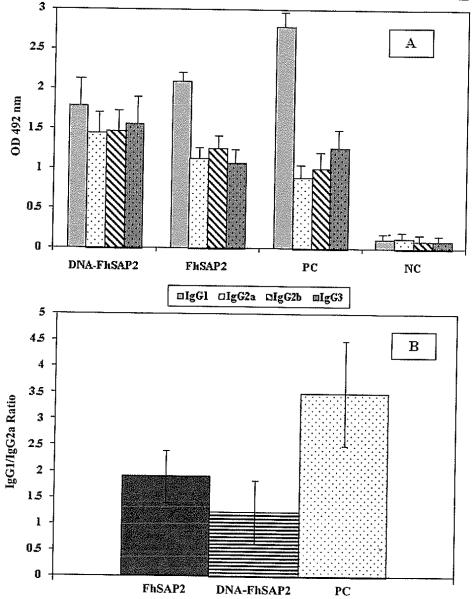
Figure 2A shows the mean levels of IgG1, IgG2a, IgG2b and IgG3 detected in sera from vaccinated and control animals at challenge. IgG1 was the predominating antibody isotype in the control groups (P<.001), followed by the group vaccinated with FhSAP2. Although in the group vaccinated with cDNA-FhSAP2 the levels of IgG1 were higher than the other isotypes, the mean values were not significantly different (P>.05). When the IgG1 to IgG2a ratios between groups was compared it was observed that the control groups had the highest IgG1 to IgG2a ratio (Fig. 2B). The control groups had 3-5-fold more IgG1 vs IgG2a. The group vaccinated with FhSAP2 had 1.9-fold more IgG1 vs IgG2a, and the group vaccinated with cDNA-FhSAP2 had only 1.23-fold more IgG1 IgG2a. No statistical differences were found between the mean IgG1 to IgG2a ratio obtained for the FhSAP2-vaccinated group and the mean IgG1 to IgG2a ratio obtained for the cDNA-FhSAP2-vaccinated group. However, significant differences (P<.0036) were found between the IgG1 to IgG2a ratios from the group vaccinated with FhSAP2 and the positive control group and between the group vaccinated with cDNA-FhSAP2 compared to positive controls (P<.0013).
Fig 2.
(A) Levels of specific anti-FhSAP2 IgG1, IgG2a, IgG2b and IgG3 elicited by immunization with FhSAP2, or with cDNA-FhSAP2 45 days after challenge. Values represent the mean ± SD of triplicate ELISA readings of each vaccinated group. PC represent the serum samples collected from animals vaccinated with PBS-adjuvant and empty pFLAG-CMV−2 plasmid. NC represent the serum samples collected at day 0 from naïve mice. (B) IgG1 to IgG2a ratios obtained for FhSAP2- and cDNA-FhSAP2-vaccinated groups compared to the PC group. Values represent the mean ± SD of IgG1 to IgG2a ratio ELISA readings. Significant differences (P<.0036) were found between the IgG1 to IgG2a ratios from the group vaccinated with recombinant FhSAP2 and the PC group and between the group vaccinated with DNA-SAP2 compared with the PC group (P<.0013)
Discussion
Although sheep or cattle are the most important hosts to which a vaccine against F. hepatica should be addressed, in most occasions the evaluation of novel antigens as well as all combinations of vaccines, routes, doses and schedules cannot be determined in these animals and usually these parameters are evaluated in smaller experimental models. A mouse is a permissive host for infection with F. hepatica, as are humans, rabbits, rats and ruminants and therefore, is a good model for preliminary exploratory protection studies as described in the current study. Although the immune response elicited in the mouse model of fascioliasis cannot be directly extrapolated to the ruminant model, it could serve as framework to understand the immune mechanisms that occurs during the vaccination and the challenge infection.
A mouse is highly susceptible to F. hepatica infection and usually dies when infected with more than 3 mc F. hepatica. Our study demonstrates that mortality was prevented by a successful immunization either with the protein FhSAP2 or a DNA construct expressing FhSAP2. This is consistent with the reports of other authors who reduced significantly the mortality among mice vaccinated with other Fasciola antigen.31 The reduction of fluke burdens and the reduction in liver damage are the most important criteria of protection in experimental infections against F. hepatica.32-38 According to the results of this study, the reduction of fluke burdens combined with the smaller gross-pathological lesions observed in the livers of vaccinated animals suggest that both protein and cDNA forms of the FhSAP2 may induce some level of protection to subsequent infection with the fluke mc. Some inconsistencies were observed in the group vaccinated with empty vector in which the same lesion score was found with 1, 2 or 3 recovered flukes but this can be explained by the differences in size of flukes recovered. The pathology induced by Fasciola is related to damage induced by cuticle and this is turn is dependent on the size of the flukes.
