Abstract
Use of the rabbit as disease model has long been hampered by a lack of immunological assays specific to this species. In the present study we developed a SYBR Green-based, real-time RT-PCR protocol to quantitate cytokine mRNA in freshly harvested rabbit peripheral mononuclear cells. The method was validated in the course of a vaccination trial in which animals vaccinated with the recombinant antigen FhSAP2 were challenged with Fasciola hepatica metacercariae. Changes in the levels of rabbit interleukin (IL)-2, IL-4, IL-6, IL-10, tumor necrosis factor-alpha (TNFα), and interferon-gamma (IFNγ) mRNA were determined. Messenger RNA from the universally expressed housekeeping gene GAPDH was used as an amplification control and allowed for correction of variations in the efficiencies of RNA extraction and reverse transcription. Rabbits vaccinated with FhSAP2 showed an 83.3% reduction in liver fluke burden after challenge infection when compared to non-vaccinated controls. All cytokine mRNAs were found at detectable levels; however, the levels of IFNγ, TNFα, IL-2 and IL-10 were significantly higher in the vaccinated group compared to the non-vaccinated group. These results suggest that protection conferred by FhSAP2 protein could be associated with a mixed Th1/Th2 immune response in which Th1 cytokines are dominant. The real-time RT-PCR method described herein can be a useful tool for monitoring changes in basic immune functions in the rabbit model of fascioliasis and may also aid in studies of human diseases for which the rabbit is an important experimental model.
Keywords: Cytokine array, Fasciola hepatica, Real-time RT-PCR, Saposin-like proteins
1. Introduction
Cytokines are regulatory proteins that play a central role in the immune system by modulating cellular responses, including lymphocyte activation, proliferation, differentiation, survival and apoptosis (Giulietti et al., 2001). They are low-molecular weight proteins secreted by many different cells, most prominently by lymphocytes, antigen-presenting cells, monocytes, endothelial cells and fibroblasts. Cytokines are classified into different groups such as interleukins, interferons, tumor growth factors, and chemokines. Interacting with one another, cytokines exert polarizing effects on effector T cells and are pivotal in tuning immune responses (Boeuf et al., 2005). The best-defined subsets of effector T cells are the CD4+ helper lineages Th1 and Th2. Th1 cells secrete interferon-gamma (IFNγ), lymphotoxin, and interleukin (IL)-2, which stimulate inflammatory immune responses such as Delayed-Type Hypersensitivity, and also the production of complement-fixing antibodies and cytotoxic T lymphocytes. Th2 cells secrete IL-4, IL-5 and IL-10, which regulate the humoral immune response (Abbas et al., 1996; Mena et al., 2002). The cytokines of each T cell subtype are counter-regulatory to the reciprocal subset, resulting in polarization of the immune response to either type-1 or type-2. Numerous studies indicate that the outcome of an infection depends greatly on the balance between Th1 and Th2 cells (Abbas et al., 1996; Dinarello, 1997; Mena et al., 2002). Thus, cytokine profiling should be done through simultaneous quantification of cytokines characteristic of both T-helper subsets.
The quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) is widely used to quantify mRNA expression levels in cells, body fluids, tissues, or tissue biopsies (Overbergh et al., 2003). Because of its specificity and sensitivity, qRT-PCR is the method of choice for quantifying mRNA expression levels of cytokines, which normally are expressed at very low levels (Giulietti et al., 2001). Most qRT-PCR protocols have been developed to quantify cytokines in mice and humans, owing to the availability of a complete collection of leukocyte expressed sequences tags (ESTs) and complete genomic sequences for those species (Bilate et al., 2008; Castilho et al., 2008; Dantas et al., 2009; Fang et al., 2009; Gaertner et al., 2009; Suksumek et al., 2008; Walsh et al., 2009). In contrast, very few assays have been developed for quantification of cytokines in domestic animals (Ledger et al., 2004; Leutenegger et al., 1999a,b).
Rabbits are used as models in a wide variety of studies, including for evaluation of novel antibiotic formulations as therapy against bacterial or parasitic infections (el-Bahy, 1997; Force et al., 2008), for production of high-quality antiserum, in studies of immunoglobulin structure and regulation (Kindt, 1975), in studies of B cell development (Yang et al., 2005), in studies of myxomatosis, coccidioidomicosis and trematodiasis (Clemons et al., 2007; Fouchet et al., 2008; Hussein and Khalifa, 2008; Silva et al., 2004), and in vaccination studies against parasites and viral infections (Espino and Hillyer, 2004; Palmer et al., 2006). The main goal of our laboratory is to identify Fasciola hepatica antigens with potential for use in vaccines, and we often use a rabbit model of fascioliasis to evaluate the effectiveness of these antigens. One of these antigens is the protein termed FhSAP2, which is a member of the F. hepatica saposin-like protein family (Espino and Hillyer, 2003). As a vaccine, FhSAP2 was shown to induce significant anti-pathological and anti-fecundity effects on F. hepatica as well as a reduction in parasite burden of ∼81.2% (Espino and Hillyer, 2004). In the present paper we describe a newly developed, highly sensitive and reproducible qRT-PCR assay for six key rabbit cytokines. The method was validated during the course of a vaccination trial that replicated the protection results previously obtained with FhSAP2 in a rabbit model of fascioliasis. The signal for rabbit glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is used to normalize against differences in RNA extraction, extent of RNA degradation, and variability in reverse transcription and PCR efficiencies. The observed changes in cytokine gene expression of the rabbit immune response measured by this qRT-PCR led us to postulate that the protection conferred by FhSAP2 in the rabbit model of fascioliasis could be associated with a mechanism driven by CD4+ Th1 cells.
2. Materials and methods
2.1. Animals
Nine male 3-month-old New Zealand White (NZW) rabbits (Harland Inc., Indianapolis, IN) and 5 female mixed breeder rabbits reared at a local farm in San Juan, Puerto Rico were used in the study. Animals were housed, fed and kept under conventional germ-free conditions in the animal care facility of the University of Puerto Rico, School of Medicine and treated according to international regulations for the care of laboratory animals.
2.2. Recombinant FhSAP2 and preparation of adult F. hepatica excretory secretory (ES) products
cDNA coding for recombinant FhSAP2 (GenBank AF286903) was cloned into pBAD-HisB, expressed in Escherichia coli TOP10 cells as a fusion protein with a His-tag, and the protein was purified by Ni2+ column affinity chromatography as previously described (Espino and Hillyer, 2003). Purified recombinant protein was used as the immunogen in the vaccination trial.
ES products were collected from living adult F. hepatica parasites freshly isolated from bovine livers at local abattoirs. The flukes were carefully washed and incubated in RPMI medium with 100 IU of penicillin and 100 μg streptomycin/ml of medium (1 fluke per 5 ml) at 37 °C for 24 h as previously described (Espino et al., 1987). After centrifugation at 5000 × g for 30 min at 4 °C, the supernatant was dialyzed for 24 h against phosphate-buffered saline (PBS) and then concentrated 10-fold by using an AMICON ultrafiltration membrane YM-3. This ES preparation was used as the antigen for antibody determinations. The protein concentrations of recombinant FhSAP2 and ES antigen were estimated by using the bicinchoninic acid method (Thermo Scientific, Rockford, IL, USA) according to the manufacturer's instructions.
2.3. Vaccination and assessment of protection
Four NZW rabbits received subcutaneous injections of 100 μg of purified FhSAP2 mixed with Freund's complete adjuvant (FCA) followed by two similar booster injections at 2-week intervals. The other five NZW rabbits received the same number of injections but with PBS in the adjuvant. Four weeks after the last injection all animals were orally infected with 30 fresh F. hepatica metacercariae obtained from Baldwin Aquatics (Monmouth, OR), and 10 weeks after the challenge they were euthanized and necropsied for collection of organs. Livers were macroscopically examined for organ pathology by three different blinded investigators. The observers summarized the grade of lesions as follows: +, mild; ++, moderate; +++, intense; and ++++, severe. Flukes found in the main bile ducts were removed. The livers were then cut into 1-cm long pieces, soaked in water at 37 °C for 30 min, squeezed, and forced through a 300-μm mesh sieve; the retained material was analyzed for immature or mature flukes. Total worm burdens were summarized for each vaccinated and control group by arithmetic means. The numbers of eggs in the feces and gall bladders were also estimated.
