INTRODUCTION
In 1990 we asked clinicians attending a symposium during the annual meeting of the American Gastroenterological Association how many were using psychopharmacological agents, specifically antidepressants, to treat functional gastrointestinal disorders.1 Very few raised their hands. Over the subsequent 15 years, these agents increasingly have become used in the management of functional gastrointestinal symptoms, despite a limited amount of scientific information supporting this practice. It is now estimated that at least 1 in 8 patients with irritable bowel syndrome (IBS) is offered an antidepressant.2,3 Nearly every comprehensive current review of management strategies for IBS and other mainstream functional gastrointestinal disorders mentions their use, and prescribing among gastroenterologists has become commonplace.3–10 In parallel with this change, primary care physicians have become sufficiently comfortable in using antidepressants for treating not only anxiety and depression but also a host of somatic symptoms and syndromes that their use has nearly tripled in the past decade.11
Enhanced appreciation for the relative importance of central mechanisms (for example, signal processing alterations) in many if not most patients with IBS and other painful functional gastrointestinal disorders is an important factor responsible for prescribing shifts among gastroenterologists—more so than a new or improved understanding of the relationship of symptoms to anxiety or depression.8 The composite body of investigation over the past 15 years involving visceral stimulation, brain imaging, multidimensional clinical studies, and treatment trials designed to answer mechanistic questions has led us closer to understanding the pathways by which psychopharmacological agents may interrupt the symptomatic process in these common gastrointestinal disorders. Complemented by clinical observations, improved recommendations for their use have evolved.6,12 This review describes the types of psychopharmacological agents used in functional gastrointestinal disorders, new models for their potential benefits, a summary of the reported efficacy, and practical aspects surrounding treatment. The majority of the available information has been accrued in patients with IBS, although findings are similar when other pain or discomfort based functional gastrointestinal disorders have been studied.
BACKGROUND
Recounting observations instigating use of psychopharmacological agents in functional gastrointestinal disorders remains a useful exercise because their mechanism of effect remains unclear. The most conspicuous explanation for experimentation has been the high rate of psychiatric illness in patients with these gastrointestinal disorders.13,14 Interview methods demonstrate that nearly 70% of patients seeking care at secondary or tertiary locations meet diagnostic criteria for a psychiatric disorder, particularly anxiety states and major depression.14 These early observations have not changed in recent years. However, approximately one third of subjects conspicuously do not meet criteria, and response to psychopharmacological agents, particularly antidepressants, is neither predicated on the presence of an anxiety or depressive disorder nor consistently dependent on the intended psychiatric effects of the medications.14,15 Such findings indicate that the relevance of psychiatric comorbidity is in its reflection of another underlying disorder or phenomenon that is responsive to psychopharmacological intervention. In this regard, several other observations have supported the use of psychopharmacological treatments (table 1▶), although none stands out as providing a singular dominant rationale for their need.
Table 1.
Observations supporting the testing of psychopharmacological agents for functional gastrointestinal disorders
| ▸ High lifetime and current rates of anxiety disorders and depression |
| ▸ High self reported distress |
| ▸ Increased sexual and physical abuse histories |
| ▸ Response of other functional syndromes to antidepressants |
| ▸ Response of neuropathic pain syndromes to antidepressants |
Recent advances have focused on the high degree of comorbidity with psychiatric disorders and non-gastrointestinal functional somatic syndromes in clarifying the role of psychopharmacological agents.16 Although gastroenterologists have been slow to embrace the concept that functional gastrointestinal disorders may be but one component of a broader polysymptomatic process with a unifying underlying mechanism, two lines of thought are developing that expand the psychopharmacological rationale. One incorporates the prevalent comorbidity of medically unexplained somatic syndromes with the functional gastrointestinal disorders under the umbrella of somatisation.16–19 This construct hypothesises that a central neurophysiological process, separate from anxiety and depression, promotes distorted afferent signal processing, reporting of various distinct functional syndromes, and endorsement of many medically unexplained symptoms across multiple organ systems on symptom checklists.
In support of this, high rates of somatisation disorder, an extreme example of somatisation defined by DSM-IV criteria, were detected recently in two studies of female IBS patients seeking care at a university clinic.17,18,20 The rates (30–42% of patients meeting or nearly meeting criteria) were substantially higher than reported previously because physician interviewers and carefully executed chart review were used for establishing the diagnosis. Likewise, somatisation tendency, as measured by somatic symptom ratings on self report measures, is prevalent among patients with functional gastrointestinal disorders and has proved to be an important independent predictor of a variety of clinical and experimental characteristics associated with them, including sensitivity to balloon distension in the gut, linkage of abuse history to subsequent functional gastrointestinal and non-gastrointestinal symptoms, and likelihood of lingering IBS symptoms following gut infection.16,21–24 The relevance of these observations to the “average” patient is highlighted by the remarkably high prevalence rates of multiple gastrointestinal and non-gastrointestinal functional syndromes in patients with functional gastrointestinal disorders.15,16 A minority of patients has a single functional gastrointestinal disorder with no associated comorbidity.
Another recent observation demonstrates that the excess anxiety and affective disorders previously associated with IBS also segregate predominantly to the subgroup showing high degrees of somatisation.17 Thus this group of self report based syndromes may be reflective of an underlying symptom reporting tendency, being “psychoform” rather than precisely representing the primary psychiatric disorders.17,18,27 These intriguing possibilities may provide support for the modest dosages of psychopharmacological agents commonly employed for functional gastrointestinal disorders, as the response may differ compared with management of primary affective or anxiety disorders encountered in psychiatric practices.28 Management of somatisation, therefore, may represent another rationale for the use of psychopharmacological agents. Determining its importance to the individual patient with a functional gastrointestinal disorder is an area of flourishing investigation.
A second line of thought links specific medical and psychiatric syndromes, including IBS, fibromyalgia, and migraine, as representing affective spectrum disorders.29 The disorders are not thought to be manifestations of depression; the term is meant to imply their responsiveness to antidepressant therapy or possibly their representation of a unique type of affective disorder with heritable characteristics.29–31 Again, a unifying central neurophysiological process is speculated, a process that differs potentially from somatisation and segregates this cluster from somatisation disorder. Both lines of thought have merit in expanding previously narrow vistas of the rationale for psychopharmacological agents in functional gastrointestinal disorders.
New observations
▸ Psychopharmacological agents, particularly antidepressants, commonly are used today for patients with functional gastrointestinal disorders
▸ Central nervous system neurophysiological processes, such as somatisation, help explain the high prevalence of comorbid functional somatic syndromes and psychiatric illnesses with functional gastrointestinal disorders
▸ Such central processes participate in global distress and morbidity associated with functional gastrointestinal disorders and are responsive to psychopharmacological treatment
▸ Psychopharmacological agents have greater effect on global measures than specific gastrointestinal symptoms
Although treatment is selected today using conventional clinical indicators, including refractoriness of symptoms, severity of manifestations, and risk-benefit ratios, it seems likely that better characterisation of the functional gastrointestinal disorder patient within broader constructs reflecting activity of these suspected central mechanisms will refine management algorithms. Functional brain imaging eventually may complement clinical indicators (such as medical histories of other functional syndromes or self report symptom checklists) in further improving the identification of subjects most likely to benefit from psychopharmacological interventions. At a minimum, investigative use of these techniques may refine the clinically based selection process. Finally, better stratification of patients based on the extent and type of comorbidities may improve selection of existing psychopharmacological agents as well as assist in the development of new medications specifically directed at central mechanisms behind functional symptoms rather than being targeted primarily at anxiety and depression.