FhSAP2 delivered as cDNA appeared to be a little more efficient in the prevention of the infection than the recombinant protein in Freund’s adjuvant. The level of protection induced by our DNA constructs is similar to other DNA constructs expressing F. hepatica antigens such as glutathione-S-transferase39 and cysteine protease40,41 and the protection induced by the recombinant FhSAP2 is also comparable to other recombinant or native antigens assayed in mice, including ES antigens (86%),42 partially purified F. hepatica crude extract (FhSmIII[M]),43 and recombinant FABP-Fh15.44
The magnitude of antibodies’ response stimulated by the recombinant FhSAP2 or DNA construct are consistent with those obtained in previous studies,25 and confirm that FhSAP2 is highly immunogenic and that the antigen expressed by the transfected cells is able to stimulate antibody production by the host. The high levels of antibodies relative to ES antigens observed in the vaccinated groups demonstrates that the antibodies induced by FhSAP2 are able to recognize the native antigen and that the antigen expressed by the host cells following DNA immunization has a similar structure and antigenicity to the native antigen expressed by F. hepatica. This result again confirms the presence of FhSAP2 in the excretion/secretion material of the parasite and is consistent with predicted secretory signal-sequences at the N-terminal end of FhSAP2.13
There are few reports on cDNA vaccination against helminth infections and most have been conducted in laboratory animal models. Results depend on the host-parasite system tested, the cDNA fragments used, the type of vector and the route of the DNA delivery.39-41,45,46 Smooker et al investigated the humoral response to DNA vaccination of mice with F. hepatica cathepsin-L5 delivered as secretory and found that the humoral response was sustained for at least 20 weeks.46 Antibody isotype analysis demonstrated a Th2-dependent response characterized by lower titer to IgG1 and IgG3 but the authors failed to ascertain if this response was protective. In a previous study we demonstrated that the immune responses stimulated by cDNA-FhSAP2 is sustained for at least 16-weeks25 and herein we demonstrated that this construct induced a mixed Th1/Th2-dependent antibody response characterized by higher IgG2a levels than elicited during the natural infection. It has been reported that, in mice IgG2a, IgG2b and IgG3 antibodies are Th1 regulated, while IgG1 response is Th2-dependent.47 It was not surprising that our non-vaccinated, challenged controls had the highest levels of IgG1 and the highest IgG1 to IgG2a ratios since it has been reported that the F. hepatica infection down regulates the Th1 response in mice.48 The observation that the lowest IgG1 to IgG2a ratios were found in rhe group vaccinated with the cDNA-FhSAP2, which acquired the highest protection levels, suggest that FhSAP2 in the form of cDNA may induce a stronger Th1 antibody dependent immune response. However, these observation needs to be confirmed by measurement of Th1 and Th2 secreted cytokines responses. It should be stressed that the final outcome of the immune response depends on a very delicate and complicated interaction between various cytokines.
The lack of a vaccine and inadequate control measurements could cause fascioliasis, along with many other tropical diseases, to contribute to the health disparities in undeveloped countries. The preparation of a vaccine against F. hepatica may contribute to the solution of this problem. The current exploratory study is a modest contribution to prepare a protein vaccine against this parasite. Further studies are in progress in which the protein FhSAP2 will be tested in a variety of vaccine formulations that include adjuvants other than Freund’s adjuvant. It is a powerful adjuvant eliciting immune response but its use is restricted to laboratory use and could never be used for practical purposes even in the veterinary medicine arena. Vaccination routes other than IM with the DNA construct will be also assayed and the humoral and cellular arms of the immune response will also be analyzed.
Acknowledgments
This study was supported by grants from the Minority Biomedical Research Support of the Competitive Research program (MBRS-SCORE, S06 GM 8224) and the Research Center for Minority Institutions program (RCMI, G12-RR-03051).