2.4. Sampling
During the experiment, blood samples were collected by ear venipuncture using vacuntainers with and without heparin. Samples that were collected prior to the first immunization were used as negative controls, and samples collected at different points during the experiment were used as experimental samples. Samples collected in vacuntainers with heparin were gently mixed with RNAlater in 2-ml tubes and used for total RNA extraction either immediately or after freezing at −20 °C for several weeks. In order to obtain serum, samples collected without heparin were left overnight at 4 °C and the serum was separated by centrifugation at 3000 rpm for 5 min and stored at −20 °C.
2.5. Analysis of the antibody response
Fluke antigen-specific antibodies in sera were detected by using ELISA. Wells of 96-well polystyrene plates (Costar, Corning Inc., Corning, NY, USA) were coated with 20 μg/ml F. hepatica ES products by adding the antigen in carbonate–bicarbonate buffer, pH 9.6, to the wells and incubating overnight at 4 °C. The excess binding sites were blocked with 3% bovine serum albumin in PBS containing 0.05% Tween-20 (PBST) at 37 °C for 1 h. After washing with PBST, sera (100 μl; 1:100 dilutions) were added to the wells, and the plates were incubated at 37 °C for 1 h. Bound antibodies were detected by using peroxidase-conjugated anti-rabbit immunoglobulin (BioRad, Hercules, CA, USA) and the substrate O-phenylenediamine as previously described (Espino and Hillyer, 2004).
2.6. RNA and genomic DNA extraction
Total RNA was extracted from RNAlater-conserved blood by using a RiboPure™-Blood Kit according to the manufacturer's guidelines. RNA was then treated with 5 U of amplification grade DNase I (Invitrogen, Carlsbad, CA) according to the manufacturer's specifications to remove any contaminating genomic DNA. RNAs were quantified by measuring the optical density at 260 nm (OD260) using a Nanodrop-1000 spectrophotometer (Thermo Scientific) and the purity was assessed by determining the OD260/OD280 ratio, which was between 1.9 and 2. Genomic DNA (gDNA) was prepared from peripheral blood mononuclear cells (PBMC), purified by Ficoll Hypaque gradient centrifugation from heparin-treated blood samples, by using a standard phenol-chloroform extraction method (Sambrook et al., 1989). One hundred nanograms of gDNA (determined by photometry at 260/280 nm) was used as template for amplification in the SYBR-Green-based real-time RT-PCR assay.
2.7. Reverse transcription
To synthesize cDNA, 2 μl of oligo (dT) primer, 2 μl of 2.5 mM stock of dNTPs (Invitrogen) and 16 μl of DNase-treated RNA in DEPC-treated water were incubated at 65 °C for 5 min and then put on ice for 3 min. The following reagents were added: 4 μl of 10× Superscript buffer (Invitrogen), 8 μl of 25 mM stock of MgCl2 (Invitrogen) and 4 μl of 0.1 M dithiothreitol (Invitrogen). The contents were mixed gently and incubated for 2 min at room temperature. The tubes were briefly centrifuged and incubated for another 10 min at room temperature after adding 2 μl of 50 μg/ml Superscript II (Invitrogen) followed by incubation at 42 °C for 50 min. Superscript II was heat-inactivated for 15 min at 72 °C. Four units of RNase H were added and the reaction mixture was incubated at 37 °C for 30 min. cDNA samples were diluted 1:5 in DEPC-treated water prior to amplification.
2.8. Design of primers and production of amplicons
Table 1 lists the six rabbit cytokine and housekeeping genes that were selected after searching the publicly available databases (GenBank). Primers for all target sequences were designed by using Primer Express software (Applied Biosystems). We selected sense and antisense primers annealing to sequences in exons on opposite sides of an intron to secure primer specificity for target DNA and to avoid the amplification of pseudogenes or other related genes. All primers were from 19 to 22 bp in size with annealing temperatures between 56 and 58 °C. The percentage of G+C content was kept in the 18–32% range and runs of identical nucleotides were avoided. The amplicon lengths ranged from 138 to 160 bp.
Table 1.
Primers for PCR amplification of specific rabbit cytokines.
| Cytokine | Accession no. | Amplicon size (bp) | Sense primer 5′–3′ | Anti-sense primer 5′–3′ |
|---|---|---|---|---|
| IL-2 | AF068057 | 138 | GGAAACACAGGAACAACTGGA | TTCAATTCTGTGACCTTCTTGG |
| IL-4 | DQ852343 | 140 | AGAGCTCGGTGACCTCAGAC | CTTGCATGGCGGTCTTTAG |
| IL-6 | AF169176 | 153 | GAAAACACCAGGGTCAGCAT | CAGCCACTGGTTTTTCTGCT |
| IL-10 | AF0680058 | 145 | GAACTCCCTGGGGGAAAAC | GGCTTTGTAGACGCCTTCCT |
| IFN( | AB010386 | 160 | TTCCCAAGGATAGCAGTGGT | TGAAGCCAGAAGTCCTCAAAA |
| TNFα | M12845 | 138 | CTCCTACCCGAACAAGGTCA | CGGTCACCCTTCTCCAACT |
| GAPDH | L23961 | 160 | GAATCCACTGGCGTCTTCAC | CGTTGCTGACAATCTTGAGAGA |
2.9. Quantitation of cytokine transcript by real-time PCR
Real-time PCR was performed with a LightCycler 480 SYBR Green I Master kit (Roche Applied Science, Indianapolis, IN, USA) using 2 μl of the 1:5 cDNA dilutions (in DEPC) in a volume of 20 μl with the specific primer mix. Quantitative PCR was performed in the LightCycler 480 for 38 cycles with the following parameters: denaturation at 95 °C for 10 s, annealing at the primer-specific annealing temperature for 20 s, and extension at 72 °C for 10–15 s. Melting curve analysis was performed after the amplification phase to eliminate the possibility of nonspecific amplification or primer-dimer formation. Specificity of the SYBR Green-based real-time PCR for rabbit cytokines was verified by cloning and sequencing PCR amplicons. The cytokine system was tested at least once for amplification of a single amplicon, visualized by agarose gel electrophoresis.
2.10. The comparative CT method
Each well was screened for fluorescence every 7 s and signals were regarded as positive if the fluorescence intensity exceeded 10 times the standard deviation of the baseline fluorescence (threshold cycle, CT). Of the two methods for quantitation of gene transcription (absolute quantitation by the standard curve method and relative quantitation by the comparative CT method), the latter was selected due to its ease and speed for setup and analysis. For relative quantitation by the comparative CT method, values are expressed relative to a reference sample, called the calibrator, i.e. PBMC from naïve rabbits. Each test or control sample was run in duplicate. First, the CT for the target amplicon and the CT for the internal control (GAPDH as an endogenous housekeeping gene) were determined for each sample. Differences in the CT for the target and the CT for the internal control, called ΔCT, were calculated to normalize for differences in the amount of total nucleic acid added to each reaction and the efficiency of the RT step. The ΔCT for each experimental sample was subtracted from the ΔCT of the calibrator. This difference is called the ΔΔCT value. Finally, the amount of target, normalized to GAPDH and relative to the calibrator, was calculated by 2−ΔΔCT. Thus, all the experimental samples are expressed as an n-fold change relative to the calibrator.
2.11. Statistical analysis
All antibody detections by ELISA were performed in triplicate on two different days and the results are expressed as the mean OD492 for each determination. Values yielding an OD492 nm ≥0.16 were considered positive. This cut-off value represents the mean OD ± 3SD 3SD of sera of naïve rabbits. The weekly differences in OD492 values between groups were analyzed by using Student's t-test. Percentage of protection for immunized animals was calculated by the formula (C − E)/C × 100, where C is the average number of flukes in the control group and E is the average number of flukes in the vaccinated group of animals. The following comparisons between the relative changes in levels of cytokines (n-fold change) were made: (1) cytokine levels after the last FhSAP2-vaccination (Tv) relative to the cytokine levels in the same group before vaccination and after challenge; (2) cytokine levels in the non-vaccinated group at weeks 2, 4, 6 and 10 after the challenge infection with respect to the levels of cytokines in the same group before infection; (3) cytokine levels of the FhSAP2-vaccinated group with respect to the cytokine levels of the non-vaccinated group at 10 weeks after challenge; (4) the levels of IFNγ and IL-4 in both experimental groups with respect to the number of liver flukes. The comparative analyses were done by analysis of variance (ANOVA). When global differences were detected in this analysis, we applied a post-ANOVA test using Fisher's protected least significant differences analysis. All the statistical analyses were performed with the statistical software STATA, version 10.