PSYCHOPHARMACOLOGICAL AGENTS USED FOR FUNCTIONAL GASTROINTESTINAL DISORDERS
As a result of the above observations and their perceived utility in clinical practice, anxiolytics and antidepressants are the psychopharmacological agents most commonly used for functional gastrointestinal disorders. Early reports described some success with phenothiazines and related antipsychotic agents.18,32 Benefits were not related conspicuously to antipsychotic effects of the drugs, side effects from long term use appeared unwarranted, and such agents are no longer recommended. Contemporary antipsychotic agents have not been tested. Anxiolytics, including benzodiazepines and buspirone, have also been used both for their anxiolytic properties and for effects that may be targeted more specifically at functional gastrointestinal symptoms.33–35 Clinical experience suggests that benzodiazepines share many benefits observed from antidepressants but their pharmacokinetics and potential for dependency make them undesirable but for short term symptom management.
By far the greatest reported experience has been with antidepressants, especially the tricyclic antidepressants (TCAs) (table 2▶).36 TCAs have broad effects on neurotransmitter physiology, a feature that potentially explains their non-antidepressant benefit in functional gastrointestinal disorders as well as their expanded side effect profile. Direct comparisons of TCAs in managing functional gastrointestinal symptoms are lacking, and no agent appears conspicuously superior to another in open label observation.28 More recent studies report outcomes from selective serotonin reuptake inhibitors (SSRIs) in functional gastrointestinal syndromes.36–38 Although one approach had been to reserve SSRIs for TCA failures, for patients with suspected primary anxiety or affective disorders that might be influencing symptom presentation, or for patients with anxiety or depression symptoms that persist despite improvement in functional gastrointestinal symptoms with TCAs, the agents are being used increasingly for the initial antidepressant trial in suitable candidates.37,38 Antidepressants that more closely share the neurotransmitter effects of TCAs and their analgesic effects, such as duloxetine, are also being examined for their benefits in functional gastrointestinal symptoms,39,40 and other newer antidepressants are being employed anecdotally (table 2▶).
Table 2.
Common antidepressants and usual psychiatric dosages
| Class | Agent | Proposed antidepressant mechanism | Usual daily &;dosage (mg)* |
|---|---|---|---|
| TCAs | |||
| Tertiary amine | Amitriptyline | NE and 5-HT reuptake inhibition | 75–300 |
| Imipramine | NE and 5-HT reuptake inhibition | 75–300 | |
| Doxepin | NE and 5-HT reuptake inhibition | 75–300 | |
| Trimipramine | NE and 5-HT reuptake inhibition | 75–300 | |
| Clomipramine | Mixed action | 75–300 | |
| Secondary amine | Nortriptyline | NE reuptake inhibition | 40–200 |
| Desipramine | NE reuptake inhibition | 75–300 | |
| SSRIs | Citalopram | 5-HT reuptake inhibition | 20–60 |
| Escitalopram | 5-HT reuptake inhibition | 10–20 | |
| Fluoxetine | 5-HT reuptake inhibition | 10–40 | |
| Paroxetine | 5-HT reuptake inhibition | 20–50 | |
| Sertraline | 5-HT reuptake inhibition | 50–150 | |
| Other newer antidepressants | Bupropion | Dopamine reuptake inhibition | 200–400 |
| Duloxetine | NE and 5-HT reuptake inhibition | 20–60 | |
| Nefazodone | Pre- and postsynaptic activity | 300–600 | |
| Mirtazapine | Pre- and postsynaptic activity | 15–30 | |
| Trazodone | Mixed action | 150–600 | |
| Venlafaxine | NE and 5-HT reuptake inhibition | 150–375 |
TCAs, tricyclic antidepressants; SSRIs, selective serotonin reuptake inhibitors; NE, norepinephrine; 5-HT, 5-hydroxytryptamine.
*Dosage ranges for adults in good health not taking confounding medications; geriatric dosages may be required.
Adapted from Rush AJ. Mood disorders: treatment of depression. In: Sadock BJ, Sadock VA. Kaplan and Sadock’s comprehensive textbook of psychiatry, 8th edn. Philadelphia: Lippincott Williams and Wilkins, 2005:1652–61.
Acceptability of antidepressants varies considerably from patient to patient when they are used for psychiatric purposes, and the same holds true when they are prescribed for functional gastrointestinal disorders. Consequently, clinicians are advised to familiarise themselves with several TCAs and non-TCA antidepressants. Knowledge of recommended dosage ranges for managing psychiatric disorders is important (table 2▶). Reaching this target is advised when the goal is management of a comorbid anxiety or depressive disorder; a low dose regimen initially employed for managing functional gastrointestinal symptoms should be escalated to this therapeutic range in the unresponsive patient.41,42
Given the current limitations in understanding the pathogenetic mechanisms behind functional gastrointestinal symptoms and the non-systematic often empirical use of psychopharmacological agents in symptom management, selection of the specific medication is a crude process. Interaction of the side effect profile with presenting gastrointestinal symptoms often dictates the initial choice. For example, TCAs with dominant anticholinergic properties may seem better chosen for IBS patients denying constipation predominant patterns.6 Such concerns are not substantiated fully in clinical practice, and fears that side effects of psychopharmacological agents will exacerbate dysmotility symptoms may be overrated.6,28 Instability in motility features, such as bowel habit subtype within IBS populations, is partly responsible for this observation.43
The bulk of additional information on psychopharmacological agents in functional gastrointestinal disorders is related to antidepressants. Consequently, the remainder of this review will focus on antidepressant mechanisms, antidepressant efficacy, and practical aspects of antidepressant use in functional gastrointestinal disorders.
POTENTIAL BENEFITS OF ANTIDEPRESSANTS IN FUNCTIONAL GASTROINTESTINAL DISORDERS
Antidepressants have a spectrum of actions that potentially could help patients with functional gastrointestinal disorders (table 3▶). Effects on gut motility, including transit, are probably of secondary importance in many patients, considering that most patients who are candidates for antidepressants already have failed conventional treatments.6,14,44 Although recognised for their analgesia in management of neuropathic pain, impressive effects of antidepressants on visceral hypersensitivity have not been demonstrated.45–47 Meta-analyses and systematic reviews identify both global improvement and pain reduction as potential benefits of antidepressants in IBS and other functional gastrointestinal disorders.48–51 However, a recent well designed trial of desipramine in women with painful functional bowel disorders found that treatment satisfaction realised by TCA treatment was not related significantly to a reduction in pain ratings.41 Similarly, longitudinal evaluation of paroxetine (an SSRI) in IBS demonstrated improved functional outcomes, yet no distinct advantage of the antidepressant in pain relief.37 These findings were corroborated in a subsequent double blind placebo controlled trial of paroxetine in IBS patients who failed to respond to fibre supplementation alone.38
Table 3.