References
- 1.Spithill TW, Smooker PM, Copeman DB. Fasciola gigantica: epidemiology, control, immunology and molecular biology. In: Dalton JP, editor. Fascioliasis. CABI Publishing; Oxon, UK: 1999. pp. 465–525. [Google Scholar]
- 2.Mas-Coma S. Epidemiology of fascioliasis in human endemic areas. J Helminthol. 2005;79(3):207–216. doi: 10.1079/joh2005296. [DOI] [PubMed] [Google Scholar]
- 3.Gulsen MT, Savas MC, Koruk M, Kadayifci A, Demirci F. Fascioliasis: a report of five cases presenting with common bile duct obstruction. Neth J Med. 2006;64(1):17–19. [PubMed] [Google Scholar]
- 4.Hopkins DR. Homing in on helminths. Am. J Trop Med Hyg. 1992;46(6):626–34. doi: 10.4269/ajtmh.1992.46.626. [DOI] [PubMed] [Google Scholar]
- 5.Rim HJ, Farag HF, Sornmani S, Cross JH. Food-borne trematodes: ignored or emerging? Parasitol Today. 1994;10:207–209. [Google Scholar]
- 6.Esteban JG, Flores A, Angles R, Mas-Coma S. High endemicity of human fascioliasis between Lake Titicaca and La Paz valley, Bolivia. Trans R. Soc Trop Med Hyg. 1999;93(2):151–156. doi: 10.1016/s0035-9203(99)90289-4. [DOI] [PubMed] [Google Scholar]
- 7.Esteban JG, Gonzalez C, Curtale F, et al. Hyperendemic fascioliasis associated with schistosomiasis in villages in the Nile Delta of Egypt. Am J Trop Med Hyg. 2003;69(4):429–437. [PubMed] [Google Scholar]
- 8.Mas-Coma MS, Esteban JG, Bargues MD. Epidemiology of human fascioliasis: a review and proposed new classification. Bull WHO. 1999;77(4):340–346. [PMC free article] [PubMed] [Google Scholar]
- 9.Torgerson P, Claxton J. Epidemiology and control. In: Dalton JP, editor. Fascioliasis. CABI Publishing; Oxon, UK: 1999. pp. 113–149. [Google Scholar]
- 10.Fairweather I. Triclabendazole: new skills to unravel an old(ish) enigma. J Helminthol. 2005;79(3):227–234. doi: 10.1079/joh2005298. [DOI] [PubMed] [Google Scholar]
- 11.Suhardono, Widjajanti S, Stevenson P, Carmichael IH. Control of Fasciola gigantica with triclabendazole in Indonesian cattle. Trop Anim Health Prod. 1991;23(4):217–220. doi: 10.1007/BF02357103. [DOI] [PubMed] [Google Scholar]
- 12.Overend DJ, Bowen FL. Resistance of Fasciola gigantica to triclabendazole. Aust Vet J. 1995;72(7):275–276. doi: 10.1111/j.1751-0813.1995.tb03546.x. [DOI] [PubMed] [Google Scholar]
- 13.Espino AM, Hillyer GV. Molecular cloning of a member of the Fasciola hepatica saposin-like protein family. J Parasitol. 2003;89(3):545–552. doi: 10.1645/GE-3113. [DOI] [PubMed] [Google Scholar]
- 14.Grams R, Adisakwattana P, Ritthisunthorn N, Eursitthichai V, Vichasri-Grams S, Viyanant V. The saposin-like proteins 1, 2, and 3 of Fasciola gigantica. Mol Biochem Parasitol. 2006;148(2):133–143. doi: 10.1016/j.molbiopara.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Andersson M, Gunne H, Agerberth B, et al. NK-lysin, a novel effector peptide of cytotoxic T and NK cells. Structure and cDNA cloning of the porcine form, induction by interleukin 2, antibacterial and antitumour activity. EMBO J. 1995;14(8):1615–1625. doi: 10.1002/j.1460-2075.1995.tb07150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leippe M, Andra J, Muller-Eberhard HJ. Cytolytic and antibacterial activity of synthetic peptides derived from amoebapore, the pore-forming peptide of Entamoeba histolytica. Proc Nat Acad Sci USA. 1994;91(7):2602–2606. doi: 10.1073/pnas.91.7.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leippe M, Andra J, Nickel R, Tannich E, Muller-Eberhard HJ. Amoebapores, a family of membranolytic peptides from cytoplasmic granules of Entamoeba histolytica: isolation, primary structure, and pore formation in bacterial cytoplasmic membranes. Mol Microbiol. 1994;14(5):895–904. doi: 10.1111/j.1365-2958.1994.tb01325.x. [DOI] [PubMed] [Google Scholar]