3. Results
3.1. Fluke burden and egg output
The fluke recovery data, egg count, and results of the qualitative evaluation of liver damage following challenge of rabbits with F. hepatica metacercariae are shown in Table 2. An 83.3% reduction in the fluke count was found in the FhSAP2-vaccinated rabbits versus the non-vaccinated controls. Significant differences (P < 0.001) between vaccinated and control animals were seen in the total number of eggs found in the feces or gall bladder.
Table 2.
Parasite burden and egg output in vaccinated rabbits compared to controls 10 weeks after challenge.
| Rabbit ID | Vaccinated, infected | Egg output in feces | Egg output in gall bladder (× 103) | Rabbit ID | Non-vaccinated, infected | Egg output in feces | Egg output in gall bladder (×103) | ||
|---|---|---|---|---|---|---|---|---|---|
| No. flukes | Liver damage | No. flukes | Liver damage | ||||||
| R #1 | 3 | ++ | 4.5 | 6 | R #6 | 19 | ++++ | 20 | 54 |
| R #2 | 0 | − | 0 | 0 | R #7 | 13 | ++++ | 14 | 150 |
| R #3 | 2 | + | 1 | 2.1 | R #8 | 8 | ++++ | 13 | 166 |
| R #4 | 3 | + | 5 | 18.5 | R #9 | 9 | +++ | 20 | 60 |
| R #10 | 11 | ++++ | 17 | 177 | |||||
| Average | 2.0 ± 1.22 | 2.6 ± 2.1 | 6.7 ± 7.1 | Average | 12 ± 3.9 | ++++ | 16.8 ± 2.92 | 118.5 ± 58.5 | |
| Reduction in parasite burden: 83.3%; reduction of egg output: feces, 84.5%, gall bladder, 94.3% | |||||||||
(−) Indicates no liver damage, (+) minor liver damage, (++) moderate liver damage, (+++) intense liver damage and (++++) severe liver damage.
3.2. Antibody responses against ES antigens
The results of total specific antibodies to ES antigen are shown in Fig. 1. All FhSAP2-vaccinated animals developed high levels of antibody 2 weeks after the first vaccination, which were significantly different from the control level; absorbance values in the FhSAP2-vaccinated animals ranged from 0.99 to 1.72, with a mean value of 1.38 ± 0.26. Antibody levels reached their maximum values 4 weeks after the last challenge; absorbance values ranged from 1.78 to 2.26, with a mean value of 1.96 ± 0.19. Subsequent to the challenges, antibody levels in the vaccinated animals remained at the same level (mean OD 2.0 ± 0.22) until necropsy. Prior to infection, absorbance values in the animals that were vaccinated with PBS in adjuvant remained under the cut-off value. However, the values were significantly increased 4 weeks after challenge (P < 0.001); absorbance values ranged from 1.26 to 1.43 with a mean of 1.32 ± 0.06. Antibody levels in the control group reached the maximum value 8 weeks after challenge (mean OD 1.55 ± 0.22).
Fig. 1.
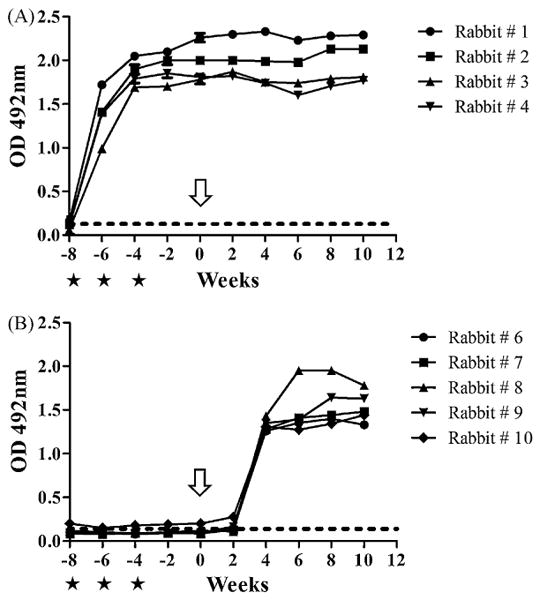
Kinetics of total antibody to F. hepatica ES antigens during the course of the experiment. The starts indicate the vaccination times and arrow indicates the time of challenge infection. The dashed line indicates the cut off value (OD ≥ 0.16), which was calculated as the mean OD ± 3 SD of the negative sera. (A) Representation of the kinetic of production of anti-ES antibodies for rabbits vaccinated with FhSAP2 (rabbits 1–4). (B) Representation of the kinetics of production of anti-ES antibodies for the non-vaccinated, infected rabbits (rabbits 6–10).
3.3. Primer validation
Samples collected from normal NZW rabbits and from mixed breeder rabbits were used as reference samples (calibrators) and to validate the primers. Primer concentrations were optimized to determine the minimum primer concentrations giving the lowest threshold cycle (CT) and the maximum signal-to-noise fluorescence ratio while minimizing nonspecific amplification (data not shown). Among the final concentrations of 50, 300, 400 and 600 nM tested for each primer, the optimal final concentration was set at 400 nM for every primer. Melting curves were generated in each run to determine the specificity of each primer pair. The presence of a unique peak confirms the specificity of the primer pair for the target DNA. Fig. 2 shows typical melting peaks obtained for all cytokine systems applied to samples collected from a rabbit at different time points during the experiment.
Fig. 2.
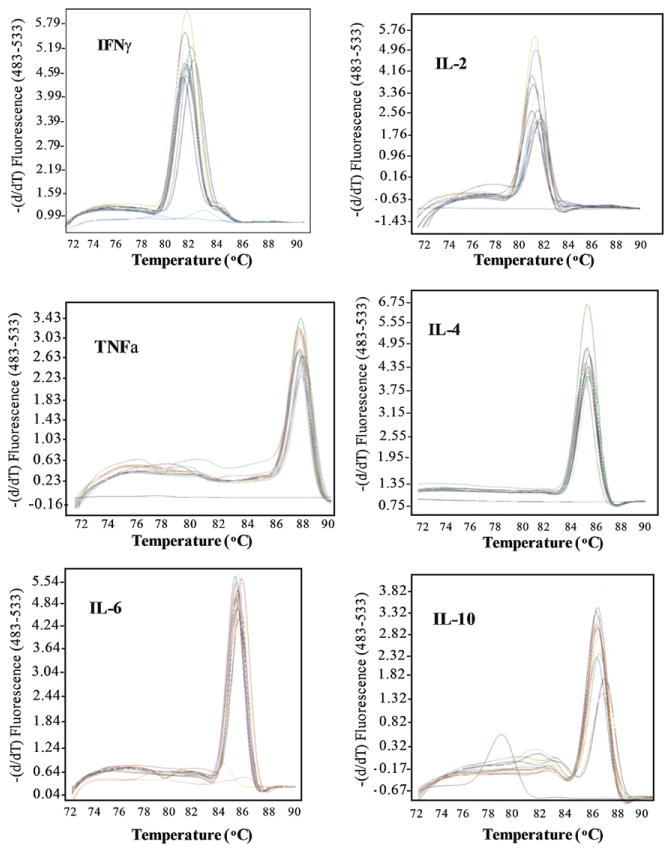
Typical melting peaks obtained for each cytokine system aplied to samples collected from rabbit #-1 at different points during the experiment. Each set of curves represents amplifications in duplicate of samples collected before vaccination (To), after completing the vaccination protocol (Tv), and at 2 weeks (T2), 4 weeks (T4), 6 weeks (T6) and 10 weeks (T10) after the challenge infection.