Antidepressant actions that could influence symptom reporting in functional gastrointestinal disorders
| Central actions |
| Depression remission |
| Anxiolysis |
| Generalised effect on unexplained symptom reporting (anti-somatisation effect) |
| Sleep restoration |
| Analgesia |
| Modulation of visceral pain perception (inconsistent findings) |
| Peripheral actions |
| Anticholinergic effects |
| Altered gastrointestinal transit |
| Gastric fundic relaxation |
| Peripheral analgesic effect |
The fact that antidepressants are more consistent in improving global measures than specific gastrointestinal symptoms has raised some concerns.2 Do antidepressants provide purely a “band-aid” approach to management or are mechanisms of action more directly targeted at the underlying pathophysiology? A recent advance towards understanding factors responsible for global well being in various gastrointestinal disorders addresses this point. Preliminary data from patients with functional and non-functional gastrointestinal disorders attending a university based clinic revealed discrepancies in the contribution of principle gastrointestinal symptoms towards global well being.52 In patients with non-functional disorders, principle gastrointestinal symptoms were strong predictors of pretreatment global well being, and symptom changes with treatment correlated well with changes in global measures. In contrast, principle gastrointestinal symptoms in patients with functional gastrointestinal disorders were poor predictors of global well being at baseline, and changes in their ratings were only weak predictors of changes in well being following treatment with TCAs. Drossman et al also found that treatment induced changes in abdominal pain in patients with painful functional bowel disorders did not contribute significantly to treatment satisfaction.41 Most recently, Spiegel et al showed that non-gastrointestinal symptoms rather than traditionally elicited gastrointestinal symptoms were the strongest predictors of health related quality of life in patients with IBS.53 These authors discouraged a focus on physiological features such as stool characteristics and subtypes of IBS in favour of gauging global symptom severity in planning management.
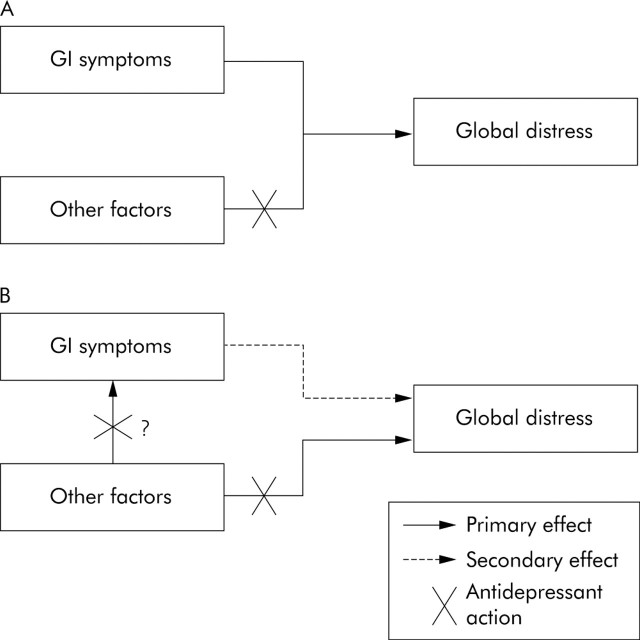
Consequently, antidepressants may address mechanisms more specifically related to global distress than to correction of gut physiological abnormalities or individual gastrointestinal complaints, mechanisms presumably of more importance in functional gastrointestinal disorders than has been appreciated (fig 1▶). The relevance of this lies in the fact that morbidity associated with functional gastrointestinal disorders is linked to social impairment, work absenteeism, and other functional limitations—morbidity often resulting from the degree of global distress. Although gut events (for example, infection, inflammation, noxious stimuli) presumably are responsible for the initiation and possibly perpetuation of the syndromes, they become overshadowed by antidepressant responsive features in many subjects.
Figure 1.
New paradigms for understanding global impairment in functional gastrointestinal disorders and the potential effects of antidepressants. Factors other than gastrointestinal (GI) symptoms alone (for example, somatisation, other underlying neurophysiological mechanisms) may (A) coexist with functional gastrointestinal disorders or (B) underlie the presentation of functional gastrointestinal symptoms and have important independent effects on global well being. Antidepressants could block the independent effects of other factors on global well being or influence both the manifestation of functional gastrointestinal symptoms (for example, pain) and global well being through somewhat separate mechanisms. (Dual actions may be more representative of tricyclic antidepressants than selective serotonin reuptake inhibitors.) The effect of antidepressants on global well being is not mediated through an action on gastrointestinal symptoms alone.
EFFICACY OF ANTIDEPRESSANTS IN FUNCTIONAL GASTROINTESTINAL DISORDERS
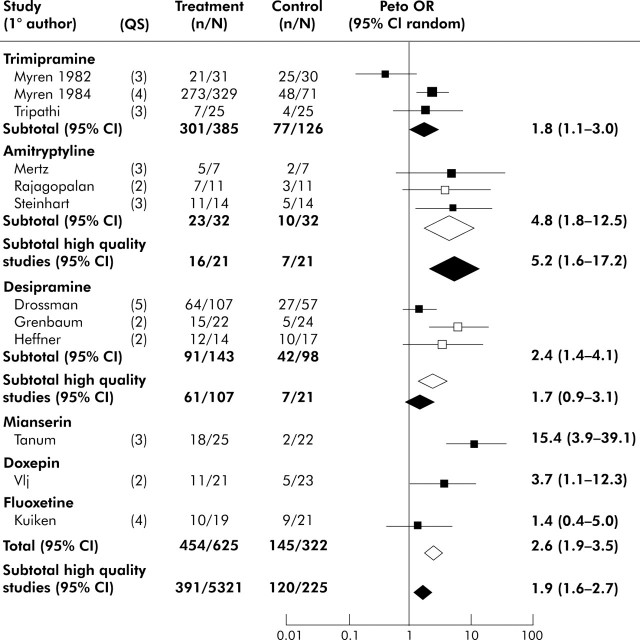
A small number of placebo controlled antidepressant trials has been reported.50,54 One limiting factor restricting the body of information has been the lack of pharmaceutical industry support for large scale or multicentre investigations. Meta-analyses of existing trials acknowledge the limited quality of many investigations. Three separate meta-analyses, either restricted to IBS or encompassing all functional gastrointestinal disorders, concluded that antidepressants demonstrate efficacy on at least one outcome measure, typically a global rating or pain.15,49,50 The most recent meta-analysis by Lesbros-Pantoflickova et al found a significant effect of antidepressants in IBS on overall improvement, with an odds ratio of 2.6 (confidence interval (CI) 1.9–3.5).50 When the analysis was restricted to higher quality studies, the odds ratio was 1.9 (CI 1.3–2.7) in favour of antidepressants (fig 2▶). Odds ratios are greater for antidepressants when other functional gastrointestinal disorders are included in the evaluation but many of these additional studies are of low quality.49 The number needed to treat (NNT) estimate for antidepressant efficacy in functional gastrointestinal disorders has been as low as 3.2; as many as 80% of IBS patients appear to have moderate or greater physician rated benefits in open label clinical practice, and adherence to antidepressants is higher than for other treatments.3,15,28
Figure 2.