- 18.Leippe M, Muller-Eberhard HJ. The pore-forming peptide of Entamoeba histolytica, the protozoan parasite causing human amoebiasis. Toxicol. 1994;87(1-3):5–18. doi: 10.1016/0300-483x(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 19.Sano A, Mizuno T, Kondoh K, et al. Saposin-C from bovine spleen; complete amino acid sequence and relation between the structure and its biological activity. Biochim Biophys Acta. 1992;1120(1):75–80. doi: 10.1016/0167-4838(92)90426-e. [DOI] [PubMed] [Google Scholar]
- 20.Stenger S, Hanson DA, Teitelbaum R, et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282(5386):121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 21.Espino AM, Hillyer GV. A novel Fasciola hepatica saposinlike recombinant protein with immunoprophylactic potential. J Parasitol. 2004;90(4):876–879. doi: 10.1645/GE-215R. [DOI] [PubMed] [Google Scholar]
- 22.Cook RM, Carvalho-Queiroz C, Wilding G, LoVerde PT. Nucleic acid vaccination with Schistosoma mansoni antioxidant enzyme cytosolic superoxide dismutase and the structural protein filamin confers protection against the adult worm stage. Inf Immu. 2004;72(10):6112–6124. doi: 10.1128/IAI.72.10.6112-6124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romcih MH, Hassan HM, Shousha TS, Saber MA. Immunization against Egyptian Schistosoma mansoni infection by multivalent DNA vaccine. Acta Biochim Biophys Sin. (Shanghai) 2008;40(4):327–338. doi: 10.1111/j.1745-7270.2008.00404.x. [DOI] [PubMed] [Google Scholar]
- 24.Shalaby KA, Yin L, Thakur A, Christen L, Niles EG, LoVerde PT. Protection against Schistosoma mansoni utilizing DNA vaccination with genes encoding Cu/Zn cytosolic superoxide dismutase, signal peptide-containing superoxide dismutase and glutathione peroxidase enzymes. Vaccine. 2003;22(1):130–136. doi: 10.1016/s0264-410x(03)00535-8. [DOI] [PubMed] [Google Scholar]
- 25.Espino AM, Osuna A, Gil R, Hillyer GV. Fasciola hepatica: humoral and cytokine responses to a member of the saposin-like protein family following delivery as a DNA vaccine in mice. Exp Parasitol. 2005;110(4):374–383. doi: 10.1016/j.exppara.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 26.Espino AM, Dumenigo BE, Fernandez R, Finlay CM. Immunodiagnosis of human fascioliasis by enzyme-linked immunosorbent assay using excretory-secretory products. Am J Trop Med Hyg. 1987;37(3):605–608. doi: 10.4269/ajtmh.1987.37.605. [DOI] [PubMed] [Google Scholar]
- 27.Harness E, Doy TG, Hughes DL. The early migratory behaviour of young Fasciola hepatica in sensitized mice. Int J Parasitol. 1977;7(1):51–54. doi: 10.1016/0020-7519(77)90024-8. [DOI] [PubMed] [Google Scholar]
- 28.Meddis R. Statistics Using Ranks. A Unified Approach. Basil Blackwell; New York: 1984. [Google Scholar]
- 29.Sheskin DJ. Hanbook of Parametric and Non-Parametric Procedures. 3rd ed. Chapman & Hall / CRC; Boca Raton: 2004. [Google Scholar]
- 30.Kaplan EL, Meier P. Nonparametric estimation for incomplete observations. J Am Statist Asoc. 1958;53:457–481. [Google Scholar]
- 31.Muro A, Casanucva P, Lopez-Aban J, Ramajo V, Martinez-Fernandez AR, Hillyer GV. Identification of Fasciola hepatica recombinant 15-kDa fatty acid-binding protein T-cell epitopes that protect against experimental fascioliasis in rabbits and mice. J Parasitol. 2007;93(4):817–823. doi: 10.1645/GE-1050R1.1. [DOI] [PubMed] [Google Scholar]
- 32.Acosta D, Cancela M, Piacenza L, Roche L, Carmona C, Tort JF. Fasciola hepatica leucine aminopeptidase, a promising candidate for vaccination against ruminant fasciolosis. Mol Biochem Parasitol. 2008;158(1):52–64. doi: 10.1016/j.molbiopara.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Fernandez AR, Nogal-Ruiz JJ, Lopez-Aban J, et al. Vaccination of mice and sheep with Fh12 FABP from Fasciola hepatica using the new adjuvant/immunomodulator system ADAD. Vet Parasitol. 2004;126(3):287–298. doi: 10.1016/j.vetpar.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 34.Muro A, Ramajo V, Lopez J, Simon F, Hillyer GV. Fasciola hepatica: vaccination of rabbits with native and recombinant antigens related to fatty acid binding proteins. Vet Parasitol. 1997;69(3–4):219–229. doi: 10.1016/s0304-4017(96)01131-4. [DOI] [PubMed] [Google Scholar]