To address the question of whether contaminating gDNA present in the total RNA samples could influence the cytokine cDNA quantitation, RNA preparations without prior cDNA synthesis were subjected to PCR. None of the samples gave a signal for any of the cytokines (results not shown). To investigate whether contaminating gDNA was co-amplified or could have interfered with the quantitation of cytokine cDNAs, both cDNA and 100 ng of genomic DNA were subjected to PCR. All cytokine sequences were amplified with cDNA but not with gDNA as template. Therefore, all reactions were able to differentiate between cDNA and gDNA, confirming the specificity of the primer combinations used for the selective amplification of cDNA. When PCR products were examined by agarose gel electrophoresis (4% gel), all cytokine systems produced a single band of the expected length. In addition, the specificity of the primers was evaluated by melting-curve analysis. PCR products were sequenced after cloning and found to have >98% identity with the expected sequences (data not shown).
To address the question of whether the genetic background of the animals could modify the homogeneity of the reference samples, total RNA was extracted from PBMC derived from 10 NZW rabbits and from 5 mixed breeder rabbits reared in local farms and then subjected to the test protocol. All of the targets DNAs were amplified. Overall, no differences were observed between CT values obtained for each cytokine in the NZW rabbit samples versus the mixed breeder animals. However, the melting curving analysis indicated that in a few cases the CT obtained resulted from a primer-dimer effect.
3.4. Amplification efficiencies of GAPDH and cytokine cDNA
For the comparative CT method (ΔΔCT method) to be valid, the efficiencies of the target amplifications must be approximately equal. To determine the amplification efficiencies of GAPDH and the cytokine cDNAs, different dilutions of cDNA preparations were amplified, and the resulting CT values were plotted against the dilution of input total RNA and a regression line was calculated. Six or seven dilutions were amplified in triplicate for 38 cycles. Differences in the slopes of cytokines versus GAPDH were: IFNγ: 0.09; TNFα: 0.08; IL-2: 0.06; IL-4: 0.09; IL-6: 0.08; and IL-10: 0.1.
3.5. Quantitation of cytokines after FhSAP2 vaccination and after F. hepatica challenge infection
To address the question of whether vaccination with FhSAP2 could modify the levels of cytokines, the cytokine levels after the last FhSAP2-vaccination (Tv) were compared to the relative levels of cytokines before vaccination. It was observed that, after vaccination, the animals expressed 2.5-fold more IFNγ, 1.8-fold more TNFα, 1.6-fold more IL-2, and 2-fold more IL-10 than before vaccination. These changes were found to be statistically significant (P < 0.05). Conversely, the changes in the levels of IL-6 and IL-4 were not significant. No further changes were observed in the levels of cytokines after the challenge infection (Fig. 3A).
Fig. 3.
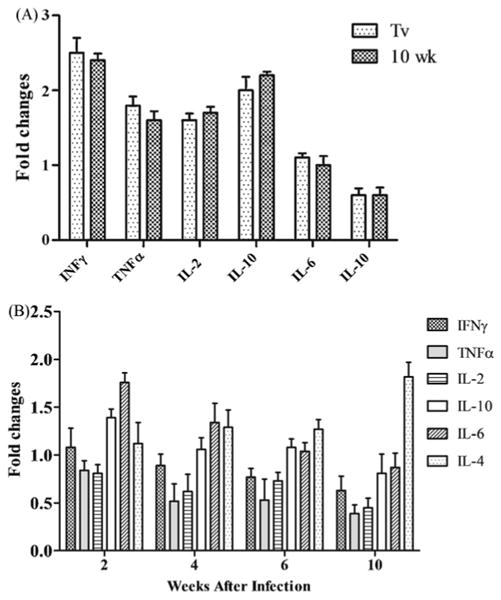
Comparison of the levels of rabbit cytokines as determined by SYBR-Green qRT-PCR. (A) Histograms representing the fold-changes (2−ΔΔCT) in the quantitation of cytokines in the FhSAP2-vaccinated group compared to the negative controls (samples collected before immunization). Light gray bar: samples collected 4 weeks after the last vaccination (Tv). Dark gray bar: samples collected 10 weeks after the challenge (T10). (B) Histograms representing the fold-changes (2−ΔΔCT) in the quantitation of cytokines in the non-vaccinated, infected rabbits at 2 weeks (T2), 4 weeks (T4), 6 weeks (T6) and 10 weeks (T10) after the challenge infection.
To ascertain how the challenge infection modifies the levels of cytokines in the non-vaccinated group, the levels of cytokines at different points after the challenge infection were compared to the levels of cytokines before infection. The infected rabbits showed a progressive diminution in the levels of IFNγ, TNFα, IL-2, IL-6 and IL-10, which reached their lowest values at 10 weeks post-infection. In contrast, the level of IL-4 progressively increased until it reached a maximum value at 10 weeks after infection (Fig. 3B).
When both experimental groups were compared at necropsy it was found that the FhSAP2-vaccinated group had 1.8-fold more IFNγ, 1.56-fold more TNFα, 1.47-fold more IL-2 and 2.25-fold more IL-10 than the non-vaccinated, infected group (Fig. 4). The fold changes in the levels of these cytokines between the two groups were found to be statistically significant (P < 0.05). Changes in the relative amounts of IL-6 and IL-4 in the vaccinated group were only 1.16-fold and 0.8-fold more than those observed in the non-vaccinated group, and these changes were not significantly different.
Fig. 4.
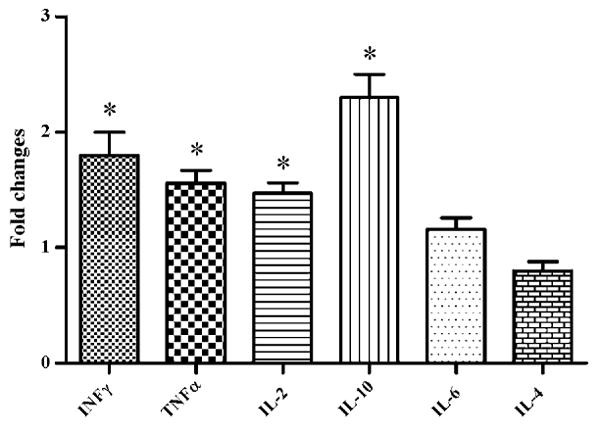
Fold-changes in the quantitation of rabbit cytokines determined by SYBR-Green qRT-PCR between group of rabbits vaccinated with FhSAP2 and the non-vaccinated group 10 weeks after the challenge infection. An asterisk over the bar indicates a significant differences (P < 0.05).
To address the question of whether the observed changes in cytokine levels are associated with fluke burden, the levels of IFNγ and IL-4 of each experimental rabbit were compared with the corresponding fluke recovery values. As shown in Fig. 5, rabbits that had large number of flukes in the liver had low levels of IFNγ accompanied by high levels of IL-4, and rabbits that had few or no flukes in the liver had relatively low levels of both IFNγ and IL-4.
Fig. 5.
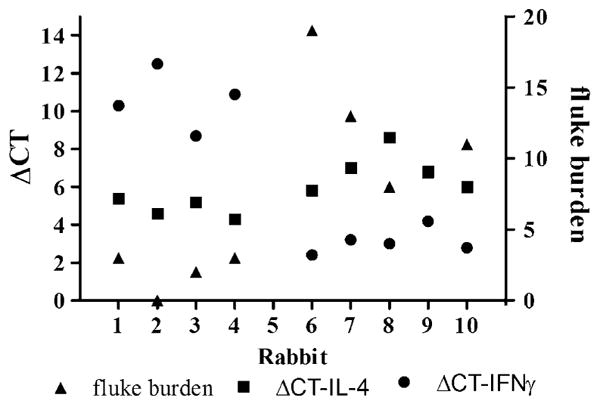
Differences in the CT for the target cytokines (IFNγ and IL-4) and the CT for GAPDH as the internal control (ΔCT) obtained for each experimental rabbit in relation to their corresponding liver fluke burden. Rabbits #-1 to #-4 were vaccinated with FhSAP2 in Freund's adjuvant. Rabbits #-6 to #-10 were vaccinated with PBS in adjuvant. All animals were challenged with 30 F. hepatica metacercariae and necropsied 10 weeks after the challenge.