Effects of antidepressants on global improvement in patients with irritable bowel syndrome (IBS). Odds ratio (OR) and associated 95% confidence interval (CI ) for each study are plotted on a logarithmic scale. Box sizes are proportional to the study’s weight in the analysis, based on study size and variance. Diamonds represents the point estimate and 95% CI for the pooled data. Open boxes represent low quality studies (quality score <3); closed boxes represent high quality studies (quality score >3). Open diamonds represent the point estimate of all studies (high and low quality); closed diamonds represent the point estimate of high quality studies only. Quality score (QS): double blind study (yes 1, no 0); sufficient number of subjects (yes 1, no 0); crossover (0) or parallel design (1); adequate definition of IBS symptoms (yes 1, no 0); presence (1) or absence (0) of intention to treat analysis. Modified from Lesbros-Pantoflickova and colleagues.50 Citations for the individual studies listed in the figure are provided in the original report.50
Recent treatment advances
▸ Success with antidepressants is best measured in terms of global well being, improved quality of life, and treatment satisfaction.
▸ Active depression symptoms interfere with outcome, represent either primary psychiatric comorbidity or neurophysiological processes underlying functional gastrointestinal disorders, and may need specific attention.
▸ Many antidepressant side effects reflect these same symptom promoting mechanisms and can be attenuated with low initial dosages and slow incrementation, especially in patients with prominent features of somatisation.
Although cumulative findings from mixed quality trials, meta-analyses, and clinical experience almost uniformly support the value of antidepressants, important recent advances come from examining a well constructed two centre trial of the TCA desipramine in women with painful functional bowel disorders.41 The large number of participants had criteria defined IBS or functional abdominal pain syndrome and were offered up to 150 mg desipramine per day in a placebo controlled study. In the intention to treat analysis, desipramine was not significantly superior to placebo, although there was a 13% margin in treatment satisfaction (p = 0.13). Inclusion of patients with functional abdominal pain syndrome, a rare and more refractory functional gastrointestinal disorder, may have attenuated the response to the TCA. Of note, 28% of subjects in the desipramine arm failed to complete the trial, most commonly because of side effects. An additional 12% of subjects had undetectable blood levels of desipramine, suggesting that they did not take the medication. When a post hoc analysis was performed in subjects who actually adhered to the study protocol, the agent was indeed effective with a 24% margin over placebo (p<0.01). This study demonstrates the limitations imposed by side effects and possibly the stigmata associated with the use of psychopharmacological agents for somatic symptoms. It underscores the importance of a pre-emptive plan towards side effect reduction and education regarding use of antidepressants for their ability to reverse global impairments associated with functional gastrointestinal disorders independent of traditional psychiatric effects (anxiolytic, antidepressant).42
Patient characteristics that indicate a high degree of somatisation
▸ History of multiple functional disorders and drug sensitivities.
▸ Positive response to at least seven symptoms on a 15 item screening tool for somatisation (find this tool at http://psy.psychiatryonline.org/cgi/content/full/39/3/263).*
▸ Features of somatisation disorder (complicated medical history beginning before the age of 30; history of pain related to at least four sites or functions; two gastrointestinal symptoms; one sexual symptom; and one symptom suggestive of a neurological condition, none of which are feigned or can be explained adequately by the medical evaluation).†
▸ Endorsement of many symptoms on a review of systems checklist (>15 on a comprehensive checklist exceeds the mean for functional gastrointestinal patients and has a high specificity for a functional diagnosis).‡
Kruenke K, Spitzer RL, deGruy FV III, et al. A symptom checklist to screen for somatoform disorders in primary care. Psychosomatics 1998;39:263–72.
Pseudoneurological symptoms include paralysis, loss of coordination, imbalance, localised weakness, etc. American Psychiatric Association.20
See Brown and colleagues.22
INITIATING ANTIDEPRESSANT THERAPY
Antidepressants are indicated for patients who meet criteria for a functional gastrointestinal disorder and who fail conventional low risk interventions, including reassurance and pharmacological and non-pharmacological therapies (for example, diet, fibre) directed at specific gut symptoms.6 Sufficient interference with global well being and functional capacity is also expected such that the risk/benefit ratio is acceptable. The clinician should feel confident in offering an antidepressant in this situation.
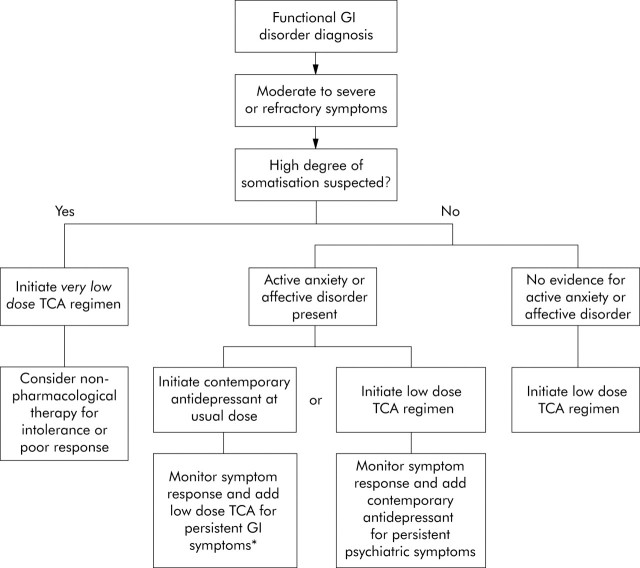
Although candidate selection is not predicated on the presence or absence of anxiety or depression, the initiation protocol can be affected by the degree of comorbidity and complexity of functional complaints expressed by the patient (that is, the degree of suspected somatisation) (fig 3▶). Patients with high degrees of somatisation by clinical evaluation tolerate medication side effects poorly, and the effectiveness of the intervention ultimately is impaired.17 Because there is some evidence that unexplained somatic complaints, even in patients with high degrees of somatisation, will respond to antidepressants, an initial trial is warranted.54 However, the trial should begin with a very low dose of the antidepressant (for example, 10 mg/day of a TCA) and dose escalation should be slow. A systematic review found that TCAs are more successful than SSRIs across the spectrum of unexplained somatic symptoms and syndromes, further supporting the initial trial with a TCA even in this situation.55 They also have an analgesic advantage that may have value.36,56,57 A moderately low daily dose of a TCA (25–50 mg per day) is the reasonable initiation step for patients in whom lower degrees of somatisation are evident. This approach is particularly warranted in subjects without any conspicuous symptoms of an active anxiety or depressive disorder.
Figure 3.
An algorithm for the appropriate initiation of antidepressants in selected patients with functional gastrointestinal disorders. High degrees of somatisation can be detected by patient endorsement of many symptoms on a review of systems checklist or from features of somatisation disorder in the medical history. Contemporary antidepressants include the selective serotonin reuptake inhibitors (SSRIs). *Monitoring for toxicity with tricyclic antidepressant (TCA) levels is required if the TCA is used in conjunction with medications that interfere with normal cytochrome p450 activity, such as SSRIs. Modified from Clouse.12
The choice of initial antidepressant in the subset of patients exhibiting significant anxiety and depression symptoms remains debated. Because these symptoms may represent further manifestations of the neurophysiology behind functional gastrointestinal symptoms, as mentioned above, an initial trial with a TCA at low daily dosage (for example, escalated to 50–100 mg/day) is not unreasonable, as long as monitoring of psychiatric symptoms is included in the management plan. Alternatively, an SSRI or other contemporary antidepressant may be initiated primarily and outcome on the functional gastrointestinal symptoms monitored (fig 3▶).