- 35.Piacenza L, Acosta D, Basmadjian I, Dalton JP, Carmona C. Vaccination with cathepsin L proteinases and with leucine aminopeptidase induces high levels of protection against fascioliasis in sheep. Inf Immu. 1999;67(4):1954–1961. doi: 10.1128/iai.67.4.1954-1961.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramajo V, Oleaga A, Casanueva P, Hillyer GV, Muro A. Vaccination of sheep against Fasciola hepatica with homologous fatty acid binding proteins. Vet Parasitol. 2001 May 9;97(1):35–46. doi: 10.1016/s0304-4017(01)00388-0. [DOI] [PubMed] [Google Scholar]
- 37.Sexton JL, Milner AR, Panaccio M, et al. Glutathione S-transferase. Novel vaccinc against Fasciola hepatica infection in sheep. J Immunol. 1990;l45(11):3905–3910. [PubMed] [Google Scholar]
- 38.Sexton JL, Wilce MC, Colin T, et al. Vaccination of sheep against Fasciola hepatica with glutathione S-transferase. Identification and mapping of antibody epitopes on a three-dimensional model of the antigen. J Immunol. 1994;152(4):1863–1872. [PubMed] [Google Scholar]
- 39.Kofta W, Wedrychowicz H. c-DNA vaccination against parasitic infections: advantages and disadvantages. Vet Parasitol. 2001;100(1–2):3–12. doi: 10.1016/s0304-4017(01)00478-2. [DOI] [PubMed] [Google Scholar]
- 40.Wedrychowicz H, Lamparska M, Kesik M, et al. The immune response of rats to vaccination with the cDNA or protein forms of the cysteine proteinase of Fasciola hepatica. Vet Immunol Immunopathol. 2003;94(1–2):83–93. doi: 10.1016/s0165-2427(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 41.Wedrychowicz I, Jedlina-Panasiuk L, Szymanski P, Bienkowska-Szewczyk K. Blood leukocyte responses in rats vaccinated widi cDNA encoding glutathioinie-S-transferase of Fasciola hepatica. Wiad Parazytol. 2001;47(4):551–557. [PubMed] [Google Scholar]
- 42.Lang BZ, Hall RF. Host-parasite relationships of Fasciola hepatica in the white mouse. VIII. Successful vaccination with culture incubate antigens and antigens from sonic disruption of immature worms. J Parasitol. 1977;63(6):1046–1049. [PubMed] [Google Scholar]
- 43.Hillyer GV. Induction of immunity in mice to Fasciola hepatica with a Fasciola/Schistosoma cross-reactive defined immunity antigen. Am J Trap Med Hyg. 1985;34(6):1127–1131. doi: 10.4269/ajtmh.1985.34.1127. [DOI] [PubMed] [Google Scholar]
- 44.Lopez-Aban J, Casanueva P, Nogal J, et al. Progress in the development of Fasciola hepatica vaccine using recombinant fatty acid binding protein with the adjuvant adaptation system ADAD. Vet Parasitol. 2007;145(3–4):287–296. doi: 10.1016/j.vetpar.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 45.Sagodira S, Iochmann S, Mevelec MN, Dimier-Poisson I, Bout D. Nasal immunization of mice with Cryptosporidium parvum DNA induces systemic and intestinal immune responses. Parasite Immunol. 1999;21(10):507–516. doi: 10.1046/j.1365-3024.1999.00247.x. [DOI] [PubMed] [Google Scholar]
- 46.Smooker PM, Kennedy NJ, Steeper KR, Christopoulos H, Spithill TW. Fasciola: kinetics and quality of humoral responses to fatty acid binding protein and cathepsin I following delivery as DNA vaccines in mice. Exp Parasitol. 2001;97(3):154–160. doi: 10.1006/expr.2001.4601. [DOI] [PubMed] [Google Scholar]
- 47.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Ann Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 48.O’Neill SM, Brady MT, Callanan JJ, et al. Fasciola hepatica infection downregulates Th1 responses in mice. Parasite Immunol. 2000;22(3):147–155. doi: 10.1046/j.1365-3024.2000.00290.x. [DOI] [PubMed] [Google Scholar]