4. Discussion
We previously demonstrated that FhSAP2 emulsified in TiterMax adjuvant induced in rabbits both anti-pathological and anti-fecundity effects as well as an 81.2% reduction in parasite burden after challenge with F. hepatica metacercariae (Espino and Hillyer, 2004). In the current experiment, FhSAP2 emulsified in Freund's adjuvant induced an 83.3% reduction in liver fluke count and a significant reduction in egg output and liver damage, confirming the protective role of FhSAP2. The presence of high levels of antibodies to F. hepatica ES antigens in the animals that acquired immunity against infection suggests that antibodies could have contributed to the protection. This is consistent with observations made by other authors who, using immunogens other than FhSAP2, found associations between protection and the levels of antibodies (Acosta et al., 2008; Almeida et al., 2003; Law et al., 2003; Martinez-Fernandez et al., 2004; Meeusen and Piedrafita, 2003; Ramajo et al., 2001; Reszka et al., 2005; Sexton et al., 1994; Smooker et al., 2004; Vilar et al., 2003; Zafra et al., 2008). Because FhSAP2 is a lytic protein, FhSAP2 could be involved in acquiring nutrition for the liver fluke and/or its migration through the liver parenchyma (Espino and Hillyer, 2003). Although the mechanism of protection induced by FhSAP2 is unknown, we speculate that it may be related to the antibodies produced after vaccination, which may act by blocking nutrient acquisition, reducing fluke migration through host tissues, or activating cellular effector mechanisms that directly affect the parasite, or a combination of these. Interference with any of these activities by neutralizing antibodies would be expected to blunt the virulence of liver flukes.
Due to the lack of rabbit-specific reagents and assays, the cellular effector mechanisms induced by FhSAP2 have not been studied. To overcome this deficiency, in the current study we developed a qRT-PCR assay for quantifying cytokine mRNA levels, which lets us correlate cytokine levels with protection results. The qRT-PCR protocol uses SYBR Green as a dsDNA-binding dye together with the LightCycler 480 system, which combines rapid thermal cycling (30–40 cycles in <30 min) in a multiwell-plate format. The system has the further advantages of permitting viewing of the data while the PCR amplifications are still in progress and allowing analysis of the results by calculating melting curves (Abdul-Careem et al., 2008; Brisbin et al., 2008; Sarson et al., 2008). The greatest advantage of SYBR Green is that it can be used with any pair of primers for any target, thus providing significant cost savings compared to traditional protocols that use TaqMan probes. However, its major disadvantage is that SYBR Green binds to any dsDNA, detecting not only the specific target but also nonspecific PCR products (Giulietti et al., 2001). The way to rectify this problem is by comparing melting curves (Ririe et al., 1997). As our results show, by comparing melting curves we can distinguish the products that resulted from specific amplification from those that resulted from primer-dimer formation. Therefore, it was possible to set the software to acquire fluorescence above the melting temperature of the primer dimer but below that of the product. As shown in Fig. 2, the specificity of each primer set was high, as judged by the presence of a unique peak. In addition, our system was able to strictly differentiate between gDNA and cDNA.
Accurate cytokine cDNA quantitation was made possible by normalizing the cytokine signals using the GAPDH signals. This normalization requires that the efficiencies of both the cytokine and GAPDH amplifications are approximately equal. A sensitive method for comparing the amplification efficiencies of two amplicons is to determine the slope of the CT value variation obtained from a cDNA dilution series for GAPDH and the respective cytokine. We found that the amplification efficiencies for both GAPDH and cytokine cDNA were approximately equal (<10% difference), thus allowing GAPDH-based normalization of cytokine transcription.
As our results show (Fig. 3A), after vaccination, all animals developed detectable levels of all cytokines, with significant predominance of IFNγ over IL-4. These findings indicate that FhSAP2 induced a strong cellular immune response. The fact that the levels of all cytokines as well as the antibodies did not increase further after the challenge infection was not surprising; rather, it suggest that the doses and the frequency of injections used induced an immune response of maximum intensity that was not modified by the presence of more antigen in the form of a challenge.
The levels of IFNγ, TNFα and IL-2 cytokine mRNAs after the challenge in the non-vaccinated group showed sequential diminution accompanied by significant increases of IL-4 as the infection progressed (Fig. 3B). This indicates that the infection stimulated a switch to the Th2 immune response, which is consistent with previous observations in other fascioliasis models (Brown et al., 1994a; Cervi et al., 2004; O'Neill et al., 2000). In well-characterized murine and ruminant models, soon after infection with F. hepatica the immune response is proinflammatory, lasting about 4–6 weeks. This immune response is switched off at around the time the adult flukes begin to enter the bile duct. It is likely that liver-fluke secretions modify macrophage-based signaling events to switch off the proinflammatory immune response before the infection has been appropriately controlled. This is consistent with evidence that a non-Th1 response is dominant in animals chronically infected with F. hepatica. For example, a Th2-cytokine profile was detected in spleen cells and in draining lymph node cells during experimental fascioliasis in rats (Cervi et al., 2004, 2001), and high levels of IgE (a Th2-dependent isotype) to Fasciola antigens was identified in infected cattle (Pfister et al., 1984). Moreover, systemic eosinophilia was observed for 3–13 weeks after primary infection of sheep with F. hepatica (Chauvin et al., 1995) and F. gigantica (Roberts et al., 1997a,b). These results suggest an inverse correlation between F. hepatica infection and induction of parasite-specific Th1 cells, and are consistent with the hypothesis that Th2 responses are promoted in chronic fascioliasis.
The results show that the vaccinated rabbits which had the lowest numbers of flukes in the liver combined with smaller gross-pathological lesions and the lowest parasite egg outputs (Table 2) expressed the highest levels of IFNγ, IL-2 and TNFα and the lowest levels of IL-4 and IL-6 (Fig. 5). IL-6 is a multifunctional Th2 cytokine produced by a variety of cell types, including macrophages, dendritic cells, T cells, endothelial cells, and hepatocytes (Akira et al., 1993; Silvennoinen et al., 1996). IL-6 has been shown to be involved in Th2 differentiation by promoting IL-4 production by precursor T helper cells (Rincon et al., 1997). Therefore, it was not surprising that IL-6 was present at relatively low levels in those animals that developed a stronger Th1 immune response. FhSAP2 possesses two Th1 epitopes and three Th0 epitopes (Espino et al., 2007), all of which overlap with two dominant B cell epitopes (Torres and Espino, 2006). Apparently, the Th1 epitopes are dominant over the B cell and Th0 epitopes, influencing the repertoire and specificity of the cytokine- and antibody-producing cells toward a Th1 phenotype that is able to curtail progression of the infection. An interesting finding from this work is that the high levels of IFNγ, IL-2 and TNFα were accompanied by high levels of IL-10 (Fig. 4). Given that IL-10 is a regulatory cytokine that typically down-regulates the expression of Th1 cytokines, MHC class II antigens, and costimulatory molecules on macrophages (Moore et al., 2001), the finding of IL-10 at levels similar to those of IFNγ, TNFα and IL-2 in the group of animals that developed protection could seem contradictory. However, it is likely that FhSAP2 it able to induce differentiated T cell clones to secrete simultaneously IL-10 and IFNγ, as has been described to occur in bovines and humans (Brown et al., 1994b; Windhagen et al., 1996). This could indicate that FhSAP2 stimulates an immune response that do not fit into reciprocal Th1- and Th2-cell subsets. In support of this possibility, studies in vitro with PBMC from cattle that had been stimulated with F. hepatica antigens demonstrated the simultaneous presence of IL-4 and IFNγ in bulk cultures (Brown et al., 1994a). Moreover, we previously found a mix of IgG2a and IgG1 in mice vaccinated with FhSAP2 (Espino et al., 2005), which indicates a mixed Th1/Th2 immune response.