An important clinical error is failure to escalate antidepressant dosages when patients respond inadequately to the initial intervention. Consensus recommendations for increasing dosages are unavailable, but most suggest TCA increments by 10–25 mg/day at 5–7 day intervals. Patients who tolerate the medications yet see limited initial benefits should be instructed to continue incrementing the dosage well into the psychiatric dosing range (table 2▶) before declaring the trial a failure. In most instances, SSRIs and other contemporary antidepressants are prescribed according to recommended psychiatric starting dosages from the outset, but daily dosages of these agents should also be escalated appropriately in non-responders. Unsatisfactory response after four weeks at the full psychiatric or maximum tolerated dose indicates a change in medication or approach.
FACTORS AFFECTING OUTCOME
Patients resisting psychopharmacological therapy, those who fail to take the prescribed medications, and subjects with intolerable side effects obviously do poorly. Acceptance is enhanced by educating the patient of potential side effects of antidepressants and acknowledging that their primary benefits often are independent of anxiolytic or antidepressant effects of the drugs. Offering plausible mechanistic explanations to the patient in the presence of a relative or significant other may also enhance treatment adherence. This technique can avoid unfounded assumptions when dialogues continue beyond the office visit that the physician simply suspects anxiety or depression. Comparing antidepressants with other medications that have benefits extending beyond their original indications can be helpful (for example, to aspirin when used for heart disease versus fever or arthritis), although most patients today are familiar with off label prescribing. Despite the long list of potential adverse effects from antidepressants, a high level of disclosure is recommended. Trust in the prescribing physician will increase, and the patient will be in a better position to compare advantages and disadvantages of therapy at follow up visits.
Who has a good outcome from antidepressants? This is not predicted easily from clinical characteristics, and presently no biological marker is used for selecting optimal candidates. With IBS patients, pain or diarrhoea predominance may predict a better response to TCAs compared with a constipation predominant pattern.6,36,41 Discriminating characteristics in other functional gastrointestinal disorders have not been identified. One might predict that subjects with more diffuse functional symptom presentations (that is, those with higher degrees of somatisation) might be better candidates because a greater central contribution to global impairment is suspected. Contrast this with the patient with little comorbidity and presumably a simpler form of gastrointestinal disorder.17 However, patients with higher degrees of somatisation are more sensitive to medication side effects, diminishing the efficacy of the intervention. Consequently, the “good responder” typically can only be identified post hoc following the therapeutic trial.
Little information is available regarding the long term outcome in patients with functional gastrointestinal disorders who initially are managed successfully with antidepressants. In open label TCA treatment of patients with functional chest pain who were unresponsive to antireflux therapy, 81% had at least a moderate initial response to a TCA.58 Of these, more than two thirds had sustained improvement (defined as at least six months of treatment satisfaction). During maintenance therapy, 41% were successfully treated continuously or for symptom relapses over an average of 2.6 years; 29% discontinued successful treatment after >0.5 years with sustained benefits; and the remaining 29% eventually discontinued successful treatment for side effects or uncertain reasons. Other maintenance treatment data are lacking. Recommending at least six months of antidepressants for the successfully managed patient seems prudent, and very long treatment courses are common.2,6
Who responds poorly to antidepressants? Poorer outcomes are predicted for patients with objective delays in gastrointestinal motility or either specific gastrointestinal symptoms or comorbid medical conditions that could be exacerbated by antidepressant side effects, although therapeutic trials are not necessarily contraindicated.6,28,59 Of the antidepressants, TCAs are most likely to interact negatively. Patients with multiple medication sensitivities and other features of somatisation disorder also tolerate antidepressants poorly, even in very low dosages.17,20 Substituting non-pharmacological interventions (for example, psychotherapy, other psychological and behavioural therapies) for the psychopharmacological approach should be considered earlier in this group.
Active depression symptoms were shown in early TCA trials and confirmed in the more recent desipramine study of women with functional bowel disorders to reduce antidepressant efficacy.41,42 This irony can be explained away through several mechanisms. Firstly, the daily dosages used for treating functional gastrointestinal symptoms, particularly with TCAs, often fall below the usual recommendations for depression management. Escalating dosages into the psychiatric therapeutic range when depression symptoms are evident may re-establish the efficacy of antidepressants, although the benefits of this approach have not been documented. The manoeuvre seems reasonable, if only to reduce the diffuse effects comorbid depression has on medical illnesses in general.60 A second possibility is that depression symptom reporting heralds a more significant degree of central dysfunction common across functional disorders, a theme reiterated throughout this review, and thereby predicts greater resistance to antidepressants. To this point, North et al found that a recent history of depression (current or within the past one year) was one of the best clinical predictors of somatisation disorder in a study of IBS patients attending a university clinic.17 Thus the presence of depression symptoms requires more careful monitoring of treatment response, as suggested in the initiation algorithm of fig 3▶.
SIDE EFFECTS OF ANTIDEPRESSANT THERAPY FOR FUNCTIONAL GASTROINTESTINAL DISORDERS
Side effects of antidepressants are significant and common, emphasising the importance of sufficient morbidity from the functional gastrointestinal disorder to establish patient candidacy. The NNT for serious side effects from TCA treatment of neuropathic pain has been calculated at 22 and for minor side effects at 3–4.61 Rates of side effects may be even higher when TCAs are used for functional gastrointestinal disorders, despite low daily dosages.28,62,63 Clinicians using antidepressants should become very familiar with side effect profiles of the one or two TCA and non-TCA antidepressants that they choose to prescribe. Although some side effects are peculiar to specific antidepressants, typical side effects and their relative frequencies by antidepressant class are shown in table 4▶.
Table 4.
Relative occurrence of common side effects of selected antidepressants
| Drug class or drug | Anticholinergic‡ | Side effect* |
|||||
|---|---|---|---|---|---|---|---|
| Central nervous system† | Cardiovascular | Other | |||||
| Drowsiness | Insomnia/&;agitation | Orthostatic &;hypotension | Cardiac &;arrhythmia | Gastrointestinal &;distress | Weight gain &;(>6 kg) | ||
| TCAs | |||||||
| Tertiary amines | 3–4 | 3–4 | 0–1 | 4 | 3 | 0–1 | 3–4 |
| Secondary amines | 1–2 | 1–2 | 0–1 | 2 | 2 | 0–1 | 1 |
| SSRIs | 0–2 | 1 | 2 | 0 | 0 | 3 | 0 |
| Other newer antidepressants | |||||||
| Bupropion | 0 | 0 | 2 | 0 | 1 | 1 | 0 |
| Duloxetine | 2 | 1 | 1 | 0 | 0 | 3 | 0 |
| Nefazodone | 0 | 1 | 0 | 1 | 0 | 3 | 0 |
| Mirtazapine | 1 | 2 | 1 | 1 | 0 | 0 | 4 |
| Trazodone | 0 | 4 | 0 | 1 | 1 | 1 | 1 |
| Venlafaxine | 0 | 1 | 2 | 0 | 0 | 3 | 0 |
*Relative occurrence of side effects among agents listed: ranked from 0 (absent or rare) to 4 (relatively common).
†A reduction of seizure threshold can occur with all antidepressants and is most pronounced with bupropion.
‡Includes dry mouth, blurred vision, urinary hesitancy, constipation.
Modified from Lustman PJ, Clouse RE, Alrakawi A, et al. Treatment of major depression in adults with diabetes: a primary care perspective. Clin Diabetes 1997;15:122–6.
Early side effects most commonly interfering with successful TCA treatment include sedation, other CNS side effects (sleep disturbance, nervousness, agitation, nightmares), and anticholinergic side effects (xerostomia, tachycardia, palpitations, urinary dysfunction, visual disturbances). Weight gain and sexual dysfunction are common patient complaints when antidepressants are continued for maintenance therapy.64,65 A host of other potential adverse effects are possible, and medication interactions are significant considerations.66 TCAs are metabolised by the cytochrome P450 (2D6) system. Medications that interfere with their metabolism, such as SSRIs, can induce supra-therapeutic TCA blood levels even when low dose TCA regimens are used.67 Combinations antidepressant regimens also increase the risk of the uncommon serotonin syndrome.68
Recent observations that SSRI use can precipitate hostile behaviours and possibly suicide in adolescents and young adults are relevant.69 The phenomenon may have generalisation across antidepressant classes and can occur in adults. Details of recent US Food and Drug Administration releases on this topic can be found at http://www.fda.gov/cder/drug/antidepressants/default.htm. Increased awareness of withdrawal syndromes, including the potential for suicide, is also occurring.70,71 Gradual introduction and withdrawal of antidepressants is recommended with forewarning of these potential adverse affects, especially in young subjects. Although gradual introduction is already commonplace in antidepressant management of functional gastrointestinal disorders, many clinicians are unaware of the importance of tapered withdrawal over two to four weeks depending on the antidepressant and daily dose at the time the decision for discontinuation is made.66
Detailed assessment of side effect reporting when antidepressants are used for functional gastrointestinal disorders has fuelled further the interest in central processes potentially underlying these disorders. Many side effects eventually attributed to the antidepressant and leading to its discontinuation are present at baseline, before the antidepressant is initiated.72,73 This observation has been made previously in some patients with major depression74 but the dropout rate with antidepressant therapy in functional gastrointestinal disorders appears to exceed that seen in depression, even in the face of medical illness.41,63,75 In functional gastrointestinal disorders, higher degrees of somatisation predict worsening of existent symptoms or new side effects and subsequent antidepressant intolerance.73 Thus the same neurophysiological processes suspected as explaining the comorbidities associated with functional gastrointestinal disorders, underlying many of the physiological phenomena that characterise these disorders, and responsible for a large portion of the associated global distress, may also define the propensity for side effects from psychopharmacological interventions.
Awareness of this is helpful in management. Very gradual introduction of the antidepressant and use of agents with lowest side effect profiles is recommended for patients with multiple functional comorbidities, diffusely positive system review, complicated histories of symptoms poorly explained by the degree of objective findings, histories of multiple medication sensitivities, or other indicators of high degrees of somatisation. Within the TCA class, the secondary amines may be better tolerated (for example, nortriptyline, desipramine). Counselling the patient regarding the distinction between favourable effects and side effects, your intention to slowly increment the dose to limit side effects while beneficial effects take hold, and your sensitivity to the patient’s side effect predisposition may be helpful. Likewise, describing the gradual benefits that might occur over four weeks or more of treatment may improve adherence. If suspicions regarding mechanisms of side effects in many patients with functional gastrointestinal disorders are correct, then this is one unusual situation in which a medication has the therapeutic potential to negate its own initial side effects.74
NEW DIRECTIONS IN PSYCHOPHARMACOLOGICAL TREATMENT OF FUNCTIONAL GASTROINTESTINAL DISORDERS
Optimal use of psychopharmacological agents in functional gastrointestinal disorders undoubtedly has not been realised. Continued definition of the response mechanism is required and should enhance development or testing of new agents targeted more specifically to these disorders. Early attempts are being made to use functional brain imaging for this purpose.76 In the interim, better identification of response predictors should improve initial candidate selection and reduce dependence on therapeutic trials. Some interest in studying combination therapy with both TCAs and other antidepressants is surfacing, especially in the subset with active anxiety or depression symptoms.2,12 Preliminary data suggest that addition of a low dose TCA to a daily SSRI regimen in patients being managed with the latter for psychiatric symptoms may improve functional gastrointestinal symptoms, the TCA response being predicated by the degree of psychiatric symptom control.67 Overall, investigation of psychopharmacological agents for functional gastrointestinal disorders will likely increase, as they may take a more primary position in management algorithms.30 For the reasons outlined in this review, it is unlikely that concerns about limited direct effects of antidepressants on functional gastrointestinal symptoms will deter this effort.
Conflict of interest: None declared.
REFERENCES
- 1.Clouse RE. Esophageal spasm syndrome. Presented at the annual meeting of the American Gastroenterological Association, San Antonio, May 1990.
- 2.Talley NJ. Antidepressants in IBS: are we deluding ourselves? Am J Gastroenterol 2004;99:921–3. [DOI] [PubMed] [Google Scholar]
- 3.Whitehead WE, Levy RL, Von Korff M, et al. The usual medical care for irritable bowel syndrome. Aliment Pharmacol Ther 2004;20:1305–15. [DOI] [PubMed] [Google Scholar]
- 4.Ringel Y, Sperber AD, Drossman DA. Irritable bowel syndrome. Annu Rev Med 2001;52:319–38. [DOI] [PubMed] [Google Scholar]
- 5.Camilleri M, Heading RC, Thompson WG. Consensus report: clinical perspectives, mechanisms, diagnosis and management of irritable bowel syndrome. Aliment Pharmacol Ther 2002;16:1407–30. [DOI] [PubMed] [Google Scholar]
- 6.Mertz HR. Irritable bowel syndrome. N Engl J Med 2003;349:2136–46. [DOI] [PubMed] [Google Scholar]
- 7.Talley NJ. Evaluation of drug treatment in irritable bowel syndrome. Br J Clin Pharmacol 2003;56:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drossman DA, Camilleri M, Mayer EA, et al. AGA technical review on irritable bowel syndrome. Gastroenterology 2002;123:2108–31. [DOI] [PubMed] [Google Scholar]
- 9.Spiller RC. Treatment of irritable bowel syndrome. Curr Treat Options Gastroenterol 2003;6:329–37. [DOI] [PubMed] [Google Scholar]
- 10.Tack J, Fass R. Review article: Approaches to endoscopic-negative reflux disease: part of the GERD spectrum or a unique acid-related disorder? Aliment Pharmacol Ther 2004;19 (suppl 1) :28–34. [DOI] [PubMed] [Google Scholar]
- 11.Hollinghurst S, Kessler D, Peters TJ, et al. Opportunity cost of antidepressant prescribing in England: analysis of routine data. BMJ 2005;330:999–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clouse RE. Antidepressants for irritable bowel syndrome. Gut 2003;52:598–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker EA, Roy-Byrne PP, Katon WJ, et al. Psychiatric illness and irritable bowel syndrome: a comparison with inflammatory bowel disease. Am J Psychiatry 1990;147:1656–60. [DOI] [PubMed] [Google Scholar]
- 14.Clouse RE. Antidepressants for functional gastrointestinal syndromes. Dig Dis Sci 1994;39:2352–63. [DOI] [PubMed] [Google Scholar]
- 15.Jackson JL, O’Malley PG, Tomkins G, et al. Treatment of functional gastrointestinal disorders with antidepressant medication: a meta-analysis. Am J Med 2000;108:65–72. [DOI] [PubMed] [Google Scholar]
- 16.Whitehead WE, Palsson O, Jones KR. Systematic review of the comorbidity of irritable bowel syndrome with other disorders: what are the causes and implications? Gastroenterology 2002;122:1140–56. [DOI] [PubMed] [Google Scholar]
- 17.North CS, Downs D, Clouse RE, et al. The presentation of irritable bowel syndrome in the context of somatization disorder. Clin Gastroenterol Hepatol 2004;2:787–95. [DOI] [PubMed] [Google Scholar]
- 18.Miller AR, North CS, Clouse RE, et al. The association of irritable bowel syndrome and somatization disorder. Ann Clin Psychiatry 2001;13:25–30. [DOI] [PubMed] [Google Scholar]
- 19.Kroenke K, Spitzer RL, deGruy FV III, et al. Multisomatoform disorder: an alternative to undifferentiated somatoform disorder for the somatizing patient in primary care. Arch Gen Psychiatry 1997;54:352–8. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association Press, 1994.