Although the presence of IFNγ, TNFα and IL-2 in rabbits that developed protection clearly indicates that FhSAP2 elicited an effective cellular immune response, it is not obvious how a cellular mechanism could exert a protective effect against an extracelular parasite like F. hepatica. One possible mechanism of action could involve effective stimulation of innate immunity through activation of appropriate Toll-like receptors (TLRs). Indeed, the potency of Freund's adjuvants to elicit an immune response has been reported to lie in their ability to induce expression of TLR2, brought on by the active ingredient in FCA, killed Mycobacterium tuberculosis bacilli (Mtb) (Sai-Kiang, 2003). Because FCA is essentially made of Mtb dissolved in Freund's incomplete adjuvant, it is the solvent in FCA that induces increased TLR2 expression, leading to enhanced recognition of killed Mtb by host cells. Therefore, a probable explanation for the levels of protection induced by FhSAP2-FCA achieved in this work could be related to effective stimulation of TLR2, which is important for the production of inflammatory cytokines and interferon-stimulated genes (Vanhoutte et al., 2008). Supporting this hypothesis, differences in the sizes of spleen and lymph nodes were observed in immunized animals. Rabbits immunized with FhSAP2-FCA showed marked hyperplasia of the spleen and lymph nodes compared to non-vaccinated rabbits. Furthermore, it has been demonstrated that components of innate immunity participate in the resistance to F. hepatica infection (Baeza et al., 1994). For example, Piedrafita et al. (2001) reported that monocytes/macrophages and nitric oxide are involved in the killing of migrating juvenile flukes in the rat peritoneal cavity.
On the other hand, it has been suggested that the mechanism of action of repository adjuvants such as TiterMax and Freund's adjuvants is primarily the slow release of antigen into draining lymph nodes (likely to the dendritic cells), which stimulates continued antibody production by follicular B cells (Brewer and Alexander, 1997). Therefore, based on this model, the success of immunization with FhSAP2-FCA could be related to the persistence of a specific immune response against the parasite.
The cytokine profile described herein could be quite different depending on whether FA is substituted by other, less inflammatory adjuvants. FCA is an acceptable adjuvant for experimental purposes but it is not permitted for commercial vaccine formulations for human or veterinary use. For this reason it will be necessary to test the effectiveness of FhSAP2 emulsified in safer, commercially acceptable adjuvants like aluminium salts, Montanide™ or immunostimulating (ISCOM) particles supplemented with immunostimulatory molecules. It is well known that aluminium salts, which are highly immunomodulatory and form short-term depots, induce very strong Th2-type responses (Cox and Coulter, 1999). It is likely that FhSAP2 in Alumin will stimulate the production of high levels of IL-4; but if our hypothesis is correct, that the Th1 immune response is determinant for inducing protection against F. hepatica infection, then vaccination with FhSAP2 in Alum could fail to induce protection. However, it is likely that FhSAP2 emulsified in water in an oil-based adjuvant like Montanide™ or into ISCOMs supplemented with monophosphoril lipid A (MPL) or oligodeoxynucleotides (ODN) containing the CpG motif characteristic of bacterial DNA could mimic the Th1-polarizing properties of FCA and induce protective immunity. In those hypothetical scenarios the possible immune mechanism of action could be diverse. For formulations containing CpG-ODN, the immune mechanism could be related to efficient stimulation of TLR-9 (Hemmi et al., 2000), which is required for monocytes and dendritic cells to produce IL-12. This cytokine is involved in activating Th1 cells which are needed for protection against numerous pathogens (Hemmi et al., 2000). For formulations containing MPL, the immune mechanism could be through activation of TLR-4, as occurs with lipopolysaccharide (LPS) and its derivates (Thompson et al., 2005). Studies of these different adjuvant formulations for FhSAP2 are in progress and will be expedited in the rabbit model now thanks to the optimized quantitative real-time RT-PCR protocol described herein.
In conclusion, this paper reproduced the protection results previously achieved with FhSAP2 in rabbits against the extracellular parasite F. hepatica and described and validated a new real-time RT-PCR protocol for examining cytokine responses in the rabbit. The use of this technique led us to determine that protection induced by FhSAP2 is associated with high levels of IFNγ, TNFα, IL-2 and IL-10, which suggests a mixed Th1/Th2 mechanism with dominance of Th1-cytokines. Because FhSAP2 was emulsified with FCA, it is likely that the protection was achieved via an effective stimulation of TLR2, which is important for the production of inflammatory cytokines and interferon-stimulated genes. Further studies are in progress in which the innate immune response elicited by FhSAP2 is being investigated. The quantitative real-time RT-PCR approach described herein will enable studies of basic immune function in the rabbit model of fascioliasis as well as of human diseases for which the rabbit is an important experimental model.
Acknowledgments
This work was supported by grants from the Minority Biomedical Research Support of the Competitive Research program (MBRS-SCORE, SO6 GM 8224) and the Research Center for Minority Institutions program (RCMI, G12-RR-03051). Authors wish to thank the R & D division of Capital Biosciences, Inc. for its invaluable collaboration in optimization of the real-time-RT-PCR. The authors also wish to thank Dr. Daryl Henderson for editing and proofreading.
Footnotes
Patent application: Results disclosed in this manuscript have been protected in the USA by provisional patent application 611-08263.
References
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- Abdul-Careem MF, Hunter DB, Lambourne MD, Read LR, Parvizi P, Sharif S. Expression of cytokine genes following pre- and post-hatch immunization of chickens with herpesvirus of turkeys. Vaccine. 2008;26:2369–2377. doi: 10.1016/j.vaccine.2008.02.069. [DOI] [PubMed] [Google Scholar]
- Acosta D, Cancela M, Piacenza L, Roche L, Carmona C, Tort JF. Fasciola hepatica leucine aminopeptidase, a promising candidate for vaccination against ruminant fasciolosis. Mol Biochem Parasitol. 2008;158:52–64. doi: 10.1016/j.molbiopara.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- Almeida MS, Torloni H, Lee-Ho P, Vilar MM, Thaumaturgo N, Simpson AJ, Tendler M. Vaccination against Fasciola hepatica infection using a Schistosoma mansoni defined recombinant antigen, Sm14. Parasite Immunol. 2003;25:135–137. doi: 10.1046/j.1365-3024.2003.00619.x. [DOI] [PubMed] [Google Scholar]
- Baeza E, Poitou I, Delers F, Boulard C. Influence of anti-inflammatory treatments on experimental infection of rats with Fasciola hepatica: changes in serum levels of inflammatory markers during the early stages of fasciolosis. Res Vet Sci. 1994;57:172–179. doi: 10.1016/0034-5288(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Bilate AM, Teixeira PC, Ribeiro SP, Brito T, Silva AM, Russo M, Kalil J, Cunha-Neto E. Distinct outcomes of Trypanosoma cruzi infection in hamsters are related to myocardial parasitism, cytokine/chemokine gene expression, and protein expression profile. J Inf Dis. 2008;198:614–623. doi: 10.1086/590347. [DOI] [PubMed] [Google Scholar]
- Boeuf P, Vigan-Womas I, Jublot D, Loizon S, Barale JC, Akanmori BD, Mercereau-Puijalon O, Behr C. CyProQuant-PCR: a real time RT-PCR technique for profiling human cytokines, based on external RNA standards, readily automatable for clinical use. BMC Immunol. 2005;6:5. doi: 10.1186/1471-2172-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JM, Alexander J. Cytokines and the mechanisms of action of vaccine adjuvants. Cytokines Cell Mol Ther. 1997;3:233–246. [PubMed] [Google Scholar]
- Brisbin JT, Zhou H, Gong J, Sabour P, Akbari MR, Haghighi HR, Yu H, Clarke A, Sarson AJ, Sharif S. Gene expression profiling of chicken lymphoid cells after treatment with Lactobacillus acidophilus cellular components. Dev Comp Immunol. 2008;32:563–574. doi: 10.1016/j.dci.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Brown WC, Davis WC, Dobbelaere DA, Rice-Ficht AC. CD4+ T-cell clones obtained from cattle chronically infected with Fasciola hepatica and specific for adult worm antigen express both unrestricted and Th2 cytokine profiles. Inf Immun. 1994a;62:818–827. doi: 10.1128/iai.62.3.818-827.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WC, Woods VM, Chitko-McKown CG, Hash SM, Rice-Ficht AC. Interleukin-10 is expressed by bovine type 1 helper, type 2 helper, and unrestricted parasite-specific T-cell clones and inhibits proliferation of all three subsets in an accessory-cell-dependent manner. Inf Immun. 1994b;62:4697–4708. doi: 10.1128/iai.62.11.4697-4708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho TM, Camargo LM, McMahon-Pratt D, Shaw JJ, Floeter-Winter LM. A real-time polymerase chain reaction assay for the identification and quantification of American Leishmania species on the basis of glucose-6-phosphate dehydrogenase. Am J Trop Med Hyg. 2008;78:122–132. [PubMed] [Google Scholar]
- Cervi L, Cejas H, Masih DT. Cytokines involved in the immunosuppressor period in experimental fasciolosis in rats. Int J Parasitol. 2001;31:1467–1473. doi: 10.1016/s0020-7519(01)00275-2. [DOI] [PubMed] [Google Scholar]
- Cervi L, Borgonovo J, Egea M, Chiapello L, Masih D. Immunization of rats against Fasciola hepatica using crude antigens conjugated with Freund's adjuvant or oligodeoxynucleotides. Vet Immunol Immunopathol. 2004;97:97–104. doi: 10.1016/j.vetimm.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Chauvin A, Bouvet G, Boulard C. Humoral and cellular immune responses to Fasciola hepatica experimental primary and secondary infection in sheep. Int J Parasitol. 1995;25:1227–1241. doi: 10.1016/0020-7519(95)00039-5. [DOI] [PubMed] [Google Scholar]
- Clemons KV, Capilla J, Stevens DA. Experimental animal models of coccidioidomycosis. Ann N Y Acad Sci. 2007;1111:208–224. doi: 10.1196/annals.1406.029. [DOI] [PubMed] [Google Scholar]
- Cox J, Coulter A. Prospects for the development of new vaccine adjuvants. Bio Drugs. 1999;12:439–453. doi: 10.2165/00063030-199912060-00004. [DOI] [PubMed] [Google Scholar]
- Dantas SF, Vieira de Rezende TC, Bailao AM, Taborda CP, da Silva Santos R, Pacheco de Castro K, Maria de Almeida Soares C. Identification and characterization of antigenic proteins potentially expressed during the infectious process of Paracoccidioides brasiliensis. Microbes Infect. 2009;11:895–903. doi: 10.1016/j.micinf.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Role of pro- and anti-inflammatory cytokines during inflammation: experimental and clinical findings. J Biol Regul Homeost Agents. 1997;11:91–103. [PubMed] [Google Scholar]
- el-Bahy MM. Comparative studies on the effect of bithionol, praziquantel and triclabendazole in rabbit's fascioliasis. J Egyptian Soc Parasitol. 1997;27:615–616. [PubMed] [Google Scholar]
- Espino AM, Hillyer GV. Molecular cloning of a member of the Fasciola hepatica saposin-like protein family. J Parasitol. 2003;89:545–552. doi: 10.1645/GE-3113. [DOI] [PubMed] [Google Scholar]
- Espino AM, Hillyer GV. A novel Fasciola hepatica saposin-like recombinant protein with immunoprophylactic potential. J Parasitol. 2004;90:876–879. doi: 10.1645/GE-215R. [DOI] [PubMed] [Google Scholar]
- Espino AM, Dumenigo BE, Fernandez R, Finlay CM. Immunodiagnosis of human fascioliasis by enzyme-linked immunosorbent assay using excretory-secretory products. Am J Trop Med Hyg. 1987;37:605–608. doi: 10.4269/ajtmh.1987.37.605. [DOI] [PubMed] [Google Scholar]
- Espino AM, Osuna A, Gil R, Hillyer GV. Fasciola hepatica: humoral and cytokine responses to a member of the saposin-like protein family following delivery as a DNA vaccine in mice. Exp Parasitol. 2005;110:374–383. doi: 10.1016/j.exppara.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Espino AM, Torres D, Morales A, Delgado B, Quetel J, Osuna A. Fasciola hepatica: identification of CD4+ T-helper epitopes from the 11.5 kDa saposin-like protein SAP-2 using synthetic peptides. Exp Parasitol. 2007;117:65–73. doi: 10.1016/j.exppara.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R, Nie H, Wang Z, Tu P, Zhou D, Wang L, He L, Zhou Y, Zhao J. Protective immune response in BALB/c mice induced by a suicidal DNA vaccine of the MIC3 gene of Toxoplasma gondii. Vet Parasitol. 2009;164:134–140. doi: 10.1016/j.vetpar.2009.06.012. [DOI] [PubMed] [Google Scholar]
- Force E, Taberner F, Cabellos C, Ribes S, Domenech A, Tubau F, Viladrich PF, Gudiol F. Experimental study of meropenem in the therapy of cephalosporin-susceptible and -resistant pneumococcal meningitis. Eur J Clin Microbiol Infect Dis. 2008;27:685–690. doi: 10.1007/s10096-008-0492-8. [DOI] [PubMed] [Google Scholar]
- Fouchet D, Guitton JS, Marchandeau S, Pontier D. Impact of myxomatosis in relation to local persistence in wild rabbit populations: the role of waning immunity and the reproductive period. J Theor Biol. 2008;250:593–605. doi: 10.1016/j.jtbi.2007.10.037. [DOI] [PubMed] [Google Scholar]
- Gaertner FC, Rohde F, Mueller J, Blechert B, Janssen KP, Essler M. Endogenous expression of the sodium iodide symporter mediates uptake of iodide in murine models of colorectal carcinoma. Int J Cancer. 2009;125:2783–2791. doi: 10.1002/ijc.24705. [DOI] [PubMed] [Google Scholar]
- Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods. 2001;25:386–401. doi: 10.1006/meth.2001.1261. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Tolllike receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Hussein AN, Khalifa RM. Experimental infections with Fasciola in snails, mice and rabbits. Parasitol Res. 2008;102:1165–1170. doi: 10.1007/s00436-008-0888-5. [DOI] [PubMed] [Google Scholar]
- Kindt TJ. Rabbit immunoglobulin allotypes: structure, immunology, and genetics. Adv Immunol. 1975;21:35–86. doi: 10.1016/s0065-2776(08)60218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law RH, Smooker PM, Irving JA, Piedrafita D, Ponting R, Kennedy NJ, Whisstock JC, Pike RN, Spithill TW. Cloning and expression of the major secreted cathepsin B-like protein from juvenile Fasciola hepatica and analysis of immunogenicity following liver fluke infection. Inf Immun. 2003;71:6921–6932. doi: 10.1128/IAI.71.12.6921-6932.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledger TN, Pinton P, Bourges D, Roumi P, Salmon H, Oswald IP. Development of a macroarray to specifically analyze immunological gene expression in swine. Clin Diagn Lab Immunol. 2004;11:691–698. doi: 10.1128/CDLI.11.4.691-698.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutenegger CM, Mislin CN, Sigrist B, Ehrengruber MU, Hofmann-Lehmann R, Lutz H. Quantitative real-time PCR for the measurement of feline cytokine mRNA. Vet Immunol Immunopathol. 1999a;71:291–305. doi: 10.1016/S0165-2427(99)00100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutenegger CM, von Rechenberg B, Huder JB, Zlinsky K, Mislin C, Akens MK, Auer J, Lutz H. Quantitative real-time PCR for equine cytokine mRNA in nondecalcified bone tissue embedded in methyl methacrylate. Calcif Tissue Int. 1999b;65:378–383. doi: 10.1007/s002239900717. [DOI] [PubMed] [Google Scholar]