- 21.Vandvik PO, Wilhelmsen I, Ihlebaek C, et al. Comorbidity of irritable bowel syndrome in general practice: a striking feature with clinical implications. Aliment Pharmacol Ther 2004;20:1195–203. [DOI] [PubMed] [Google Scholar]
- 22.Brown WH, Chey WD, Elta GH. Number of responses on a review of systems questionnaire predicts the diagnosis of functional gastrointestinal disorders. J Clin Gastroenterol 2003;36:222–7. [DOI] [PubMed] [Google Scholar]
- 23.Riley JL 3rd, Robinson ME, Kvaal SA, et al. ffects of physical and sexual abuse in facial pain: direct or mediated? Cranio 1998;16:259–66. [DOI] [PubMed] [Google Scholar]
- 24.Gwee KA, Leong YL, Graham C, et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut 1999;44:400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locke GR III, Zinsmeister AR, Fett SL, et al. Overlap of gastrointestinal symptom complexes in a US community. Neurogastroenterol Motil 2005;17:29–34. [DOI] [PubMed] [Google Scholar]
- 26.Aaron LA, Buchwald D. A review of the evidence for overlap among unexplained clinical conditions. Ann Intern Med 2001;134:868–81. [DOI] [PubMed] [Google Scholar]
- 27.Lenze EL, Miller A, Munir Z, et al. Psychiatric symptoms endorsed by somatization disorder patients in a psychiatric clinic. Ann Clin Psychiatry 1999;11:73–9. [DOI] [PubMed] [Google Scholar]
- 28.Clouse RE, Lustman PJ, Geisman RA, et al. Antidepressant therapy in 138 patients with irritable bowel syndrome: A five-year clinical experience. Aliment Pharmacol Ther 1994;8:409–16. [DOI] [PubMed] [Google Scholar]
- 29.Hudson JI, Mangweth B, Pope HG, et al. Family study of affective spectrum disorder. Arch Gen Psychiatry 2003;60:170–7. [DOI] [PubMed] [Google Scholar]
- 30.Castle MZD, Silk DBA, Libby GW. Review article: the rationale for antidepressant therapy in functional gastrointestinal disorders. Aliment Pharmacol Ther 2004;19:969–79. [DOI] [PubMed] [Google Scholar]
- 31.Gruber AJ, Hudson JI, Pope HG Jr. The management of treatment-resistant depression in disorders on the interface of psychiatry and medicine. Fibromyalgia, chronic fatigue syndrome, migraine, irritable bowel syndrome, atypical facial pain, and premenstrual dysphoric disorder. Psychiatr Clin North Am 1996;19:351–69. [DOI] [PubMed] [Google Scholar]
- 32.Ritchie JA, Truelove SC. Comparison of various treatments for irritable bowel syndrome. BMJ 1980;281:1317–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baume P, Buthbert J. The effect of medazepam in relieving symptoms of gastrointestinal distress. Aust NZJ Med 1973;3:457–60. [DOI] [PubMed] [Google Scholar]
- 34.Tack J. Functional dyspepsia: impaired fundic accommodation. Curr Treat Options Gastroenterol 2003;3:287–94. [DOI] [PubMed] [Google Scholar]
- 35.Shrivastava RK, Siegel H. The role of tricyclics and benzodiazepine compounds in the treatment of irritable gut syndrome and peptic ulcer disease. Psychopharm Bull 1984;20:616–21. [PubMed] [Google Scholar]
- 36.Clouse RE, Lustman PJ. Antidepressants for irritable bowel syndrome. In: Camilleri M, Spiller RC, eds. Irritable bowel syndrome: diagnosis and treatment. London: WB Saunders, 2002:161–71.