- Martinez-Fernandez AR, Nogal-Ruiz JJ, Lopez-Aban J, Ramajo V, Oleaga A, Manga-Gonzalez Y, Hillyer GV, Muro A. Vaccination of mice and sheep with Fh12 FABP from Fasciola hepatica using the new adjuvant/immunomodulator system ADAD. Vet Parasitol. 2004;126:287–298. doi: 10.1016/j.vetpar.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Meeusen EN, Piedrafita D. Exploiting natural immunity to helminth parasites for the development of veterinary vaccines. Int J Parasitol. 2003;33:1285–1290. doi: 10.1016/s0020-7519(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Mena A, Ioannou XP, Van Kessel A, Van Drunen Little-Van Den Hurk S, Popowych Y, Babiuk LA, Godson DL. Th1/Th2 biasing effects of vaccination in cattle as determined by real-time PCR. J Immunol Methods. 2002;263:11–21. doi: 10.1016/s0022-1759(02)00029-7. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- O'Neill SM, Brady MT, Callanan JJ, Mulcahy G, Joyce P, Mills KH, Dalton JP. Fasciola hepatica infection downregulates Th1 responses in mice. Parasite Immunol. 2000;22:147–155. doi: 10.1046/j.1365-3024.2000.00290.x. [DOI] [PubMed] [Google Scholar]
- Overbergh L, Giulietti A, Valckx D, Decallonne R, Bouillon R, Mathieu C. The use of real-time reverse transcriptase PCR for the quantification of cytokine gene expression. J Biomol Technol. 2003;14:33–43. [PMC free article] [PubMed] [Google Scholar]
- Palmer KE, Benko A, Doucette SA, Cameron TI, Foster T, Hanley KM, McCormick AA, McCulloch M, Pogue GP, Smith ML, Christensen ND. Protection of rabbits against cutaneous papillomavirus infection using recombinant tobacco mosaic virus containing L2 capsid epitopes. Vaccine. 2006;24:5516–5525. doi: 10.1016/j.vaccine.2006.04.058. [DOI] [PubMed] [Google Scholar]
- Pfister K, Daveau C, Ambroise-Thomas P. Partial purification of somatic and excretory-secretory products of adult Fasciola hepatica and their application for the serodiagnosis of experimental and natural fascioliasis using an ELISA. Res Vet Sci. 1984;37:39–43. [PubMed] [Google Scholar]
- Piedrafita D, Parsons JC, Sandeman RM, Wood PR, Estuningsih SE, Partoutomo S, Spithill TW. Antibody-dependent cell-mediated cytotoxicity to newly excysted juvenile Fasciola hepatica in vitro is mediated by reactive nitrogen intermediates. Parasite Immunol. 2001;23:473–482. doi: 10.1046/j.1365-3024.2001.00404.x. [DOI] [PubMed] [Google Scholar]
- Ramajo V, Oleaga A, Casanueva P, Hillyer GV, Muro A. Vaccination of sheep against Fasciola hepatica with homologous fatty acid binding proteins. Vet Parasitol. 2001;97:35–46. doi: 10.1016/s0304-4017(01)00388-0. [DOI] [PubMed] [Google Scholar]
- Reszka N, Cornelissen JB, Harmsen MM, Bienkowska-Szewczyk K, de Bree J, Boersma WJ, Rijsewijk FA. Fasciola hepatica procathepsin L3 protein expressed by a baculovirus recombinant can partly protect rats against fasciolosis. Vaccine. 2005;23:2987–2993. doi: 10.1016/j.vaccine.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ririe KM, Rasmussen RP, Wittwer CT. Product differentiation by analysis of DNA melting curves during the polymerase chain reaction. Anal Biochem. 1997;245:154–160. doi: 10.1006/abio.1996.9916. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Estuningsih E, Widjayanti S, Wiedosari E, Partoutomo S, Spithill TW. Resistance of Indonesian thin tail sheep against Fasciola gigantica and F. hepatica. Vet Parasitol. 1997a;68:69–78. doi: 10.1016/s0304-4017(96)01027-8. [DOI] [PubMed] [Google Scholar]
- Roberts JA, Estuningsih E, Wiedosari E, Spithill TW. Acquisition of resistance against Fasciola gigantica by Indonesian thin tail sheep. Vet Parasitol. 1997b;73:215–224. doi: 10.1016/s0304-4017(97)00119-2. [DOI] [PubMed] [Google Scholar]
- Sai-Kiang L. Freund adjuvant induces TLR3 but not TLR4 expression in the liver of mice. Int Immunopharm. 2003;3:115–118. doi: 10.1016/s1567-5769(02)00256-4. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd. Cold Spring Harbor Laboratory; New York: 1989. [Google Scholar]
- Sarson AJ, Parvizi P, Lepp D, Quinton M, Sharif S. Transcriptional analysis of host responses to Marek's disease virus infection in genetically resistant and susceptible chickens. Anim Genet. 2008;39:232–240. doi: 10.1111/j.1365-2052.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- Sexton JL, Wilce MC, Colin T, Wijffels GL, Salvatore L, Feil S, Parker MW, Spithill TW, Morrison CA. Vaccination of sheep against Fasciola hepatica with glutathione S-transferase. Identification and mapping of antibody epitopes on a three-dimensional model of the antigen. J Immunol. 1994;152:1861–1872. [PubMed] [Google Scholar]
- Silva E, Castro A, Lopes A, Rodrigues A, Dias C, Conceicao A, Alonso J, Correia da Costa JM, Bastos M, Parra F, Moradas-Ferreira P, Silva M. A recombinant antigen recognized by Fasciola hepatica-infected hosts. J Parasitol. 2004;90:746–751. doi: 10.1645/GE-136R. [DOI] [PubMed] [Google Scholar]
- Silvennoinen O, Nishigaki H, Kitanaka A, Kumagai M, Ito C, Malavasi F, Lin Q, Conley ME, Campana D. CD38 signal transduction in human B cell precursors. Rapid induction of tyrosine phosphorylation, activation of syk tyrosine kinase, and phosphorylation of phospholipase C-gamma and phosphatidylinositol 3-kinase. J Immunol. 1996;156:100–107. [PubMed] [Google Scholar]
- Smooker PM, Rainczuk A, Kennedy N, Spithill TW. DNA vaccines and their application against parasites—promise, limitations and potential solutions. Biotech Annu Rev. 2004;10:189–236. doi: 10.1016/S1387-2656(04)10007-0. [DOI] [PubMed] [Google Scholar]
- Suksumek N, Leelawat K, Leelawat S, Russell B, Lek-Uthai U. TaqMan real-time PCR assay for specific detection of Opisthorchis viverrini DNA in Thai patients with hepatocellular carcinoma and cholangiocarcinoma. Exp Parasitol. 2008;119:217–224. doi: 10.1016/j.exppara.2008.01.018. [DOI] [PubMed] [Google Scholar]
- Thompson BS, Chilton PM, Ward JR, Evans JT, Mitchell TC. The low-toxicity versions of LPS, MPL adjuvant and RC529, are efficient adjuvants for CD4+ T cells. J Leukoc Biol. 2005;78:1273–1280. doi: 10.1189/jlb.0305172. [DOI] [PubMed] [Google Scholar]
- Torres D, Espino AM. Mapping of B-cell epitopes on a novel 11.5-kilodalton Fasciola hepatica-Schistosoma mansoni cross-reactive antigen belonging to a member of the F. hepatica saposin-like protein family. Inf Immun. 2006;74:4932–4938. doi: 10.1128/IAI.00442-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte F, Paget C, Breuilh L, Fontaine J, Vendeville C, Goriely S, Ryffel B, Faveeuw C, Trottein F. Toll-like receptor (TLR)2 and TLR3 synergy and cross-inhibition in murine myeloid dendritic cells. Immunol Lett. 2008;116:86–94. doi: 10.1016/j.imlet.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Vilar MM, Barrientos F, Almeida M, Thaumaturgo N, Simpson A, Garratt R, Tendler M. An experimental bivalent peptide vaccine against schistosomiasis and fascioliasis. Vaccine. 2003;22:137–144. doi: 10.1016/s0264-410x(03)00300-1. [DOI] [PubMed] [Google Scholar]
- Walsh R, Seth R, Behnke J, Potten CS, Mahida YR. Epithelial stem cell-related alterations in Trichinella spiralis-infected small intestine. Cell Prolif. 2009;42:394–403. doi: 10.1111/j.1365-2184.2009.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windhagen A, Anderson DE, Carrizosa A, Williams RE, Hafler DA. IL-12 induces human T cells secreting IL-10 with IFN-gamma. J Immunol. 1996;157:1127–1131. [PubMed] [Google Scholar]
- Yang G, Obiakor H, Sinha RK, Newman BA, Hood BL, Conrads TP, Veenstra TD, Mage RG. Activation-induced deaminase cloning, localization, and protein extraction from young VH-mutant rabbit appendix. Proc Natl Acad Sci USA. 2005;102:17083–17088. doi: 10.1073/pnas.0501338102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafra R, Buffoni L, Martinez-Moreno A, Perez-Ecija A, Martinez-Moreno FJ, Perez J. A study of the liver of goats immunized with a synthetic peptide of the Sm14 antigen and challenged with Fasciola hepatica. J Compar Pathol. 2008;139:169–176. doi: 10.1016/j.jcpa.2008.06.004. [DOI] [PubMed] [Google Scholar]