- 37.Creed F, Fernandes L, Guthrie E, et al. The cost-effectiveness of psychotherapy and paroxetine for severe irritable bowel syndrome. Gastroenterology 2003;124:303–17. [DOI] [PubMed] [Google Scholar]
- 38.Tabas G, Beaves M, Wang J, et al. Paroxetine to treat irritable bowel syndrome not responding to high-fiber diet: a double-blind placebo-controlled trial. Am J Gastroenterol 2004;99:914–20. [DOI] [PubMed] [Google Scholar]
- 39.Raskin J, Goldstein DJ, Mallinckrodt CH, et al. Duloxetine in the long-term treatment of major depressive disorder. J Clin Psychiatry 2003;64:1237–44. [DOI] [PubMed] [Google Scholar]
- 40.Briley M. Clinical experience with dual action antidepressants in different chronic pain syndromes. Hum Psychopharmacol 2004;19 (suppl 1) :S21–5. [DOI] [PubMed] [Google Scholar]
- 41.Drossman DA, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy vs. education and desipramine vs. placebo for moderate to severe functional bowel disorders. Gastroenterology 2003;125:19–31. [DOI] [PubMed] [Google Scholar]
- 42.Clouse RE. Managing functional bowel disorders from the top down: lessons from a well-designed treatment trial. Gastroenterology 2003;125:249–53. [DOI] [PubMed] [Google Scholar]
- 43.Drossman DA, Morris CB, Hu Y, et al. A prospective assessment of bowel habit in irritable bowel syndrome in women: defining an alternator. Gastroenterology 2005;128:580–9. [DOI] [PubMed] [Google Scholar]
- 44.Gorard DA, Libby GW, Farthing MJG. Influence of antidepressants on orocaecal and whole gut transit times in health and irritable bowel syndrome. Aliment Pharmacol Ther 1994;8:159–66. [DOI] [PubMed] [Google Scholar]
- 45.Fioramonti J, Bueno L. Centrally acting agents and visceral sensitivity. Gut 2002;51 (suppl I) :i91–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siproudhis L, Dinasquet M, Sebille V, et al. Differential effects of two types of antidepressants, amitriptyline and fluoxetine, on anorectal motility and visceral perception. Aliment Pharmacol Ther 2004;20:689–95. [DOI] [PubMed] [Google Scholar]
- 47.Kuiken SD, Tytgat GN, Boeckxstaens GE. The selective serotonin reuptake inhibitor fluoxetine does not change rectal sensitivity and symptoms in patients with irritable bowel syndrome: a double-blind, randomized, placebo-controlled study. Clin Gastroenterol Hepatol 2003;1:219–28. [DOI] [PubMed] [Google Scholar]
- 48.Jackson JL, O’Malley PG, Tomkins G, et al. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med 2000;108:65–72. [DOI] [PubMed] [Google Scholar]
- 49.Clouse RE, Prakash C, Anderson RJ, et al. Antidepressants for functional gastrointestinal symptoms and syndromes: a meta-analysis. Gastroenterology 2001;120 (suppl 1) :A642. [Google Scholar]
- 50.Lesbros-Pantoflickova D, Michetti P, Fried M, et al. Meta-analysis: the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 2004;20:1253–69. [DOI] [PubMed] [Google Scholar]
- 51.Jailwala J, Imperiale TF, Kroenke K. Pharmacologic treatment of the irritable bowel syndrome: a systematic review of randomized, controlled trials. Ann Intern Med 2000;133:136–47. [DOI] [PubMed] [Google Scholar]
- 52.Prakash C, Owens MM, Freedland KE, et al. Contributors to global symptom status in patients with functional GI disorders and the impact of tricyclic antidepressants. Gastroenterology 2000;118:2069. [Google Scholar]
- 53.Spiegel BMR, Gralnek IM, Bolus R, et al. Clinical determinants of health-related quality of life in patients with irritable bowel syndrome. Arch Intern Med 2004;164:1773–80. [DOI] [PubMed] [Google Scholar]
- 54.O’Malley PG, Jackson JL, Santoro J, et al. Antidepressant therapy for unexplained symptoms and syndromes. J Fam Pract 1999;48:980–90. [PubMed] [Google Scholar]
- 55.O’Malley PG, Jackson JL, Tomkins GE, et al. Efficacy of antidepressants for physical symptoms: a critical review. J Gen Intern Med 1999;14 (suppl 2) :60.9893093 [Google Scholar]
- 56.Bomholt SF, Mikkelsen JD, Blackburn-Munro G. Antinociceptive effects of the antidepressants amitriptyline, duloxetine, mirtazapine and citalopram in animal models of acute, persistent and neuropathic pain. Neuropharmacology 2005;48:252–63. [DOI] [PubMed] [Google Scholar]
- 57.McQuay HJ, Moore RA. Antidepressants and chronic pain: effective analgesia in neuropathic pain and other syndromes. BMJ 1997;314:763–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prakash C, Clouse RE. Long-term outcome from tricyclic antidepressant treatment of functional chest pain. Dig Dis Sci 1999;44:2373–9. [DOI] [PubMed] [Google Scholar]
- 59.Sawhney M, Prakash C, Lustman PJ, et al. Tricyclic antidepressants for persistent or recurrent vomiting in diabetic patients. Gastroenterology 2001;120:A243. [Google Scholar]
- 60.Freedland KE, Carney RM, Lustman PJ. The impact of psychiatric disorders on medical illness. In: Rubin EH, Zorumski CF, eds. Adult psychiatry, 2nd edn. Oxford: Blackwell Publishing, 2005; (in press).
- 61.McQuay HJ, Tramer M, Nye BA, et al. A systematic review of antidepressants in neuropathic pain. Pain 1996;68:217–27. [DOI] [PubMed] [Google Scholar]
- 62.Lembo A. Irritable bowel syndrome medications side effects survey. J Clin Gastroenterol 2004;38:776–81. [DOI] [PubMed] [Google Scholar]
- 63.Prakash C, Lustman PJ, Freedland KE, et al. Tricyclic antidepressants for functional nausea and vomiting: clinical outcome in 37 patients. Dig Dis Sci 1998;43:1951–6. [DOI] [PubMed] [Google Scholar]
- 64.Aronne LJ, Segal KR. Weight gain in the treatment of mood disorders. J Clin Psychiatry 2003;64 (suppl 8) :22–9. [PubMed] [Google Scholar]
- 65.Montgomery SA, Baldwin DS, Riley A. Antidepressant medications: a review of the evidence for drug-induced sexual dysfunction. J Affect Disord 2002;69:119–40. [DOI] [PubMed] [Google Scholar]
- 66.Kaplan HI, Sadock BJ. Sadock VA, eds. In: Kaplan and Sadock’s pocket handbook of psychiatric drug treatment, 3rd edn. Philadelphia: Lippincott, Williams and Wilkins 2001:1–28.
- 67.Nair D, Prakash C, Lustman PJ, et al. Added value of tricyclic antidepressants for functional gastrointestinal symptoms in patients on selective serotonin re-uptake inhibitors (SSRIs). Am J Gastroenterol 2001;96 (suppl) :S316. [Google Scholar]
- 68.Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med 2005;352:1112–20. [DOI] [PubMed] [Google Scholar]
- 69.Brent DA. Antidepressants and pediatric depression—the risk of doing nothing. N Engl J Med 2004;351:1598–601. [DOI] [PubMed] [Google Scholar]
- 70.Haddad PM. Antidepressant discontinuation syndromes. Drug Saf 2001;24:183–97. [DOI] [PubMed] [Google Scholar]
- 71.Yerevanian BI, Koek RJ, Feusner JD, et al. Antidepressants and suicidal behaviour in unipolar depression. Acta Psychiatr Scand 2004;110:452–8. [DOI] [PubMed] [Google Scholar]
- 72.Dalton C, Diamant NE, Morris CB, et al. Are side effects of tricyclic antidepressants (TCAs) really side effects? Gastroenterology 2004;126 (suppl 2) :A28. [Google Scholar]
- 73.Thiwan SM, Dalton C, Morris CB, et al. Factors predicting symptom reports of “side effects” when using tricyclic antidepressants. Gastroenterology 2005;128 (suppl 2) :A66. [Google Scholar]
- 74.Rollman BL, Block MR, Schulberg HC. Symptoms of major depression and tricyclic side effects in primary care patients. J Gen Intern Med 1997;12:284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gill D, Hatcher S. Antidepressants for depression in people with physical illness. Cochrane Database Syst Rev 2004; (4) :CD001312. [DOI] [PubMed]
- 76.Mertz H, Pickens D, Morgan V. Amitriptyline reduces activation of the anterior cingulated cortex in irritable bowel syndrome patients during rectal pain. Gastroenterology 2003;124:A47. [DOI] [PMC free article] [PubMed] [Google Scholar]